Abstract
Specimens of an egg parasitoid wasp, Telenomuscristatus Johnson (Platygastroidea, Scelionidae), were reared from stink bug egg masses collected in the wild, in Maryland, United States. The egg masses were identified morphologically as Halyomorphahalys (Stål), Banasa Stål and Euschistus Dallas (Hemiptera, Pentatomidae). Molecular tools were used to further identify the Euschistus egg masses as E.servus (Say) and E.tristigmus (Say). All of these are new host associations for Te.cristatus. We also provide data to contribute to future identification of Te.cristatus: images of the holotype specimen and COI sequences from two disparate localities.
Keywords: egg parasitoid, BMSB, stink bug, new host associations
New information
First record of Te.cristatus in Maryland and first records of it parasitizing eggs of H.halys, E.servus, E.tristigmus and Banasa sp. Images of the holotype specimen and DNA barcode data are provided.
Introduction
Halyomorphahalys (Stål) (Hemiptera, Pentatomidae), also known as the brown marmorated stink bug (BMSB), is an invasive insect native to China, Japan, South Korea and Taiwan. Halyomorphahalys was first detected in the United States in 1996 in Allentown, Pennsylvania (Hoebeke and Carter 2003), speculated to have been brought over through bulk container shipping from Beijing, China. Halyomorphahalys quickly spread and is currently found in 47 U.S. states and four Canadian provinces in North America (Northeastern IPM Center 2021). This pest bug feeds on a wide range of economically important crops and woody trees with over 100 known host plants (Rice et al. 2014) and is also a nuisance for homeowners, aggregating on the outside of buildings in late autumn and overwintering inside buildings (Inkley 2012). Halyomorphahalys was first detected in Maryland in Washington County in 2003 (Sargent et al. 2011). In Maryland, it was initially a nuisance pest inside homes and buildings, but, as the populations grew, serious injury was reported in fruit and vegetable crops. Halyomorphahalys has also been reported damaging ornamental trees and shrubs, greenhouse plants and cut flowers in Maryland (Gill et al. 2010). Chemical control is currently the most widely used method for managing H.halys, but the broad-spectrum insecticides generally used can cause secondary pest outbreaks and compromise existing integrated pest management programs (Hull et al. 2011, Leskey et al. 2012, Rice et al. 2014).
A more sustainable approach is the use of biological control agents. Since the detection of H.halys in the United States, numerous studies have identified indigenous natural enemies associated with this species, the most prominent being egg parasitoids. Currently, there are 19 species of hymenopteran endoparasitoids in the genera Anastatus Motchulsky (Eupelmidae), Trissolcus Ashmead, Telenomus Haliday and Hadronotus Förster (reported as Gryonobesum Masner) (Scelionidae) and Ooencyrtus Ashmead (Encyrtidae) reported to parasitize eggs of H.halys in the United States (Rice et al. 2014, Abram et al. 2014, Balusu et al. 2019). To expand knowledge about the life history, geographic distribution and host associations of these parasitoids, insect egg surveys were conducted in 2020 and 2021 throughout Maryland, focused on rearing parasitoids from wild stink bug egg masses. In addition, we documented the host plant associations of the stink bug egg masses. Here, we report on new host associations of Te.cristatus Johnson and provide data to aid future identifications.
Material and methods
Insect egg surveys: Surveys to collect naturally-laid insect eggs were conducted throughout Maryland, United States, in 2020 and 2021. In 2020, surveyors collected eggs ad hoc from commercial tree nurseries and urban woody landscapes (June through September). In 2021, fifty community scientist volunteers from the University of Maryland Extension Master Gardener Program were recruited from five Maryland counties (Allegany, Frederick, Garrett, Montgomery and Washington) and trained to help survey for eggs. Community scientists searched for and collected eggs from various habitat types (agricultural, urban herbaceous, urban vegetable garden, urban woody and woods/wooded edge) from March through September. Eggs were placed in labelled Petri dishes, which were transported in a cooler to the Shrewsbury laboratory (University of Maryland) for further processing.
Parasitoid rearing: Petri dishes with collected eggs were sealed with parafilm and placed into a growth chamber maintained at 23.3–25.4°C, 58–87% relative humidity (RH) and a 16L:8D photoperiod. The eggs were checked every one to six days for any emergence of stink bug nymphs or parasitoid adults from June through October 2020 and March through September 2021. Emerged parasitoids were counted and placed in labelled vials of 70% ethanol for later identification.
Morphological identification: All parasitoids that emerged from the eggs of Pentatomidae (stink bug) were identified to genus or species. Telenomuspodisi Ashmead and Te.cristatus were identified using the key in Johnson (1984). Pentatomidae egg masses were identified using Herbert et al. (2015), a guide by Dieckhoff (2014) and voucher specimens provided by R. A. Waterworth (USDA EPA, Washington, D.C.). Voucher specimens of Te.cristatus are deposited in the Florida State Collection of Arthropods, Gainesville, Florida (Table 1).
Table 1.
Data associated with specimens used for COI barcoding.
| Collecting unit identifier | Species | Host | GenBank accession | BOLD BIN |
| Stink bug | ||||
| FSCA 00094026 | Euschistusservus | Quercusalba | OQ605865 | BOLD:AAE0845 |
| FSCA 00094027 | Euschistustristigmus | Celtisoccidentalis | OQ605866 | BOLD:AAG8876 |
| FSCA 00094028 | Euschistustristigmus | Cerciscanadensis | OQ605867 | BOLD:AAG8876 |
| Parasitoid | ||||
| FSCA 00060141 | Telenomuscristatus | Halyomorphahalys | OP801505 | |
| FSCA 00060144 | Telenomuscristatus | OP801506 |
Photography: Images were produced with a Macropod microphography system using 10x and 20x Mitutoyo objective lenses and were rendered in Helicon focus. Images of the holotype specimen are deposited in Zenodo (https://zenodo.org/record/7709039#.ZAi_rnbMJaR). Images of molecular voucher specimens are deposited in BOLD (Barcode of Life Database), in association with their sequence and collection data.
COI barcoding: Genomic DNA was non-destructively isolated from entire specimens (stink bug egg masses and Te.cristatus) using a Qiagen DNeasy Blood and Tissue kit (Hilden, Germany). The barcode region of the mitochondrial Cytochrome c Oxidase Subunit I (CO1) was amplified using the universal barcoding primer sets LCO1490/HCO2198 (Folmer et al. 1994). PCRs used the following thermocycle: 1) initial denaturation at 95°C for 2 minutes, 2) 98°C for 30 seconds, 3) 50°C for 30 seconds, 4) 72°C for 40 seconds [32x steps 2–4] and a final extension at 72°C for 7 minutes. Egg masses from Euschistustristigmus required use of the primer set PENT_F2/HCO2198 (Gariepy et al. 2014). Samples were then sequenced bidirectionally on the ABI SeqStudio platform with BigDye v.3.1 chemistry. Sequences were trimmed and assembled into contigs using Geneious Prime 2023.03.
Results
Telenomuscristatus
The key to species of the Te.podisi group in Johnson (1984) made it a straightforward task to identify the specimens that emerged from egg masses in Maryland (Figs 1, 2). These specimens are from some of the northernmost localities for Te.cristatus, making it worthwhile to corroborate the identification via direct comparison with the holotype specimen (Fig. 3) and comparing its COI sequences to a specimen collected in Tampa (Hillsborough County), Florida, which is relatively close to the type locality (Duval County, Florida). The high sequence identity, 99.85%, provides additional evidence that these specimens are conspecific. By providing images of the specimens used for COI sequencing (Table 1) and the holotype specimen, we expanded the available data that can be used for future identifications of Te.cristatus.
Figure 1.
Telenomuscristatus (FSCA 00060141), reared from egg of Halyomorphahalys.
Te.cristatus (FSCA 00060141), reared from egg of H.halys in Maryland.
Figure 2a.
Figure 2b.
Figure 2c.
Figure 2d.
Te.cristatus, holotype female (FSCA 00060143).
Figure 3a.
Figure 3b.
Figure 3c.
Figure 3d.
Hosts
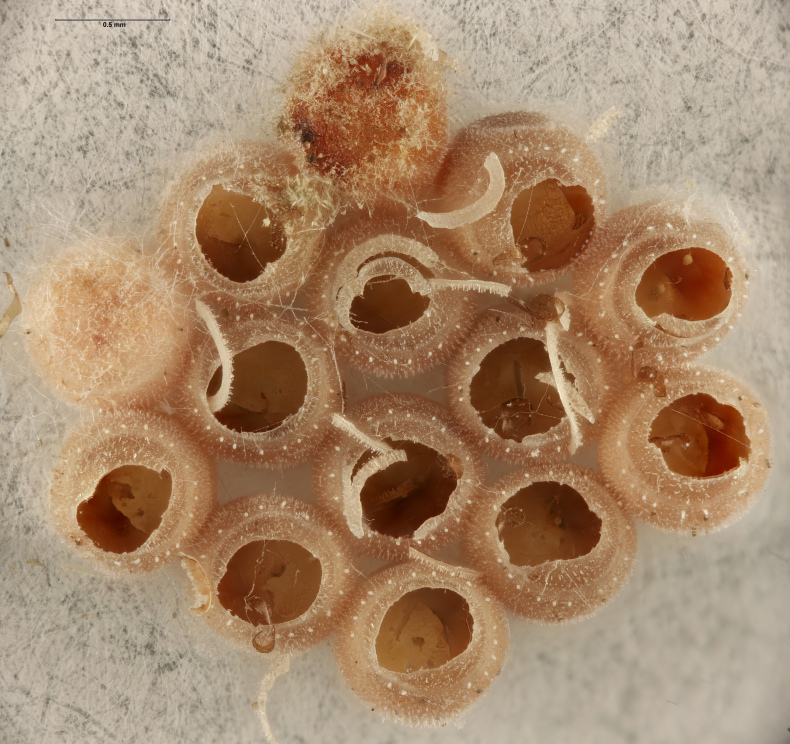
In previous studies, Te.cristatus was reported to parasitize the eggs of Chinaviahilaris (Say), Podisusmaculiventris (Say) (Orr et al. 1986) and Nezaraviridula (Linnaeus) (Johnson 1984). We here add four new host associations, from naturally-laid eggs, for Te.cristatus. These were identified morphologically as H.halys (Stål), Banasa Stål, and Euschistus Dallas (Hemiptera, Pentatomidae) (Fig. 4). We further identified the Euschistus egg masses as E.servus (Say) and E.tristigmus (Say) (Table 1) by comparing COI from these egg masses with sequences in BOLD. The number of males and females of Te.cristatus that emerged from each egg mass are provided in Table 2.
Stink bug egg masses parasitized by Te.cristatus. Exit holes indicate eggs from which Te.cristatus emerged.
Figure 4a.
Egg mass of Halyomorphahalys (EM8MM);
Figure 4b.
Egg mass of Banasa sp. (FSCA 00094204);
Figure 4c.
Egg mass of E.servus (FSCA 00094026);
Figure 4d.
Egg mass of E.tristigmus (FSCA 00094028).
Table 2.
Emergence data for Te.cristatus from naturally-laid stink bug egg masses.
| Collecting unit identifier (egg mass) | Genus/Species |
Total Number
of Eggs |
Te.cristatus
males |
Te.cristatus females | unsexed |
| FSCA 00094024 | Banasa sp. | 14 | 1 | 11 | 0 |
| FSCA 00094025 | Euschistus sp. | 20 | 2 | 3 | 0 |
| FSCA 00094026 | E.servus | 28 | 0 | 1 | 0 |
| FSCA 00094027 | E.tristigmus | 14 | 1 | 1 | 1 |
| FSCA 00094028 | E.tristigmus | 15 | 1 | 11 | 0 |
| EM8MM | H.halys | 28 | 0 | 1 | 0 |
Stink bug egg masses
We amplified and sequenced COI from three of the four egg masses that were morphologically identified as Euschistus. In BOLD, two of these matched E.tristigmus. Images of voucher specimens in BOLD depicted the distinctive shape of the humeral spines that characterize this species (Joe Eger, personal communication). The third specimen matched a BIN that contained sequences identified as both E.servus and E.variolarius. Many of the images associated with this BIN show the mandibular plates extending past the tylus, which is is common in northern specimens of E.servus and not E.variolarius (Joe Eger, personal communication). We therefore treat this BIN as E.servus. Details are provided in Table 1.
Distribution
In the United States, Te.cristatus has been reported from Florida and Lousiana (Johnson 1984, Orr et al. 1986,Orr and Boethel 1990). In addition to the specimens reared in Maryland, we identified specimens of Te.cristatus from yellow sticky card surveys from Kentucky, North Carolina, West Virginia, Virginia and New Jersey (unpublished records). To the south, the range of this species extends at least to Mexico (Tamaulipas) and Trinidad (Johnson 1984, Orr et al. 1986, Ramirez-Ahuja et al. 2019).
Diagnosis
Among Nearctic species of the podisi species group, Te.cristatus can be identified by the following combination of characters: hyperoccipital carina present; occiput coriaceous near hyperoccipital carina, otherwise smooth; frontal depression well developed; frons slightly bulging between antennal insertions and inner orbits; ocellar setae absent; lack of longitudinal elements in the mesoscutal sculpture; mesoscutellum with submarginal foveae smaller than metascutellar (dorsellar) punctures; greatest length of basal costae on T2 less than medial length of T1 (Figs 1, 2, 3), (Johnson 1984).
Discussion
Numerous surveys in the United States have been conducted to assess natural enemies of H.halys, employing sentinel egg masses, collecting wild egg masses or a combination of both (Abram et al. 2017). Our study shows that new associations remain to be discovered and that community engagement can be a useful tool for advancing biological knowledge. By the keen eyes of Master Gardeners, we were able to collect a larger number of samples, which are needed to more thoroughly characterize parasitoid-host associations. In conjunction with the technical aspects of taxonomy and molecular diagnostics, this enabled us to advance our knowledge about the biology and distribution of Te.cristatus.
To date, the most dominant species of Telenomus associated with H.halys has been Te.podisi, which is mainly associated with field/vegetable crops and orchard habitats (Abram et al. 2017). We recovered Te.cristatus from a tree production nursery and the difference in the plant composition may be indicative of a habitat preference. Given that H.halys feeds in a variety of habitats, it is essential to select sampling sites for potential natural enemies that are equally diverse. Specimens of Te.cristatus in Virginia, West Virgina and North Carolina were recorded from yellow sticky cards used to survey for egg parasitoids of H.halys. Although these yellow sticky card surveys do not provide host information, these records have greater significance now that Te.cristatus is known to parasitize H.halys eggs. Further testing is needed to determine if these records are simply incidental and if Te.cristatus can parasitize H.halys eggs at a rate that would contribute to biological control.
We also note that the use of molecular diagnostics to identify organisms is only as accurate as the assocation between the taxon name and DNA sequence(s) used as a reference. In this study, we relied on publicly available COI sequences for species-level identification of Euschistus egg masses. The digital morphology framework of BOLD enabled us to enlist the help of a specialist who could interpret images associated with Euschistus sequences that had ambiguous identifications. In turn, we have striven to provide reliable identifications for Te.cristatus and associated sequences with high resolution images of vouchers.
Acknowledgements
This work was supported in part by the USDA National Institute of Food and Agriculture (NIFA), Specialty Crop Research Initiative, award # 2016-51181-25409; USDA NIFA McIntire-Stennis, Project # MD-ENTM-22001; USDA NIFA EIP, award # 2021-70006-35473; USDA ARS Areawide IPM Program; and the 2021 Maryland Native Plant Society Research Grant. Elijah Talamas and Matthew Moore were supported by the Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI). We thank Joe Eger for taxonomic input on stink bug identification, and the University of Maryland Extension Master Gardeners and coordinators. Cheryl Roberts and Lynn Combee (FDACS-DPI), Nancy Harding, Adelaide Figurskey and Cassie Herman (UMD) provided laboratory and field support.
Conflicts of interest
No conflict of interest to declare
Disclaimer: This article is (co-)authored by any of the Editors-in-Chief, Managing Editors or their deputies in this journal.
References
- Abram P. K., Gariepy T. D., Boivin G., Brodeur J. An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biological Invasions. 2014;16:1387–1395. doi: 10.1007/s10530-013-0576-y. [DOI] [Google Scholar]
- Abram P. K., Hoelmer K. A., Acebes-Doria A., Andrews H., Beers E. H., Bergh C. J., Bessin R., Biddinger D., Botch P., Buffington M. L., Cornelius M. L., Costi E., Delfosse E. S., Dieckhoff C., Dobson R., Donais Z., Grieshop M., Hamilton G., Haye T., Hedstrom C., Herlihy M. V., Hoddle M. S., Hooks C. R.R., Jentsch P., Joshi N. K., Kuhar T. P., Lara J., Lee J. C., Legrand A., Leskey T. C., Lowenstein D., Maistrello L., Mathews C. R., Milnes J. M., Morrison W. R., Nielson A. L., Ogburn E. C., Pickett C. H., Poley K., Pote J., Radl J., Shrewsbury P. M., Talamas E., Tavella L., Walgenbach J. F., Waterworth R., Weber D. C., Welty C., Wiman N. G. Indigenous arthropod natural enemies of the invasive brown marmorated stink bug in North America and Europe. Journal of Pest Science. 2017;90:1009–1020. doi: 10.1007/s10340-017-0891-7. [DOI] [Google Scholar]
- Balusu R. R., Cottrell T. E., Talamas E. J., Toews M. D., Blaauw B. R., Sial A. A., Buntin D. G., Vinson E. L., Fadamiro H. Y., Tillman G. P. New record of Trissolcussolocis (Hymenoptera: Scelionidae) parasitizing Halyomorphahalys (Hemiptera: Pentatomidae) in the United States of America. Biodiversity Data Journal. 2019;7:e30124. doi: 10.3897/BDJ.7.e30124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckhoff Christine. Eggs of pentatomidae found in the Eastern USA Northeastern IPM. 2014. https://www.northeastipm.org/neipm/assets/File/BMSB-Resources/BMSB-Biocontrol-Workshop-Jun-2014/Eggs-of-Pentatomidae-Found-in-the-Eastern-USA-Dieckhoff.pdf https://www.northeastipm.org/neipm/assets/File/BMSB-Resources/BMSB-Biocontrol-Workshop-Jun-2014/Eggs-of-Pentatomidae-Found-in-the-Eastern-USA-Dieckhoff.pdf Manual from BMSB parasitoid worksho.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3(5):294–299. [PubMed] [Google Scholar]
- Gariepy TD, Haye T, Zhang J. A molecular diagnostic tool for the preliminary assessment of host-parasitoid associations in biological control programmes for a new invasive pest. Molecular Ecology. 2014;23:3912–3924. doi: 10.1111/mec.12515. [DOI] [PubMed] [Google Scholar]
- Gill S., Klick S., Kenney S. Brown marmorated stink bug (Halyomorphahalys): IPM pest alert, October 2010. University of Maryland Extension.; 2010. [Google Scholar]
- Herbert D. A., Kamminga K., Malone S., Kuhar T. P., Day E., Greene J., Bundy C. S., Brown L., Ellsworth P. Field guide to stink bugs of agricultural importance in the United States. Virginia IPM.; 2015. [Google Scholar]
- Hoebeke E. R., Carter M. E. Halyomorphahalys (Stål) (Heteroptera: Pentatomidae): a polyphagous plant pest from Asia newly detected in North America. Proceedings of Entomological Society of Washington. 2003;105(1):225–237. [Google Scholar]
- Hull L. A., Krawczyk G., Biddinger D. Maintaining the integrity of IPM in Pennsylvania while battling the brown marmorated stink bug. Penn State Fruit Research and Extension Center.; 2011. [Google Scholar]
- Inkley D. Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae) Journal of Entomological Science. 2012;47(2):125–130. doi: 10.18474/0749-8004-47.2.125. [DOI] [Google Scholar]
- Johnson N. F. Systematics of Nearctic Telenomus: classification and revisions of the podisi and phymatae species groups (Hymenoptera: Scelionidae) Ohio Biological Survey Bulletin New Series. 1984;6(3):10–113. [Google Scholar]
- Leskey T. C., Short B. D., Butler B. R., Wirght S. E. Impact of the invasive brown marmorated stink bug, Halyomorphahalys (Stål), in Mid-Atlantic tree fruit orchards in the United States: case studies of commercial management. Psyche. 2012;2012:1–13. doi: 10.1155/2012/535062. [DOI] [Google Scholar]
- Center Northeastern IPM. State-by-state. https://www.stopbmsb.org/where-is-bmsb/state-by-state/ [2022-09-21T00:00:00+03:00]. https://www.stopbmsb.org/where-is-bmsb/state-by-state/
- Orr D. B., Russin J. S., Boethel D. J., Jones W. A. Stink bug (Hemiptera: Pentatomidae) egg parasitism in Louisiana Soybeans. Environmental Entomology. 1986;15:1250–1254. doi: 10.1093/ee/15.6.1250. [DOI] [Google Scholar]
- Orr D. B., Boethel D. J. Reproductive potential of Telenomuscristatus and T.podisi (Hymenoptera: Scelionidae), two egg parasitoids of pentatomids (Heteroptera) Annals of the Entomological Society of America. 1990;83(5):902–905. doi: 10.1093/aesa/83.5.902. [DOI] [Google Scholar]
- Ramirez-Ahuja M., Lopez-Arroyo J. I., Rodriguez-Sanchez I. P., Gonzalez-Hernandez A. Hymenoptera parasitoids associated with Stink Bugs in Mexico. Society of Southwestern Entomologists. 2019;44(1):35–49. doi: 10.3958/059.044.0104. [DOI] [Google Scholar]
- Rice K. B., Bergh C. J., Bergmann E. J., Biddinger D. J., Dieckhoff C., Dively G., Fraser H., Gariepy T., Hamilton G., Haye T., Herbert A., Hoelmer K., Hooks C. R., Jones A., Krawczyk G., Kuhar T., Martinson H., Mitchell W., Nielsen A. L., Pfeiffer D. G., Raupp M. J., Rodriguez-Saona C., Shearer P., Shrewsbury P., Venugopal P. D., Whalen J., Wiman N. G., Leskey T. C., Tooker J. F. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae) Journal of Integrated Pest Management. 2014;5(3):1–13. doi: 10.1603/ipm14002. [DOI] [Google Scholar]
- Sargent C., Martinson H. M., Raupp M. J. The orient express in Maryland: the brown marmorated stink bug, Halyomorphahalys (Stål) (Hemiptera: Pentatomidae) The Maryland Entomologist. 2011;5(3):2–21. [Google Scholar]