Abstract
NADH:nitrate reductase (EC 1.6.6.1) and NAD(P)H:nitrate reductase (EC 1.6.6.2) were purified from wild-type soybean (Glycine max [L.] Merr., cv Williams) and nr1-mutant soybean plants. Purification included Blue Sepharose- and hydroxylapatite-column chromatography using acetone powders from fully expanded unifoliolate leaves as the enzyme source.
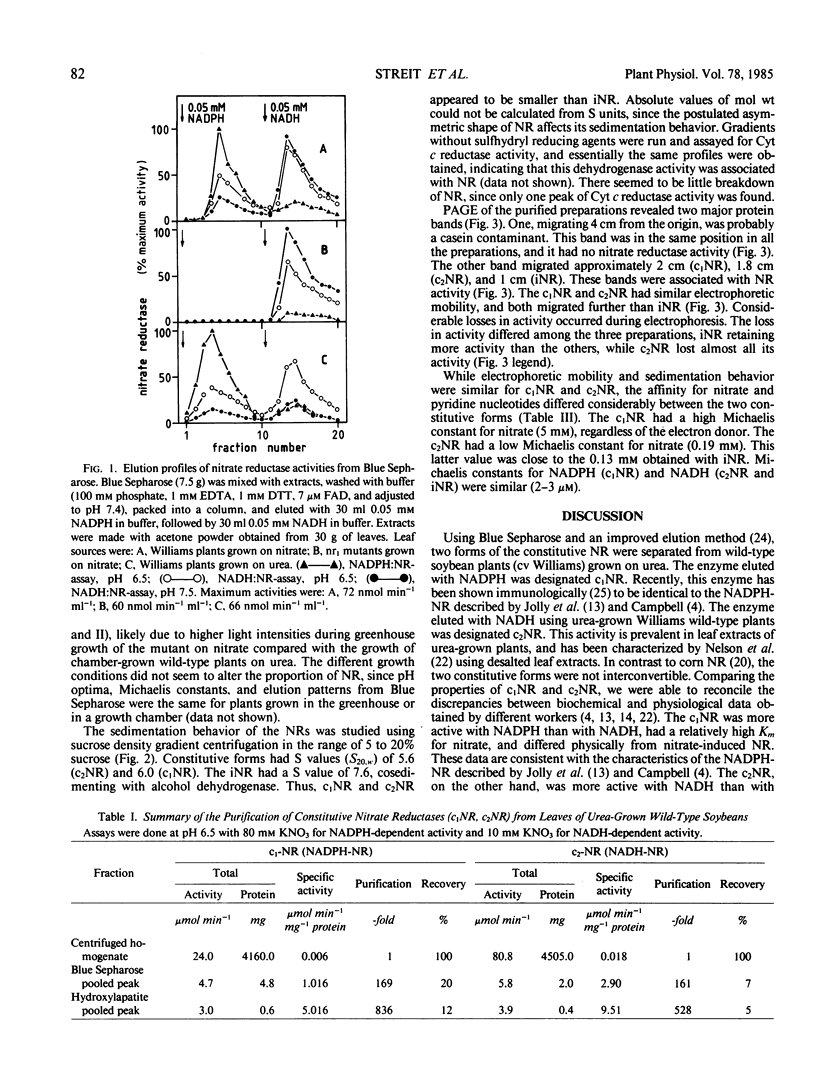
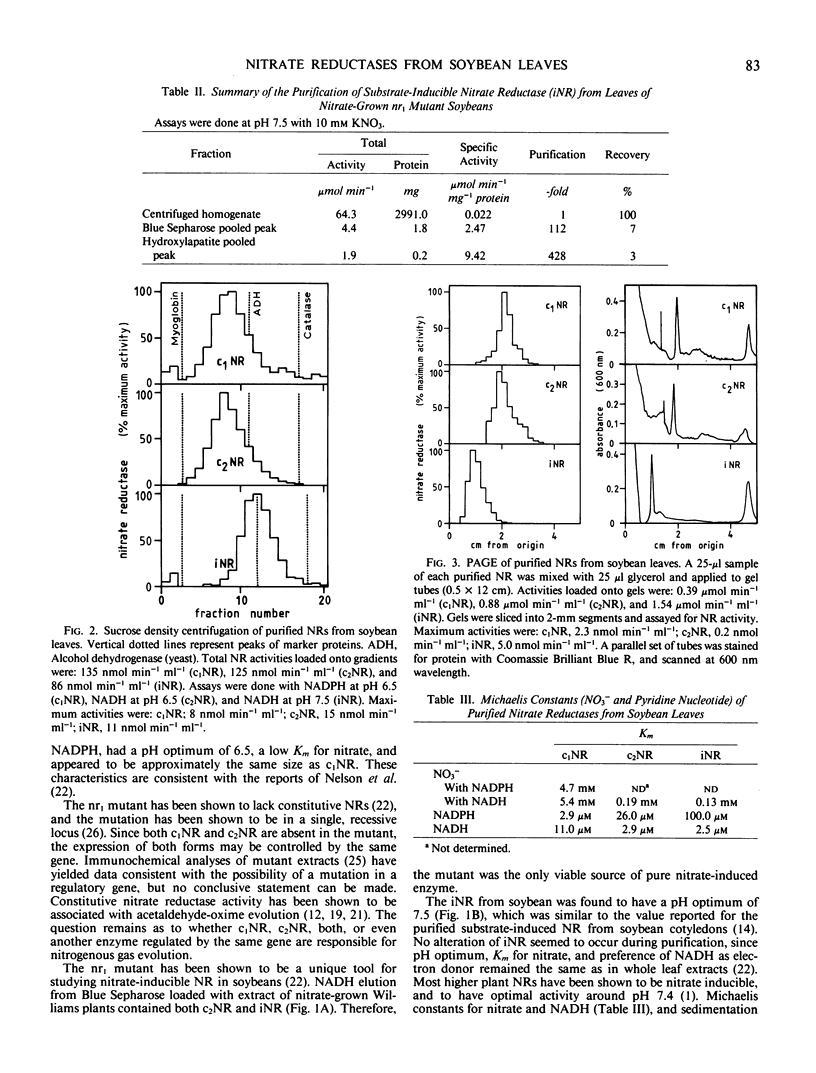
Two forms of constitutive nitrate reductase were sequentially eluted with NADPH and NADH from Blue Sepharose loaded with extract from wild-type plants grown on urea as sole nitrogen source. The form eluted with NADPH was designated c1NR, and the form eluted with NADH was designated c2NR. Nitrate-grown nr1 mutant soybean plants yielded a NADH:nitrate reductase (designated iNR) when Blue Sepharose columns were eluted with NADH; NADPH failed to elute any NR form from Blue Sepharose loaded with this extract. Both c1NR and c2NR had similar pH optima of 6.5, sedimentation behavior (s20,w of 5.5-6.0), and electrophoretic mobility. However, c1NR was more active with NADPH than with NADH, while c2NR preferred NADH as electron donor. Apparent Michaelis constants for nitrate were 5 millimolar (c1NR) and 0.19 millimolar (c2NR). The iNR from the mutant had a pH optimum of 7.5, s20,w of 7.6, and was less mobile on polyacrylamide gels than c1NR and c2NR. The iNR preferred NADH over NADPH and had an apparent Michaelis constant of 0.13 millimolar for nitrate.
Thus, wild-type soybean contains two forms of constitutive nitrate reductase, both differing in their physical properties from nitrate reductases common in higher plants. The inducible nitrate reductase form present in soybeans, however, appears to be similar to most substrateinduced nitrate reductases found in higher plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Dailey F. A., Kuo T., Warner R. L. Pyridine nucleotide specificity of barley nitrate reductase. Plant Physiol. 1982 May;69(5):1196–1199. doi: 10.1104/pp.69.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E. Evolution of Nitrogen Oxide(s) during In Vivo Nitrate Reductase Assay of Soybean Leaves. Plant Physiol. 1981 Dec;68(6):1488–1493. doi: 10.1104/pp.68.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Campbell W., Tolbert N. E. NADPH- and NADH-nitrate reductases from soybean leaves. Arch Biochem Biophys. 1976 Jun;174(2):431–439. doi: 10.1016/0003-9861(76)90371-4. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mulvaney C. S., Hageman R. H. Acetaldehyde Oxime, A Product Formed during the In Vivo Nitrate Reductase Assay of Soybean Leaves. Plant Physiol. 1984 Sep;76(1):118–124. doi: 10.1104/pp.76.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Poulle M., Oaks A. Characterization of Nitrate Reductase from Corn Leaves (Zea mays cv W64A x W182E) : Two Molecular Forms of the Enzyme. Plant Physiol. 1984 Jun;75(2):285–289. doi: 10.1104/pp.75.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. S., Ryan S. A., Harper J. E. Soybean mutants lacking constitutive nitrate reductase activity : I. Selection and initial plant characterization. Plant Physiol. 1983 Jun;72(2):503–509. doi: 10.1104/pp.72.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin P., Streit L., Campbell W. H., Harper J. E. Immunochemical Characterization of Nitrate Reductase Forms from Wild-Type (cv Williams) and nr(1) Mutant Soybean. Plant Physiol. 1985 Jan;77(1):232–236. doi: 10.1104/pp.77.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. A., Nelson R. S., Harper J. E. Soybean Mutants Lacking Constitutive Nitrate Reductase Activity : II. Nitrogen Assimilation, Chlorate Resistance, and Inheritance. Plant Physiol. 1983 Jun;72(2):510–514. doi: 10.1104/pp.72.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T. C., Funkhouser E. A., Guerrero M. G. NADH- and NAD(P)H-Nitrate Reductases in Rice Seedlings. Plant Physiol. 1976 Sep;58(3):292–294. doi: 10.1104/pp.58.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W., Johnson C. B. Nitrate Reductase and Soluble Cytochrome c Reductase(s) in Higher Plants. Plant Physiol. 1978 May;61(5):748–752. doi: 10.1104/pp.61.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]


