Abstract
The timely diagnosis and treatment of elevated intracranial pressure (ICP) reduces morbidity rates and prevents mortality. The aim of the present systematic review and meta-analysis was to determine the diagnostic accuracy of optic nerve sheath diameter (ONSD) vs. standard invasive ICP measurements in patients with traumatic brain injury (TBI). The PubMed, Embase, Web of Science and Cochrane Library databases were systematically searched for studies including adult patients with TBI with suspected elevated ICP, and the sonographic ONSD measurements were compared with those from a standard invasive method. The quality of the studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 tool by two independent authors. A bivariate random effects model was used to summarize the pooled sensitivity, specificity and diagnostic odds ratio (DOR). A total of eight prospective studies with 222 patients with TBI were included. The pooled sensitivity was 0.82 [95% confidence interval (CI), 0.75-0.88], the specificity was 0.82 (95% CI, 0.71-0.90) and the DOR was 17.75 (95% CI, 7.02-44.83) with partial evidence of heterogeneity. The accuracy of the area under the summary ROC was 0.87. An ultrasound-determined elevated ICP has reasonable performance indicators with high sensitivity and specificity in patients with TBI. As such, this method may be a useful complementary monitoring tool in acute care.
Keywords: ultrasound, intracranial pressure, traumatic brain injury, optic nerve sheath diameter
Introduction
The intracranial volume often increases following trauma owing to a hemorrhage, cerebral edema or hydrocephalus. This can lead to an injurious shift in the brain, termed herniation (1). In addition, an increased volume within the rigid skull can elevate intracranial pressure (ICP), leading to compartment syndrome, which blocks or prevents blood flow to the brain (2). Brain ischemia can eventually lead to disability or mortality. Therefore, it is critical to dynamically monitor the changes in ICP (2).
Intracerebroventricular catheterization, intraparenchymal probing and lumbar puncture are considered the gold standard protocols for the measurement of ICP. However, they are associated with various risks, including bleeding, infection and malfunction, and are contraindicated in individuals requiring prolonged monitoring or having a predisposition to coagulation disorders or platelet disorders (3). Additionally, lumbar puncture is strongly discouraged in patients with brain herniation (4). This has prompted to the investigation of an appropriate ICP assessment method for bedside applications. Ultrasound has been used as a method for measuring the optic nerve sheath diameter (ONSD) owing to its portability, feasibility, safety, reproducibility and the lack of exposure to radiation hazards or well-known side-effects (5). The optic nerve is wrapped by a sheath, originating from the meninges and extending toward the orbit (5). This communication permits cerebrospinal fluid (CSF) transfer and therefore, similar pressure changes between the intracranial and orbital subarachnoid spaces (6,7). Therefore, the ultrasound detection of a high ICP based on the ONSD is becoming increasingly popular in trauma, neurosurgery and emergency medicine, although it does not permit continuous measurement and priming (8).
Meta-analysis studies on ultrasound measurements of the ONSD for the assessment of ICP have been previously published. However, there are limitations, including an increased heterogeneity or a limited number of included studies in the literature (9,10). This affects the accuracy of the ultrasound assessment of ICP. In the present systematic review and meta-analysis, the accuracy of ultrasound for measuring the ONSD and standard invasive methods for measuring the ICP in patients with traumatic brain injury (TBI) are discussed. The aim of the present meta-analysis was to examine the accuracy of ONSD ultrasonography in the diagnosis of ICP.
Materials and methods
Literature search
The PubMed, Embase, Web of Science and Cochrane Library databases were systematically searched from database inception to November, 2022 to identify relevant articles using the following search terms: ‘Optic nerve’, ‘optic nerve sheath’, ‘optic nerve sheath diameter’, ‘ONSD’, ‘ultrasound’, ‘ultrasonography’, ‘sonography’, ‘intracranial pressure’, ‘raised intracranial pressure’, ‘high intracranial pressure’, ‘ICP’, ‘high ICP’ and ‘raised ICP’ (the search strategy used herein is presented in the file entitled Data S1). The present meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11).
Study selection criteria
The included studies assessed adult patients with TBI who underwent an ultrasound for the measurement of ONSD and invasive intracranial monitoring for the measurement of ICP, without restrictions in language or year of publication. The exclusion criteria were the following: i) Studies that included patients aged <18 years; ii) case reports, reviews and meta-analyses; iii) studies that included pediatric patients or animals; iv) studies that included non-TBI patients; and v) studies that did not contain sufficient information (in the supplementary material and/or original article) to construe the 2x2 contingency table (true positive, true negative, false positive and false negative results). If it was not possible to build a table based on the existing published data, the corresponding author was contacted to clarify the issue. If no response was obtained, the study was excluded from the primary outcome analysis. Any disagreements regarding the included specific studies were solved by reaching a consensus amongst all the authors involved in the study.
Data abstraction and quality assessment
From each study, baseline characteristics were extracted, including the year of publication, author list, study design, country, sample size, diagnosis, invasive ICP measurement methods, high ICP thresholds, and the specificity and sensitivity of index tests. Invasive intracranial monitoring is the gold standard for measuring ICP (3). One investigator extracted the data, while another independently verified the data to construct a 2x2 contingency table. The two investigators extracted the data from the original study, and any disagreements were resolved through consultation with a third independent author. The methodological quality of the studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. Of note, two authors performed the quality assessments, and disagreements were resolved by consensus in the presence of a third author. An assessment of reporting bias was attempted using funnel plots (data not shown); however, this did not proceed due to the lack of relevant studies.
Quantitative data synthesis
Data synthesis was performed using the methods recommended in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. A bivariate random effects model was used to analyze and pool the statistics of the diagnostic tests (sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds of ultrasound). A diagnostic test statistic in the present study referred to the ability of ultrasound to detect high ICP. Heterogeneity was assessed using the I2 statistics. Values of P<0.05 or I2>50% indicated significant heterogeneity. Hierarchical summary receiver operating characteristics (SROC) analysis was performed, and an area under the curve of >0.9 was considered highly accurate in assessing the summary accuracy of ultrasound. All analyses were performed using Review Manager 5.3 or Meta-DiSc software 1.4(12).
Results
Search results and study characteristics
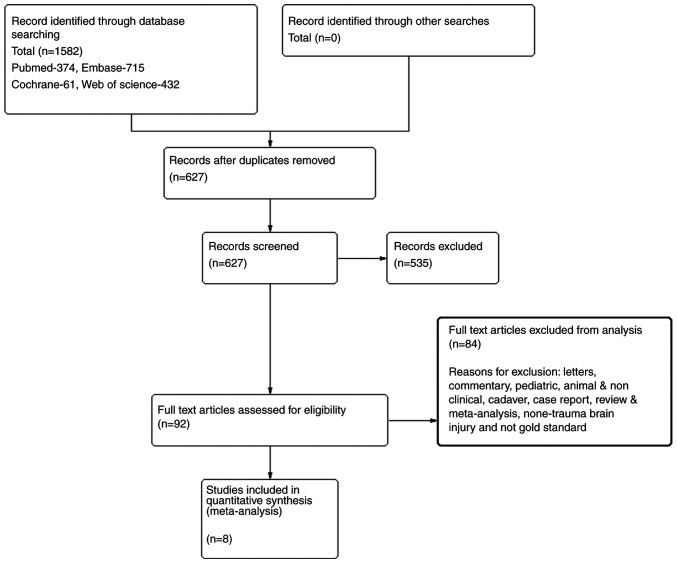
The flow diagram presented in Fig. 1 provides a summary of the PRISMA format of the literature search (13-20). A total of 1,582 studies were identified during the preliminary search. Following the removal of 955 duplicates, the abstracts of the 627 remaining studies were evaluated by two separate authors. The full text of 92 articles was reviewed based on the eligibility criteria, and 84 articles were rejected based on the exclusion criteria. Ultimately, eight studies with 222 patients with TBI were included.
Figure 1.
PRISMA flow chart of the literature search and selection of studies that reported accuracy of optic nerve sheath diameter for confirmation of a raised intracranial pressure in patients with traumatic brain injury. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of the included studies
The characteristics of the eight included studies (13-20) are summarized in Table I. The studies were performed between 2007 and 2020, and included 10-49 patients. These studies included in the primary outcome analysis presented with threshold values of ONSD ranging from 5-6.4 mm, indicating an elevated ICP ranging from 5.0-6.4 mm. In all the studies analyzed, invasive ICP monitoring was used, including brain parenchyma and ventricles.
Table I.
Characteristic of studies included in the meta-analysis.
| First author/s, year of publication | Country | No. of Patients | Age (Years) | Diagnosis | Cut-off of raised ICP | ICP measurement | Cut-off of ONSD (mm) | Interval between ICP and ONSD measurement | Exclusion criteria | Sensitivity | Specificity | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geeraerts et al, 2007 | France | 31 | 38 | TBI, GCS<8 | 20 mmHg | Intraparenchymal catheter inserted into the frontal lobe | 5.9 | Within 1 h | Ocular trauma and/or pathology | 0.87 | 0.94 | (15) |
| Maissan et al, 2015 | The Netherlands | 18 | 38 | TBI | 20 mmHg | Intraparenchymal probe to monitor ICP | 5 | Simultaneous | Ocular trauma | 0.94 | 0.98 | (16) |
| Soldatos et al, 2008 | Greece | 32 | 49 | Moderate-severe TBI | 20 mmHg | Invasive measurements of ICP | 5.7 | Simultaneous | Ocular pathology | 0.74 | 1 | (17) |
| Soliman et al, 2018 | Kingdom of Saudi Arabia | 40 | 37 | Severe TBI, GCS<8 | 20 mmHg | A Camino intraparenchymal catheter to monitor the ICP | 6.4 | Simultaneous | Ocular trauma and/or pathology | 0.85 | 0.83 | (18) |
| Strumwasser et al, 2011 | USA | 10 | 43 | Severe TBI, GCS<8 | 20 mmHg | Insertion of the intracranial device | 6 | Within 1 h | Ocular pathology, pregnant, decompressive craniotomy | 0.36 | 0.38 | (19) |
| Širanović et al, 2011 | Croatia | 20 | 31 | TBI | 20 mmHg | An intraven tricular catheter to monitor the ICP | 6.1 | Simultaneous | Ocular trauma and/or pathology | 1 | 0.83 | (20) |
| Du et al, 2020 | P.R. China | 49 | 50 | TBI | 20 mmHg | An ICP probe was placed in the lateral ventricle | 5.53 | Within 1 h | Previous ocular and optic nerve diseases or injuries at admission | 0.80 | 0.79 | (13) |
| Altayar et al, 2021 | Kingdom of Saudi Arabia | 22 | 40 | TBI | 22 mmHg | External ventricular drainage system | 6.1 | Within 1 h | Penetrating head injuries or any associated ocular injuries | 0.85 | 0.67 | (14) |
ICP, intracranial pressure; ONSD, optic nerve sheath diameter; TBI, traumatic brain injury; GCS, Glasgow Coma Scale.
Quality assessment
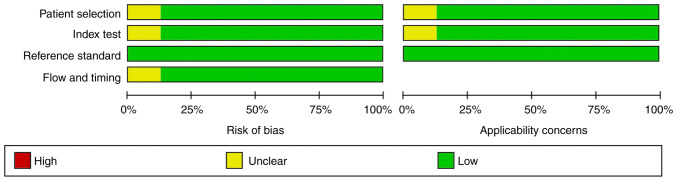
Quality assessment analysis of all the included studies was performed using the QUADAS-2 tool (Table II). No studies exhibited any risk of bias or applicability areas. Of note, one study was considered to be of low quality, since five unclear concerns were documented (20). The quality assessment of the included studies is presented in Figs. 2 and 3.
Table II.
Quality assessment of the included studies using QUADAS-2 tool.
| Risk of bias | Applicability concerns | |||||||
|---|---|---|---|---|---|---|---|---|
| First author/s, Year of publication | Patient selection | Index test | Reference standard | Flow timing | Patient selection | Index test | Reference standard | (Refs.) |
| Geeraerts et al, 2007 | Low | Low | Low | Low | Low | Low | Low | (15) |
| Maissan et al, 2015 | Low | Low | Low | Low | Low | Low | Low | (16) |
| Soldatos et al, 2008 | Low | Low | Low | Low | Low | Low | Low | (17) |
| Soliman et al, 2018 | Low | Low | Low | Low | Low | Low | Low | (18) |
| Strumwasser et al, 2011 | Low | Low | Low | Low | Low | Low | Low | (19) |
| Širanović et al, 2011 | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Low | (20) |
| Du et al, 2020 | Low | Low | Low | Low | Low | Low | Low | (13) |
| Altayar et al, 2021 | Low | Low | Low | Low | Low | Low | Low | (14) |
Figure 2.
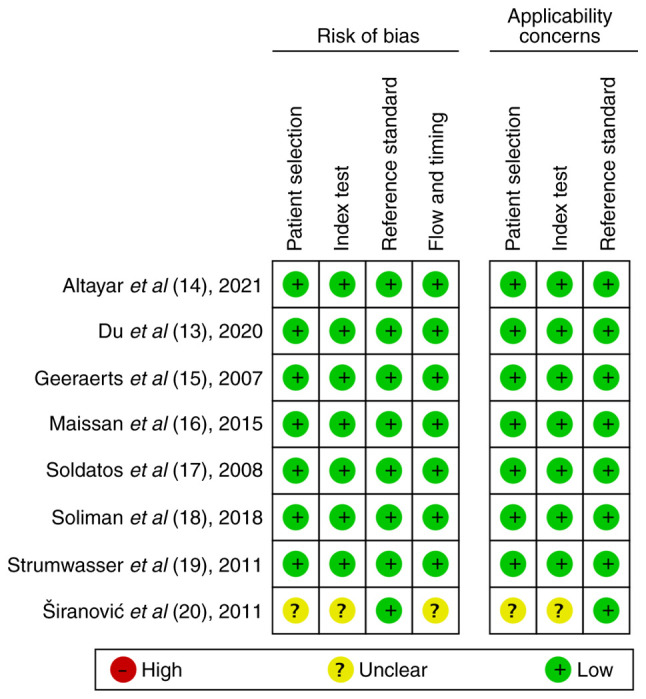
Risk of bias and applicability concerns summary. Evaluation by the authors regarding each domain for each one of the included studies.
Figure 3.
Graph of risk of bias and applicability concerns: Evaluation by the authors regarding each domain are presented as percentages across the included studies.
Quantitative data synthesis results
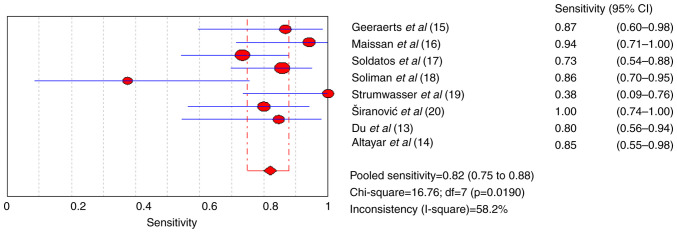
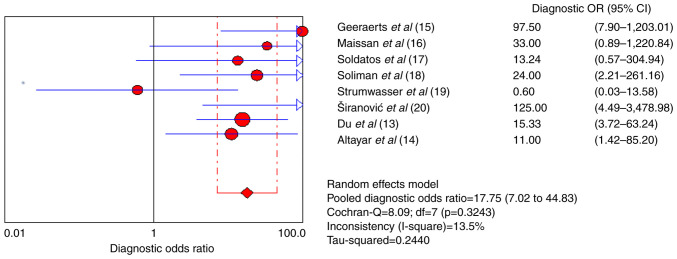
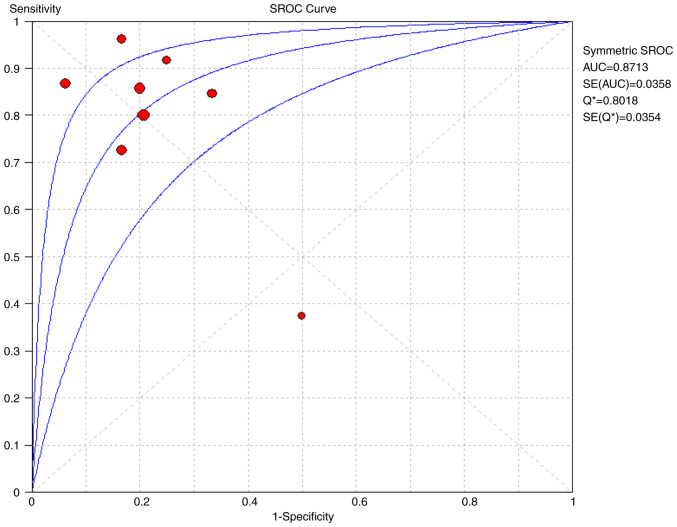
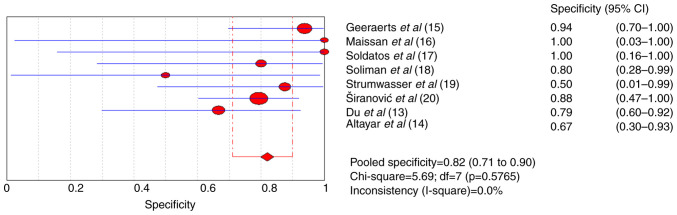
The pooled sensitivity and specificity for increased ICP detected by ultrasound were 0.82 [95% confidence interval (CI), 0.75-0.88] and 0.82 (95% CI, 0.71-0.90), respectively (Figs. 4 and 5). Furthermore, the diagnostic odds ratio (DOR) for ultrasound was 17.75 (95% CI: 7.02-44.83; Fig. 6). The area under the SROC curve analysis revealed an appropriate accuracy of 0.87 (Fig. 7).
Figure 4.
Forest plots of the sensitivity of ultrasonography for a raised intracranial pressure.
Figure 6.
Forest plots of the diagnostic odds ratio of ultrasonography for a raised intracranial pressure.
Figure 7.
Summary plots of five studies investigating the diagnostic ability of ultrasonography to detect a raised intracranial pressure. SROC, summary receiver operating characteristics.
Discussion
The present systematic review and meta-analysis of 222 patients with TBI revealed that ultrasonography performed well in detecting an increased ICP, with an overall pooled sensitivity of 0.82 (95% CI: 0.75-0.88) and a specificity of 0.82 (95% CI: 0.71-0.90). The DOR of ultrasonography was 17.75, indicating that the odds of a positive test in patients with an elevated ICP are >17-fold higher compared with those of a negative test in patients with normal ICP. The area under the SROC curve, which was based on predefined criteria, was relatively high (0.87). The present study confirms the effectiveness of ultrasound as a complementary method to assess the ICP in patients with TBI. Moreover, these results are notable, as invasive ICP monitoring is associated with various risks, including bleeding, infection and malfunction, and are contraindicated in individuals who require prolonged monitoring or have a predisposition to coagulation disorders or platelet disorders (3).
Aletreby et al (9) reported high sensitivity and specificity of the ONSD method (0.90 and 0.85, respectively), which were higher than the results of the present study. Due to the inclusion of various diagnoses, the data in the study by Aletreby et al (9) are heterogeneous both clinically and epidemiologically. Lee et al (10) reported the sensitivity and specificity of the ONSD method (0.91 and 0.77, respectively). However, the number of studies included was relatively small and included studies on the computed tomography measurement of optic nerve sheath diameter (10). In another review study it was revealed that the combined area under the ROC curve of ONSD ultrasound was 0.94 (0.91-0.96) (6). The present systematic review and meta-analysis revealed that ultrasonography performed well in confirming an elevated ICP, with an overall pooled sensitivity of 0.82 and an overall pooled specificity of 0.82. Robba et al (21) reported the pooled DOR for ONSD from the bivariate diagnostic random-effects model return was 67.5 (95% CI 29-135), and the area under the HSROC curve was 0.938, while the partial AUC was 0.916. This finding is in accord with the results of previously published studies (6,21). The conclusions of these studies demonstrated that ONSD can accurately predict ICP despite the lack of consensus on ONSD thresholds and operator-dependent issues.
The results of the present study revealed a high sensitivity and specificity of ONSD in predicting intracranial hypertension. However, the thresholds for ONSD were inconsistent, ranging from 5.0-6.4 mm. The results of the meta-analysis by Montorfano et al (22) revealed a mean ONSD of 5.82 mm (95% CI 5.58-6.06 mm) in patients with an increased ICP. The observed differences may be attributed to differences in the etiology of intracranial hypertension, the clinical setting in which the ONSD was measured, and the criteria used to diagnose intracranial hypertension. Experts suggest following the ONSD trend of the same patient over time, rather than a fixed threshold; this is crucial, considering the variability of ONSD described and the different thresholds under pathological conditions (23). However, in order for it to be useful at the bedside, it is necessary to define a percentage change or threshold (24). The ONSD does not shrink immediately following a marked increase in ICP, since an enlarged ONSD requires drainage of CSF from the intracranial compartment, which does not occur immediately in all cases. Thus, this makes the assessment of trends problematic. Future studies are thus required to focus on both defined thresholds and the behavior of the ONSD with increased ICP.
In recent years, the use of ultrasound has markedly increased due to various factors, including technological advances, a minimally invasive nature, affordability and the ease of use (25,26). The use of optic nerve ultrasound as an accurate, non-invasive, safe, reproducible and cost-effective ICP tool has recently been validated by the measurement of ONSD, thereby reducing the potentially deleterious consequences of invasive transcranial measurements (14,27,28). Ultrasound of the optic nerve can be used to differentiate between both normal and elevated ICP and can be a useful screening tool in resource-limited practices (29).
The present systematic review has several methodological limitations. The total sample of patients with TBI was relatively small, with only eight studies with 222 patients having been included in the analysis. All included studies used invasive ICP monitoring, possibly combined with debridement decompression. However, Gao et al (30) reported that the accuracy of invasive ICP monitoring after debridement decompression was low, which may have affected the accuracy of the present study. In addition, a standardized ONSD cut-off value as a criterion for diagnosing an elevated ICP was not established, since different studies used various cut-off points and ultrasound techniques. An ONSD detected by ultrasound does not accurately reflect the ICP values above or below the threshold of 20 cm H2O; however, the method can be used to monitor ICP trends and evaluate the response to therapeutic interventions.
In conclusion, based on the available evidence, ultrasound determined elevated ICP has reasonable performance indicators with high sensitivity and specificity in patients with TBI. This method is a useful complementary monitoring tool in acute care. The use of ultrasound to assess ICP in TBI is less risky, more cost-effective and quicker than invasive methods, aiding towards more accurate clinical decision-making, particularly in resource-limited settings.
Supplementary Material
Figure 5.
Forest plots of the specificity of ultrasonography for a raised intracranial pressure.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
WC and PY conceived and designed the study. WC and XZ wrote and prepared the draft of the manuscript. XY and XZ collected data. All authors contributed to manuscript revision and have read and approved the final version of the manuscript. WC and XZ confirm authenticity all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hawryluk GWJ, Citerio G, Hutchinson P, Kolias A, Meyfroidt G, Robba C, Stocchetti N, Chesnut R. Intracranial pressure: Current perspectives on physiology and monitoring. Intensive Care Med. 2022;48:1471–1481. doi: 10.1007/s00134-022-06786-y. [DOI] [PubMed] [Google Scholar]
- 2.Cucciolini G, Motroni V, Czosnyka M. Intracranial pressure for clinicians: It is not just a number. J Anesth Analg Crit Care. 2023;3(31) doi: 10.1186/s44158-023-00115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavakoli S, Peitz G, Ares W, Hafeez S, Grandhi R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017;43(E6) doi: 10.3171/2017.8.FOCUS17450. [DOI] [PubMed] [Google Scholar]
- 4.Lane BC, Scranton R, Cohen-Gadol AA. Risk of brain herniation after craniotomy with preoperative lumbar spinal drainage: A single-surgeon experience of 365 patients among 3000 major cranial cases. Oper Neurosurg (Hagerstown) 2021;20:E77–E82. doi: 10.1093/ons/opaa262. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery SP, Moore B, Hampton SM, Macy G, Li W, Bronshteyn YS. Optic nerve sheath point of care ultrasound: Image acquisition. J Vis Exp. 2023 doi: 10.3791/64929. [DOI] [PubMed] [Google Scholar]
- 6.Fernando SM, Tran A, Cheng W, Rochwerg B, Taljaard M, Kyeremanteng K, English SW, Sekhon MS, Griesdale DEG, Dowlatshahi D, et al. Diagnosis of elevated intracranial pressure in critically ill adults: Systematic review and meta-analysis. BMJ. 2019;366(l4225) doi: 10.1136/bmj.l4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, Shook AC, Smielewski P, Czosnyka M, Gupta AK, Menon DK. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014;40:1267–1274. doi: 10.1007/s00134-014-3392-7. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez Restrepo JN, León OJ, Quevedo Florez LA. Ocular ultrasonography: A useful instrument in patients with trauma brain injury in emergency service. Emerg Med Int. 2019;2019(9215853) doi: 10.1155/2019/9215853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aletreby W, Alharthy A, Brindley PG, Kutsogiannis DJ, Faqihi F, Alzayer W, Balhahmar A, Soliman I, Hamido H, Alqahtani SA, et al. Optic nerve sheath diameter ultrasound for raised intracranial pressure: A literature review and meta-analysis of its diagnostic accuracy. J Ultrasound Med. 2022;41:585–595. doi: 10.1002/jum.15732. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Kim HS, Yun SJ. Optic nerve sheath diameter measurement for predicting raised intracranial pressure in adult patients with severe traumatic brain injury: A meta-analysis. J Crit Care. 2020;56:182–187. doi: 10.1016/j.jcrc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1) doi: 10.1186/2046-4053-4-1. PRISMA-P Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6(31) doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du J, Deng Y, Li H, Qiao S, Yu M, Xu Q, Wang C. Ratio of optic nerve sheath diameter to eyeball transverse diameter by ultrasound can predict intracranial hypertension in traumatic brain injury patients: A prospective study. Neurocrit Care. 2020;32:478–485. doi: 10.1007/s12028-019-00762-z. [DOI] [PubMed] [Google Scholar]
- 14.Altayar AS, Abouelela AZ, Abdelshafey EE, Mohammed KSS, Hassan AA, Khattab MA, Alhabashy W, Gomaa W, Mohammed AF, Umerani MS. Optic nerve sheath diameter by ultrasound is a good screening tool for high intracranial pressure in traumatic brain injury. Ir J Med Sci. 2021;190:387–393. doi: 10.1007/s11845-020-02242-2. [DOI] [PubMed] [Google Scholar]
- 15.Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B, Duranteau J, Benhamou D. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. 2007;33:1704–1711. doi: 10.1007/s00134-007-0797-6. [DOI] [PubMed] [Google Scholar]
- 16.Maissan IM, Dirven PJAC, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123:743–747. doi: 10.3171/2014.10.JNS141197. [DOI] [PubMed] [Google Scholar]
- 17.Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. 2008;12(R67) doi: 10.1186/cc6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman I, Johnson GGRJ, Gillman LM, Zeiler FA, Faqihi F, Aletreby WT, Balhamar A, Mahmood NN, Ahmad Mumtaz S, Alharthy A, et al. New optic nerve sonography quality criteria in the diagnostic evaluation of traumatic brain injury. Crit Care Res Pract. 2018;2018(3589762) doi: 10.1155/2018/3589762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strumwasser A, Kwan RO, Yeung L, Miraflor E, Ereso A, Castro-Moure F, Patel A, Sadjadi J, Victorino GP. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. 2011;170:265–271. doi: 10.1016/j.jss.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Širanović M, Magdić Turković T, Gopčević A, Kelečić M, Kovač N, Kovač J, Rode B, Vučić M. Comparison of ultrasonographic measurement of optic nerve sheath diameter (ONSD) versus direct measurement of intracranial pressure (ICP) in traumatic brain injury patients. Signa Vitae. 2011;6:33–35. [Google Scholar]
- 21.Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, Taccone FS, Citerio G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2018;44:1284–1294. doi: 10.1007/s00134-018-5305-7. [DOI] [PubMed] [Google Scholar]
- 22.Montorfano L, Yu Q, Bordes SJ, Sivanushanthan S, Rosenthal RJ, Montorfano M. Mean value of B-mode optic nerve sheath diameter as an indicator of increased intracranial pressure: A systematic review and meta-analysis. Ultrasound J. 2021;13(35) doi: 10.1186/s13089-021-00235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen HC, Helmke K. Optic nerve sheath responses to pressure variations. Intensive Care Med. 2019;45:1840–1841. doi: 10.1007/s00134-019-05746-3. [DOI] [PubMed] [Google Scholar]
- 24.Robba C, Santori G, Czosnyka M, Corradi F, Citerio G. Optic nerve sheath diameter: The next steps. Intensive Care Med. 2019;45:1842–1843. doi: 10.1007/s00134-019-05769-w. [DOI] [PubMed] [Google Scholar]
- 25.Liao SF, Chen PJ, Chaou CH, Lee CH. Top-cited publications on point-of-care ultrasound: The evolution of research trends. Am J Emerg Med. 2018;36:1429–1438. doi: 10.1016/j.ajem.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Wu XL, Wang JJ, Yuan DQ, Chen WT. Ultrasound-guided radial artery catheterization at different sites: A prospective and randomized study. Eur Rev Med Pharmacol Sci. 2022;26:415–421. doi: 10.26355/eurrev_202201_27865. [DOI] [PubMed] [Google Scholar]
- 27.Bhandari D, Udupi Bidkar P, Adinarayanan S, Narmadhalakshmi K, Srinivasan S. Measurement of changes in optic nerve sheath diameter using ultrasound and computed tomography scan before and after the ventriculoperitoneal shunt surgery in patients with hydrocephalus-A prospective observational trial. Br J Neurosurg. 2019;33:125–130. doi: 10.1080/02688697.2019.1576856. [DOI] [PubMed] [Google Scholar]
- 28.Cho BI, Lee H, Shin H, Kim C, Choi HJ, Kang BS. The prognostic value of optic nerve sheath diameter/eyeball transverse diameter ratio in the neurological outcomes of out-of-hospital cardiac arrest patients. Medicina (Kaunas) 2022;58(1233) doi: 10.3390/medicina58091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aduayi OS, Asaleye CM, Adetiloye VA, Komolafe EO, Aduayi VA. Optic nerve sonography: A noninvasive means of detecting raised intracranial pressure in a resource-limited setting. J Neurosci Rural Pract. 2015;6:563–567. doi: 10.4103/0976-3147.165347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Li Q, Wu C, Liu S, Zhang M. Diagnostic and prognostic value of the optic nerve sheath diameter with respect to the intracranial pressure and neurological outcome of patients following hemicraniectomy. BMC Neurol. 2018;18(199) doi: 10.1186/s12883-018-1202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.