Abstract
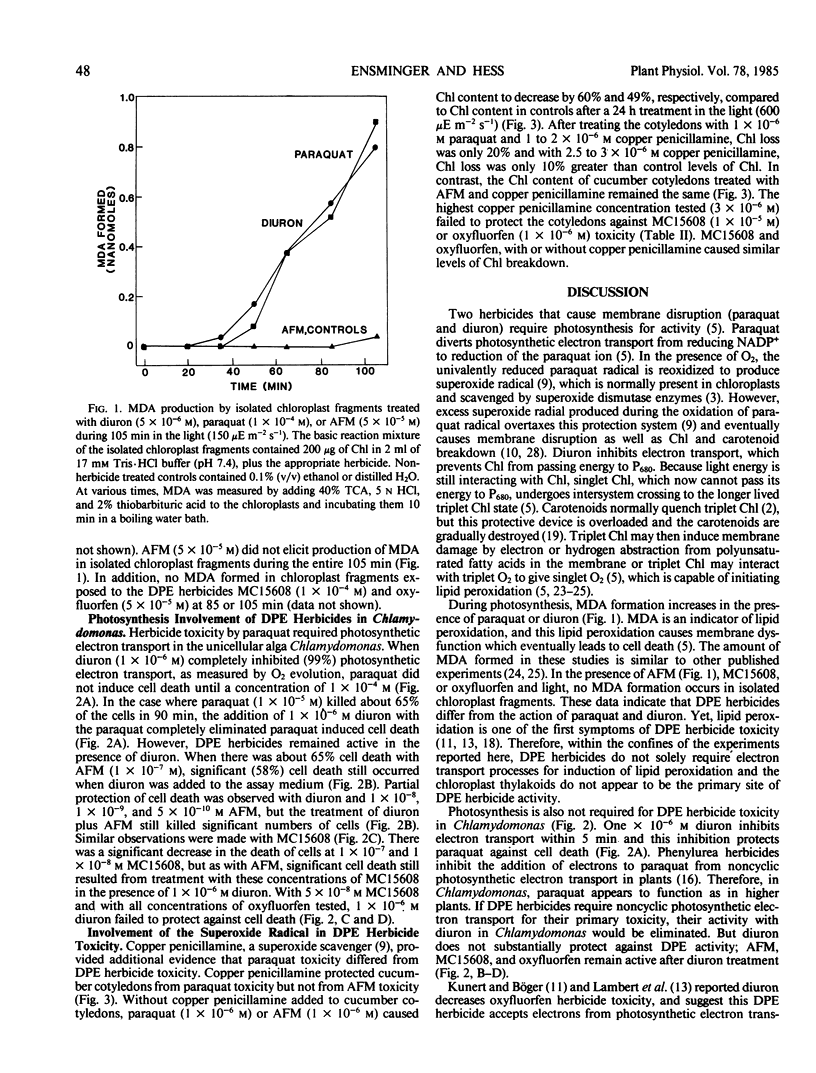
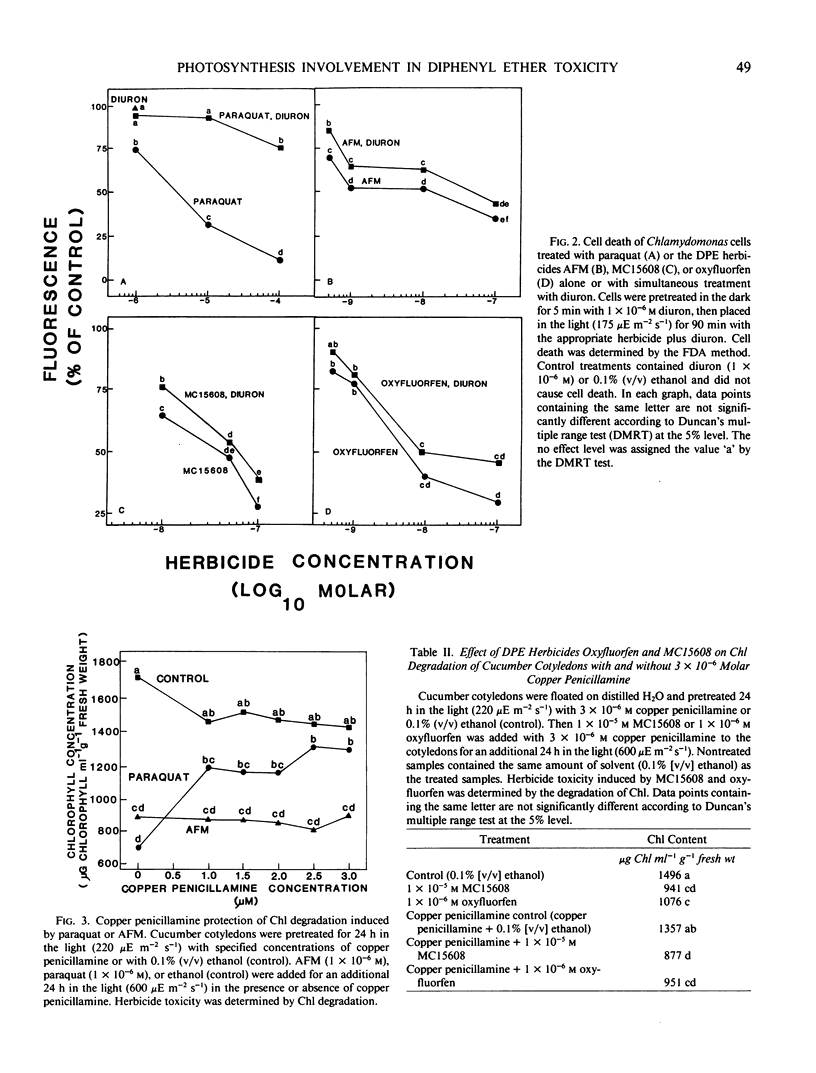
Photosynthesis is not required for the toxicity of diphenyl ether herbicides, nor are chloroplast thylakoids the primary site of diphenyl ether herbicide activity. Isolated spinach (Spinacia oleracea L.) chloroplast fragments produced malonyl dialdehyde, indicating lipid peroxidation, when paraquat (1,1′-dimethyl-4,4′-bipyridinium ion) or diuron [3-(3,4-dichlorophenyl)-1,1-dimethylurea] were added to the medium, but no malonyl dialdehyde was produced when chloroplast fragments were treated with the methyl ester of acifluorfen (methyl 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid), oxyfluorfen [2-chloro-1-(3-ethoxy-4-nitrophenoxy)-4-(trifluoromethyl)benzene], or MC15608 (methyl 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-chlorobenzoate). In most cases the toxicity of acifluorfen-methyl, oxyfluorfen, or MC15608 to the unicellular green alga Chlamydomonas eugametos (Moewus) did not decrease after simultaneous treatment with diuron. However, diuron significantly reduced cell death after paraquat treatment at all but the highest paraquat concentration tested (0.1 millimolar). These data indicate electron transport of photosynthesis is not serving the same function for diphenyl ether herbicides as for paraquat. Additional evidence for differential action of paraquat was obtained from the superoxide scavenger copper penicillamine (copper complex of 2-amino-3-mercapto-3-methylbutanoic acid). Copper penicillamine eliminated paraquat toxicity in cucumber (Cucumis sativus L.) cotyledons but did not reduce diphenyl ether herbicide toxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Robertson D. S. Role of Carotenoids in Protecting Chlorophyll From Photodestruction. Plant Physiol. 1960 Jul;35(4):531–534. doi: 10.1104/pp.35.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Urano M., Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem. 1973 Jul 2;36(1):257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Birker P. J., Freeman H. C. Structure, properties, and function of a copper(I)-copper(II) complex of D-penicillamine: pentathallium(I) mu8-chloro-dodeca (D-penicillaminato)-octacuprate(I)hexacuprate(II) n-hydrate. J Am Chem Soc. 1977 Oct 12;99(21):6890–6899. doi: 10.1021/ja00463a019. [DOI] [PubMed] [Google Scholar]
- Dodge A. D. Oxygen radicals and herbicide action. Biochem Soc Trans. 1982 Apr;10(2):73–75. doi: 10.1042/bst0100073. [DOI] [PubMed] [Google Scholar]
- Farrington J. A., Ebert M., Land E. J., Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973 Sep 26;314(3):372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- Orr G. L., Hess F. D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons : LIGHT ACTIVATION AND THE SUBSEQUENT FORMATION OF LIPOPHILIC FREE RADICALS. Plant Physiol. 1982 Feb;69(2):502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placer Z. A., Cushman L. L., Johnson B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., O'Neal F. O., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation. Photochem Photobiol. 1978 Oct-Nov;28(4-5):803–809. doi: 10.1111/j.1751-1097.1978.tb07022.x. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Youngman R. J., Dodge A. D., Lengfelder E., Elstner E. F. Inhibition of paraquat phytotoxicity by a novel copper chelate with superoxide dismutating activity. Experientia. 1979 Oct 15;35(10):1295–1296. doi: 10.1007/BF01963967. [DOI] [PubMed] [Google Scholar]



