Abstract
Background
Humidity control measures in the home environment of patients with asthma have been recommended, since a warm humid environment favours the growth of house dust mites. However, there is no consensus about the usefulness of these measures.
Objectives
To study the effect of dehumidification of the home environment on asthma control.
Search methods
The clinical trials registers of the Cochrane Collaboration and Cochrane Airways Group were searched. Searches were current as of March 2013.
Selection criteria
Randomised controlled trials on the use of humidity control measures in the home environment of patients with asthma were evaluated for inclusion.
Data collection and analysis
Data were extracted independently using a pre‐designed data extraction form by two review authors.
Main results
A second trial has been added for the 2013 update of this review. The original open‐label trial compared an intervention consisting of mechanical ventilation heat recovery system with or without high efficiency vacuum cleaner fitted in 40 homes of patients with asthma who had positive tests for sensitivity to house dust mite. The new double‐blind trial also compared a mechanical ventilation heat recovery system with a placebo machine in the homes of 120 adults with allergy to house dust mite. The new trial, which was at low risk of bias, showed no significant difference in morning peak flow (mean difference (MD) 13.59; 95% confidence interval (CI) ‐2.66 to 29.84), which was the primary outcome of the trial. However, there was a statistically significant improvement in evening peak flow only (MD 24.56; 95% CI 8.97 to 40.15). There was no significant difference in quality of life, rescue medication, requirement for oral corticosteroids, visits to the GP, emergency department (ED) or hospitalisations for asthma. There was no significant difference in the house dust mite count and the antigen levels in the new trial, in contrast to the previous trial.
Authors' conclusions
Evidence on clinical benefits of dehumidification using mechanical ventilation with dehumidifiers remains scanty, and the addition of a new double blind trial to this review does not indicate significant benefit in most measure of control of asthma from such environmental interventions.
Plain language summary
Dehumidifiers in the home for asthma
The health benefits of dehumidification of the home environment of patients with asthma were studied. Only two studies qualified to be included in the review. Current evidence shows little clinical benefit from the use of dehumidification using mechanical devices on the clinical status of asthma patients.
Summary of findings
Summary of findings for the main comparison. MHRV compared to placebo for asthmatics with sensitivity to house dust mite.
| MHRV compared with placebo for asthmatics with sensitivity to house dust mite | ||||||
| Patient or population: asthmatics with sensitivity to house dust mite Settings: Community Intervention: MHRV Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | MHRV | |||||
|
Change in Morning PEF after 12 months (% predicted) Follow‐up: 12 months |
Change of ‐7% on placebo | Change of +6.4% on MHRV | MD 13.59 % (‐2.66 to 29.84) | 100 (1 study) | ⊕⊕⊕⊝ moderate1 | |
|
Change in Evening PEF after 12 months (% predicted) Follow‐up: 12 months |
Change of ‐12% on placebo | Change of +12% on MHRV | MD 24.56 % (8.97 to 40.15) | 100 (1 study) | ⊕⊕⊕⊝ moderate1 | |
|
Change in FEV1 after 12 months (% predicted) Follow‐up: 12 months |
Change of +1.8% on placebo | Change of +1.0% on MHRV | MD 1.32 % (‐2.55 to 5.19) | 100 (1 study) | ⊕⊕⊕⊝ moderate1 | |
|
Quality of life SGRQ Scale from 0 to 100 (0 on the scale is better) Follow‐up: 12 months |
Change of ‐2.1 units on placebo | Change of ‐5.2 units on MHRV |
MD ‐2.83 units (‐7.82 to 2.16) |
100 (1 study) |
⊕⊕⊕⊝ moderate1 | |
| Exacerbations needing oral steroids Follow‐up: 12 months | 362 per 1000 | 228 per 1000 (111 to 413) | OR 0.52 (0.22 to 1.24) | 100 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Exacerbations needing ED visit Follow‐up: 12 months | 43 per 1000 | 76 per 1000 (14 to 319) | OR 1.84 (0.32 to 10.52) | 100 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Exacerbations needing hospitalisation Follow‐up: 12 months | 85 per 1000 | 8 per 1000 (0 to 138) | OR 0.09 (0 to 1.72) | 100 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk is the mean control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Single study with wide confidence interval MD: mean difference MHRV: mechanical heat recovery ventilation system OR: odds ratio PEF: peak expiratory flow
Background
Moisture content or humidity of inspired air has been variously studied in relation to asthma symptoms, control of the disease and airway response to exercise. There are studies that demonstrate attenuation of broncho‐provocative response to exercise when administered along with humidity increase in the inspired air (Boulet 1991), whereas an increase in humidity of the ambient air in the natural habitat of individuals with asthma has been shown to increase asthma symptoms due to an increase in the mould and house dust mite content in the environment (Korsgaard 1983). Humidity control measures in the form of provision of mechanical ventilation to the houses have been used to decrease the house dust mite content (Crane 1998).
There is however, no consensus on whether such measures help in the control of asthma. The consensus statements on management of asthma recommend the reduction of indoor humidity to less than 50% as a desirable action to control dust mite population, which is an important allergen source causing and leading to increase in symptoms of asthma, in sensitised individuals.
Humidity plays an important role in determining the house dust mite count in the indoor environment. House dust mite has been shown to be a very important allergen. Indoor humidity also leads to breeding of fungi in the home, which can also cause asthma. Recent times have seen measures being introduced to control indoor humidity in order to decrease the prevalence of these respiratory allergens. These measures include provision of healthy and well‐ventilated homes, use of portable and fixed dehumidifiers, mechanical ventilation and behavioural intervention. However, it is not known how useful these measures are in the management of patients with chronic asthma.The present review studies the effect of using dehumidification of ambient air in the home environment of patients with asthma using various devices.
Description of the condition
Asthma is a chronic disease characterised by recurrent attacks of breathlessness and wheezing, which vary in severity and frequency from person to person. Symptoms may occur several times in a day or a week in affected individuals, and for some people become worse during physical activity or at night. Recurrent asthma symptoms frequently cause sleeplessness, daytime fatigue, reduced activity levels and school and work absenteeism. Asthma has a relatively low fatality rate compared to other chronic diseases. The World Health Organization (WHO) estimates that 235 million people currently suffer from asthma. Asthma is the most common chronic disease among children. Asthma is a public health problem not just for high‐income countries; it occurs in all countries regardless of the level of development. Most asthma‐related deaths occur in low‐ and lower‐middle income countries. Asthma is under‐diagnosed and under‐treated. It creates a substantial burden to individuals and families and often restricts individuals’ activities for a lifetime.
Description of the intervention
Higher humidity levels may worsen asthma as a warm humid environment favours the growth of house dust mite, fungus and moulds which can act as allergens and trigger asthma.
Here we have studied dehumidification i.e. decreasing the humidity levels of ambient air as an additional intervention to control the symptoms of asthma. Dehumidification is achieved by using mechanical ventilation and heat recovery systems.
How the intervention might work
The intervention might work by decreasing the humidity levels and all its attendant risks such as breeding of dust mite and proliferation of fungal spores.
Why it is important to do this review
Many studies have been conducted using dehumidifiers in the homes of patients with asthma. However, there is no consensus whether this intervention is clinically useful. These studies are difficult to do and are reasonably expensive. Hence there is a need to appraise the evidence on this topic.
Objectives
To study the effect of dehumidification of the home environment on asthma control.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials (randomised and quasi‐randomised) in chronic asthma (in adults and children), in which humidification control using dehumidifiers had been used.
Types of participants
Individuals of any age with asthma. Children and adults with recurrent or chronic asthma or increased bronchial hyper‐reactivity. All concurrent therapies were allowed, provided they were documented.
Types of interventions
Intervention
Controlled humidity of the home environment (mechanical ventilation, both portable and fixed). The review only considered environmental remediation as an intervention, provided that the provision of dehumidification was standardised within the intervention group.
Control (no intervention)
Placebo device or no intervention.
Types of outcome measures
Airway function (forced expiratory flow (FEV1) and peak expiratory flow (PEF))
Asthma symptoms
Use of rescue bronchodilator medication
Daily steroid use
Exacerbations
Emergency room attendance/unscheduled clinic visits
Hospital admissions
Health status (quality of life)
Bronchial hyper‐responsiveness
Skin reactivity to moulds or house dust mite
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
humid* OR water vapour OR water vapor* OR water‐vapour* OR water‐vapor* or moisture*
Searches were current as of March 2013.
Searching other resources
The publications of references identified as randomised controlled trials (RCTs) or unclear, clearly or potentially relevant trials, were obtained and reviewed. Secondly, reference lists of all identified RCTs were checked to identify potentially relevant citations. Thirdly, we contacted the international headquarters of manufacturing companies producing humidity control equipment. Inquiries regarding other published or unpublished studies known and/or supported by these companies or their subsidiaries were made so that these results may be included in our review. Finally, personal contact with colleagues and trialists working in the field of paediatric asthma was made to identify potentially relevant trials.
Data collection and analysis
Data collection
Each abstract was reviewed and annotated as (1) RCT (2) clearly not an RCT or (3) unclear.
Data were extracted independently by three persons during the current update (Meenu Singh, Nishant Jaiswal and Harpreet Kaur). The author of the first included controlled trial was contacted to verify the accuracy of extracted data.
Data synthesis
The planned data analyses focused on the following comparisons.
Humidity control of ambient air using dehumidifiers with no humidity control.
Humidity control of ambient air + anti‐asthma medications versus placebo or no humidity control + anti‐asthma medications
We planned to summarise the difference in groups in event rates such as number of exacerbations in a specified period of time by a ratio of rates.
We intended to analyse continuous outcomes such as pulmonary function tests or quality of life scores of the patients using the mean difference (MD) or the standardised mean difference (SMD), if different scales were used.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The systematic search for studies yielded 178 potentially relevant studies. Ten studies were considered for inclusion into the review. There were five studies in which randomisation had been used (Hyndman 2000; Warner 2000; Morgan 2004; Burr 2007; Wright 2009). However, studies which emphasised the environmental control of humidity and its effect mainly on the house dust mite were excluded.The Hyndman 2000 study was examined closely by review authors, but was eventually excluded as the overall focus of the study was on the impact of portable humidifiers on the domestic environment, rather than on the effect of air humidification on asthma symptoms.
An updated search in November 2011 identified a study which was excluded on the grounds that the environmental intervention provided was a number of measures aimed at reducing domestic moulds (Kercsmar 2006). Another trial Morgan 2004 was excluded as they had used a specific educational intervention for environmental control. A subsequent search did not add any more studies.
Included studies
One study was included in the original review (Warner 2000) and a second study has been added for this update (Wright 2009).
Warner 2000 studied 40 patients in a parallel randomised control trial over 12 months. They had used mechanical ventilation with heat recovery (MVHR) and high efficiency vacuum cleaning (HEVC) in one group, MVHR alone in the other and HEVC alone in the third and no intervention in the fourth group. The MVHR units consisted of a heat exchanger and two fans with a manually operated boost switch for the bathroom and an EU4 filter on the supply (the MVHR units were purchased by EA Technology and installed by ADM Indux). The system was a pleated filter, which had a greater than 90% efficiency of trapping particles of greater than 5 µm and was aimed at trapping pollen grains and larger inhalable particulates. Patients with moderate to severe asthma who were using prophylactic medication were recruited from asthma clinics at Southampton University Trust Hospitals. A housing questionnaire was filled in to determine the suitability of the participants home for installation of the MVHR unit. Daily symptom diary cards and twice daily peak expiratory flow (PEF) rate with a Wright's peak flow meter were recorded before the study started and for one month prior to domiciliary visits. Patients with FEV1 less than 80% of predicted values were not subjected to histamine challenge. Twenty‐seven patients had an assessment of histamine challenge. The study consisted of four treatment arms. Group 1 was allocated to receive a MVHR in bedrooms and bathrooms plus HEVC. Group 2 was allocated to receive MVHR alone, group three received HEVC alone. Group 4 received no intervention and served as the control group. Initially 40 homes were to be included in the trial, of which 10 were subsequently deemed unfit to accommodate the MVHR unit. Thirty homes were then randomised between all four groups, and the 10 deemed unsuitable for the MVHR were randomly allocated to the last two non‐MVHR groups. Detailed information on clinical symptoms and lung functions was not provided.
Wright 2009 studied 119 asthmatic patients who were allergic to house dust mite, in a parallel, double‐blind, randomised trial over 12 months. They used MHRV in one group and no intervention in the other group. MHRV units were fitted in the roof space or hallway cupboard in 120 suitable homes by 'Vent‐Axia (Crawley,UK). These energy efficient units extract air continuously from the kitchen and bathroom and deliver pre‐warmed air via insulated ducts into the bedroom and living room. The system provided an additional 0.5 air exchanges per hour to the living room and bedroom. Patients between 16 to 60 years of age were recruited from general practice and hospital clinics in Lanarkshire, Scotland, U.K. One hundred and nineteen homes were randomised between two groups out of which 18 were protocol violators and one withdrew from the study; therefore, 53 houses were installed with an active MHRV and 47 were in the control group, in which the MHRV was installed but not activated. Patients were included on the basis of variable airflow obstruction of ≥12 % on spirometry or ≥15% on PEF readings or a symptom score of ≥0.86 on Asthma Control Questionnaire, Minimum FEV1 of the patients was more than 50% predicted at baseline and they did not have an exacerbation in the previous month. Allergy to Der P was determined according to a positive skin prick test. After surveying, houses were installed with MHRV system, participants were followed up at three, six, nine and 12 months after randomisation. A daily symptom diary was recorded and participants measured morning and evening PEF for two weeks before each visits. Spirometry was performed at each visit.
Risk of bias in included studies
The methodological quality of the eligible controlled trials was assessed as per the 'Risk of bias' tables which evaluate the reported quality of randomisation, blinding, and description of withdrawals and dropouts. This quality assessment was carried out independently by two review authors (Meenu Singh and Nishant Jaiswal).
Warner 2000 was deemed as being of low quality by both review authors. The reporting in the study was not explicit enough to have obtained a higher score, and the absence of blinding by not introducing a dummy humidifier as a control measure also prevented the authors from conferring it a higher score. Moreover, the trial was not fully randomised.
Wright 2009 was deemed good quality study as the study was double‐blinded with a good sample size.
Effects of interventions
See: Table 1
Warner 2000: Due to the absence of data for the 30 households that were randomised over the four groups, we were unable to impute any numerical data from the study. The trial authors report that no significant differences were found between any of the four randomised groups for any of the patient outcomes, including symptom scores, pulmonary function tests and histamine challenge test. The patients in intervention group (MVHR) showed higher PC20 values in a histamine challenge test, than those without intervention (control) but it did not reach significance (P = 0.085). Allergen and house dust mite levels in mattresses and sofas did not decrease significantly. However, allergen and house dust mite levels were significantly reduced in the bedroom carpets of trial participants allocated to the MVHR and the MVHR plus HEVC groups (P = 0.05). All humidity analysis were performed by using Absolute Humidity (AH) values rather than Relative Humidity (RH) values because this permitted a direct comparison between indoor and outdoor conditions. Analysis of covariance showed a highly significant difference in humidity ( P < 0.001) between the MVHR and non‐MVHR groups. The houses with MVHR had a lower bedroom humidity than the non‐MVHR houses over the whole test period, both winter and summer. The difference was greater in winter, being 0.73 g/kg at an outdoor humidity of 5g/kg and 0.38 g/kg at an outdoor humidity of 10g/kg. Efforts to obtain extraneous data for the 30 households randomised across the four groups have not been successful.
Wright 2009: A total of 100 patients in 100 houses were analysed in this trial (53 on MHRV and 47 on placebo). The trial authors reported that change in mean morning percent predicted PEF, from baseline to 12 months, did not differ between the MHRV group and the control group (mean difference (MD) 13.59; 95% confidence interval (CI) ‐2.66 to 29.84, Analysis 1.1) when compared using an adjusted difference (ANCOVA). However; there was a significant improvement in the MHRV group compared with control group in the mean evening PEF (MD 24.56; 95% CI 8.97 to 40.15, Analysis 1.2). There was no significant difference in change from baseline in percent predicted FEV1 (MD 1.32; 95% CI ‐2.55 to 5.19, Analysis 1.3) or daily symptoms including use of rescue medicines (MD ‐0.04; 95% CI ‐1.00 to 0.92, Analysis 1.4), Asthma Contol Score (MD ‐0.25; 95% CI ‐0.58 to 0.08, Analysis 1.5), or St George’s Respiratory Questionare score (MD ‐2.83; 95% CI ‐7.82 to 2.16, Analysis 1.6), There was also no significant difference in the number of participants who suffered an exacerbation requiring oral corticosteroids (odds ratio (OR) 0.52; 95% CI 0.22 to 1.24, Analysis 1.7), GP visits (OR 0.29; 95% CI 0.01 to 7.28, Analysis 1.8) or emergency department (ED) visits (OR 1.84; 95% CI 0.32 to 10.52, Analysis 1.10) or hospitalisations (OR 0.09; 95% CI 0.00 to 1.72, Analysis 1.11). The ‘per protocol’ analysis provided similar results to the intention‐to‐treat analysis. No adverse event was reported relating to the installation of the MHRV unit. The median (interquartile range) per cent of time homes achieved a reduction in the indoor relative humidity below 50% was greater in the MHRV group than in the control group in the bedroom [45.1% (30.0 to 55.1) versus 21.0(8.5 to 49.0), P = 0.001] but not in the living room [51.5% (35.4 to 58.7) versus 40.6% (12.8 to 63.5), P = 0.26]. At 12 months the changes in the mean Der P 1 and Der P 2 concentration as compared with the baseline concentrations, did not differ between the MHRV group and control group, nor were there differences in total or house dust mite specific immunoglobulin E (IgE) levels.
1.1. Analysis.

Comparison 1 MHRV versus placebo, Outcome 1 Change in morning PEF at 12 months (% predicted).
1.2. Analysis.
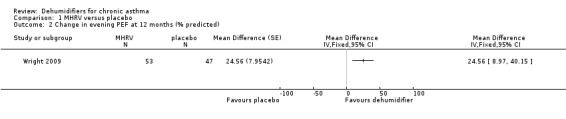
Comparison 1 MHRV versus placebo, Outcome 2 Change in evening PEF at 12 months (% predicted).
1.3. Analysis.
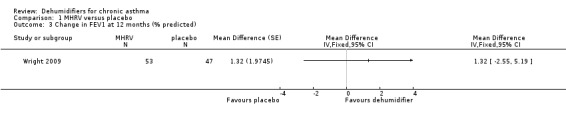
Comparison 1 MHRV versus placebo, Outcome 3 Change in FEV1 at 12 months (% predicted).
1.4. Analysis.
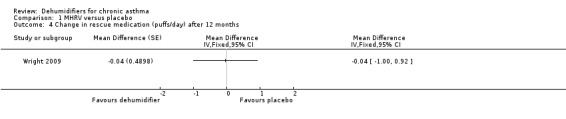
Comparison 1 MHRV versus placebo, Outcome 4 Change in rescue medication (puffs/day) after 12 months.
1.5. Analysis.
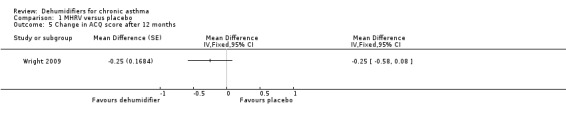
Comparison 1 MHRV versus placebo, Outcome 5 Change in ACQ score after 12 months.
1.6. Analysis.
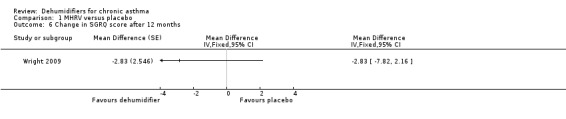
Comparison 1 MHRV versus placebo, Outcome 6 Change in SGRQ score after 12 months.
1.7. Analysis.
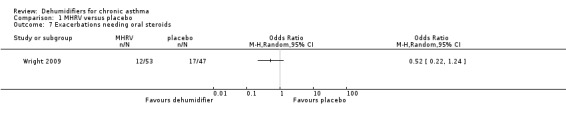
Comparison 1 MHRV versus placebo, Outcome 7 Exacerbations needing oral steroids.
1.8. Analysis.
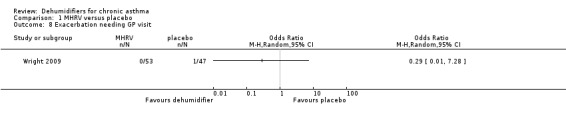
Comparison 1 MHRV versus placebo, Outcome 8 Exacerbation needing GP visit.
1.10. Analysis.
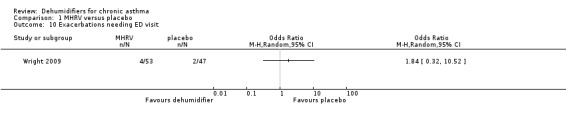
Comparison 1 MHRV versus placebo, Outcome 10 Exacerbations needing ED visit.
1.11. Analysis.
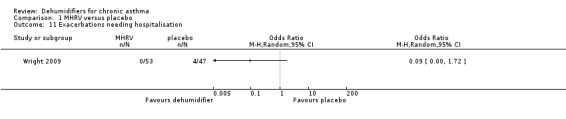
Comparison 1 MHRV versus placebo, Outcome 11 Exacerbations needing hospitalisation.
Discussion
Dehumidifiers have been used in the environmental control of homes of patients with asthma (Warner 2000; Wright 2009). Other studies have looked at the levels of dust mite concentrations, mould eradication and other non‐patient centred outcomes as primary outcome measures (Harving 1994; Hyndman 2000; Morgan 2004; Burr 2007). The focus of this review was oriented towards patient outcomes. Therefore, whilst data reported in those studies may have a relevance to the issue of reducing allergen exposure, they could not be used in our review.
The Warner 2000 study that has studied clinical outcomes in patients with asthma has not reported the results in detail. Their reported results do not reveal any benefit from using dehumidification on patient outcomes. The randomisation procedure was inadequate for the purpose of this review, as 10 homes were randomised only between the non‐MVHR groups (groups three and four). The authors did not specify how many children from the subsequent 30 homes were allocated between all groups. Hence, the evidence from randomised controlled trials is scarce and is of low quality, preventing us from commenting on the usefulness of dehumidifiers in control of chronic asthma.
Adherence to the treatment regimens in these studies has also proved difficult to determine. Unlike in drug trials where levels of a drug can be determined by laboratory tests, domiciliary visits by the trial investigators represent possibly the only way to ensure that the treatment regimens are maintained (Wood 1998). One possible solution would be to incorporate timing devices into the dehumidifying devices themselves. Warner 2000 did in fact incorporate such a device in the vacuum cleaner, but not the dehumidifiers. If participants in the study were not running their dehumidifiers for long stretches of time, this was not reflected in the data reported in the published study, and may have affected the MVHR's efficacy. Warner 2000 also reported that not all participants completed diary cards, and this may be indicative of poor adherence to the study protocol.
The Wright 2009 study studied clinical outcomes in patients with asthma and was considered to be at low risk of bias Figure 1. Daily symptoms including use of rescue medicines, Asthma Control Score, St George’s Respiratory Questionare score, requirement for oral corticosteroids, GP or ED visits or hospitalisations with asthma did not differ significantly in two groups. The results showed an improvement in the evening PEF readings of the MHRV group as compared with the control group, and there was no significant difference in FEV1. The morning PEF changes were internally consistent with these evening changes of PEF but did not achieve statistical significance, possibly because the study was insufficiently powered to demonstrate a clinical response as only 100 out of 119 could complete follow‐up. There was significant number of dropouts i.e. out of 120 houses only 100 could be evaluated at the end of the trial as one participant defaulted , six were protocol violators from MHRV group and 12 from the control group. One from the MHRV group withdrew consent. It was clear that reduced relative humidity was insufficient to impact on Der P 1 burden & there was no difference between the groups in change in serum house dust mite specific IgE antibody. Wright 2009 also reported that with MHRV intervention, there was a more prolonged and more significant reduction in relative humidity in bedrooms, and that Der P 1 levels were lower in bedding, which is arguably the most important exposure.
1.
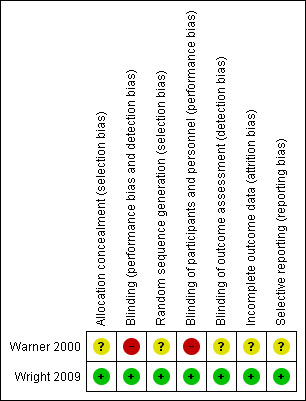
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Authors' conclusions
Implications for practice.
There is currently scanty evidence (only one trial originating from Scotland) to indicate whether dehumidifiers are of clinical benefit to patients with asthma.
Implications for research.
Randomised controlled trials (RCTs) with portable or fixed domestic dehumidification measures are difficult to perform. The results from a high quality RCT leave considerable uncertainty about dehumidifiers in the UK. The results cannot be generalised to hot climatic conditions where similar trials may be needed. The adherence to treatment regimens should, where possible, be followed up and reported. Double‐blinding should be used, using dummy dehumidifiers. Asthma outcomes being measured should include clinical parameters, pulmonary functions, quality of life measures and adverse events, including financial burden.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2014 | Amended | PLS title amended |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 6 March 2013 | New citation required and conclusions have changed | One new double‐blind randomised trial has been added to the review (Wright 2009). Feedback was incorporated. Title changed. New author team. |
| 6 March 2013 | New search has been performed | New literature search run |
| 28 July 2008 | Amended | Converted to new review format. |
| 28 September 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The author would like to extend her thanks to the Hospital Saturday Fund Charitable Trust, who kindly provided a travel grant which enabled Dr Meenu Singh to visit the editorial base. Also thanks to Toby Lasserson, Anna Bara, Karen Blackhall and Steve Milan of the Cochrane Airways Group for the review when it was published for the first time. Also thanks to Roy Buffery for providing consumer feedback on the synopsis.
The updated review was conducted at the ICMR Centre for Evidence Based Child Health, PGIMER, Chandigarh, funded by Indian Council of Medical Research, New Delhi. Ms Harpreet Kaur, from this centre, helped in data extraction and quality characterisation of trials.
Data and analyses
Comparison 1. MHRV versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in morning PEF at 12 months (% predicted) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Change in evening PEF at 12 months (% predicted) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Change in FEV1 at 12 months (% predicted) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Change in rescue medication (puffs/day) after 12 months | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Change in ACQ score after 12 months | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Change in SGRQ score after 12 months | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Exacerbations needing oral steroids | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Exacerbation needing GP visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Exacerbation needing GP out of hours | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Exacerbations needing ED visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Exacerbations needing hospitalisation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.9. Analysis.
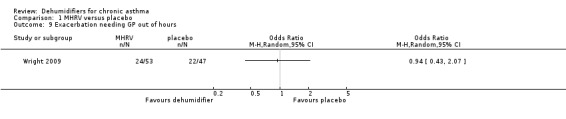
Comparison 1 MHRV versus placebo, Outcome 9 Exacerbation needing GP out of hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Warner 2000.
| Methods | Design of study: randomised controlled trial, parallel group. Method of randomisation: not stated. Concealment of randomisation: not stated. Blinding: none. Description of withdrawals or dropouts: not stated. | |
| Participants | Total number of participants enrolled into trial = 40 (13 adults and 27 children). Total number of participants in each randomised group: not given; final number of adults in each group is given (group 1 = 5; group 2 = 4; group 3 = 2; group 4 = 2). Age: 13 adults (range 20‐67 years, mean 40.1 years). 27 children (range 4‐16 years, mean 9.7 years). Sex: Adults ‐ 9 men and 4 women. Children ‐ 17 boys and 10 girls. Physician diagnosed asthma, with moderate to severe asthma. Inclusion criteria: Ability to perform peak flow measurements, flow‐volume loop, histamine bronchial challenge, and reacting positive to a skin‐prick test at least 5 mm in diameter for D pteronyssinus. Moderate to severe asthmatics requiring prophylactic medications. Participants also had to live in homes fulfilling pre‐determined inclusion criteria, information for which was gathered by questionnaires. Source of participants: hospital asthma clinics. | |
| Interventions | Setting: home. Interventions: (four randomised groups): Group 1: Mechanical ventilation and heat recovery (MVHR) and high efficiency vacuum cleaning (HEVC). Group 2: MVHR only. Group 3: HEVC only. Group 4: Control group (no intervention). Duration of trial: 12 months. | |
| Outcomes | Daily symptom diaries recording: wheeze, cough and activity; medication use; PEF (am and pm). FEV1 and histamine challenge tests. | |
| Notes | Randomisation was inadequate due to re‐allocation of homes which were not considered suitable for installation of mechanical ventilators. Authors have been contacted for additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | None |
| Random sequence generation (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available. |
| Selective reporting (reporting bias) | Unclear risk | Information not available. |
Wright 2009.
| Methods | Design of study: randomised controlled trial, parallel group, placebo control, double‐blind Method of randomisation: Random number generator Concealment of randomisation : concealed from patient and clinical research team. Blinding : Double‐blinding Description of withdrawals and dropouts: 18 protocol violators and 1 withdrawal. |
|
| Participants | Total no. of participants included: 119 Total no. of participants in each randomised group: MHRV (Mechanical heat recovery ventilation system)‐60 & Placebo control‐59 Age: 16‐60 yrs (Mean age for MHRV (41.6) and Placebo control (42.3) if had asthma for more than 1 year and on regular inhaled corticosteroids and had daily symptoms. Gender : No. of males in MHRV:41 and in placebo control:32 No. of females in MHRV :19 and in placebo control :27 Inclusion criteria: Variable air flow obstruction of >=12% on spirometry or >= 15% on peak expiratory flow (PEF) or symptom score of >=0.86% on Asthma Control Questionnaire (ACQ). Participants had a minimum forced expiratory volume (FEV 1) of >50% and had not had an exacerbation in the previous month. Allergy to D. pteronyssinus was determined by positive skin test defined as a wheal diameter of >=3mm greater than that of negative control at 15 min. Subjects also had to live in homes fulfilling pre‐determined inclusion criteria. Source of participants: general practice and hospital clinics. |
|
| Interventions | Setting : home Group I: MHRV (Mechanical heat recovery ventilation system) Group II: Placebo control Duration of trial: 12 months |
|
| Outcomes | Daily symptom dairies recording : sneezing, nasal blockage and nasal discharge. PEF (morning and evening) FEV1 : Baseline and 12 months |
|
| Notes | The study was insufficiently powered to demonstrate a clinical response; only 100 of 119 participants completed follow‐up. The reduced relative humidity was insufficient to impact on Der p1 burden, and there was also no difference between the groups in change in serum house dust mite specific IgE antibody. Domestic dehumidification has reduced mite allergen burden in some. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Unit activation device was concealed from the patient and the clinical research team. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded study. |
| Random sequence generation (selection bias) | Low risk | Randomisation was created using the random number generator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical research team & the patients were unaware of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are complete. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
FEV 1: forced expiratory volume PEF: peak expiratory flow
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arlian 1999 | Laboratory based controlled observation |
| Burr 2007 | Randomised study of strategies to reduce indoor mould & complications of asthma. Humidification was not provided as standard across the intervention group |
| Cabrera 1995 | Not a randomised controlled trial |
| Chivato 1997 | Not a randomised controlled trial but an observational study. |
| Crane 1998 | Not a randomised controlled trial. |
| Emenius 1993 | Not a randomised controlled trial, observational study reported as an abstract. |
| Harving 1994 | Not a randomised controlled trial. |
| Hyndman 2000 | A randomised study where dehumidification has been used to control house dust mite. No patient outcomes have been studied. |
| Kercsmar 2006 | Randomised study of numerous environmental remediation strategies to reduce indoor mould. Humidification was not provided as standard across the intervention group. |
| Korsgaard 1983 | Not a randomised controlled trial |
| Morgan 2004 | The focus of study was in the reduction of level of cockroach allergen & dust mite & complication of asthma. No outcome was recorded regarding dehumidification. |
| Mosbech 1988 | Controlled study with no mention about randomisation. |
Differences between protocol and review
No modifications were made to the protocol for this review. The following changes were made in the update.
Risk of bias updated to Cochrane 'Risk of bias' tool.
Ongoing study from the original review Thomsom 2005 (Thompson NC. Randomized controlled trial to evaluate the effect of domestic mechanical heat recovery ventilation on asthma control of patients allergic to house dust mite. National Research Register 2005) was identified as being the same as Wright 2009 and was therefore deleted from this version.
'Summary of findings' table added.
Contributions of authors
MS conceived of the protocol, which had additional input from PG who suggested changes. MS, NJ and HK extracted data during this update, assessed the quality of the included studies and developed the analysis. MS developed the Results, Discussion and Conclusions sections. These also had further input from PG.
Anna Bara opted out of the updated review.
Sources of support
Internal sources
Advanced Pediatric Centre, Post Graduate institute of Medical Education and Research, Chandigarh, India.
The Hospital Saturday Fund Charitable Trust, UK.
External sources
Garfield Weston Foundation, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Warner 2000 {published data only}
- Warner JA, Fredrick JM, Bryant TN, Weisch C, Raw GJ, Hunter C, et al. Mechanical ventilation and high frequency vacuum cleaning: A combined strategy of mite and mite allergen reduction in the control of mite sensitive asthma. Journal of Allergy & Clinical Immunology 2000;105(1):75‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wright 2009 {published data only}
- Wright GR, Howieson S, McSharry C, McMahon AD, Chaudhuri R, Thompson J, et al. Effect of improved home ventilation on asthma control and house dust mite allergen levels. Allergy 2009;64(11):1671‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arlian 1999 {published data only}
- Arlian LG, Neal JS, Vyszenski‐Moher DL. Reducing relative humidity to control the house dust mite Dermatophagoides farinae. Journal of Allergy & Clinical Immunology 1999;104(4):852‐6. [DOI] [PubMed] [Google Scholar]
Burr 2007 {published data only}
- Burr ML, Mathews IP, Arthur RA, Watson HL, Gregory CJ, Dunstan FDJ, et al. Effects on patients with asthma of eradicating visible indoor mould: a randomised controlled trial. Thorax 2007;62:767‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cabrera 1995 {published data only}
- Cabrera P, Julia‐Serda G, Rodriguez de Castro F, Caminero J, Barber D, Carrillo T. Reduction of house dust mite allergens after dehumidifier use. Journal of Allergy & Clinical Immunology 1995;95:635‐6. [DOI] [PubMed] [Google Scholar]
Chivato 1997 {published data only}
- Chivato T, Montoro A, Martínez D, Gil P, Zubeldia J, De Barrio M, et al. Clinical tolerance, parasitological efficacy and environmental effects of dehumidifiers in stable asthmatics sensitized to house dust mites. Allergologia et immunopathologia (Madr) 1997;25(2):67‐72. [PubMed] [Google Scholar]
Crane 1998 {published data only}
- Crane J, Ellis I, Siebers R, Grimmet D, Lewis S, Fitzharris P. A pilot study of the effect of mechanical ventilation and heat exchange on house‐dust mites and Der p 1 in New Zealand homes. Allergy 1998;53(8):755‐62. [DOI] [PubMed] [Google Scholar]
Emenius 1993 {published data only}
- Emenius G, Egmar L, Axelsson G, Pershagen G, Wickman M. Protective effect of balanced mechanical ventilation on mite allergen levels. Journal of Allergy & Clinical immunology 1993;91(1(2)):353. [Google Scholar]
Harving 1994 {published data only}
- Harving H, Korsgaard J, Dahl R. House dust mite exposure reduction in specially designed, mechanically ventilated "healthy" homes. Allergy 1994;49:713‐8. [DOI] [PubMed] [Google Scholar]
Hyndman 2000 {published data only}
- Hyndman SJ, Vickers LM, Htut T, Maunder JW, Peock A, Higenbottam TW. A randomized trial of dehumidification in the control of house dust mite. Clinical and Experimental Allergy 2000;30:1172‐80. [DOI] [PubMed] [Google Scholar]
Kercsmar 2006 {published data only}
- Kercsmar CM, Dearborn DG, Schluchter M, Xue L, Kirchner HL, Sobolewski J, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environmental Health Perspectives 2006;114(10):1574‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korsgaard 1983 {published data only}
- Korsgaard J. Preventive measures in mite asthma: a controlled trial. Allergy 1983;38:93‐102. [DOI] [PubMed] [Google Scholar]
Morgan 2004 {published data only}
- Morgan WJ, Crain EF, Gruchalla RS, O' Connor GT, Kattan M, Evans R III, et al. Result of home‐based environmental intervention among urban children with asthma. New England Journal of Medicine 2004;351:1068‐80. [DOI] [PubMed] [Google Scholar]
Mosbech 1988 {published data only}
- Mosbech H, Korsgaard J, Lind P. Control of house dust mites by electrical heating blankets. Journal of Allergy & Clinical Immunology 1988;81(4):706‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Boulet 1991
- Boulet LP, Turcotte H. Influence of water content of inspired air during and after exercise on induced bronchoconstriction. European Respiratory Journal 1991;4(8):979‐84. [PubMed] [Google Scholar]
Wood 1998
- Wood R, Johnson E, Natta M, Hua Chen P, Eggleston P. A placebo‐controlled trial of a HEPA air cleaner in the treatment of cat allergy. American Journal of Respiratory and Critical Care Medicine 1998;158(1):115‐20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Singh 2002
- Singh M, Bara A, Gibson PG. Humidity control for chronic asthma. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003563] [DOI] [PubMed] [Google Scholar]


