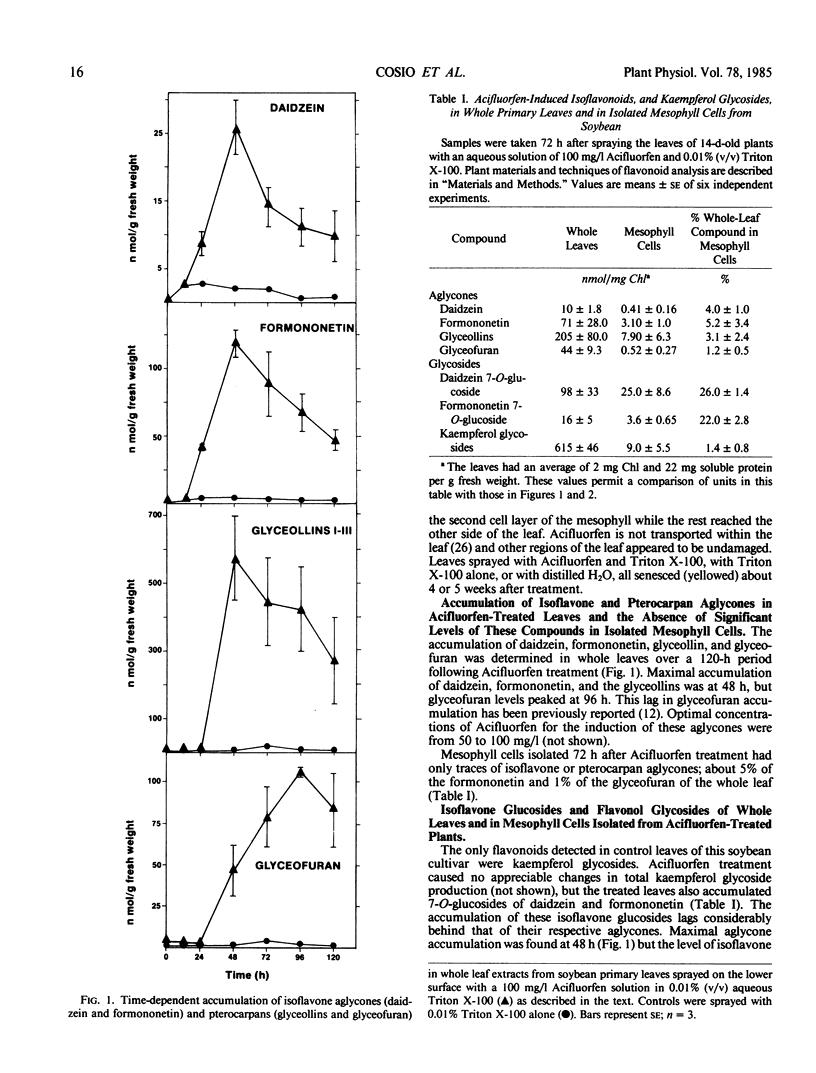
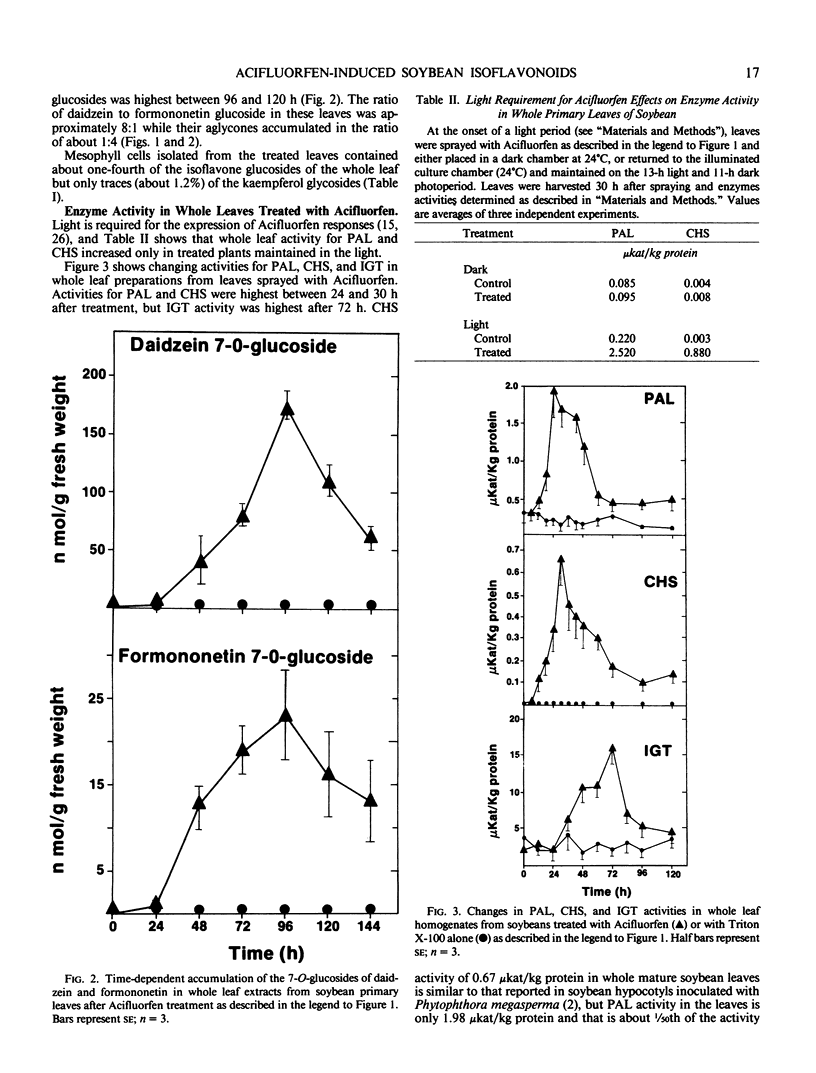
Abstract
Mature soybean (Glycine max L. cv Harosoy 63) leaves normally contain kaempferol-3-glycosides but they accumulate no other flavonoids. Whole leaves sprayed with the diphenyl ether herbicide Acifluorfen and maintained in the light developed small necrotic lesions and accumulated isoflavone aglycones, isoflavone glucosides, and pterocarpans. Isoflavonoid accumulation was preceded by induced activity for chalcone synthase (CHS) and by increased activity for phenylalanine ammonia-lyase (PAL) and UDP-glucose:isoflavone 7-O-glucosyl transferase (IGT). PAL and CHS activity was highest between 24 and 30 hours after treatment, isoflavone aglycones and pterocarpans at 48 hours, IGT at 72 hours, and isoflavone glucosides at 96 hours.
Mesophyll cells isolated from control leaves contained no activity for PAL, CHS, or IGT and no flavonoids of any class. Cells isolated from treated leaves at the stage of maximal enzyme activity or isoflavonoid content contained PAL (12% of the whole leaf activity), CHS (24%), IGT (20%), and 25% of the whole leaf isoflavone glucosides, but only traces, presumably as contaminants, of the other flavonoids. We suggest that the isoflavone glucosides were synthesized and accumulated in intact mesophyll cells as soluble detoxification products, while the isoflavone aglycones and pterocarpans accumulated in the epidermis or extracellularly within the mesophyll. To our knowledge this is the first report of tissue-specific induction of isoflavonoid glucosides and key enzymes of their biosynthesis in any plant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Börner H., Grisebach H. Enzyme induction in soybean infected by Phytophthora megasperma f.sp. glycinea. Arch Biochem Biophys. 1982 Aug;217(1):65–71. doi: 10.1016/0003-9861(82)90479-9. [DOI] [PubMed] [Google Scholar]
- Cosio E. G., McClure J. W. Kaempferol glycosides and enzymes of flavonol biosynthesis in leaves of a soybean strain with low photosynthetic rates. Plant Physiol. 1984 Apr;74(4):877–881. doi: 10.1104/pp.74.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Marx G. A., Hoch H. C. Distribution of Secondary Plant Metabolites and Their Biosynthetic Enzymes in Pea (Pisum sativum L.) Leaves : Anthocyanins and Flavonol Glycosides. Plant Physiol. 1982 Sep;70(3):745–748. doi: 10.1104/pp.70.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster J., Barz W. UDP-glucose:isoflavone 7-O-glucosyltransferase from roots of chick pea (Cicer arietinum L.). Arch Biochem Biophys. 1981 Nov;212(1):98–104. doi: 10.1016/0003-9861(81)90347-7. [DOI] [PubMed] [Google Scholar]
- Moesta P., Grisebach H. Investigation of the mechanism of glyceollin accumulation in soybean infected by Phytophthora megasperma f. sp. glycinea. Arch Biochem Biophys. 1981 Dec;212(2):462–467. doi: 10.1016/0003-9861(81)90388-x. [DOI] [PubMed] [Google Scholar]
- Naim M., Gestetner B., Zilkah S., Birk Y., Bondi A. Soybean isoflavones. Characterization, determination, and antifungal activity. J Agric Food Chem. 1974 Sep-Oct;22(5):806–810. doi: 10.1021/jf60195a031. [DOI] [PubMed] [Google Scholar]
- Stöckigt J., Zenk M. H. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch C. 1975 May-Jun;30(3):352–358. doi: 10.1515/znc-1975-5-609. [DOI] [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- Zähringer U., Ebel J., Mulheirn L. J., Lyne R. L., Grisebach H. Induction of phytoalexin synthesis in soybean. Dimethylallylpyrophosphate:trihydroxypterocarpan dimethylallyl transferase from elicitor-induced cotyledons. FEBS Lett. 1979 May 1;101(1):90–92. doi: 10.1016/0014-5793(79)81301-0. [DOI] [PubMed] [Google Scholar]


