Abstract
Background
Arthritis of the knee is a common problem causing pain and disability. If severe, knee arthritis can be surgically managed with a total knee arthroplasty. Rehabilitation following knee arthroplasty often includes continuous passive motion (CPM). CPM is applied by a machine that passively and repeatedly moves the knee through a specified range of motion (ROM). It is believed that CPM increases recovery of knee ROM and has other therapeutic benefits. However, it is not clear whether CPM is effective.
Objectives
To assess the benefits and harms of CPM and standard postoperative care versus similar postoperative care, with or without additional knee exercises, in people with knee arthroplasty. This review is an update of a 2003 and 2010 version of the same review.
Search methods
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 12), MEDLINE (January 1966 to 24 January 2013), EMBASE (January 1980 to 24 January 2013), CINAHL (January 1982 to 24 January 2013), AMED (January 1985 to 24 January 2013) and PEDro (to 24 January 2013).
Selection criteria
Randomised controlled trials in which the experimental group received CPM, and both the experimental and control groups received similar postoperative care and therapy following total knee arthroplasty in people with arthritis.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data and assessed risk of bias. The primary outcomes of interest were active knee flexion ROM, pain, quality of life, function, participants' global assessment of treatment effectiveness, incidence of manipulation under anaesthesia and adverse events. The secondary outcomes were passive knee flexion ROM, active knee extension ROM, passive knee extension ROM, length of hospital stay, swelling and quadriceps strength. We estimated effects for continuous data as mean differences or standardised mean differences (SMD), and effects for dichotomous data as risk ratios; all with 95% confidence intervals (CI). If appropriate, we performed meta‐analyses using random‐effects models.
Main results
We identified 684 papers from the electronic searches after removal of duplicates and retrieved the full reports of 62 potentially eligible trials. Twenty‐four randomised controlled trials of 1445 participants met the inclusion criteria; four of these trials were new to this update.
There was moderate‐quality evidence to indicate that CPM does not have clinically important short‐term effects on active knee flexion ROM: mean knee flexion was 78 degrees in the control group, CPM increased active knee flexion ROM by 2 degrees (95% CI 0 to 5) or absolute improvement of 2% (95% CI 0% to 4%). The medium‐ and long‐term effects are similar although the quality of evidence is lower.
There was low‐quality evidence to indicate that CPM does not have clinically important short‐term effects on pain: mean pain was 3 points in the control group, CPM reduced pain by 0.4 points on a 10‐point scale (95% CI ‐0.8 to 0.1) or absolute reduction of ‐4% (95% CI ‐8% to 1%).
There was moderate‐quality evidence to indicate that CPM does not have clinically important medium‐term effects on function: mean function in the control group was 56 points, CPM decreased function by 1.6 points (95% CI ‐6.1 to 2.0) on a 100‐point scale or absolute reduction of ‐2% (95% CI ‐5% to 2%). The SMD was ‐0.1 standard deviations (SD) (95% CI ‐0.3 to 0.1).
There was moderate‐quality evidence to indicate that CPM does not have clinically important medium‐term effects on quality of life: mean quality of life was 40 points in the control group, CPM improved quality of life by 1 point on a 100‐point scale (95% CI ‐3 to 4) or absolute improvement of 1% (95% CI ‐3% to 4%).
There was very low‐quality evidence to indicate that CPM reduces the risk of manipulation under anaesthesia; risk of manipulation in the control group was 7.2%, risk of manipulation in the experimental group was 1.6%, CPM decreased the risk of manipulation by 25 fewer manipulations per 1000 (95% CI 9 to 64) or absolute risk reduction of ‐4% (95% CI ‐8% to 0%). The risk ratio was 0.3 (95% CI 0.1 to 0.9).
There was low‐quality evidence to indicate that CPM reduces the risk of adverse events; risk of adverse events in the control group was 16.3%, risk of adverse events in the experimental group was 15%, CPM decreased the risk of adverse event by 13 fewer adverse events per 1000 or absolute risk reduction of ‐1% (95% CI ‐5% to 3%). The risk ratio was 0.9 (95% CI 0.6 to 1.3). The estimates for risk of manipulation and adverse events are very imprecise and the estimate for the risk of adverse events does not distinguish between a clinically important increase and decrease in risk.
There was insufficient evidence to determine the effect of CPM on participants' global assessment of treatment effectiveness.
Authors' conclusions
CPM does not have clinically important effects on active knee flexion ROM, pain, function or quality of life to justify its routine use. It may reduce the risk of manipulation under anaesthesia and risk of developing adverse events although the quality of evidence supporting these findings are very low and low, respectively. The effects of CPM on other outcomes are unclear.
Plain language summary
Continuous passive motion after knee replacement surgery
Background
Knee replacement surgery is common for the management of arthritis but can cause knee stiffness. Knee stiffness can make it difficult to perform certain activities including standing up from a seated position. Continuous passive motion (CPM) is a way of providing regular movement to the knee using a machine. This Cochrane review presents what we know about the effects of CPM following knee surgery. After searching for all relevant studies in January 2013, we found 24 studies with 1445 participants who had knee replacement surgery primarily for knee arthritis. CPM was started from the first to the fourth day post surgery and applied for 1.5 to 24 hours a day, over 1 to 17 days. The review showed that CPM following knee replacement surgery probably improves the ability to bend the knee slightly and the person's quality of life but may not improve pain or function. We are uncertain about the effects of CPM on need for manipulation under anaesthesia, participants' perceptions of treatment effectiveness or risk of complications.
Best estimates of what happens to people who have CPM after knee replacement surgery are:
Range of motion ‐ active knee flexion (i.e. ability to bend the knee)
People who had CPM were able to bend their knees an average of 2 degrees more (0 to 5 degrees more) than those who did not have CPM at six weeks (2% absolute improvement, 0 to 4% absolute improvement)
‐ People who had CPM were able to bend their knees an average of 80 degrees.
‐ People who did not have CPM were able to bend their knees an average of 78 degrees.
Pain (higher scores means worse or more severe pain)
People who had CPM rated their pain an average of 0.4 points lower (0.8 points lower to 0.1 points higher) on a 0 to 10 point scale at six weeks (4% absolute reduction, 8% reduction to 1% increase)
‐ People who had CPM rated their pain an average of 2.6 points on a 0 to 10 scale.
‐ People who did not have CPM rated their pain an average of 3 points on a 0 to 10 scale.
Function (higher scores means better function)
People who had CPM had a loss in function equivalent to an average of 1.6 points on a 0‐ to 100‐point scale at six months (2% absolute reduction, 5% reduction to 2% increase).
‐ People who had CPM had function equivalent to an average of 56 points on a 0‐ to 100‐point scale.
‐ People who did not have CPM had function equivalent to an average of 57.6 points on a 0‐ to 100‐points scale.
Quality of life (higher scores means better quality of life)
People who had CPM had an increase in quality of life equivalent to an average of 1 point on a 0‐ to 100‐point scale at six months (1% absolute improvement, 3% reduction to 4% increase).
‐ People who had CPM had a quality of life equivalent to an average of 41 points on a 0‐ to 100‐point scale.
‐ People who did not have CPM had function equivalent to an average of 40 points on a 0‐ to 100‐points scale.
Manipulation under anaesthesia
People who had CPM had a decrease in the risk of requiring manipulation under anaesthesia equivalent to an average of 25 fewer manipulations per 1000 patients (4% absolute risk reduction, 8% risk reduction to 0% risk reduction).
‐ People who had CPM had on average a 1.6% risk of requiring manipulation under anaesthesia.
‐ People who did not have CPM had on average a 7.2% risk of requiring manipulation under anaesthesia.
Adverse events
People who had CPM had a decrease in the risk of developing adverse events equivalent to an average of 13 fewer adverse events per 1000 patients (1% absolute risk reduction, 5% risk reduction to 3% risk increase).
‐ People who had CPM had on average a 15% risk of developing adverse events.
‐ People who did not have CPM had on average a 16.3% risk of developing adverse events.
Summary of findings
Summary of findings for the main comparison. Primary comparison for continuous passive motion (CPM) versus no CPM.
| Primary comparison for continuous passive motion (CPM) versus no CPM | ||||||
| Patient or population: hospitalised patients who have undergone knee replacement surgery Settings: hospital Intervention: Continuous passive motion (CPM) versus no CPM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Primary comparison | |||||
| Active knee flexion ROM Goniometer. Scale: 0 to 130 Follow‐up: 6 weeks | The mean active knee flexion ROM in the control groups was 78 degrees | The mean active knee flexion ROM in the intervention groups was 2 higher (0 to 5 higher) | ‐ | 470 (10 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference 2% (0 to 4); relative percent change 0% (2 to 5); not statistically significant2 |
| Pain Visual analogue scale. Scale: 0 to 10 (lower score better) Follow‐up: 6 weeks | The mean pain in the control groups was 3 points | The mean pain in the intervention groups was 0.4 lower (0.8 lower to 0.1 higher) | ‐ | 414 (8 studies) | ⊕⊕⊝⊝ low1,3,4 | Absolute risk difference ‐4% (‐8 to 1); relative percent change ‐80% (‐36 to 8); not statistically significant5 |
| Function Various. Scale: 0 to 100 (higher score better) Follow‐up: 6 months | The mean function in the control groups was 56 points6 | The mean function in the intervention groups was 1.6 lower (6.1 lower to 2 higher)7 | ‐ | 405 (6 studies) | ⊕⊕⊕⊝ moderate1,3 | SMD ‐0.1 (‐0.3 to 0.1); absolute risk difference ‐2% (‐5 to 2); relative percent change ‐4 (‐12 to 5)8 |
| Quality of life SF‐12. Scale from: 0 to 100 (higher score better). Follow‐up: 6 months | The mean quality of life in the control groups was 40 points | The mean quality of life in the intervention groups was 1 higher (3 lower to 4 higher) | 156 (2 studies) | ⊕⊕⊕⊝ moderate1,3 | Absolute risk difference 1% (‐3 to 4) ; Relative percent change ‐8% (‐8 to 13); non‐statistically significant9 | |
| Participants' global assessment of treatment effectiveness Questionnaire. Scale: 0 to 7 Follow‐up: 6 weeks | See comment | See comment | Not estimable | 211 (3 studies) | ⊕⊝⊝⊝ very low1,3,10,11 | Not estimable12 |
| Manipulation under anaesthesia Counts of manipulation under anaesthesia Follow‐up: 6 weeks | 72 per 1000 | 25 per 1000 (9 to 64) | RR 0.34 (0.13 to 0.89) | 581 (8 studies) | ⊕⊝⊝⊝ very low4,13,14 | Absolute risk difference ‐4% (‐8 to 0); relative percent decrease ‐67% (‐87 to 11); NNTB 21 (16 to 126) |
| Adverse events Counts of adverse events Follow‐up: 6 weeks | 163 per 1000 | 150 per 1000 (103 to 216) | RR 0.92 (0.63 to 1.33) | 1040 (16 studies) | ⊕⊕⊝⊝ low1,15 | Absolute risk difference ‐1% (‐5 to 3); relative percent decrease ‐9% (‐37 to 33); not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; ROM: range of motion; RR: risk ratio; SF‐12: Short‐Form 12‐Item Health Survey. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The methodological quality of at least some of the included trials were susceptible to bias. 2 Relative percentage change based on baseline mean of Sahin 2006 (105 degrees). 3 This outcome included self‐report from participants who were not blinded to the intervention. This can cause bias. 4 There are inconsistencies between the trials. 5 Relative percentage change based on baseline mean of Lenssen 2008 (1.05 points on a 0‐ to 10‐point scale converted from a 0‐ to 100‐points scale for consistency with other trials). 6 This is the mean final score of the control participants of Bennett 2005 (mean 56; standard deviation (SD) 21.63). Results are expressed in relationship to Knee Society Score (0‐ to 100‐point scale). 7 Estimate of the effect is back‐translated from standardised mean difference (SMD) ‐0.08 (95% confidence interval (CI) ‐0.27 to 0.12) using the baseline SD of Bennett 2005 (SD 20.19). Results are expressed in relationship to Knee Society Score (0‐ to 1000‐point scale). 8 Absolute risk difference is based on the mean and SD of the control group at baseline in Bennett 2005 (mean 45.3; standard error 2.8; SD 20.19). Results are expressed in relationship to Knee Society Score (0‐ to 100‐point scale). 9 Relative percentage change based on baseline mean of Maniar 2012 (30.58 points). 10 The point estimate of 1 trial favours the continuous passive motion (CPM) group and the point estimates of the other 2 trials favours the control group. 11 The 95% CI of the estimate of each trial is wide. 12 Data were not pooled. 13 It is not clear whether those making the decisions about the need for manipulation were blinded. 14 The CI for the risk ratio is wide. 15 The 95% CI associated with the risk ratio includes the possibility of an important increase and decrease in the risk of adverse events with CPM.
Background
Description of the condition
Total knee arthroplasties (TKA) are surgical procedures that involve replacing the knee joint with artificial components. They are commonly performed to reduce pain and increase function in people with severe arthritis such as osteoarthritis (OA) and rheumatoid arthritis (RA). A common problem post surgery is joint stiffness.
Description of the intervention
Continuous passive motion (CPM) was first introduced by Salter et al in the 1960s (Salter 1989). It is a way of providing regular movement to the knee using an external motorised device that passively moves the joint through a pre‐set arc of motion (Sheppard 1995).
How the intervention might work
CPM is primarily advocated in the belief that it increases knee range of motion (ROM) (McCarthy 1992; Salter 1989). This is supported by animal studies, which have demonstrated that early movement minimises joint stiffness and improves ROM (Videman 1987). However, some scientists also claim that it reduces pain (Harms 1991), length of hospital stay (Fisher 1985; Schnebel 1989), and the need for manipulation under anaesthesia (a procedure that involves forcefully flexing the knee while the patient is anaesthetised to increase knee ROM (Fox 1981).
Why it is important to do this review
There remains uncertainty about whether CPM is therapeutic following TKA. For example, CPM continues to be prescribed as part of postoperative care following TKA, commercial companies continue to advertise CPM machines as a way to improve outcomes following TKA and research attention continues to be directed at determining the effects of CPM. Therefore, the purpose of this systematic review was to update two earlier versions of the same review with the overall aim of providing an objective synthesise of the evidence about the effectiveness of CPM following TKA for people with arthritis.
Objectives
To assess the benefits and harms of CPM and standard postoperative care versus similar postoperative care, with or without additional knee exercises, in people with knee arthroplasty. This review is an update of a 2003 and 2010 version of the same review.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCT), regardless of language. We accepted abstracts. We did not exclude trials based on quality assessment.
Types of participants
Participants could be of any age provided they were hospitalised following TKA. All participants needed to have a pre‐surgery diagnosis of arthritis.
Types of interventions
We included trials if CPM and standard postoperative care were compared with similar postoperative care with or without additional knee exercises. Standard postoperative care could include muscle‐strengthening exercises (isometric or dynamic), functional exercises, gait training, immobilisation or ice, provided both groups received the same intervention. Additional knee exercises could include instructions or supervised active or passive knee ROM exercises. They could not include knee exercises provided with any type of CPM device.
Types of outcome measures
Major outcomes
The primary outcomes of interest were active knee flexion ROM, pain, function, quality of life, participants' global assessment of treatment effectiveness, need for manipulation under anaesthesia and adverse events. If study authors did not distinguish between active and passive knee flexion ROM then it was assumed that the measurement was passive. Only direct measures of pain intensity were of interest. These included pain scales but not pain medication. These outcomes are presented in Table 1.
Secondary outcomes
Secondary outcomes were passive knee flexion ROM, active knee extension ROM, passive knee extension ROM, length of hospital stay, swelling and quadriceps strength. If authors did not distinguish between active and passive knee ROM then it was assumed that the measurement was passive. We interpreted "knee extension lag" as active knee extension ROM and "fixed deformity" as passive knee extension ROM.
All results, except need for manipulation and adverse events, were categorised into short‐term effects of CPM (reflected in outcomes taken less than six weeks after randomisation), medium‐term effects of CPM (reflected in outcomes taken six weeks to six months after randomisation) and long‐term effects of CPM (reflected in outcomes taken more than six months after randomisation). Where trials collected data at multiple time periods within one of these categories, we used the data collected at the longest time since randomisation. Need for manipulation and adverse events were extracted regardless of time since randomisation.
Each of the primary outcomes were used in the 'Summary of findings' table. We used short‐term effects except for function and quality of life where we used medium‐term effects because either there were no data for the short‐term effects or the data could not be pooled because of heterogeneity.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 12);
MEDLINE (January 1966 to January 24, 2013);
EMBASE (January 1980 to January 24, 2013);
CINAHL (January 1982 to January 24, 2013);
AMED (January 1985 to January 24, 2013);
PEDro (to January 24, 2013).
We applied no language restrictions. The database details and results of all the searches are recorded in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; and Appendix 6.
Searching other resources
We checked the reference lists of all included trials for other potentially relevant trials.
Data collection and analysis
Selection of studies
Two independent review authors examined the titles and abstracts of trials identified by the search strategy for trials that potentially met the inclusion criteria. We retrieved all trials classified as potentially eligible by at least one of the review authors. We re‐examined the retrieved articles to ensure that they met the inclusion criteria.
Data extraction and management
Two review authors independently extracted characteristics of included studies and results from each of the included trials. We resolved discrepancies by consensus. We addressed questions regarding the relative effectiveness of different dosages of CPM in a meta‐regression (see 'Data synthesis' for details). We used standardised mean differences (SMDs) when different scales were used to measure the same construct (e.g. function). SMDs were calculated by dividing the difference between treated and control means by the pooled estimate of the SD. If authors of trials provided both intention‐to‐treat and per‐protocol data, we used the intention‐to‐treat data. We did not impute missing data.
Assessment of risk of bias
Two review authors independently assessed the risk of bias in each trial using the method recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following methodological domains:
random sequence generation;
allocation sequence concealment;
blinding of participants;
blinding of therapists;
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting; and
other potential sources of bias.
We rated each potential source of bias as high, low or unclear (either lack of information or uncertainty over the potential for bias). We attempted to contact all study authors to clarify any ambiguities.
We resolved disagreements in ratings by discussion or, where necessary, by consulting a third review author.
Measures of treatment effect
For continuous data, we presented summary estimates as mean differences (MD) and 95% confidence intervals (CI) for pain, ROM and quality of life (weighted by the inverse of the variances of the estimates) or SMD and 95% CI for outcomes measured on different scales (function). SMDs were back‐translated to a typical scale (e.g. 0 to 100 for function) by multiplying the SMD by a typical among‐person SD (e.g. the SD of the control group at baseline from the most representative trial).
For dichotomous outcomes, we presented results as risk ratios (RR) and 95% CI.
For outcomes where it was desirable to have a lower score (e.g. pain); a negative value indicated a beneficial treatment effect of CPM. Conversely, for outcomes where it was desirable to have a larger score (e.g. knee flexion ROM); a positive value indicates a beneficial treatment effect of CPM. For some outcomes, such as function, different outcomes were used in different trials and the direction of beneficial treatment effect was not consistent such that higher numbers sometimes indicated a better outcome while in other trials it was the opposite. To overcome this problem, we inverted some data to ensure all outcomes were consistent within the one analysis.
Unit of analysis issues
In trials with more than two groups, we extracted only data from the two groups with the most contrasting interventions and used these for analyses. For example, in trials with one control and two CPM groups, we included only the results of the control group and CPM group with the highest dosage in analyses. We adopted this approach to reduce complexity and increase the readability of the systematic review.
Dealing with missing data
If outcomes were only reported graphically, we estimated the mean scores and SDs from the graphs.
Data synthesis
We estimated the effect of CPM by taking the difference in the mean outcome of the groups that did and did not receive CPM. For the primary analysis, the standard postoperative care may or may not have included additional knee exercises to one or both groups. We conducted a secondary analysis on just those trials in which the standard postoperative care for the control group included additional knee exercises. These trials provide a head‐to‐head comparison of the effectiveness of CPM and additional knee exercises. We pooled the MDs in outcomes from each trial to obtain a summary estimate of the effectiveness of CPM provided the I2 statistic was not greater than 50%. We used random‐effects models throughout. We used random‐effects meta‐regression to explore the effect of mean total CPM time (hours) on passive knee flexion ROM in the short term. We used the user‐written metareg routine in the Stata software package (version 10) for this purpose.
'Summary of findings' tables
We used the GRADE approach to summarise the quality of evidence about the effect of CPM on each of the primary outcomes (active knee flexion ROM, pain, function, quality of life, participants' global assessment of treatment effectiveness, need for manipulation under anaesthesia and adverse events). We used the short‐term effects in the analyses except for function and quality of life where we used the medium‐term effects because either there were no data for the short‐term effects or the data could not be pooled because of heterogeneity. We defined levels of quality as follows:
high quality: randomised trials;
medium quality: downgraded randomised trials;
low quality: double‐downgraded randomised trials; and
very low quality: triple‐downgraded randomised trials.
The quality of evidence was downgraded if:
there were limitations in the design and implementation of available trials suggesting high likelihood of bias;
there was only indirect evidence (indirect population, intervention, control, outcomes);
there was unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
the results were imprecise (wide CIs); and
there was a high probability of publication bias.
In the same way, the quality of evidence was upgraded if:
the effect sizes were large;
all confounding factors reduced a demonstrated effect or suggested a spurious effect in trials that showed no effect; and
there was a dose‐response gradient.
We used GRADEpro software to compile the 'Summary of findings' table.
We re‐expressed outcomes pooled using SMDs as a MD by multiplying the SMD by a representative control group baseline SD from a trial, using a familiar instrument.
In addition to the absolute and relative magnitude of effect provided in the 'Summary of findings' table, we have reported the absolute percent difference, the relative percent change from baseline, and the number needed to treat (NNT) for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH) (the NNT was only provided for outcomes that showed a statistically significant difference).
For dichotomous outcomes, we calculated the absolute risk difference using the risk difference statistic in Review Manager 5 (RevMan 2012), and expressed the result as a percentage; the relative percentage change as the RR ‐ 1 and expressed it as a percentage; and the NNT from the control group event rate and the RR were determined using the Visual Rx NNT calculator (Cates 2008).
For continuous outcomes, we calculated the absolute risk difference as the MD between intervention and control groups in the original measurement units (divided by the scale), expressed as a percentage; the relative difference as the absolute change (or MD) divided by the baseline mean of the control group from a representative trial. As no continuous outcomes were significantly different between the intervention and control group, we did not calculate the NNT.
Subgroup analyses
We planned no subgroup analyses.
Sensitivity analyses
We planned no sensitivity analyses; however, we did look for small sample bias by re‐doing all the analyses using a fixed‐effect model and comparing results between the random‐effects model and fixed‐effect model of analyses for each outcome (Higgins 2011).
Results
Description of studies
Results of the search
The electronic searches identified 931 records (see Table 2 and Figure 1). This included 516 records that were identified in 2009 and an additional 415 records that were identified in 2013. Once duplicates were removed, there were 684 records (327 from 2009 and 357 from 2013).
1. Results from each electronic database for 2009‐2013 search.
| Database name, platform and time span | Update search date | Number of results |
| The Cochrane Library, Issue 12, December 2012 from database inception | January 2009 to 24 January 2013 | 1 |
| MEDLINE(R) Ovid 1966 to January week 3 2013 and MEDLINE(R) Ovid In‐Process & Other Non‐Indexed Citations 23 January 2013 | January 2009 to 24 January 2013 | 126 |
| EMBASE Ovid 1980 to 2013 week 3 | January 2009 to 24 January 2013 | 146 |
| EBSCOhost CINAHL 1981 to 23 January 2013 | January 2009 to 24 January 2013 | 1 |
| AMED Ovid (Allied and Complementary Medicine Database) 1985 to January 2013 | January 2009 to 24 January 2013 | 140 |
| Physiotherapy Evidence Database (PEDro) | January 2009 to 24 January 2013 | 1 |
| ‐ | TOTAL | 415 |
| ‐ | Total AFTER duplicates removed | 4 |
1.
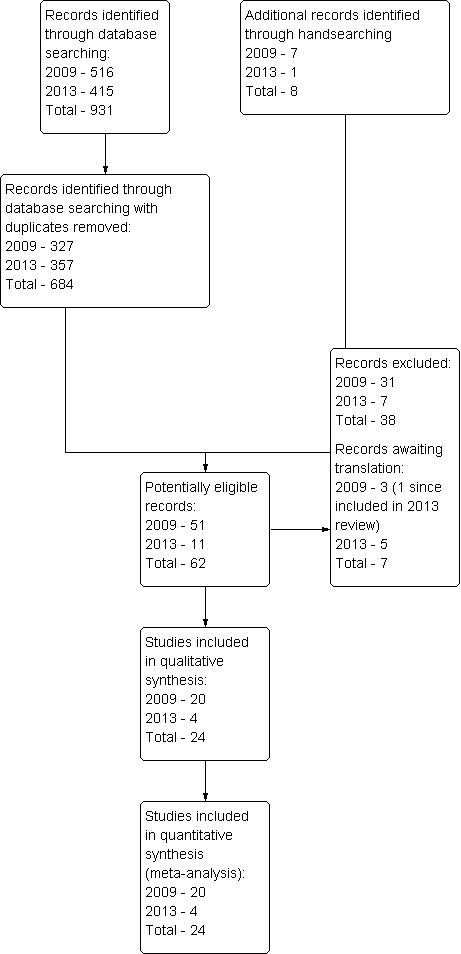
Study flow diagram. Results from the 2003 version of this systematic review are included in the 2009 search results because a full search was re‐done in 2009 using a new search strategy.
Included studies
Of the 684 records, 62 trials were potentially eligible. On inspection of the full reports, 24 met the inclusion criteria (Alkire 2010; Bennett 2005; Bruun‐Olsen 2009; Can 1995; Chiarello 1997; Colwell 1992; Denis 2006; Harms 1991; Huang 2003; Kumar 1996; Lau 2001; Lenssen 2003a; Lenssen 2008; MacDonald 2000; Maniar 2012; May 1999; McInnes 1992; Montgomery 1996; Ng 1999; Nielsen 1988; Ritter 1989; Sahin 2006; Vince 1987; Worland 1998). Four new studies were included in this update (Alkire 2010; Bruun‐Olsen 2009; Maniar 2012; Sahin 2006). All but one of the studies (Can 1995) were full papers. In the 24 included trials, CPM was administered from 1.5 to 24 hours a day (median 5.7, interquartile range 4 to 14) and for between 1 and 17 days (median 8, interquartile range 6 to 13) (see Characteristics of included studies table). A total of 1335 patients were randomised. Most patients had OA rather than RA. Treatments were initiated between the first and fourth postoperative day (POD) in all trials except one (May 1999), in which CPM treatment was initiated on transfer to a rehabilitation facility (between POD 2 and 13).
Excluded studies
Potentially eligible trials were most commonly excluded because the control group received something other than usual care with or without additional exercises (see Characteristics of excluded studies table). Seven trials are awaiting classification (see Characteristics of studies awaiting classification table).
Risk of bias in included studies
No trial blinded patients or treating therapists as this is not easily achievable in trials of this type (see Figure 2 and Figure 3). Only eight trials concealed allocation and used an adequate method to generate the random sequence. Selective reporting was potentially a problem in 16 of the 24 trials and there was incomplete reporting of data in at least eight trials. Probably the biggest threat to bias in trials of CPM come from the use of non‐blinded assessors and only 10 trials blinded assessors. Sixteen trials had complete outcome data. Four trials were highly vulnerable to bias not satisfying any of the criteria used to assess methodological quality (Can 1995; Huang 2003; Vince 1987; Worland 1998).
2.
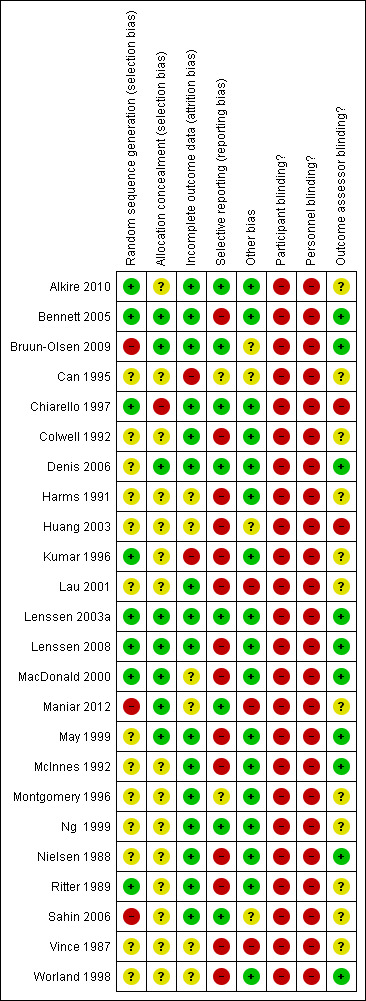
Methodological quality summary: review authors' judgements about each methodological quality item for each included trial.
3.
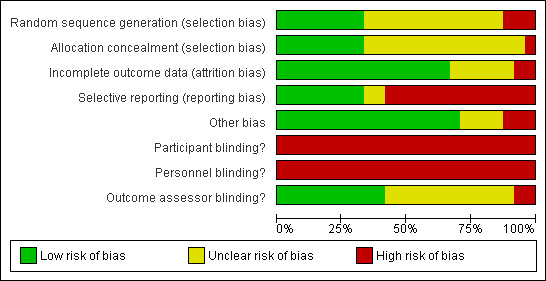
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1
The primary comparisons compared CPM combined with standard postoperative care versus standard postoperative care with or without additional knee exercises to one or both groups. These analyses included all trials for which data were available. The results are summarised below and in the Table 1.
Major outcomes
1. Active knee flexion range of motion
Short‐term effects: Twelve trials with 626 participants measured active knee flexion ROM (Bennett 2005; Bruun‐Olsen 2009; Chiarello 1997; Denis 2006; Huang 2003; Lau 2001; Lenssen 2008; Maniar 2012; May 1999; McInnes 1992; Ng 1999; Sahin 2006). Ten trials with 470 participants provided useful data (Bruun‐Olsen 2009; Chiarello 1997; Denis 2006; Huang 2003; Lau 2001; Lenssen 2008; May 1999; McInnes 1992; Ng 1999; Sahin 2006). Active knee flexion was measured with a goniometer in all trials. The MD was 2 degrees with more ROM for the CPM group (95% CI 0 to 5; P value = 0.07; I2 = 43%; Analysis 1.1).
1.1. Analysis.
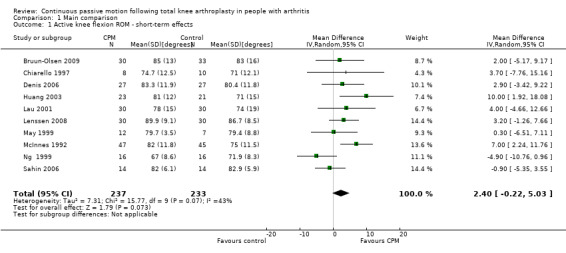
Comparison 1 Main comparison, Outcome 1 Active knee flexion ROM ‐ short‐term effects.
Medium‐term effects: Seven trials with 411 participants measured active knee flexion ROM (Bennett 2005; Bruun‐Olsen 2009; Huang 2003; Lau 2001; Lenssen 2008; Maniar 2012; Sahin 2006). Four trials with 195 participants provided useful data (Bruun‐Olsen 2009; Huang 2003; Lenssen 2008; Sahin 2006). Active knee flexion was measured with a goniometer in all trials. There was considerable between‐study heterogeneity in estimates of effect (I2 = 69%), so we did not pool the data. Point estimates of effect ranged from ‐7 to 8 degrees (Analysis 1.2).
1.2. Analysis.
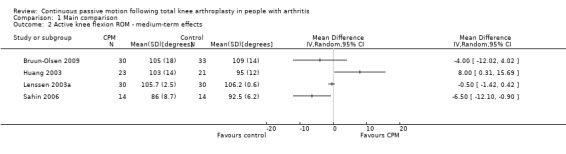
Comparison 1 Main comparison, Outcome 2 Active knee flexion ROM ‐ medium‐term effects.
Long‐term effects: Four trials with 232 participants measured active knee flexion ROM (Bennett 2005; Huang 2003; Lau 2001; Sahin 2006). Three trials with 132 participants provided useful data (Huang 2003; Lau 2001; Sahin 2006). Active knee flexion was measured with a goniometer in all trials. The MD was 3 degrees with more ROM for the CPM group (95% CI ‐1 to 7; P value = 0.16; I2 = 54%; Analysis 1.3).
1.3. Analysis.
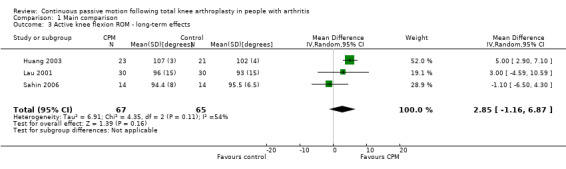
Comparison 1 Main comparison, Outcome 3 Active knee flexion ROM ‐ long‐term effects.
2. Pain
Short‐term effects: Eleven trials with 683 participants measured pain (Bennett 2005; Bruun‐Olsen 2009; Denis 2006; Harms 1991; Lenssen 2003a; Lenssen 2008; Maniar 2012; May 1999; McInnes 1992; Montgomery 1996; Sahin 2006). Eight trials with 414 participants provided useful data (Bruun‐Olsen 2009; Denis 2006; Lenssen 2003a; Lenssen 2008; May 1999; McInnes 1992; Montgomery 1996; Sahin 2006). Pain was measured on a 10‐ or 100‐point visual analogue scale but we converted all results to a 10‐point scale for this review. The MD was ‐0.4 points on a 0‐ to 10‐point scale with less pain for the CPM group (95% CI ‐0.8 to 0.1; P value = 0.1; I2 = 50%; Analysis 1.4).
1.4. Analysis.
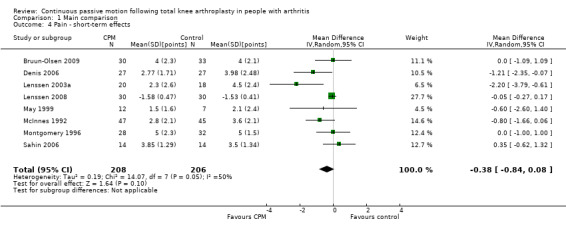
Comparison 1 Main comparison, Outcome 4 Pain ‐ short‐term effects.
Medium‐term effects: Four trials with 243 participants measured pain (Alkire 2010; Bruun‐Olsen 2009; Lenssen 2008; Maniar 2012). Three trials with 179 participants provided useful data (Bruun‐Olsen 2009; Lenssen 2008; Maniar 2012). Pain was measured on a 10‐ or 100‐point visual analogue scale but we converted all results to a 10‐point scale for this review. The MD was 0.3 points on a 0‐ to 10‐point scale with more pain for the CPM group (95% CI ‐0.4 to 0.9; P value = 0.44; I2 = 52%; Analysis 1.5).
1.5. Analysis.
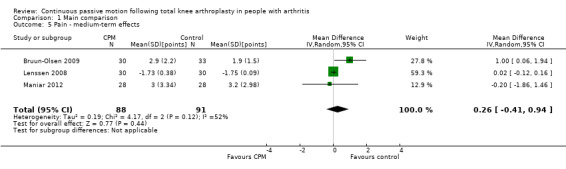
Comparison 1 Main comparison, Outcome 5 Pain ‐ medium‐term effects.
Long‐term effects: One trial with 28 participants measured pain using a 10‐point visual analogue scale (Sahin 2006). The MD was 0.1 points on a 0‐ to 10‐point scale with more pain for the CPM group (95% CI ‐0.8 to 0.9; P value = 0.87; Analysis 1.6).
1.6. Analysis.
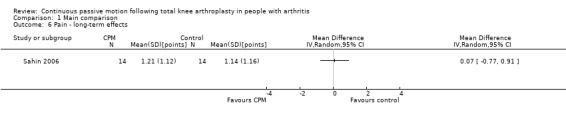
Comparison 1 Main comparison, Outcome 6 Pain ‐ long‐term effects.
3. Function
Short‐term effects: Five trials with 227 participants measured function (Denis 2006; Lenssen 2003a; Lenssen 2008; Maniar 2012; May 1999). Four trials with 171 participants provided useful data (Denis 2006; Lenssen 2003a; Lenssen 2008; May 1999). Function was measured in a variety of ways including the Knee Society Score, The Hospital for Special Surgery Score, The Timed Up and Go Test and the 10‐metre Walking Test. There was considerable between‐study heterogeneity in estimates of effect (I2 = 72%), so we did not pool the data. Point standardised estimates of effect ranged from ‐0.4 SD to 1 SD (Analysis 1.7).
1.7. Analysis.
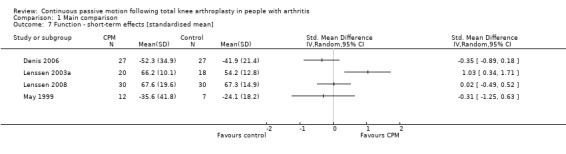
Comparison 1 Main comparison, Outcome 7 Function ‐ short‐term effects [standardised mean].
Medium‐term effects: Eight trials with 555 participants measured function (Alkire 2010; Bennett 2005; Bruun‐Olsen 2009; Kumar 1996; Lenssen 2008; Maniar 2012; McInnes 1992; Worland 1998). Six trials with 405 participants provided useful data (Bennett 2005; Bruun‐Olsen 2009; Kumar 1996; Lenssen 2008; Maniar 2012; Worland 1998). Function was measured in a variety of ways including the Knee Society Score, The Hospital for Special Surgery Score, and the Western Ontario and McMaster Universities Osteoarthritis Index Physical Function Subscore. The SMD was ‐0.1 SD with less function for the CPM group (95% CI ‐0.3 to 0.1; P value = 0.45; I2 = 0%; Analysis 1.8).
1.8. Analysis.
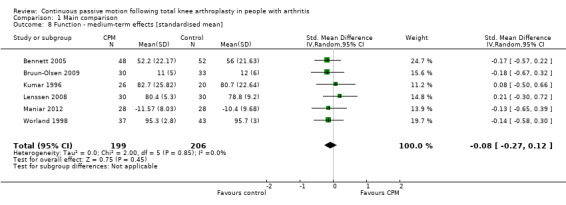
Comparison 1 Main comparison, Outcome 8 Function ‐ medium‐term effects [standardised mean].
Long‐term effects: Four trials with 288 participants measured function (Bennett 2005; MacDonald 2000; Sahin 2006; Worland 1998). All four trials provided useful data. Function was measured in a variety of ways including the Knee Society Score and The Hospital for Special Surgery Score. The SMD was 0 SD (95% CI ‐0.2 to 0.3; P value = 0.89; I2 = 0%; Analysis 1.9).
1.9. Analysis.
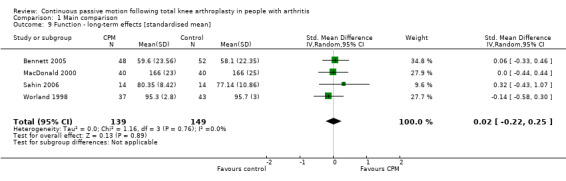
Comparison 1 Main comparison, Outcome 9 Function ‐ long‐term effects [standardised mean].
4. Quality of life
Short‐term effects: No trial measured this outcome during this time periods.
Medium‐term effects: Three trials with 197 participants measured quality of life(Bennett 2005; Kumar 1996; Maniar 2012). Two trials with 156 participants provided useful data (Bennett 2005; Maniar 2012). Quality of life was measured with the physical component subscore of the Short‐Form 12‐Item Health Survey (SF‐12). The MD was 1 point on a 0‐ to 100‐point scale with better quality of life for the CPM group (95% CI ‐3 to 4; P value = 0.66; Analysis 1.10).
1.10. Analysis.
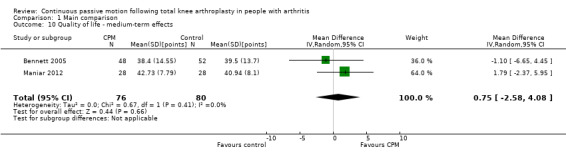
Comparison 1 Main comparison, Outcome 10 Quality of life ‐ medium‐term effects.
Long‐term effects: One trial with 100 participants measured quality of life(Bennett 2005), using the physical component subscore of the SF‐12. The MD was 2 points on a 0‐ to 100‐point scale with better quality of life for the CPM group (95% CI ‐4 to 8; P value = 0.48; Analysis 1.11).
1.11. Analysis.
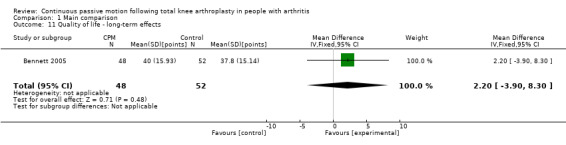
Comparison 1 Main comparison, Outcome 11 Quality of life ‐ long‐term effects.
5. Participants' global assessment of treatment effectiveness
Short‐term effects: Three trials with 212 participants measured participants' global assessment of treatment effectiveness (Harms 1991; Lenssen 2003a; Lenssen 2008). All trials provided useful data. Participants' global assessment of treatment effectiveness was measured using a 7‐point perceived effects scale or a 10‐point visual analogue scale. There was considerable between‐study heterogeneity in estimates of effect (I2 = 67%), so we did not pool the data. Point estimates of effect ranged from ‐0.5 to 0.4 points (Analysis 1.12).
1.12. Analysis.
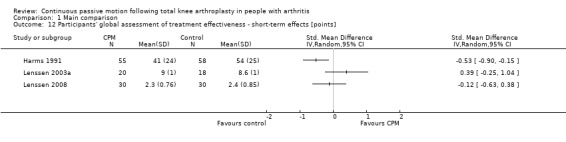
Comparison 1 Main comparison, Outcome 12 Participants' global assessment of treatment effectiveness ‐ short‐term effects [points].
Medium‐term effects: One trial with 60 participants measured participants' global assessment of treatment effectiveness (Lenssen 2008), using a 7‐point perceived effects scale. The MD was ‐0.3 points on a 0‐ to 7‐point scale with less perception of treatment effectiveness for the CPM group (95% CI ‐0.7 to 0.1; P value = 0.18; Analysis 1.13).
1.13. Analysis.
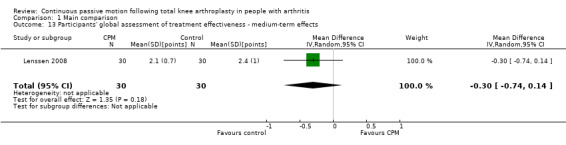
Comparison 1 Main comparison, Outcome 13 Participants' global assessment of treatment effectiveness ‐ medium‐term effects.
Long‐term effects: No trial measured this outcome during this time periods.
6. Manipulation under anaesthesia
Nine trials with 600 participants measured incidence proportion of manipulation under anaesthesia (Alkire 2010; Denis 2006; Harms 1991; Kumar 1996; Lenssen 2008; Maniar 2012; May 1999; McInnes 1992; Vince 1987). Eight trials with 581 participants provided useful data (Denis 2006; Harms 1991; Kumar 1996; Lenssen 2008; Maniar 2012; McInnes 1992; Vince 1987). There were 25 manipulations under anaesthesia. The RR was 0.34 with less risk for the CPM group (95% CI 0.13 to 0.89; P value = 0.03; I2 = 0%; Analysis 1.14).
1.14. Analysis.
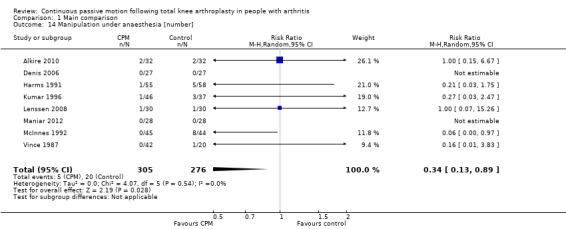
Comparison 1 Main comparison, Outcome 14 Manipulation under anaesthesia [number].
7. Adverse events
Seventeen trials with 1104 participants reported incidence proportion of adverse events (Alkire 2010; Bennett 2005; Colwell 1992; Denis 2006; Harms 1991; Huang 2003; Kumar 1996; Lau 2001; Maniar 2012; McInnes 1992; Montgomery 1996; Ng 1999; Nielsen 1988; Ritter 1989; Sahin 2006; Vince 1987; Worland 1998). Sixteen trials with 1040 participants provided useful data (Bennett 2005; Colwell 1992; Denis 2006; Harms 1991; Huang 2003; Kumar 1996; Lau 2001; Maniar 2012; McInnes 1992; Montgomery 1996; Ng 1999; Nielsen 1988; Ritter 1989; Sahin 2006; Vince 1987; Worland 1998). Adverse events included delayed healing, haemarthrosis, falls, deep venous thromboses, wound infections, pulmonary emboli, knee haematoma and a patellar rupture. There were 178 adverse events in total. The RR was 0.92 with less risk for the CPM group (95% CI 0.63 to 1.33; P value = 0.65; I2 = 39%; Analysis 1.15).
1.15. Analysis.
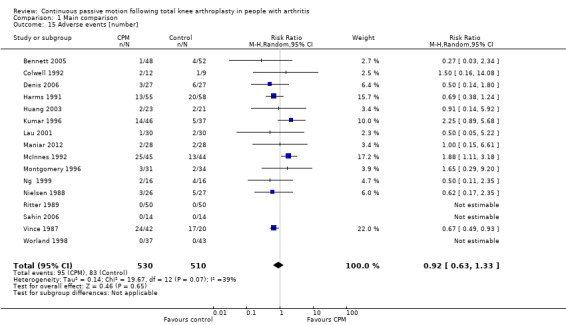
Comparison 1 Main comparison, Outcome 15 Adverse events [number].
Secondary outcomes
1. Passive knee flexion range of motion
Short‐term effects: Fifteen trials with 944 participants measured passive knee flexion ROM (Alkire 2010; Bennett 2005; Bruun‐Olsen 2009; Chiarello 1997; Colwell 1992; Harms 1991; Kumar 1996; Lenssen 2003a; Lenssen 2008; Montgomery 1996; Ng 1999; Nielsen 1988; Ritter 1989; Vince 1987; Worland 1998). Eleven trials with 697 participants provided useful data (Bruun‐Olsen 2009; Chiarello 1997; Harms 1991; Kumar 1996; Lenssen 2003a; Lenssen 2008; Montgomery 1996; Ng 1999; Nielsen 1988; Ritter 1989; Worland 1998). Passive knee flexion was measured with a goniometer in all trials. The MD was 2 degrees with more ROM for the CPM group (95% CI 0 to 4; P value = 0.03; I2 = 22%; Analysis 1.16).
1.16. Analysis.
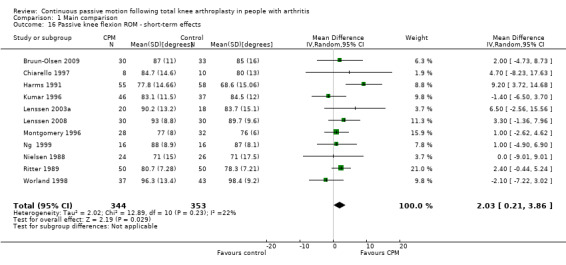
Comparison 1 Main comparison, Outcome 16 Passive knee flexion ROM ‐ short‐term effects.
Medium‐term effects: Seven trials with 447 participants measured passive knee flexion ROM (Alkire 2010; Bruun‐Olsen 2009; Colwell 1992; Kumar 1996; MacDonald 2000; Ritter 1989; Worland 1998). Four trials with 264 participants provided useful data (Bruun‐Olsen 2009; Kumar 1996; MacDonald 2000; Worland 1998). Passive knee flexion was measured with a goniometer in all trials. The MD was ‐2 degrees with less ROM for the CPM group (95% CI ‐5 to 2; P value = 0.29; I2 = 29%; Analysis 1.17).
1.17. Analysis.
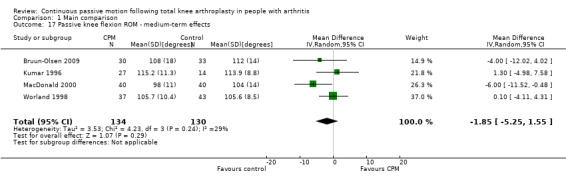
Comparison 1 Main comparison, Outcome 17 Passive knee flexion ROM ‐ medium‐term effects.
Long‐term effects: Three trials with 177 participants measured passive knee flexion ROM (Colwell 1992; MacDonald 2000; Worland 1998). Two trials with 160 participants provided useful data (MacDonald 2000; Worland 1998). Passive knee flexion was measured with a goniometer in all trials. The MD was 0 degrees (95% CI ‐2 to 2; P value = 0.96; I2 = 0%; Analysis 1.18).
1.18. Analysis.
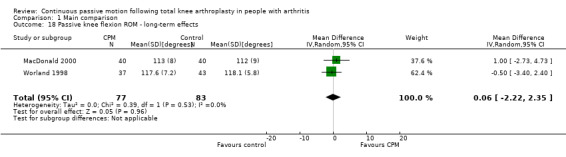
Comparison 1 Main comparison, Outcome 18 Passive knee flexion ROM ‐ long‐term effects.
2. Active knee extension range of motion
Short‐term effects: Fifteen trials with 890 participants measured active knee extension ROM (Bennett 2005; Bruun‐Olsen 2009; Chiarello 1997; Denis 2006; Harms 1991; Huang 2003; Lau 2001; Lenssen 2008; Maniar 2012; May 1999; McInnes 1992; Ng 1999; Nielsen 1988; Ritter 1989; Sahin 2006). Eleven trials with 574 participants provided useful data (Bruun‐Olsen 2009; Chiarello 1997; Denis 2006; Harms 1991; Huang 2003; Lenssen 2008; May 1999; McInnes 1992; Ng 1999; Nielsen 1988; Sahin 2006). Active knee extension was measured with a goniometer in all trials. The MD was 1 degree with more ROM for the CPM group (95% CI 0 to 2; P value = 0.17; I2 = 41%; Analysis 1.19).
1.19. Analysis.
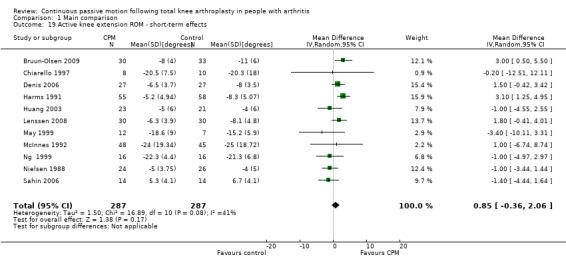
Comparison 1 Main comparison, Outcome 19 Active knee extension ROM ‐ short‐term effects.
Medium‐term effects: Six trials with 355 participants measured active knee extension ROM (Bennett 2005; Bruun‐Olsen 2009; Huang 2003; Lau 2001; Lenssen 2008; Sahin 2006). Four trials with 195 participants provided useful data (Bruun‐Olsen 2009; Huang 2003; Lenssen 2008; Sahin 2006). Active knee extension was measured with a goniometer in all trials. The MD was 1 degree with more ROM for the CPM group (95% CI ‐1 to 2; P value = 0.33; I2 = 41%; Analysis 1.20).
1.20. Analysis.
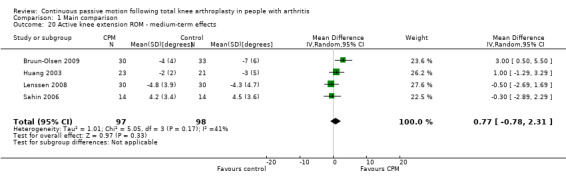
Comparison 1 Main comparison, Outcome 20 Active knee extension ROM ‐ medium‐term effects.
Long‐term effects: Five trials with 312 participants measured active knee extension ROM (Bennett 2005; Huang 2003; Lau 2001; Sahin 2006; Worland 1998). Two trials with 108 participants provided useful data (Sahin 2006; Worland 1998). Active knee extension was measured with a goniometer in all trials. The MD was 0 degrees (95% CI 0 to 0; P value = 0.33; I2 = 0%; Analysis 1.21).
1.21. Analysis.
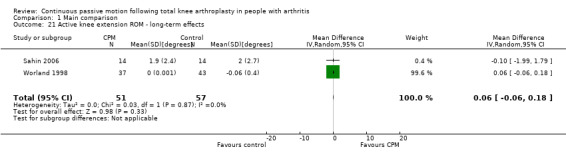
Comparison 1 Main comparison, Outcome 21 Active knee extension ROM ‐ long‐term effects.
3. Passive knee extension range of motion
Short‐term effects: Fifteen trials with 876 participants measured passive knee extension ROM (Alkire 2010; Bennett 2005; Bruun‐Olsen 2009; Chiarello 1997; Colwell 1992; Huang 2003; Kumar 1996; Lenssen 2003a; Lenssen 2008; May 1999; McInnes 1992; Ng 1999; Ritter 1989; Vince 1987; Worland 1998). Eleven trials with 629 participants provided useful data (Bruun‐Olsen 2009; Chiarello 1997; Huang 2003; Kumar 1996; Lenssen 2003a; Lenssen 2008; May 1999; McInnes 1992; Ng 1999; Ritter 1989; Worland 1998). Passive knee extension was measured with a goniometer in all trials. The MD was 1 degree with more ROM for the CPM group (95% CI 0 to 2; P value = 0.16; I2 = 47%; Analysis 1.22).
1.22. Analysis.
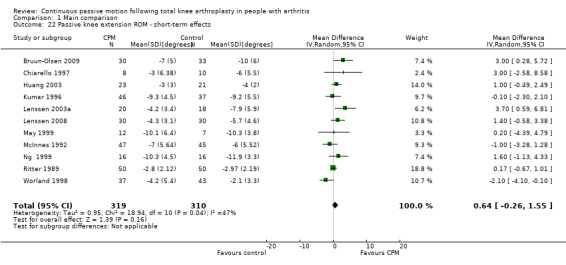
Comparison 1 Main comparison, Outcome 22 Passive knee extension ROM ‐ short‐term effects.
Medium‐term effects: Seven trials with 411 participants measured passive knee extension ROM (Alkire 2010; Bennett 2005; Bruun‐Olsen 2009; Colwell 1992; Huang 2003; Kumar 1996; Worland 1998). Four trials with 228 participants provided useful data (Bruun‐Olsen 2009; Huang 2003; Kumar 1996; Worland 1998). Passive knee extension was measured with a goniometer in all trials. There was considerable between‐study heterogeneity in estimates of effect (I2 = 80%), so we did not pool the data. Point estimates of effect ranged from ‐3 to 3 degrees (Analysis 1.23).
1.23. Analysis.
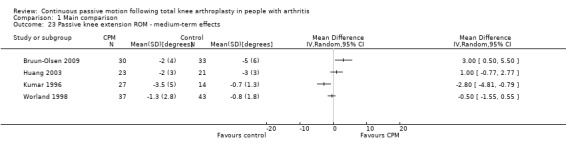
Comparison 1 Main comparison, Outcome 23 Passive knee extension ROM ‐ medium‐term effects.
Long‐term effects: Five trials with 321 participants measured passive knee extension ROM (Bennett 2005; Colwell 1992; Huang 2003; MacDonald 2000; Worland 1998). Three trials with 204 participants provided useful data (Huang 2003; MacDonald 2000; Worland 1998). Passive knee extension was measured with a goniometer in all trials. The MD was 0 degrees (95% CI 0 to 1; P value = 0.59; I2 = 0%; Analysis 1.24).
1.24. Analysis.
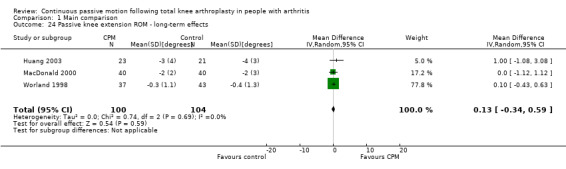
Comparison 1 Main comparison, Outcome 24 Passive knee extension ROM ‐ long‐term effects.
4. Length of hospital stay
Short‐term effects: Fourteen trials with 840 participants measured length of hospital stay (Alkire 2010; Bennett 2005; Colwell 1992; Denis 2006; Harms 1991; Huang 2003; Kumar 1996; Lenssen 2003a; MacDonald 2000; May 1999; McInnes 1992; Montgomery 1996; Sahin 2006; Vince 1987). Ten trials with 614 participants provided useful data (Colwell 1992; Denis 2006; Harms 1991; Huang 2003; Kumar 1996; Lenssen 2003a; MacDonald 2000; McInnes 1992; Montgomery 1996; Sahin 2006). The MD was ‐0.4 days with shorter length of hospital stay for the CPM group (95% CI ‐1.0 to 0.2; P value = 0.15; I2 = 39%; Analysis 1.25).
1.25. Analysis.
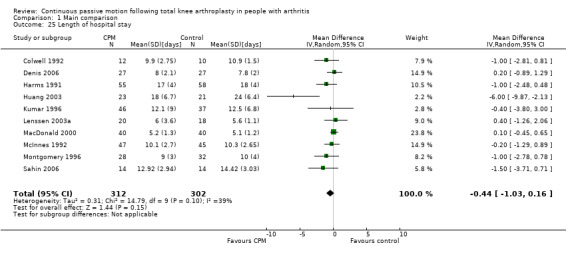
Comparison 1 Main comparison, Outcome 25 Length of hospital stay.
5. Swelling
Short‐term effects: Seven trials with 464 participants measured swelling (Alkire 2010; Bruun‐Olsen 2009; Maniar 2012; McInnes 1992; Montgomery 1996; Ritter 1989; Sahin 2006). Five trials with 300 participants provided useful data (Bruun‐Olsen 2009; Maniar 2012; McInnes 1992; Montgomery 1996; Sahin 2006). Swelling was measured with a tape measure. There was considerable between‐study heterogeneity in estimates of effect (I2 = 71%), so we did not pool the data. Point estimates of effect ranged from ‐1.9 to 1.6 cm (Analysis 1.26).
1.26. Analysis.
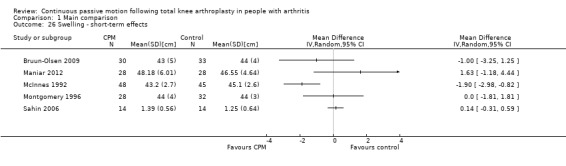
Comparison 1 Main comparison, Outcome 26 Swelling ‐ short‐term effects.
Medium‐term effects: Two trials with 119 participants measured swelling (Bruun‐Olsen 2009; Maniar 2012). Both trials provided useful data. Swelling was measured with a tape measure. The MD was 0.8 cm with more swelling for the CPM group (95% CI ‐1.1 to 2.8; P value = 0.41; I2 = 31%; Analysis 1.27).
1.27. Analysis.
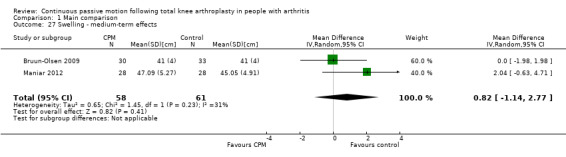
Comparison 1 Main comparison, Outcome 27 Swelling ‐ medium‐term effects.
Long‐term effects: No trial measured this outcome during this time periods.
6. Quadriceps strength
Short‐term effects: Two trials with 130 participants measured quadriceps strength (Lenssen 2003a; McInnes 1992). Both trials provided useful data. Strength was measured with a dynamometer (N) or manual muscle test (6‐point scale). The SMD was 0.3 SD with more strength for the CPM group (95% CI ‐0.1 to 0.6; P value = 0.13; I2 = 0%; Analysis 1.28).
1.28. Analysis.
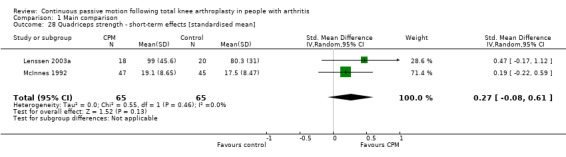
Comparison 1 Main comparison, Outcome 28 Quadriceps strength ‐ short‐term effects [standardised mean].
Medium‐ and long‐term effects: No trial measured this outcome during these time periods.
The secondary comparison compared CPM combined with standard postoperative care versus standard postoperative care plus additional knee exercises. These analyses provide a head‐to‐head comparison of the effectiveness of CPM and exercise. Analyses were performed on data from four trials (May 1999; Montgomery 1996; Ritter 1989; Worland 1998). Where it was possible to calculate pooled estimates, there was no indication of the superiority of either intervention (CPM or exercise).
The short‐term effects of mean total continuous passive motion time on passive knee flexion range of motion
Eleven trials with 697 participants measured passive knee flexion ROM and provided sufficient data to estimate mean total CPM time (Bruun‐Olsen 2009; Chiarello 1997; Harms 1991; Kumar 1996; Lenssen 2003a; Lenssen 2008; Montgomery 1996; Ng 1999; Nielsen 1988; Ritter 1989; Worland 1998). The duration of CPM had no effect on passive knee flexion ROM (mean increase of 0.01 degrees for every additional hour of CPM; 95% CI ‐0.04 to 0.06). This effect was not statistically significant (P value = 0.66).
Sensitivity analysis
Small sample size bias was not detected for any of the main comparisons.
Discussion
Summary of main results
CPM following TKA is primarily advocated for its proposed benefits on knee ROM, particularly knee flexion. Knee flexion ROM is important for mobility tasks such as walking, transferring and performing activities of daily living (Rowe 2000). It is also important for activities such as squatting and kneeling, which are activities of daily living for many cultures (Hemmerich 2006). In interpreting the results of this review, it is important to consider how much additional knee flexion ROM is required to justify the use of CPM. Few people would claim that an added benefit of less than 5 degrees is functionally important, and most people would probably agree that considerably more than 5 degrees is required to justify the added time, cost and inconvenience of CPM. This being the case, the findings of this systematic review provide moderate‐quality evidence to indicate that CPM does not have clinically important short‐term effects on active knee flexion ROM (see Table 1 and Analysis 1.1). The short‐term effects on passive knee flexion, passive knee extension and active knee extension are similar with mean effects of 1 to 2 degrees. All estimates of the short‐term effects of CPM on knee ROM are estimated with less than 5 degrees of uncertainty (as reflected in the 95% CI). The medium‐ and long‐term effects of CPM on active knee flexion ROM, passive knee flexion ROM, active knee extension ROM or passive knee extension ROM are similar to the short‐term effects ‐ all have mean effects less than 3 degrees. Importantly, the upper 95% CIs of all but one of the eight estimates of the medium‐ and long‐term effects are less than 5 degrees. The exception is the long‐term effects on active knee flexion ROM, but this estimate was only based on three trials of 132 participants and the estimate is imprecise (95% CI ‐1 to 7 degrees). Taken together, this review provides moderate‐quality evidence that CPM does not have sufficient effect on knee ROM to justify its widespread use.
Some people develop very stiff knees following TKA, irrespective of whether they receive CPM. One way to manage this problem is to manipulate the knee under anaesthesia. There is very low‐quality evidence to suggest that CPM reduces the risk of manipulation. The reported effect is large in relative terms (RR 0.34) but the incidence proportion of manipulations following TKA is low (i.e. 7%) with only 25 manipulations reported in the eight trials involving 581 participants. An RR of 0.34 with this incidence proportion corresponds to an absolute reduction in risk of manipulation under anaesthesia of 4% (see Table 1). This means that one manipulation will be prevented in every 21 people provided with CPM (95% CI 16 to 126). However, the estimate of RR is imprecise (95% CI 0.13 to 0.89) and does not rule out the possibility of a trivially small effect (i.e. one manipulation prevented in every 126 people provided CPM). In addition, these results are based on just eight trials and it is not clear in any of the included trials whether the clinicians making decisions about the need for manipulation were blinded to allocation. Interestingly, while knee stiffness is the most common indication for manipulation under anaesthesia, the results from this systematic review indicate there was little difference in knee ROM of people who did and did not receive CPM. Therefore, the findings about risk of manipulation need to be interpreted with caution (see Table 1 and Analysis 1.14).
One hundred and seventy‐eight adverse events were reported in 16 trials comprising 1040 participants. The types of adverse events reported were delayed healing, haemarthrosis, falls, deep venous thromboses, wound infections, pulmonary emboli and a patellar rupture. Some of the adverse events were unlikely to be related to CPM but nonetheless were included in the analyses because they would not bias the findings. The point estimate provides low‐quality evidence to indicate a decrease in the risk of adverse events with CPM (RR 0.92; 95% CI 0.63 to 1.33); however, the associated 95% CI includes an important increase and decrease in the risk of adverse events with CPM. It is, therefore, unclear whether CPM affects the risk of adverse events. The effect of CPM on participants' global assessment of treatment effectiveness was also unclear. The three trials that included this outcome provided contradictory results and the data were too heterogeneous to pool.
CPM clearly does not have clinically meaningful short‐, medium‐ or long‐term effects on pain (MDs range from ‐0.4 to 0.3 points on a 10‐point scale; 95% CI range from ‐0.8 to 0.9) although the medium‐ and long‐term effects are only based on three and one trials, respectively, and, therefore, need to be interpreted with caution. The short‐, medium‐ and long‐term effects of CPM on length of hospital stay, swelling and quadriceps strength are more difficult to interpret. While none of the meta‐analyses on these outcomes were statistically significant, it is not clear if they rule out clinically meaningful effects. Resolution of this issue requires clarification of what constitutes a clinically meaningful effect. For example, if a reduction in length of hospital stay of 2 days is considered clinically meaningful then the results of this systematic review clearly indicate that CPM does not have clinically meaningful effects (MD ‐0.4 days; 95% CI ‐1.0 to 0.2). However, if a reduction in length of hospital stay of 1 day is considered clinically meaningful then the data do not rule out the possibility of a clinically meaningful effect.
Perhaps the most important outcome is function because it reflects the implications of ROM, strength, swelling and pain on activities of daily living. However, function was not routinely included as an outcome measure and when it was, many different scales were used. The best estimate of the effect of CPM on function comes from trials that examined the medium‐term effect. These trials provided moderate‐quality evidence to suggest that CPM does not have clinically important effects on function unless a relative percent change in function of 5% is considered as worthwhile (relative percent change ‐4%; 95% CI ‐12 to 5; see Table 1). The precision of this estimate is surprisingly good given the multidimensional nature of the different measures used to measure function. For example, The Hospital and Knee Score used in some trials not only measures the ability of patients to walk, transfer and climb stairs but also measures knee ROM, pain, strength and instability. Future trials would benefit from some agreement on the most appropriate way to measure function in this group of patients.
Quality of life was only measured in two trials (Bennett 2005; Maniar 2012), yet together these trials provide moderate‐quality evidence to indicate that CPM does not have clinically important effects. The medium‐term effect was very small and the estimate surprisingly precise (MD of 1 point on a 0‐ to 100‐point scale; 95% CI ‐3 to 4). This estimate does not rule out the possibility of a 4/100 point improvement in quality of life with CPM but most would consider this too small to justify its use. However, more trials are required to verify these findings.
Overall completeness and applicability of evidence
Many different protocols were used to administer CPM. For example, in some trials CPM was started immediately after the TKA operation whereas in other trials it was started days later. The CPM settings were also different between trials. In some trials, the settings were dictated by a protocol whereas in other trials they were determined by patient comfort or clinician discretion. All these variables may have influenced the observed effects of CPM. A meta‐regression was used to explore the possibility that total CPM time influences passive knee flexion ROM. Passive knee flexion ROM was selected because this was the most commonly measured outcome. The meta‐regression indicated that there was little or no effect of CPM duration on short‐term passive knee flexion ROM. That is, trials that applied CPM for 24 hours a day over an extended time period did not report systematically more passive knee flexion ROM than those that only applied CPM for a few hours over one or two days.
Protocols for co‐interventions were also highly variable. In a subset of four trials, control participants also received additional knee exercises. A secondary analysis explored the effect of CPM in this subset of trials. The purpose was to compare the effectiveness of CPM and knee exercises. This analysis was inconclusive because of the small number of trials. Nonetheless, it is unlikely that CPM would be more effective than knee exercises because the primary analysis indicated CPM is no more effective than usual care, with or without additional knee exercises.
There was usually a high degree of consistency (low between‐study heterogeneity) in estimates of effects of pooled findings. Heterogeneity was only apparent in a small number of comparisons, typically where SMDs were used and when total sample sizes were small. The heterogeneity could have been due to any number of factors but was most likely due to the use of different tools to measure the same construct. This was particularly problematic for function, which was measured with outcomes as diverse as self reporting questionnaires and timed walking tests.
Interestingly, the applicability of the evidence about CPM is changing. CPM may no longer be a viable or appropriate treatment option, regardless of findings, because patients are now commonly discharged within a few days of surgery and often mobilised on the same day as surgery.
Quality of the evidence
The methodological quality of trials was variable (see Figure 2). Only 10 trials clearly blinded assessors. Not surprisingly, given the nature of the intervention, no trial blinded participants or therapists. Failure to blind assessors, participants and therapists exposes the trials to performance and detection biases. The failure to blind participants is potentially more of problem for outcomes that rely on self report (such as pain) than more objective measures (such as passive knee ROM). Only eight of the 24 trials concealed allocation and nearly all trials were selective in their reporting of data. These potential sources of bias led to a downgrading of the quality of evidence for all outcomes reported in the Table 1 on the 'Risk of bias' criterion. Pain, manipulation under anaesthesia and participants' global assessment of treatment effectiveness were further downgraded because the results across trials for these outcomes were inconsistent. Imprecision of the point estimates were considered a serious problem for two of the primary outcomes, namely participants' global assessment of treatment effectiveness and manipulation under anaesthesia. Consequently, the findings for active knee flexion ROM, function and quality of life are based on moderate‐quality evidence, the findings for pain and risk of adverse events are based on low‐quality evidence, and the findings for participants' global assessment of treatment effectiveness and manipulation under anaesthesia are based on very low‐quality evidence. These limitations notwithstanding, the main findings are probably robust because bias tends to inflate estimates of effects (Gluud 2006; Savovic 2012), and most estimates of treatment effects in this systematic review were very small.
Potential biases in the review process
The main potential source of bias in the review process arises from failure to identify all relevant trials. This may have occurred, particularly if trials were unpublished. However, retrieval bias generally tends to inflate estimates of effects (Dickersin 1993; Egger 1998), but this review reports small effects of CPM on most outcomes.
Agreements and disagreements with other studies or reviews
The original version of this systematic review was done in 2003. It was updated in 2010 and then again for this review in 2013. The main difference in conclusions between the original and the 2010 version related to length of hospital stay. In 2003, it was concluded that CPM reduces length of hospital stay. In the 2010 systematic review, it was concluded that CPM does not reduce length of hospital stay. However, in this present review it was concluded that the effects of CPM on length of hospital stay are inconclusive.
It is also worth noting that the original review performed in 2003 suggested that the effect of CPM on knee ROM may be dose dependent. Dose dependence was tested in the 2010 review and then again in this review by using a meta‐regression to examine effects of mean total CPM time (hours) on passive knee flexion ROM in the short term. The results indicated that the response to CPM is not dose dependent.
Our findings are broadly consistent with the findings of similar systematic reviews (Grella 2008; Lenssen 2003b; van Dijk 2007; Viswanathan 2010). The only discrepancy is with the conclusions of Lenssen et al (Lenssen 2003b). These authors concluded that CPM had a short‐term effect on knee flexion ROM (MD 8 degrees; 95% CI ‐2 to 18) despite the considerable uncertainty around the estimate. The difference between our estimate and that of Lenssen 2003b was probably due to differences in the trials included in the two meta‐analyses. Our pooled estimate (MD 2 degrees, 95% CI 0 to 4) was based on 11 trials (697 participants) and the estimate of Lenssen 2003b was based of five trials (317 participants). There was only one trial in common in the two meta‐analyses due to differences in the inclusion criteria and year of the reviews.
Other systematic reviews have investigated the effect of CPM following knee cartilage defect surgery (Fazalare 2010), shoulder rotator cuff repair (Du Plessis 2011), and anterior cruciate ligament reconstruction (Lobb 2012; Smith 2007). One review reported no evidence of benefit of CPM (Lobb 2012), two concluded that there was insufficient evidence (Fazalare 2010; Smith 2007), and one reported two clinical trials that demonstrated a treatment effect on ROM but this conclusion was not clearly supported from the findings of the two cited trials (Du Plessis 2011). While it is possible that the effects of CPM are different for various conditions, this would seem unlikely. Therefore, the results of the systematic reviews following different types of surgery lend further weight to the results of this systematic review indicating little if any benefit of CPM on at least knee ROM following TKA.
Summary
The findings of 24 RCTs of 1445 participants provide moderate‐quality evidence that CPM does not have clinically important short‐term effects on active knee flexion ROM or medium‐term effects on function or quality of life. There is low‐quality evidence to indicate that CPM does not have clinically important short‐term effects on pain. The effects of CPM on participants' global assessment of treatment effectiveness, risk of manipulation, risk of adverse events, length of hospital stay, swelling and quadriceps strength remain unclear although there is very low‐quality evidence to indicate that CPM reduces the risk of manipulation under anaesthesia.
Authors' conclusions
Implications for practice.
The effects of continuous passive motion (CPM) on range of motion (ROM), pain, function and quality of life are too small to justify its use and costs but the effects of CPM on participants' global assessment of treatment effectiveness are unclear. This review provides very low‐quality evidence that CPM reduces the risk of manipulation under anaesthesia; however, these findings need to be interpreted with caution because they are inconsistent with the moderate‐quality evidence indicating that CPM has no effect on knee ROM even though the main indication for manipulation under anaesthesia is joint stiffness.
Implications for research.
Future trialists should strive to improve the quality and reporting of their trials. The use of concealed allocation and blinded assessors is particularly important for reducing bias. In addition, attention should be given to stating when CPM starts and finishes, how long it is applied for and what settings are used. The accuracy of future meta‐analyses could also be substantially improved if trialists consistently reported between‐group differences with associated measures of variability for all outcomes and at all time points of data collection. In addition, researchers should strive to agree upon a standardised CPM protocol and appropriate outcome measures. This is particularly important for function and quality of life because researchers are using numerous different outcome measures, which makes pooling of results problematic. However, and regardless, we would only suggest further research to verify the effect of CPM on the need for manipulation under anaesthesia. Trials designed for this purpose should consider restricting the inclusion criteria to people at high risk of requiring manipulation under anaesthesia and ensuring those making the decision about the need for manipulation are blinded.
We would not recommend trials to determine the effect of CPM on knee joint ROM even though the evidence about the futility of CPM for improving active knee flexion is only moderate. The evidence was only downgraded from high to moderate because of the susceptibility of the included trials to bias. However, bias tends to inflate estimates of treatment effectiveness. Therefore, the real estimate is probably even less that reported in this review. In addition, the findings from passive knee flexion, active knee extension and passive knee extension were no more encouraging. Therefore, resources should not be wasted on further investigating the effect of CPM on knee joint ROM. However, without an effect of CPM on knee joint ROM there is no readily understandable way that CPM could affect any other variable. CPM is primarily prescribed for knee joint ROM. This suggests that trials designed to attain better estimates of the effects of CPM on some of the other outcomes may also be futile.
However, if trialists want to be more confident about the evidence supporting the conclusions of this trial, then they could look at improving quantification of the effects of CPM on function and quality of life because the findings on these outcomes are only based on a small number of trials and the quality of evidence is only moderate. More importantly, however, future trialists should focus on determining the effect of CPM on the need for manipulation. It is very difficult to reconcile the suggestion that CPM decreases the need for manipulation when CPM has little effect on knee joint ROM. The apparent contradiction in findings could reflect the low‐quality evidence supporting the findings on the decreased need for manipulation with CPM. Therefore, future trialists should pay particular attention to reducing bias.
This Cochrane review should only be updated if new evidence emerges that is likely to substantially change the conclusions of this review or shift the quality of evidence supporting the conclusions to high. However, it is unlikely that additional trials will change the conclusions about ROM because these estimates are reasonably precise and consistent, and we anticipate that with better quality trials the treatment effects will be smaller, not greater. If CPM does not affect knee joint ROM then it is most unlikely that CPM will affect any other outcomes because CPM is primarily prescribed on the basis of its benefits on knee joint ROM. With no effect on knee joint ROM, there are no obvious mechanisms for CPM to affect other outcomes. An update may, however, be justified if subsequent trials better clarify the effect of CPM on the need for manipulation.
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2014 | Amended | Fixed minor typing errors in plain language summary, and a calculating error in the abstract. |
History
Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 8 January 2014 | New citation required but conclusions have not changed | Four new studies were included in the update (Alkire 2010; Bruun‐Olsen 2009; Maniar 2012; Sahin 2006); bringing the total number of included studies to 24 (from 20 since the last update). Some outcomes of the review presented in the summary of findings tables were changed to align with the Cochrane Musculoskeletal Group's recommended default outcomes. Reporting of the methods and results of the review were altered to align with the reporting standards recommended by the Cochrane Collaboration's Methodological Expectations of Cochrane Intervention Reviews (MECIR) project. |
| 9 September 2013 | New search has been performed | A new search was conducted on 24 January 2013, and the review updated upon request from editors. |
| 2 May 2013 | Amended | Corrected an error ‐ Kumar was missing from the short‐term effects of passive knee flexion ROM ‐ so added. Removed studies from meta‐analysis in which SDs were imputed. Details are: Active knee extension ROM ‐ short term effects (Bennett 2005; Lau 2001; Ritter 1989); Passive knee extension ROM ‐ short term effects (Bennett 2005; Colwell 1992). Lengh of hospital stay ‐ short term effects (Bennett 2005; May 1999; Vince 1987). |
| 17 February 2010 | New citation required and conclusions have changed | Change in authors and conclusions |
| 10 June 2009 | New search has been performed | A new search was conducted and the review updated. Eight new trials were included in the update: four published since the original search was conducted in the 2003 review (Bennett 2005; Denis 2006; Lenssen 2003a; Lenssen 2008); two trials published but not identified in the original search (Ng 1999; Ritter 1989); and two additional trials previously excluded met new inclusion criteria and added to the update (Lau 2003; Worland 1998). The update also includes changes to the selection criteria: participants restricted to those with pre‐surgery diagnosis of arthritis and comparisons were CPM and standard postoperative care versus CPM and standard postoperative care with active or passive knee exercises; and methods were updated in accordance with current Cochrane Collaboration recommendations: risk of bias assessment and Summary of Findings Tables added, and used updated Cochrane search filter for identifying RCTs |
| 25 July 2008 | Amended | Converted to new review format. CMSG ID: C019‐R |
Acknowledgements
First version of the review (2003): The authors thank J McGowan for her help and expertise in designing the literature search, and C Lamothe for her help with data extraction and data entry. R Herbert, T Lessen and R DeBie provided valuable feedback in their roles as peer reviewers.
The review authors acknowledge and thank the authors of the 2003 version of the review for the following: SE Milne was responsible for writing the manuscript, extracting and analysing data, and screening potentially eligible trials. V Welch contributed to updating the analyses and interpretation of the results. GA Wells and P Tugwell contributed to designing the methodology and commenting on early drafts. MJ Noel, H Drouin and J Davis contributed to extracting data, analysing and selecting trials.
Second version of the review (2010): The review authors are grateful to C Ng and N Pontifex for their assistance with data entry and proof reading.
Third version of the review (2013): The review authors are grateful to K Higgins and J Bowden for their assistance with screening, data extraction, data entry and proof reading.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) (to January 2013)
MeSH descriptor Arthroplasty, Replacement, Knee explode all trees
MeSH descriptor Knee Prosthesis explode all trees
tkr:ti,ab
MeSH descriptor Knee explode all trees
knee*:ti,ab
(#4 OR #5)
MeSH descriptor Arthroplasty explode all trees
MeSH descriptor Joint Prosthesis explode all trees
(arthroplast* or prosthe* or replac*):ti,ab
(#7 OR #8 OR #9)
(#6 AND #10)
(#1 OR #2 OR #3 OR #11)
MeSH descriptor Exercise Therapy explode all trees
MeSH descriptor Physical Therapy Modalities explode all trees
physical NEXT therap*:ti,ab
physiotherap*:ti,ab
"continuous passive motion":ti,ab
cpm:ti,ab
gait NEXT therap*:ti,ab
exercis* NEXT therap*:ti,ab
therapeutic NEAR/3 exercis*:ti,ab
(#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21)
(#12 AND #21)
Appendix 2. MEDLINE search strategy (1966 to January 2013)
1. Arthroplasty, Replacement, Knee/
2. Knee Prosthesis/
3. tkr.tw.
4. exp Knee/
5. knee$.tw.
6. 4 or 5
7. exp arthroplasty/
8. Joint Prosthesis/
9. (arthroplast$ or prosthe$ or replac$).tw.
10. or / 7‐9
11. 6 and 10
12. or / 1‐3,11
13. exp Exercise Therapy/
14. physical therapy modalities/
15. (physical adj therap$).tw.
16. physiotherap$.tw.
17. continuous passive motion.tw.
18. cpm.tw.
19. (gait adj therap$).tw.
20. (exercis$ adj therap$).tw.
21. (therapeutic adj3 exercis$).tw.
22. or / 13‐21
23. 12 and 22
24. randomized controlled trial.pt.
25. controlled clinical trial.pt.
26. randomized.ab.
27. placebo.ab.
28. drug therapy.fs.
29. randomly.ab.
30. trial.ab.
31. groups.ab.
32. or / 24‐31
33. (animals not (humans and animals)).sh.
34. 32 not 33
35. 23 and 34
Appendix 3. EMBASE (1980 to January 2013)
exp ARTHROPLASTY/
exp Joint Prosthesis/
exp "Prostheses and Orthoses"/
exp KNEE/
or / 1‐3
4 and 5
exp Knee Arthroplasty/
exp Knee Prosthesis/
tka.tw.
(knee$ and (replace$ or arthroplast$ or prosthe$ or endoprosthe$ or implant$)).tw.
or / 6‐10
exp kinesiotherapy/
exp physiotherapy/
(physical adj therap$).tw.
physiotherap$.tw.
continuous passive motion.tw.
cpm.tw.
(gait adj therap$).tw.
(exercis$ adj therap$).tw.
(therapeutic adj3 exercis$).tw.
or / 12‐20
11 and 21
random$.ti,ab.
factorial$.ti,ab.
(crossover$ or cross over$ or cross‐over$).ti,ab.
placebo$.ti,ab.
(doubl$ adj blind$).ti,ab.
(singl$ adj blind$).ti,ab.
assign$.ti,ab.
allocat$.ti,ab.
volunteer$.ti,ab.
crossover procedure.sh.
double blind procedure.sh.
randomized controlled trial.sh.
single blind procedure.sh.
or / 23‐35
exp animal/ or nonhuman/ or exp animal experiment/
exp human/
37 and 38
37 not 39
36 not 40
22 and 41
Appendix 4. CINAHL (1982 to January 2013)
S23 S11 and S21 S22 S11 and S21
S21 S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 S20 ti therapeutic N3 exercis* or ab therapeutic N3 exercis* S19 ti exercis* therap* or ab exercis* therap* S18 ti gait therap* or ab gait therap* S17 ti cpm or ab cpm
S16 ti continuous passive motion or ab continuous passive motion S15 ti physiotherap* or ab physiotherap*
S14 ti physical therap* or ab physical therap*
S13 (MH "Physical Therapy+")
S12 (MH "Therapeutic Exercise+")
S11 S1 or S2 or S10
S10 S5 and S9
S9 S6 or S7 or S8
S8 ab arthroplast* or ab prosthe* or ab replac*
S7 ti arthroplast* or ti prosthe* or ti replac*
S6 (MH "Joint Prosthesis")
S5 S3 or S4
S4 ti knee* or ab knee*
S3 (MH "Knee")
S2 ti tkr or ab tkr
S1 (MH "Arthroplasty, Replacement, Knee")
Appendix 5. AMED (1985 to January 2013)
arthroplasty replacement knee/
Knee prosthesis/
tkr.tw.
knee/
knee$.tw.
4 or 5
exp Arthroplasty/
exp Joint prosthesis/
(arthroplast$ or prosthe$ or replac$).tw.
or / 7‐9
6 and 10
or / 1‐3,11
exp exercise therapy/
exp physical therapy modalities/
(physical adj therap$).tw.
physiotherap$.tw.
continuous passive motion.tw.
cpm.tw.
(gait adj therap$).tw.
(exercis$ adj therap$).tw.
(therapeutic adj3 exercis$).tw.
or / 13‐21
12 and 22
Appendix 6. PEDro (up to January 2013)
Search 1 Continuous Passive Motion in Abstract & Title AND Lower leg or knee in Body Part
Search 2 cpm Motion in Abstract & Title AND Lower leg or knee in Body Part
Data and analyses
Comparison 1. Main comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Active knee flexion ROM ‐ short‐term effects | 10 | 470 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐0.22, 5.03] |
| 2 Active knee flexion ROM ‐ medium‐term effects | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Active knee flexion ROM ‐ long‐term effects | 3 | 132 | Mean Difference (IV, Random, 95% CI) | 2.85 [‐1.16, 6.87] |
| 4 Pain ‐ short‐term effects | 8 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.84, 0.08] |
| 5 Pain ‐ medium‐term effects | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.41, 0.94] |
| 6 Pain ‐ long‐term effects | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Function ‐ short‐term effects [standardised mean] | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Function ‐ medium‐term effects [standardised mean] | 6 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.27, 0.12] |
| 9 Function ‐ long‐term effects [standardised mean] | 4 | 288 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.22, 0.25] |
| 10 Quality of life ‐ medium‐term effects | 2 | 156 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐2.58, 4.08] |
| 11 Quality of life ‐ long‐term effects | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐3.90, 8.30] |
| 12 Participants' global assessment of treatment effectiveness ‐ short‐term effects [points] | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Participants' global assessment of treatment effectiveness ‐ medium‐term effects | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.74, 0.14] |
| 14 Manipulation under anaesthesia [number] | 8 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.89] |
| 15 Adverse events [number] | 16 | 1040 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.33] |
| 16 Passive knee flexion ROM ‐ short‐term effects | 11 | 697 | Mean Difference (IV, Random, 95% CI) | 2.03 [0.21, 3.86] |
| 17 Passive knee flexion ROM ‐ medium‐term effects | 4 | 264 | Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐5.25, 1.55] |
| 18 Passive knee flexion ROM ‐ long‐term effects | 2 | 160 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐2.22, 2.35] |
| 19 Active knee extension ROM ‐ short‐term effects | 11 | 574 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐0.36, 2.06] |
| 20 Active knee extension ROM ‐ medium‐term effects | 4 | 195 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.78, 2.31] |
| 21 Active knee extension ROM ‐ long‐term effects | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.18] |
| 22 Passive knee extension ROM ‐ short‐term effects | 11 | 629 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.26, 1.55] |
| 23 Passive knee extension ROM ‐ medium‐term effects | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24 Passive knee extension ROM ‐ long‐term effects | 3 | 204 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.34, 0.59] |
| 25 Length of hospital stay | 10 | 614 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.03, 0.16] |
| 26 Swelling ‐ short‐term effects | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27 Swelling ‐ medium‐term effects | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.14, 2.77] |
| 28 Quadriceps strength ‐ short‐term effects [standardised mean] | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.08, 0.61] |
Comparison 2. Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Active knee flexion ROM | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Short‐term effects (< 6 wk) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Passive knee flexion ROM | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term effects (< 6 wk) | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 1.14 [‐1.09, 3.38] |
| 2.2 Medium‐term effects (6 wk to 6 mo) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.11, 4.31] |
| 2.3 Long‐term effects (> 6 mo) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐3.40, 2.40] |
| 3 Active knee extension ROM | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Short‐term effects (< 6 wk) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Long‐term effects (> 6 mo) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Passive knee extension ROM | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Short‐term effects (< 6 wk) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Medium‐term effects (6 wk to 6 mo) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Long‐term effects (> 6 mo) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Length of hospital stay | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Short‐term effect (< 6 wk) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Medium‐term effects (6 wk to 6 mo) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Long‐term effects (> 6 mo) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Pain | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Short‐term effects (6 wk to 6 mo) | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Swelling | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Short‐term effects (6 wk to 6 mo) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 1 Active knee flexion ROM.
2.2. Analysis.
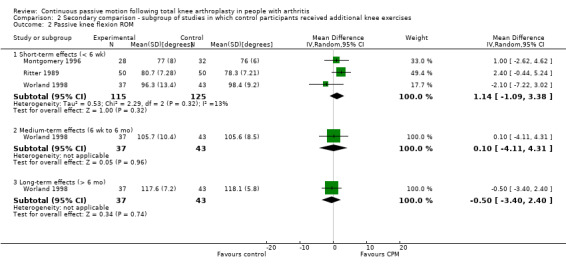
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 2 Passive knee flexion ROM.
2.3. Analysis.
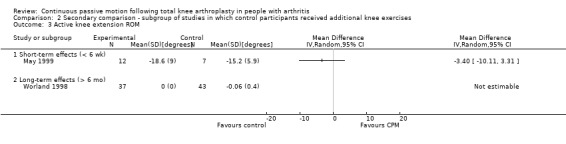
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 3 Active knee extension ROM.
2.4. Analysis.
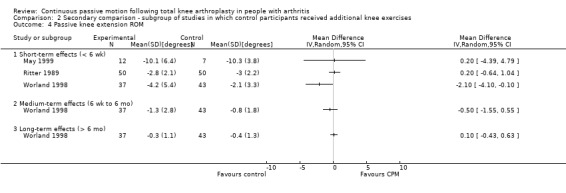
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 4 Passive knee extension ROM.
2.5. Analysis.
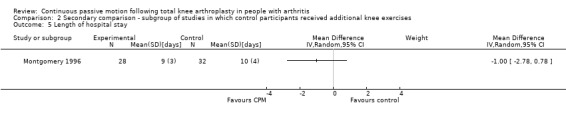
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 5 Length of hospital stay.
2.6. Analysis.
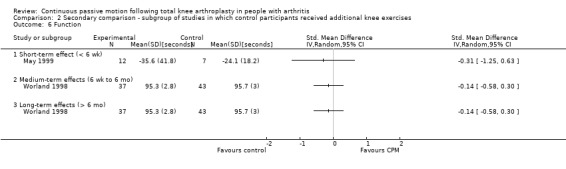
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 6 Function.
2.7. Analysis.
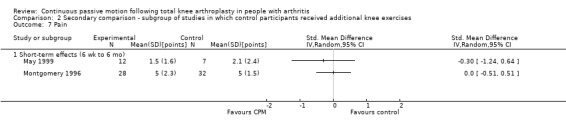
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 7 Pain.
2.8. Analysis.
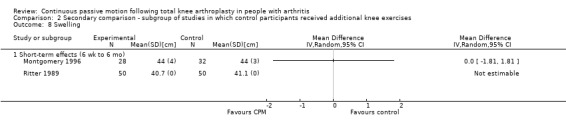
Comparison 2 Secondary comparison ‐ subgroup of studies in which control participants received additional knee exercises, Outcome 8 Swelling.
Comparison 3. Main comparison ‐ sensitivity analysis using fixed‐effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Active knee flexion ROM ‐ short‐term effects | 10 | 470 | Mean Difference (IV, Fixed, 95% CI) | 2.29 [0.39, 4.20] |
| 2 Active knee flexion ROM ‐ medium‐term effects | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Active knee flexion ROM ‐ long‐term effects | 3 | 132 | Mean Difference (IV, Fixed, 95% CI) | 4.12 [2.22, 6.02] |
| 4 Pain ‐ short‐term effects | 8 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.34, 0.05] |
| 5 Pain ‐ medium‐term effects | 3 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.10, 0.18] |
| 6 Pain ‐ long‐term effects | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Function ‐ short‐term effects [standardised mean] | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Function ‐ medium‐term effects [standardised mean] | 6 | 405 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.27, 0.12] |
| 9 Function ‐ long‐term effects [standardised mean] | 4 | 288 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.25] |
| 10 Quality of life ‐ medium‐term effects | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐2.58, 4.08] |
| 11 Quality of life ‐ long‐term effects [points] | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐3.90, 8.30] |
| 12 Participants' global assessment of treatment effectiveness ‐ short‐term effects [points] | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Participants' global assessment of treatment effectiveness ‐ medium‐term effects | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.74, 0.14] |
| 14 Manipulation under anaesthesia [number] | 8 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.64] |
| 15 Adverse events [number] | 16 | 1040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 16 Passive knee flexion ROM ‐ short‐term effects | 11 | 697 | Mean Difference (IV, Fixed, 95% CI) | 2.01 [0.50, 3.52] |
| 17 Passive knee flexion ROM ‐ medium‐term effects | 4 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐1.69 [‐4.46, 1.08] |
| 18 Passive knee flexion ROM ‐ long‐term effects | 2 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐2.22, 2.35] |
| 19 Active knee extension ROM ‐ short‐term effects | 11 | 574 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [0.33, 2.02] |
| 20 Active knee extension ROM ‐ medium‐term effects | 4 | 195 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.45, 1.92] |
| 21 Active knee extension ROM ‐ long‐term effects | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.06, 0.18] |
| 22 Passive knee extension ROM ‐ short‐term effects | 11 | 629 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐0.12, 1.00] |
| 23 Passive knee extension ROM ‐ medium‐term effects | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24 Passive knee extension ROM ‐ long‐term effects | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.34, 0.59] |
| 25 Quadriceps strength ‐ short‐term effects [standardised mean] | 2 | 130 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.08, 0.61] |
| 26 Length of hospital stay | 10 | 614 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.58, 0.19] |
| 27 Swelling ‐ short‐term effects | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 28 Swelling ‐ medium‐term effects | 2 | 119 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [‐0.87, 2.31] |
3.1. Analysis.
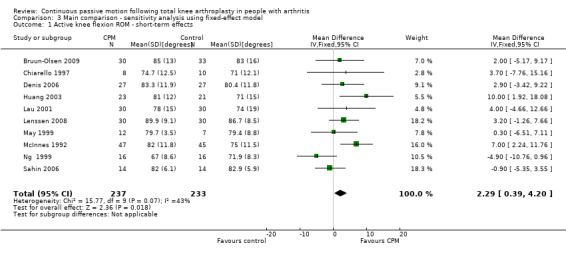
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 1 Active knee flexion ROM ‐ short‐term effects.
3.2. Analysis.
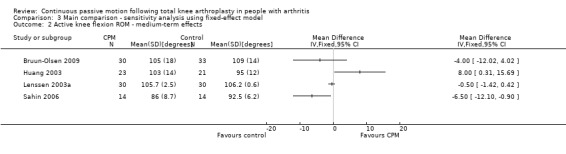
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 2 Active knee flexion ROM ‐ medium‐term effects.
3.3. Analysis.
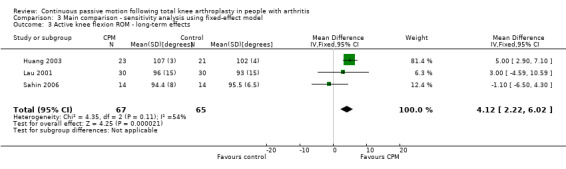
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 3 Active knee flexion ROM ‐ long‐term effects.
3.4. Analysis.
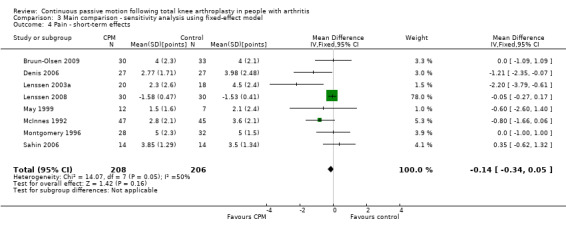
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 4 Pain ‐ short‐term effects.
3.5. Analysis.
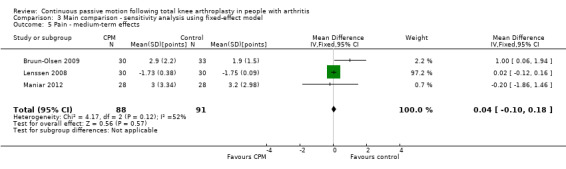
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 5 Pain ‐ medium‐term effects.
3.6. Analysis.
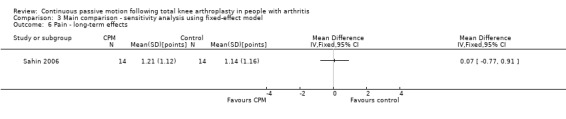
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 6 Pain ‐ long‐term effects.
3.7. Analysis.
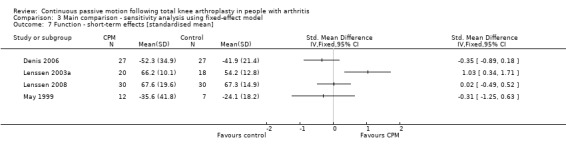
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 7 Function ‐ short‐term effects [standardised mean].
3.8. Analysis.
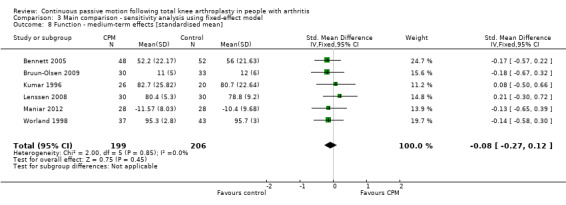
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 8 Function ‐ medium‐term effects [standardised mean].
3.9. Analysis.
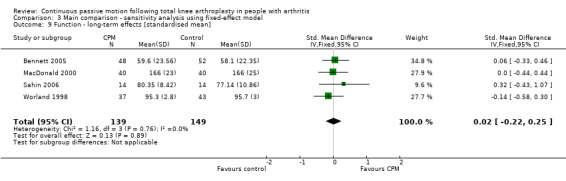
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 9 Function ‐ long‐term effects [standardised mean].
3.10. Analysis.
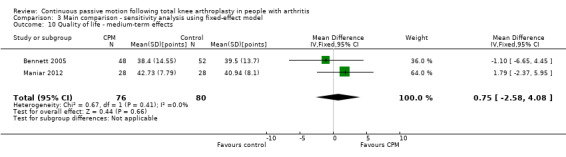
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 10 Quality of life ‐ medium‐term effects.
3.11. Analysis.
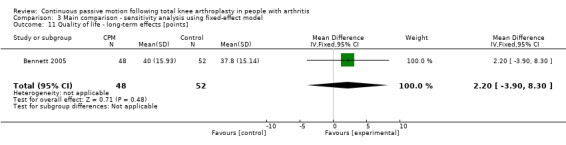
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 11 Quality of life ‐ long‐term effects [points].
3.12. Analysis.
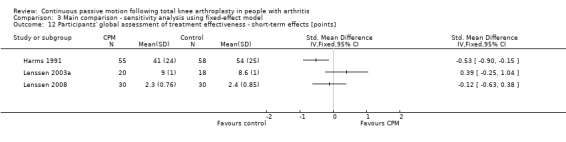
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 12 Participants' global assessment of treatment effectiveness ‐ short‐term effects [points].
3.13. Analysis.
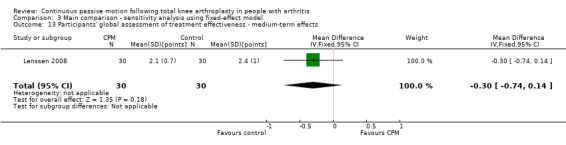
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 13 Participants' global assessment of treatment effectiveness ‐ medium‐term effects.
3.14. Analysis.
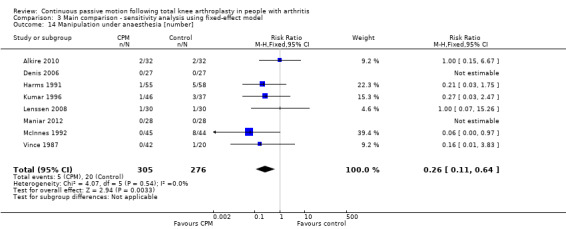
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 14 Manipulation under anaesthesia [number].
3.15. Analysis.
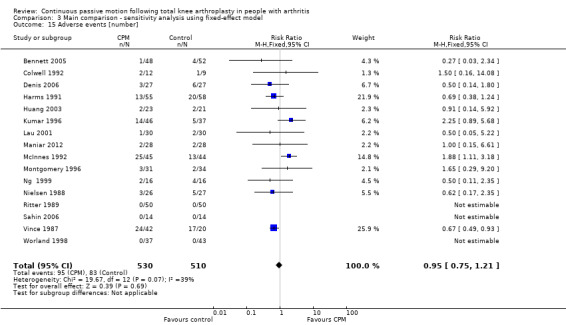
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 15 Adverse events [number].
3.16. Analysis.
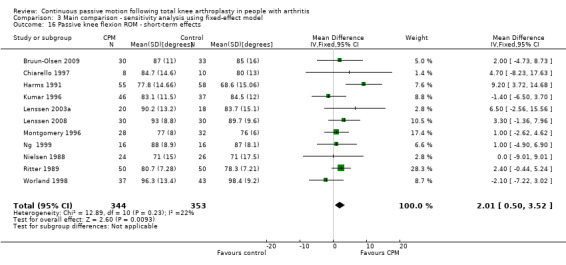
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 16 Passive knee flexion ROM ‐ short‐term effects.
3.17. Analysis.
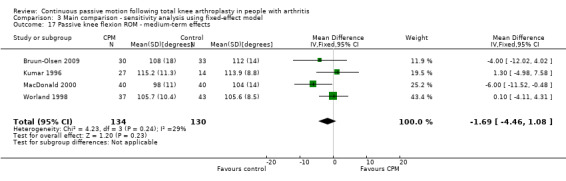
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 17 Passive knee flexion ROM ‐ medium‐term effects.
3.18. Analysis.
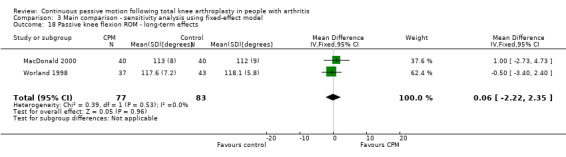
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 18 Passive knee flexion ROM ‐ long‐term effects.
3.19. Analysis.
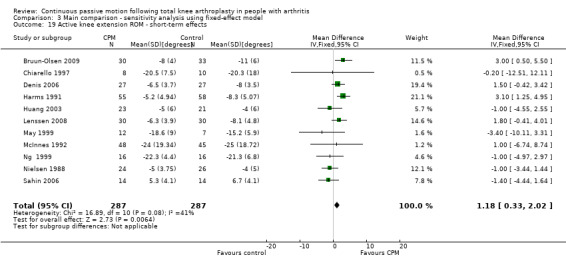
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 19 Active knee extension ROM ‐ short‐term effects.
3.20. Analysis.
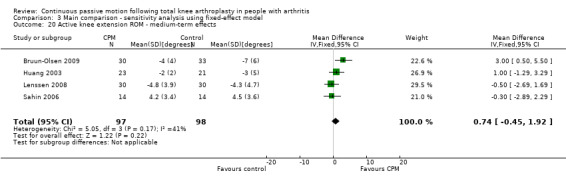
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 20 Active knee extension ROM ‐ medium‐term effects.
3.21. Analysis.
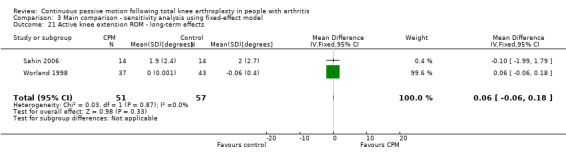
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 21 Active knee extension ROM ‐ long‐term effects.
3.22. Analysis.
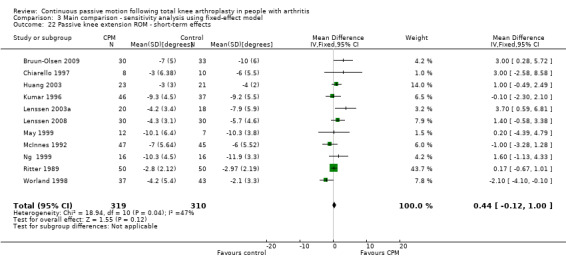
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 22 Passive knee extension ROM ‐ short‐term effects.
3.23. Analysis.
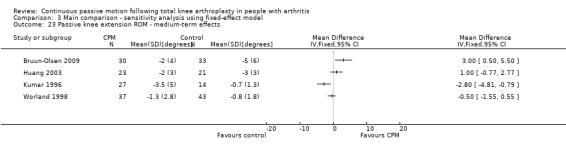
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 23 Passive knee extension ROM ‐ medium‐term effects.
3.24. Analysis.
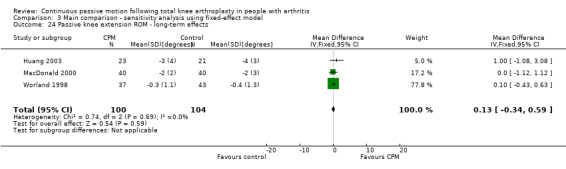
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 24 Passive knee extension ROM ‐ long‐term effects.
3.25. Analysis.
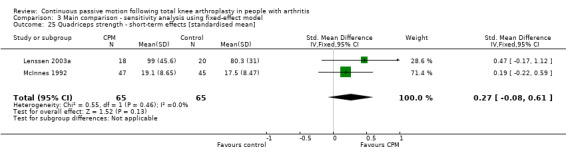
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 25 Quadriceps strength ‐ short‐term effects [standardised mean].
3.26. Analysis.
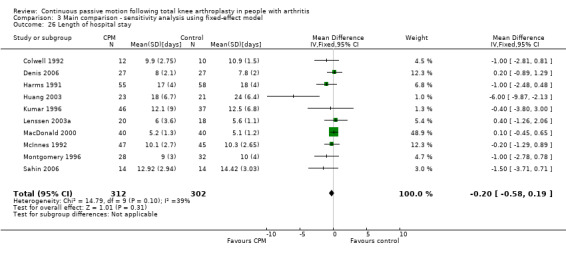
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 26 Length of hospital stay.
3.27. Analysis.
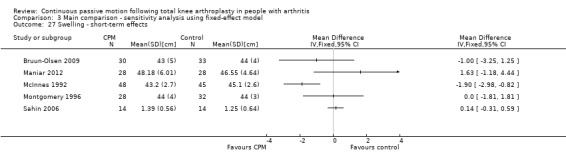
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 27 Swelling ‐ short‐term effects.
3.28. Analysis.
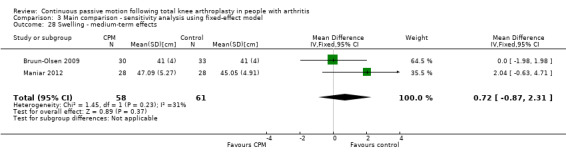
Comparison 3 Main comparison ‐ sensitivity analysis using fixed‐effect model, Outcome 28 Swelling ‐ medium‐term effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alkire 2010.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 33; Gr2: 32 Inclusion: OA or RA, > 18 years Exclusion: cognitive/sensory deficits, patients residing in skilled nursing facilities, and non‐English speaking patients. Initially participants with other co‐morbidities were also excluded but this was later amended Mean age (no measure of variance) (yr): Gr1: 65.6; Gr2: 66.9 Gender (F): Gr1: 87%; Gr2: 99% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 70‐90 dgr flexion. Provided 4 hr per session and 3 sessions per day for 3 days. Increased 10 dgr every session Gr2: standard care Standard care for Gr1 and Gr2: Provided twice a day (no details provided) |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: WOMAC‐Function, WOMAC‐Stiffness, drainage output, need for blood transfusion Timing of outcome measures: tested at POD 1, POD 2, 6 wk*, 3 mo† post randomisation |
|
| Notes | Swelling not tested at 6 wk so used POD 2 data for short‐term effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | States: "no patients lost to follow‐up". |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Bennett 2005.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 48; Gr2: 52; Gr3: 47 Inclusion: 90 dgr knee flexion ROM with CPM in recovery, OA Exclusion: bilateral TKA, revision of TKA, RA, haemophilia Mean age (variance not reported) (yr): Gr1: 71; Gr2: 72; Gr3: 71 Gender (F): Gr1: 65%; Gr2: 67%; Gr3: 72% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: early flexion CPM + standard care CPM commenced POD 0. Initially 50‐90 dgr. Provided for 6 hr/day. Increased 20‐dgr flexion/day. Continued until POD 5 Gr2: standard care Other groups: Gr3: standard CPM + standard care CPM commenced POD 0. Initially 0‐40 dgr. Provided 6 hr/day. Increased 10 dgr/day. Continued until POD 5 Standard care for Gr1, Gr2, Gr3: Commenced POD 1. Provided 1 hr/day. Included active assisted ROM, stretches, gait training, static quads, inner ROM quads, splint, transfer training, education |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: SF‐12 ‐ Mental Component Summary, length of hospital stay ‐ rehabilitation, pain: VAS daily. Timing of outcome measures: tested at POD 5*, 3 mo†, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "allocation by a block randomization". Clarified through personal communication that a random number generator was used. |
| Allocation concealment (selection bias) | Low risk | States: "surgeon and the independent assessor were blinded to group allocation". Although these people were not necessarily the people making the decisions about inclusion, it was clarified through personal communication that allocation schedule was kept in a locked cabinet and personnel responsible for recruiting did not have access to it. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | States: "one patient was subsequently excluded...results were analysed for remaining 147". |
| Selective reporting (reporting bias) | High risk | Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | States: "subjects were blinded to the study hypotheses". However, with CPM, it is not possible to blind participants to whether they did or did not receive CPM. |
| Personnel blinding? | High risk | States: "surgeon and the independent assessor were blinded to group allocation". However, with CPM, it is not possible to blind therapists to whether they did or did not administer CPM. |
| Outcome assessor blinding? | Low risk | States: "surgeon and the independent assessor were blinded to group allocation". |
Bruun‐Olsen 2009.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 30‐34 (not clear); Gr2: 33‐37 (not clear) Inclusion: OA, TKA, good cognitive function, fluent Norwegian Exclusion: RA, prosthesis in ipsilateral hip Mean age (SD) (yr): Gr1: 60 (10); Gr2: 71 (10) Gender (F): Gr1: 73%; Gr2: 67% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: CPM + active exercise CPM commenced POD 0. Initially 70‐1030 dgr flexion. Provided 2 hr per session and initially 2 sessions then 3 sessions per day. Increased to 100 dgr on POD 2. Continued until POD 7. Gr2: active exercise Active exercise for Gr1, Gr2: Commenced POD 1. Continued until discharge at 1 week. Non‐standardised physiotherapy provided following discharge. Included active and assisted active ROM, gait training and static quads |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: 40‐m walk, up/down stairs. Timing of outcome measures: tested at 1 week*, 3 mo† post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | States: "closed, opaque envelopes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/67 were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Table 2 results correspond with reported outcome measures. |
| Other bias | Unclear risk | Some outcome data missing at 1 week and not clear if collected. |
| Participant blinding? | High risk | States: "single blind". |
| Personnel blinding? | High risk | States: "single blind". |
| Outcome assessor blinding? | Low risk | States: "single blind…the physiotherapist who performed the measures did not know". |
Can 1995.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 26 knees; Gr2: 22 knees; (combined: 44 pts) Inclusion: primary TKA Exclusion: revision of TKA Age/gender: not reported Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐30 dgr. Provided for 4‐6 hr/day. Increased 5‐10 dgr/day. Continued for unreported period. Gr2: standard care Standard care for Gr1, Gr2: Commenced POD 0. Provided 2 x per day. Provided for unreported period. Included isometric exercises, assisted SLR, passive ROM, active ROM, stretches, inner ROM quad strengthening, gait training |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: none Timing of outcome measures: tested at POD 3, POD 4, D/C*, 3 mo†, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Not stated. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Chiarello 1997.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 8; Gr2: 10; Gr3: 8; Gr4: 9; Gr5: 11 Inclusion: degenerative joint disease, primary and unilateral TKA Exclusion: not reported Mean age (SD) (yr): Gr1: 74 (6); Gr2: 63 (10); Gr3: 71 (10); Gr4: 74 (9); Gr5: 71 (10) Gender (F): Gr1: 100%; Gr2: 70%; Gr3: 88%; Gr4: 56%; Gr5: 64% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 1‐3. Initial settings not reported. Provided for 10‐12 hr/day. Increased 10 dgr/day. Continued until D/C or 2 wk post surgery Gr2: standard care Other groups: Gr3: short duration CPM + standard care CPM commenced POD 1‐3. Initial settings not reported. Provided 3‐5 hr/day. Increased 10 dgr/day. Continued until D/C or 2 wk post surgery Gr4: short duration CPM + standard care CPM commenced POD 1‐3. Initial settings not reported. Provided 3‐5 hr/day. Increased as tolerated. Continued until D/C or 2 wk post surgery Gr5: long duration CPM + standard care CPM commenced POD 1‐3. Initial settings not reported. Provided 10‐12 hr/day. Increased as tolerated. Continued until D/C or 2 wk post surgery Standard care for Gr1, G2, Gr3, Gr4, Gr5: Commenced POD 1‐3. Provided daily. Included unspecified ROM exercises, gait training, transfer training, education, moist heat, strength and ROM exercises |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: none Timing of outcome measures: tested at D/C or 14 days* post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "randomly assigned...pre‐determined randomized list". |
| Allocation concealment (selection bias) | High risk | Not stated where "pre‐determined randomized list" was accessible to those recruiting participants. Clarified through personnel communication that allocation was not concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46/49 were present at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported on all outcomes at all endpoints. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | High risk | States: "treating physical therapists collected data". |
Colwell 1992.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 12; Gr2: 10 Inclusion: OA or RA, primary TKA Exclusion: TKA revisions, bilateral TKA, TKA requiring post‐surgical manipulation under anaesthesia Mean age (variance not reported) (yr): Gr1: 73; Gr2: 74 Gender (F): Gr1: 67%; Gr2: 70% Type of arthritis: combined: 95% OA, 5% RA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐40 dgr. Provided for majority of day. Increased 10 dgr/day as tolerated. Continued until D/C (criteria = 90‐dgr active ROM, independent SLR and activities of daily living) Gr2: standard care Physiotherapy and posterior splint for POD 1‐3 Standard care for Gr1, Gr2: Commenced POD 1. Provided 2 x per day. Included transfer training, gait training, SLR, active ROM, passive ROM exercises |
|
| Outcomes |
Outcomes included in this review: Primary None Secondary
Other outcomes: Hemovac output, pain medication Timing of outcome measures: tested at 1 mo*, 6 mo†, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/22 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | ‐ |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Denis 2006.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 27; Gr2: 27; Gr3: 26 Inclusion: OA, primary TKA, ambulatory, literate Exclusion: previous major lower‐limb surgery, contralateral TKA, total hip arthroplasty < 12 mo. Medical condition preclude testing, comprehension problems, concurrent surgical intervention, neuromuscular or degenerative disease, infection, major health complication Mean age (SD) (yr): Gr1: 68 (7); Gr2: 67 (8); Gr3: 70 (7) Gender (F): Gr1: 46%; Gr2: 52%; Gr3: 62% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 2. Initially 35‐45 dgr flexion. Provided for 2 hr/day. Increments determined by therapist. Continued until D/C or POD 8‐9 Gr2: standard care Other groups: Gr3: CPM + standard care CPM commenced POD 2. Initially 35‐45 dgr flexion. Provided 35 min/day. Increments determined by therapist. Continued until D/C or POD 8‐9 Standard care for Gr1, Gr2, Gr3: Commenced POD 1. Provided daily. Include passive ROM, active ROM, gait training, inner ROM quads, static quads, transfer training, splint (POD 0 to POD 1), stairs |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: WOMAC ‐ stiffness and functional difficulty, theoretical length of hospital stay, questionnaire ‐ frequency and intensity of physical activity. Timing of outcome measures: tested at D/C* (approx. POD 8‐9 post randomisation) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States: "two strata were created for an equivalent distribution...one set of prenumbered, sealed envelopes was prepared". However, does not state how randomisation schedule was generated. |
| Allocation concealment (selection bias) | Low risk | States: "prenumbered sealed envelopes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81/81 were present at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported on all outcomes at all endpoints. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "Assessment was performed…unaware of group allocation". |
Harms 1991.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 55; Gr2: 58 Inclusion: OA or RA, primary TKA, knee flexion contracture < 40 dgr, presurgical. condition ‐ able to walk 10 m within 2 min with walking aid, able to rise from chair with arm rests and seat height of 18 inches (45.7 cm) Exclusion: TKA revisions, concurrent knee surgery, condition comprising treatment Mean age (SD) (yr): Gr1: 69 (9); Gr2: 71 (10) Gender (F): Gr1: 78%; Gr2: 93% Type of arthritis: combined: 64% OA, 36% RA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐40 dgr at 2 dgr/sec. Provided for 6 hr/day. Increased 10 dgr/day as tolerated (after first 48 hr). Continued until 80 dgr of flexion achieved. Immobilised in splint or back slab while off CPM. Gr2: standard care Standard care for Gr1, Gr2: Commenced POD 1. Provided 20 min/day. Included: POD1: splint, static quads contractions progressing towards SLR, ankle and gluteal exercises POD2: mobilise with splint POD3: active knee flexion, inner ROM quads exercises, splint removed POD5: mobilise without splint if dynamic control of knee extension or proper SLR |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: wound drainage, number of participants requiring outpatient physiotherapy, pain medication Timing of outcome measures: tested at POD 7, POD 14*, D/C post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States: "random allocation table was generated". However, does not state how randomisation schedule was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Huang 2003.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 23; Gr2: 21 Inclusion: primary TKA Exclusion: not reported Mean age (range) (yr): combined: 69 (20‐92) Gender (F): combined: 82% Type of arthritis: combined: 77% OA, 23% RA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐40 dgr. Provided for 20 hr/day. Decreased on POD 3 to 16 hr/day. Continued until POD 14 Gr2: standard care Standard care for Gr1, Gr2: Commenced on POD 0. Provided for unreported period. Included isometric exercises, assisted SLR, passive ROM, active ROM, stretches, inner ROM quad strengthening |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: pain medication Timing of outcome measures: tested at POD 7, POD 14*, 6 wk, 3 mo†, 6 mo, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Unclear risk | Incomplete reporting of some outcomes. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | High risk | Not stated but not possible. |
Kumar 1996.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 40 pts (46 knees); Gr2: 33 pts (37 knees) Inclusion: OA Exclusion: not reported Mean age (range) (yr): Gr1: 69 (52‐86); Gr2: 68 (42‐88) Gender (F): Gr1: 58%; Gr2: 67% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐90 dgr. Provided for 10 hr/day. Increments not reported. Continued until D/C (criteria = dry wound, 80 dgr flexion, ambulation 300 feet 2 x per day with single crutch or walking stick, independent transfers). Immobilisation at night Gr2: standard care Immobilisation removed POD 1, passive ROM x 20 min (progressed to 30‐45 min), 2 x per day 90 dgr flexion achieved at each session Standard care for Gr1, Gr2: Commencement not reported. Provided 2 hr/day. Included isometric exercises, passive ROM, active ROM, stretches, gait training (including stairs). FES if extensor lag > 30 dgr or if no independent SLR performed on POD 3 |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: Knee Society Function Score, delay in physiotherapy due to wound drainage, length of acute stay (days), length of rehab stay (days). Timing of outcome measures: tested at POD 5*, 6 wk, 3 mo, 6 mo† post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "a random number generator". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46/77 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Not all data reported for all outcomes at all endpoints (e.g. SF‐36). |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Lau 2001.
| Methods | Randomised trial | |
| Participants | Sample size: combined: 43 pts (60 knees) Inclusion: TKA for OA or RA Exclusion: not reported Mean age (range) (yr): combined: 70 (43‐86) Gender (F): combined: 84% Type of arthritis (combined): OA: 72% pts, 75% knees; RA: 28% pts, 25% knees |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 1. Initially 0‐60 dgr. Provided for 23 hr/day. Increased as tolerated. Continued until POD 7 Gr2: standard care Immobilised until POD 7 Standard care for Gr1, Gr2: Commenced on POD 7. Provided for unreported period. Included assisted ROM, gait training |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: none Timing of outcome measures: tested at POD 3, POD 5, POD 7, POD 14*, POD 28, POD 42, 3 mo, 6 mo†, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38/43 patients (60/66 knees) were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Does not clearly state what outcomes were measured. Does not report all outcomes for all endpoints. Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | High risk | Abandoned VAS. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Lenssen 2003a.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 20; Gr2: 20 Inclusion: OA, undergoing TKA Exclusion: not reported Mean age (SD) (yr): Gr1: 65 (9); Gr2: 66 (10) Gender (F): Gr1: 71%; Gr2: 63% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 1. Initial settings individually determined. Provided for 4 hr/day. Increased as tolerated. Continued until POD 4 Gr2: standard care Standard care for Gr1, Gr2: Commenced on POD 1. Provided for 40 min/day. Included active ROM, passive ROM exercises, inner ROM and static quads strengthening, gait training, joint mobilisation |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: pain medication, satisfaction with physiotherapist's attention (11‐point scale), satisfaction with physiotherapist's treatment (11‐point scale), lowest pain in last 24 hr (points), worst pain in last 24 hr (points) Timing of outcome measures: tested at POD 4, POD 17* post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "computer‐generated table". |
| Allocation concealment (selection bias) | Low risk | States: "independent...secretary without knowledge of the randomisation schedule called up the patients for operation". Clarified through personal communication that allocation was concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38/40 were present at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported on all outcomes at all endpoints. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "independent, blinded observer". |
Lenssen 2008.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 30; Gr2: 30 Inclusion: primary unilateral TKA, < 80 dgr at POD 4, able to understand and speak Dutch, not suffering from mental disabilities, resident within the Maastricht Heuvelland region Exclusion: patients who need to stay in hospital > POD 5, showed relevant comorbidity influencing mobility (e.g. claudication, other prosthesis) or were operated upon by minimally invasive surgery, aged > 80 years, RA Mean age (SD) (yr): Gr1: 64 (8); Gr2: 65 (9) Gender (F): Gr1: 60%; Gr2: 70% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced when D/C from acute hospital care (approx. POD 4). Initial settings individually determined. Provided for 4 hr/day. Increased as tolerated. Continued until POD 17 Gr2: standard care Standard care for Gr1, Gr2: Commenced on POD 4. Provided for 20 min/day. Included active ROM, passive ROM exercises, inner ROM and static quads strengthening, gait training (including stairs), sit to stand training |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: WOMAC (measure of function), pain medication, satisfaction with treatment (11‐point Likert scale), satisfaction with treatment results (11‐point Likert scale), adherence to treatment protocol and use of CPM (hr), Knee Society Score ‐ function, WOMAC (stiffness and difficulty sub scale) Timing of outcome measures: tested at POD 17 (12 days after randomisation)*, 6 wk, 3 mo† post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "blocked and concealed randomisation with a block size of four ensured equal distribution of patients over the 2 treatment group....groups were prestratified on pre‐operative flexion mobility of the knee...randomly assigned". Clarified through personal communication that sequence was computer generated. |
| Allocation concealment (selection bias) | Low risk | States: "concealed randomisation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60/60 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Not all data reported for all outcomes at all endpoints (e.g. secondary outcomes at 3 mo). |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "blinded...assessment and analysis". |
MacDonald 2000.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 40; Gr2: 40; Gr3: 40 Inclusion: aged < 80 yr with primary OA, no previous surgery on the knee, normal functioning ipsilateral hips, ability to tolerate non‐steroidal anti‐inflammatory drugs and Marcaine (bupivacaine hydrochloride and epinephrine injection), ability to ambulate 30 m preoperatively, ability to climb 10 steps Exclusion: RA, > 15 dgr valgus or fixed flexion deformity Age/gender: not reported Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐50 dgr. Provided for 18‐24 hr/day. Increased by 10 dgr/hr as tolerated. Continued until POD 1 Gr2: standard care Other groups: Gr3: CPM + standard care CPM commenced POD 0. Initially 70‐110 dgr. Provided for 18‐24 hr/day. Not increased. Continued until POD 1 Standard care for Gr1, Gr2, G3: Commenced POD 1. Provided 2 x per day for 6 wk. Included active ROM, passive ROM exercises, mobilised as tolerated using walker or crutches |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: pain medication Timing of outcome measures: tested at D/C*, 6 wk†, 12 wk, 26 wk, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States: "computer generated randomised schedule". |
| Allocation concealment (selection bias) | Low risk | Not stated. Clarified through personal communication that allocation was concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | Not all data reported for all outcomes at all endpoints (e.g. 26 wk). |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "a blinded independent observer". |
Maniar 2012.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 28 (33 knees); Gr2: 28 (33 knees); Gr3: 28 (33 knees) Inclusion: OA or RA, primary TKA, ambulatory Exclusion: Medical condition interfering with tests, neuromuscular or neurodegenerative disease Mean age (variance not reported) (yr): Gr1: 66; Gr2: 67; Gr3: 67 Gender (F): Gr1: 93%; Gr2: 93%; Gr3: 89% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM for 3 days + standard care CPM commenced POD 2. Initially 0‐30 dgr flexion. Provided 15 min per session and 2 sessions per day. Increased 10 dgr every 5 min. Continued until POD 4 Gr2: standard care Other groups: Gr3: CPM for 1 day + standard care CPM commenced POD 2. Initially 0‐30 dgr flexion. Provided 15 min per session and 2 sessions per day. Increased 10 dgr every 5 min. Continued for 1 day Standard care for Gr1, Gr2, Gr3: Commenced POD 0. Continued POD 4. Included active ROM, gait training, static quads, inner ROM quads, gait and stair training, transfer training |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: WOMAC‐Stiffness, WOMAC‐Total, timed 'Up and Go' test, calf girth, pain at rest, pain on walking Timing of outcome measures: tested at POD 3, POD 5, POD 14*, POD 42, 90 days† post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | States: "using sealed envelopes". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States how many randomised but does not clearly state how many were available at follow‐up. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | High risk | Use of results from bilateral TKA without consideration of dependency. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
May 1999.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 12; Gr2: 7 Inclusion: unilateral primary TKA, OA Exclusion: surgical revision or manipulation under anaesthesia of the involved knee, knee flexion ROM > 80 dgr upon admission to rehabilitation, > POD 14 TKA, inability to participate in hydrotherapy due to factors such as incontinence or wound infection, RA, inability or unwillingness to provide informed consent Mean age (SD) (yr): Gr1: 73 (4); Gr2: 66 (9) Gender (F): Gr1: 67%; Gr2: 71% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced when admitted to rehabilitation facility (POD 2‐13). Initial settings individually determined. Provided for 3‐5 hr/day. Increased as tolerated. Continued until 80 dgr active knee flexion was achieved or until D/C, whichever came first Gr2: Lower Limb Mobility Board + standard care Commenced when admitted to rehabilitation facility (POD 2‐13). Provided 5‐10 min for 6 x per day, 7 days/wk Standard care for Gr1, Gr2: Commenced not reported. Provided 1‐1.5 hr/day, 5 days/wk. Included active assisted ROM, gait training, inner ROM and static quads strengthening, hydrotherapy |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: none Timing of outcome measures: tested on admission to rehabilitation facility (POD 2‐13), D/C* (POD 12‐31) post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States: "stratified random technique". However, does not state how randomisation schedule was generated. |
| Allocation concealment (selection bias) | Low risk | States: "one of two pieces of paper indicating group assignment was drawn from an envelope labelled by gender and surgeon". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/21 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "one of two assessors....blinded to the subjects' group allocation". |
McInnes 1992.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 48; Gr2: 45 Inclusion: RA or OA, primary TKA, passive knee flexion ROM of at least 90 dgr and no more than 20 dgr knee flexion contracture Exclusion: cognitive or sensory deficit, unable to understand or speak English, undergoing another surgical procedure prior or during TKA, weight > 136 kg Mean age (SD) (yr): Gr1: 66 (2); Gr2: 70 (1) Gender (F): Gr1: 65%; Gr2: 64% Type of arthritis: Gr1: 73% OA; Gr2: 89% OA; combined: 81% OA, 19% RA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initial settings individually determined. Provided on average, 9 hr/day for POD 0‐7. Increased as tolerated. Continued until D/C Gr2: standard care Standard care for Gr1, Gr2: Commenced POD 1. Provided 1‐2 x per day, 7 days/wk. Included inner ROM and static quads strengthening (from POD 1), active assisted ROM and passive ROM exercises (from POD 2), SLR, gait training, transfer training, bicycling, proning |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: quadriceps strength (probit. Nm) Timing of outcome measures: tested at POD 7*, 6 wk† post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93/102 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Does not clearly state what outcomes were measured (e.g. at 6 wk). Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "blinded to whether the patient was using CPM". |
Montgomery 1996.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 28; Gr2: 32 Inclusion: gonarthrosis, primary TKA Exclusion: not reported Mean age (SD) (yr): Gr1: 74 (5); Gr2: 76 (6) Gender (F): Gr1: 86%; Gr2: 75% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 1. Initial settings individually determined. Speed set at 2‐6 min/cycle. Provided for 9 hr/day, 7 days/wk. Increased as tolerated. Continued until D/C (criteria = active ROM minimum 70 dgr, no wound problem, ability to walk and climb stairs, independent with activities of daily living) or up to 2 wk Gr2: knee ex's + standard care Commenced POD 1. Provided for 1 hr/day, 5 days/wk. Included active ROM and passive ROM (assisted by physiotherapist) Standard care for Gr1, Gr2: Encouraged to exercise and provided with instructions on gait |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: none Timing of outcome measures: tested at POD 1, POD 3, POD 5, D/C* post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60/68 were present at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Does not clearly state what outcomes were measured. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Ng 1999.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 16; Gr2: 16; Gr3: 17
Inclusion: unilateral TKA, passive knee flexion ROM of 100 dgr or more, ambulant
Exclusion: cardiopulmonary complications, previous trauma or pathology (or both) of the hip on affected side, neurological deficits, not assessed by physiotherapist preoperatively Age/gender: not reported Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: early knee flexion CPM + standard care Commenced POD 0. Initially 70‐100 dgr. Provided for 4 hr/day. Increased to 50‐100 dgr on POD 1 and 0‐100 dgr on POD 2. Continued for unreported period Gr2: standard care Other groups: Gr3: conventional CPM + standard care Commenced POD 0. Initially 0‐40 dgr. Provided for 4 hr/day. Increased 10 dgr/day. Continued for unreported period Standard care for Gr1, Gr2, Gr3: Commencement not reported. Provided for unreported period. Included active ROM, gait training, inner ROM and static quads strengthening exercises, transfer training, gluteal exercises |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: number of days to achieve 90 dgr flexion ROM Timing of outcome measures: tested at POD 3, POD 5, POD 7* post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 49/55 were present at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All data reported on all outcomes at all endpoints. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Nielsen 1988.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 24; Gr2: 26 Inclusion: arthritis, primary TKA Exclusion: previous TKA in contralateral knee Mean age (range) (yr): Gr1: 71 (40‐83); Gr2: 72 (37‐83) Gender (F): Gr1: 70%; Gr2: 70% Type of arthritis: not reported |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 2. Initially 0‐25 dgr. Provided for 4 hr/day. Increased 5‐10 dgr/day. Continued until POD 12 Gr2: standard care Standard care for Gr1, G2: Commenced POD 2. Provided for unreported period. Included inner ROM quads and static quads strengthening, active ROM with full weight bearing |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: flexion deterioration, number of people with improvement, no change or deterioration. Timing of outcome measures: tested at POD 14* post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50/54 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Does not clearly state what outcomes were measured. Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "the evaluation was carried out...who was uninformed". |
Ritter 1989.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 50 pts (100 knees); Gr2: 50 pts (100 knees)
Inclusion: preoperative range > 90 dgr
Exclusion: not reported Mean age (range) (yr): combined: 73 (43‐85) Gender (F): combined: 34% Type of arthritis: combined: 94% OA, 6% RA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 2. Initially settings individually determined. Provided for 20‐24 hr/day. Increased as tolerated. Continued until POD 7 Gr2: standard care Knee extension splint at night until independent SLR achieved Standard care for Gr1, Gr2: Commenced POD 0. Provided 2 x per day. Included active ROM, stretches, static quads exercises, SLR |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: suprapatella and distal to patella circumference. Timing of outcome measures: tested at 1 wk*, 8 wk, 6 mo†, 1 yr‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated. Clarified through personal communication that sequence was computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Clarified through personal communication that sequence was stored on computer but not clear if concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47/50 were present at follow‐up. |
| Selective reporting (reporting bias) | High risk | Not all data reported for all outcomes at all endpoints (e.g. knee extension ROM at POD 61). Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. Clarified through personal communication that unblinded assessors were used for short‐term assessments and blinded assessors were used for medium‐ and long‐term assessments. |
Sahin 2006.
| Methods | American Knee Society | |
| Participants | Sample size: Gr1: 15; Gr2: 16 Inclusion: TKA for primary OA Exclusion: TKA for secondary OA or inflammatory joint disease, cognitive or sensorial deficit; speech problems; bilateral TKA Mean age (SD) (yr): Gr1: 61 (6.0); Gr2: 61.6 (7.5) Gender (F): Gr1: 86%; Gr2: 86% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 1. Initially 0‐40 dgr flexion. Provided 2.5 hr per session for 2 sessions per day. Increased by 10 dgr each day. Continued until POD 7 Gr2: standard care Standard care for Gr1, Gr2: Commenced POD 1. Provided for 30 min, 2 x per day for 1 wk. Continued until discharge (approx. POD 14). Home exercise programme provided following discharge until week 6. Included active and assisted active ROM, gait training, static quads, SLR, gluteal contractions and ankle pumping exercises |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: pain at rest, American Knee Society Knee score. Timing of outcome measures: tested at 2 weeks*, 6 weeks† and 6 months‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28/31 completed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per stated protocol. |
| Other bias | Unclear risk | Not stated how randomisation schedule was generated. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | States ‐ "same physician". Looks unlikely that he/she was blinded. |
Vince 1987.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 42; Gr2: 20 Inclusion: primary unilateral TKA Exclusion: obese (> 109 kg), bilateral TKA Mean age (range) (yr): Gr1: 68 (44‐80); Gr2: 66 (47‐83) Gender (F): Gr1: 69%; Gr2: 85% Type of arthritis: combined: 87% OA, 13% RA |
|
| Interventions |
Groups included in this review: Gr1: CPM + standard care CPM commenced POD 0. Initially 0‐30 dgr. Provided for 20 hr/day. Increased as tolerated. Continued until D/C Gr2: standard care Standard care for Gr1, Gr2: Commenced POD 3. Provided for unreported period. Included active assisted ROM exercises, gait training |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: time (days) to achieve 90 dgr flexion ROM Timing of outcome measures: tested at POD 4, POD 5, D/C* (POD 15‐16) post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | Not clear what was measured. Key outcomes reported incompletely preventing their contribution to the meta‐analysis. |
| Other bias | High risk | Unclear whether random allocated really was used. States: "The patients were assigned to the control or CPM group randomly". However, the imbalance in the size of the two groups was unlikely to occur by chance and nothing to suggest that blocked randomisation was used. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Unclear risk | Not stated. |
Worland 1998.
| Methods | Randomised trial | |
| Participants | Sample size: Gr1: 37 pts (49 knees); Gr2: 43 pts (54 knees) Inclusion: unilateral and bilateral TKA, OA Exclusion: concomitant medical problems, serious drainage from wounds postoperatively Mean age (range) (yr): Gr1: 69 (54‐81); Gr2: 71 (44‐86) Gender (F): Gr1: 61%; Gr2: 71% Type of arthritis: combined: 100% OA |
|
| Interventions |
Groups included in this review: Gr1: CPM CPM commenced when D/C from acute hospital care (approx. POD 3‐4). Initially 90 dgr. Provided for 3 hr/day. Increments not reported. Continued for 10 days Gr2: standard care Commenced when D/C from acute hospital care (approx. POD 3‐4). Provided for 1 hr, 3 x per wk. Continued for 2 wk. Included home exercises consisting of strengthening, stretching, gait training, SLR |
|
| Outcomes |
Outcomes included in this review: Primary
Secondary
Other outcomes: none Timing of outcome measures: tested at baseline upon randomisation (2 wk post operation)*, 6 wk†, 6 mo‡ post randomisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | Not all data reported for all outcomes at all endpoints (e.g. 2 wk). |
| Other bias | Low risk | No other biases were present. |
| Participant blinding? | High risk | Not stated but not possible. |
| Personnel blinding? | High risk | Not stated but not possible. |
| Outcome assessor blinding? | Low risk | States: "All patients were evaluated by our senior author, who did not know which therapy the patient received". |
*: endpoint included in analysis of short‐term effect; †: endpoint included in analysis of medium‐term effect; ‡: endpoint included in analysis of long‐term effect; ª: no measure of variability provided for at least one endpoint; ^: no data provided for at least one endpoint; ɫ: calculated SD implausible so not reported; approx.: approximately; cm: centimetre; CPM: continuous passive motion; D/C: discharge; dgr: degree; F: female; FES: functional electrical stimulation; Gr: group; hr: hour; kg: kilogram; m: metre; min: minutes; mo: month; Nm: newton metres; OA: osteoarthritis; POD: postoperative day; pts: patients; RA: rheumatoid arthritis; ROM: range of motion; SD: standard deviation; SF‐12: Short‐Form 12‐Item Health Survey; SF‐36: Short‐Form 36‐Item Health Survey; SLR: straight leg raise; TKA: total knee arthroplasty; VAS: visual analogue scale; wk: weeks; WOMAC: Western Ontario and McMaster Osteoarthritis Index; yr: years.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beaupré 2001 | Participants. Mixed population |
| Chen 2000 | Participants. Unclear if all OA |
| Coutts 1983 | Design. Not RCT |
| Davis 1984 | Participants. Unclear if all OA |
| Haug 1988 | Comparison. CPM + electrical stimulation versus CPM |
| Johnson 1990 | Comparison. CPM + exercises involving full extension versus splinting + SLR |
| Johnson 1992 | Comparison. CPM + full extension versus splinting + SLR |
| Kim 1995 | Comparison. CPM versus alternative flexion + extension splinting regime |
| Kim 2009 | Comparison and not true randomisation: allocated according to day of surgery. Passive ROM exercises provided by a therapist versus no passive ROM exercises |
| Leach 2006 | Design. Not RCT |
| Leonard 2007 | Comparison. One protocol of CPM versus another protocol of CPM |
| Lynch 1988 | Participants. Mixed population |
| Maloney 1990 | Design. Not RCT |
| Odenbring 1989 | Participants. Osteotomy, not TKA |
| Pope 1997 | Randomisation. Allocated according to admission |
| Simkin 1999 | Intervention. CPM for the hip |
| Ververeli 1995 | Design. Not RCT |
| Walker 1991 | Participants. Unclear if all OA |
| Woog 2008 | Comparison. One protocol of CPM versus another protocol of CPM |
| Yashar 1997 | Comparison. One protocol of CPM versus another protocol of CPM |
| Young 1984 | Design. Not RCT (retrospective study) |
CPM: continuous passive motion; OA: osteoarthritis; RCT: randomised controlled trial; ROM: range of motion; SLR: straight leg raise; TKA: total knee arthroplasty.
Characteristics of studies awaiting assessment [ordered by study ID]
Aubriot 1993.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | Only abstract in English. Manuscript in French |
Cui 2009.
| Methods | Randomised controlled trial |
| Participants | 20 participants following TKA |
| Interventions | 2 groups: CPM versus no CPM |
| Outcomes | Knee ROM |
| Notes | Only abstract in English. Manuscript in Chinese |
Ersozlu 2009.
| Methods | Randomised controlled trial |
| Participants | 86 participants following TKA |
| Interventions | 3 groups: conventional therapy versus CPM started on day 1 versus CPM started on day 3 |
| Outcomes | Knee ROM; Knee Society Rating |
| Notes | Only abstract in English. Manuscript in Turkish |
Li 2010.
| Methods | Between‐group study but not clear if randomised |
| Participants | 60 participants following TKA |
| Interventions | 2 groups: velocity of CPM determined by protocol versus velocity of CPM determined by patient comfort |
| Outcomes | Knee Hospital for Special Surgery, pain and Barthel Index |
| Notes | Only abstract in English. Manuscript in Chinese. Comparison may exclude trial from this review |
Li 2010a.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | Only abstract in English. Manuscript in Chinese |
Liu 2011.
| Methods | Between‐group study but not clear if randomised |
| Participants | 266 participants following TKA |
| Interventions | 2 groups: CPM versus active exercises |
| Outcomes | Knee Society Score, ROM, pain |
| Notes | Only abstract in English. Manuscript in Chinese. Authors concluded KSS scores and ROM were higher in active exercises group at 3 months |
Sosin 2000.
| Methods | Randomised controlled trial |
| Participants | 76 participants following TKA |
| Interventions | 2 groups: CPM versus no CPM |
| Outcomes | Knee Society Score |
| Notes | Only abstract in English. Manuscript in Polish. Authors conclude "no statistically significant differences" between the 2 groups |
CPM: continuous passive motion; ROM: range of motion; TKA: total knee arthroplasty.
Differences between protocol and review
The protocol was modified when the review was updated in 2010. The modifications were:
participants restricted to those with pre‐surgery diagnosis of arthritis;
comparisons were changed from continuous passive motion (CPM) and physiotherapy versus physiotherapy alone to CPM and standard postoperative care versus CPM and standard postoperative care with active or passive knee exercises;
updated methods based on current Cochrane Collaboration recommendations for risk of bias assessment, 'Summary of findings' table, and the updated Cochrane search filter for identifying randomised controlled trials;
endpoints were classified as short, medium and long term;
only one observation was extracted for each outcome within a particular endpoint (short, medium or long term);
in trials with more than two groups, only data from the two groups with the most contrasting interventions were extracted and used for analyses;
the comparisons were divided into primary and secondary comparisons;
pain outcomes were restricted to direct measures of pain intensity (e.g. visual analogue scale); data on pain medication were not extracted.
The protocol was modified when the review was updated in 2013. The modifications were:
missing standard deviations (SD) were not imputed. Instead, studies with missing SDs were removed from the analyses;
studies that stated that they reported outcomes but did not provide the data were included in the counts of studies that collected the outcomes; however, these studies were not included in the meta‐analyses;
primary and secondary outcomes were changed and added on request of the Editorial Committee of the Cochrane Musculoskeletal Group. It was requested that the original protocol be ignored and that the primary outcomes be changed to: active knee flexion, function, quality of life, participants' global assessment of treatment effectiveness, incidence of manipulation and adverse events. This necessitated adding outcomes that were not in the original protocol or the previous version of the review. It also required moving primary outcomes to secondary outcomes and vice versa;
only the primary outcomes were used in the 'Summary of findings' table. The choice between short, medium or long term were based on which ever had the most number of trials;
need for manipulation and adverse events were not categorised into short‐, medium‐ or long‐term effects. Instead, each of these outcomes were collated regardless of time of occurrence since randomisation.
Contributions of authors
LA Harvey: rewriting the manuscript in 2010 and 2013, conducting the updated search, screening potentially eligible trials, extracting all data reported in the original review and additional data required for the update, analysing data, interpreting results, updating reference list and creating the 'Summary of findings' table. L Brosseau: 2003 version of this review: extracting data, updating the reference list, updating the analyses and updating the interpretation of results; 2013 version of this review: interpreting the results and editing the manuscript. RD Herbert: 2010 version of this review: screening potentially eligible trials, extracting additional data required for the update, analysing data, interpreting results, conducting the mega‐regression and creating the 'Summary of findings' table; 2013 version of this review: conducting the mega‐regression, interpreting the results and editing the manuscript.
Sources of support
Internal sources
The University of Ottawa, Canada.
The Rehabilitation Studies Unit, Sydney School of Medicine/Northern, University of Sydney, Australia, Other.
External sources
-
NHMRC, Australia.
fellowship for RDH
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Alkire 2010 {published data only}
- Alkire MR, Swank M. Use of inpatient continuous passive motion versus no CPM in computer‐assisted total knee arthroplasty. Orthopaedic Nursing 2010;29:36‐40. [DOI] [PubMed] [Google Scholar]
Bennett 2005 {published data only}
- Bennett LA, Brearley SC, Hart JA, Bailey MJ. A comparison of 2 continuous passive motion protocols after total knee arthroplasty: a controlled and randomized study. Journal of Arthroplasty 2005;20:225‐33. [DOI] [PubMed] [Google Scholar]
Bruun‐Olsen 2009 {published data only}
- Bruun‐Olsen V, Heiberg KE, Mengshoel AM. Continuous passive motion as an adjunct to active exercises in early rehabilitation following total knee arthroplasty ‐ a randomized controlled trial. Disability & Rehabilitation 2009;31:277‐83. [DOI] [PubMed] [Google Scholar]
Can 1995 {published data only}
- Can F, Algun C, Alpaslan M. Effect of the CPM in total knee arthroplasty. Physiotherapy 1995;81:453. [Google Scholar]
Chiarello 1997 {published data only}
- Chiarello CM, Gundersen MS, O'Halloran T. The effect of continuous passive motion duration and increment on range of motion in total knee arthroplasty patients. Journal of Orthopaedic and Sports Physical Therapy 1997;25(2):119‐27. [DOI] [PubMed] [Google Scholar]
Colwell 1992 {published data only}
- Colwell CW, Morris BA. The influence of continuous passive motion on the results of total knee arthroplasty. Clinical Orthopaedics and Related Research 1992;276:225‐8. [PubMed] [Google Scholar]
Denis 2006 {published data only}
- Denis M, Moffet H, Caron F, Ouellet D, Paquet J, Nolet L. Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Physical Therapy 2006;86:174‐85. [PubMed] [Google Scholar]
Harms 1991 {published data only}
- Harms M, Engstrom B. Continuous passive motion as an adjunct to treatment in the physiotherapy management of the total knee arthroplasty patient. Physiotherapy 1991;7(4):301‐7. [Google Scholar]
Huang 2003 {published data only}
- Huang D, Peng Y, Su P, Ye W, Liang A. The effect of continuous passive motion after total knee arthroplasty on joint function. Chinese Journal of Clinical Rehabilitation 2003;7:1661‐2. [Google Scholar]
Kumar 1996 {published data only}
- Kumar PJ, McPherson EJ, Dorr LD, Wan Z, Baldwin K. Rehabilitation after total knee arthroplasty: a comparison of two rehabilitation techniques. Clinical Orthopaedics and Related Research 1996;331:93‐101. [DOI] [PubMed] [Google Scholar]
Lau 2001 {published data only}
- Lau SKK, Chiu KY. Use of continuous passive motion after total knee arthroplasty. Journal of Arthroplasty 2001;16(3):336‐9. [DOI] [PubMed] [Google Scholar]
Lenssen 2003a {published data only}
- Lenssen A, Bie RA, Bulstra SK, Steyn MJA. Continuous passive motion (CPM) in rehabilitation following total knee arthroplasty: a randomised controlled trial. Physical Therapy Review 2003;8:123‐9. [Google Scholar]
Lenssen 2008 {published data only}
- Lenssen TA, Steyn MJ, Crijns YH, Waltje EM, Roox GM, Geesink RJ, et al. Effectiveness of prolonged use of continuous passive motion (CPM), as an adjunct to physiotherapy, after total knee arthroplasty. BMC Musculoskeletal Disorders 2008;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2000 {published data only}
- MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW, Kramer J, Vaz M. Prospective randomized clinical trial of continuous passive motion after total knee arthroplasty. Clinical Orthopaedics and Related Research 2000;380:30‐5. [DOI] [PubMed] [Google Scholar]
Maniar 2012 {published data only}
- Maniar RN, Baviskar JV, Singhi T, Rathi SS. To use or not to use continuous passive motion post‐total knee arthroplasty presenting functional assessment results in early recovery. Journal of Arthroplasty 2012;27:193‐200. [DOI] [PubMed] [Google Scholar]
May 1999 {published data only}
- May A, Busse W, Zayac D, Withridge M. Comparison of continuous passive motion (CPM) machines and lower limb mobility boards (LLiMB) in the rehabilitation of patients with total knee arthroplasty. Canadian Journal of Rehabilitation 1999;12:257‐63. [Google Scholar]
McInnes 1992 {published data only}
- McInnes J, Larson MG, Daltroy LH, Brown T, Fossel AH, Eaton HM, et al. A controlled evaluation of continuous passive motion in patients undergoing total knee arthroplasty. JAMA 1992;268(11):1423‐8. [DOI] [PubMed] [Google Scholar]
Montgomery 1996 {published data only}
- Montgomery F, Eliasson M. Continuous passive motion compared to active physical therapy after total knee arthroplasty: similar hospitalization times in a randomized study of 68 patients. Acta Orthopaedica Scandinavica 1996;67(1):7‐9. [DOI] [PubMed] [Google Scholar]
Ng 1999 {published data only}
- Ng TS, Yeo SJ. An alternative early knee flexion regimen of continuous passive motion for total knee arthroplasty. Physiotherapy Singapore 1999;2:53‐63. [Google Scholar]
Nielsen 1988 {published data only}
- Nielsen PT, Rechnagel K, Nielsen SE. No effect of continuous passive motion after total knee arthroplasty of the knee. Acta Orthopaedica Scandinavica 1988;59(5):580‐1. [DOI] [PubMed] [Google Scholar]
Ritter 1989 {published data only}
- Ritter MA, Gandolf VS, Holston KS. Continuous passive motion versus physical therapy in total knee arthroplasty. Clinical Orthopaedics and Related Research 1989;244:239‐43. [PubMed] [Google Scholar]
Sahin 2006 {published data only}
- Sahin E, Akalin E, Bircan C, Karaoglan O, Tatari H, Alper S, et al. The effects of continuous passive motion on outcome in total knee arthroplasty. Journal of Rheumatology and Medical Rehabilitation 2006;17:85‐90. [Google Scholar]
Vince 1987 {published data only}
- Vince K, Kelly M, Beck J, Insall J. Continuous passive motion after total knee arthroplasty. Journal of Arthroplasty 1987;2:281‐4. [DOI] [PubMed] [Google Scholar]
Worland 1998 {published data only}
- Worland RL, Arredondo J, Angles F, Jimenez FL, Jessup DE. Home continuous passive motion machine versus professional physical therapy following total knee replacement. Journal of Arthroplasty 1998;13(7):784‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beaupré 2001 {published data only}
- Beaupré LA, Davies DM, Jones CA, Cinats JG. Exercise combined with continuous passive motion or slider board therapy compared with exercise only: a randomized controlled trial of patients following total knee arthroplasty. Physical Therapy 2001;81(4):1029‐37. [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen B, Zimmerman JR, Soulen L, DeLisa JA. Continuous passive motion after total knee arthroplasty: a prospective study. American Journal of Physical Medicine and Rehabilitation 2000;79(5):421‐6. [DOI] [PubMed] [Google Scholar]
Coutts 1983 {published data only}
- Coutts R, Borden L, Bryan R, Hungerford D, Stulberg B, Stulberg S. The effect of continuous passive motion on total knee rehabilitation. Orthopaedic Transactions 1983;3:535‐6. [Google Scholar]
Davis 1984 {published data only}
- Davis D. Continuous passive motion for total knee arthroplasty. Physical Therapy 1984;64:709. [Google Scholar]
Haug 1988 {published data only}
- Haug J, Wood LT. Efficacy of neuromuscular stimulation of the quadriceps femoris during continuous passive motion following total knee arthroplasty. Archives of Physical Medicine and Rehabilitation 1988;69:423‐4. [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson DP. The effect of continuous passive motion on wound‐healing and joint mobility after knee arthroplasty. Journal of Bone and Joint Surgery 1990;72‐A(3):421‐6. [PubMed] [Google Scholar]
Johnson 1992 {published data only}
- Johnson DP. Beneficial effects of continuous passive motion after total condylar knee arthroplasty. Annals of the Royal College of Surgeons of England 1992;74:412‐6. [PMC free article] [PubMed] [Google Scholar]
Kim 1995 {published data only}
- Kim J‐M, Moon M‐S. Squatting following total knee arthroplasty. Clinical Orthopaedics and Related Research 1995;313:177‐86. [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim TK, Park KK, Yoon SW, Kim SJ, Chang CB, Seong SC. Clinical value of regular passive ROM exercise by a physical therapist after total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy 2009;17:1152‐8. [DOI] [PubMed] [Google Scholar]
Leach 2006 {published data only}
- Leach W, Reid J, Murphy F. Continuous passive motion following total knee replacement: a prospective randomized trial with follow‐up to 1 year. Knee Surgery, Sports Traumatology, Arthroscopy 2006;14:922‐6. [DOI] [PubMed] [Google Scholar]
Leonard 2007 {published data only}
- Leonard GM, Tremblay LE, Chabot M, Lariviere J, Papadopoulos P. The effects of early continuous passive motion after total knee arthroplasty. Physiotherapy Canada 2007;59:111‐7. [Google Scholar]
Lynch 1988 {published data only}
- Lynch AF, Bourne RB, Rorabeck CH, Rankin RN, Donald A. Deep‐vein thrombosis and continuous passive motion after total knee arthroplasty. Journal of Bone and Joint Surgery 1988;70‐A(1):11‐4. [PubMed] [Google Scholar]
Maloney 1990 {published data only}
- Maloney WJ, Schurmann DJ, Hangen D, Goodman SB, Edworthy S, Bloch DA. The influence of continuous passive motion on outcome in total knee arthroplasty. Clinical Orthopaedics and Related Research 1990;256:162‐8. [PubMed] [Google Scholar]
Odenbring 1989 {published data only}
- Odenbring S, Lindstrand A, Egund N. Early knee mobilization after osteotomy for gonarthrosis. Acta Orthopaedica Scandinavica 1989;60(6):699‐702. [DOI] [PubMed] [Google Scholar]
Pope 1997 {published data only}
- Pope RO, Corcoran S, McCaul K, Howie DW. Continuous passive motion after primary total knee arthroplasty: does it offer any benefits?. Journal of Bone and Joint Surgery 1997;79‐B(6):914‐7. [DOI] [PubMed] [Google Scholar]
Simkin 1999 {published data only}
- Simkin PA, Lateur BJ, Alquist AD, Wuestad KA, Beardsley RM, Esselman PC. Continuous passive motion for osteoarthritis of the hip: a pilot study. Journal of Rheumatology 1999;26:1987‐91. [PubMed] [Google Scholar]
Ververeli 1995 {published data only}
- Ververeli PA, Sutton DC, Hearn SL, Booth RE, Hozack WJ, Rothman RR. Continuous passive motion after total knee arthroplasty. Analysis of costs and benefits. Clinical Orthopaedics and Related Research 1995;321:208‐15. [PubMed] [Google Scholar]
Walker 1991 {published data only}
- Walker RH, Morris BA, Angulo DL, Schneider J, Colwell CW. Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation, and continuous cooling pad following total knee arthroplasty [Phase 1]. Journal of Arthroplasty 1991;6(2):151‐6. [DOI] [PubMed] [Google Scholar]
Woog 2008 {published data only}
- Woog L, Vandeput C. Usefulness of continuous passive mobilization early after total knee arthroplasty: comparison of two rehabilitation protocols [Interet de l'utilisation precoce de la mobilisation passive continue apres une prothese totale de genou. Comparaison de deux protocoles de reeducation]. Kinesitherapie Revue 2008;77:38‐43. [Google Scholar]
Yashar 1997 {published data only}
- Yashar AA, Venn‐Watson E, Welsh T, Colwell CW, Lotke P. Continuous passive motion with accelerated flexion after total knee arthroplasty. Clinical Orthopaedics and Related Research 1997;345:38‐43. [PubMed] [Google Scholar]
Young 1984 {published data only}
- Young JS, Kroll MA. Continuous passive motion compared to active assisted range of motion. Physical Therapy 1984;64:721. [Google Scholar]
References to studies awaiting assessment
Aubriot 1993 {published data only}
- Aubriot JH, Guincestre JY, Grandbastien B. Rehabilitation following total knee arthroplasty. Role of passive motion. A randomised study about 120 subjects [Intérêt des appareils arthromoteurs dans la rééducation précoce des arthroplasties totales de genou. Étude prospective à propos de 120 dossiers]. Revue de Chirurgie Orthopédique 1993;79:586‐90. [PubMed] [Google Scholar]
Cui 2009 {published data only}
- Cui XQ, Wang HZ, Li W. Influence of time for continuous passive activities on range of motion following total knee arthroplasty. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13:4237‐40. [Google Scholar]
Ersozlu 2009 {published data only}
- Ersozlu S, Sahin O, Ozgur AF, Tuncay IC. The effects of two different continuous passive motion protocols on knee range of motion after total knee arthroplasty: a prospective analysis [Iki farkli surekli pasif hareket protokolunun total diz protezi sonrasi diz hareket acikligina etkileri: Ileriye donuk bir calisma]. Acta Orthopaedica et Traumatologica Turcica 2009;43:412‐8. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li XL. Velocity factor of continuous passive motion following knee replacement: differences between treatment and control groups. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14:2349‐52. [Google Scholar]
Li 2010a {published data only}
- Li XL, Zhao XO. Continuous passive joint motion following total knee replacement: 48 cases analysis. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14:665‐8. [Google Scholar]
Liu 2011 {published data only}
- Liu W, Wu YL, Cong RJ, Fu PL, Li XH, Wu HS. Controlled active motion and continuous passive motion are beneficial to function rehabilitation after total knee arthroplasty. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15:6509‐13. [Google Scholar]
Sosin 2000 {published data only}
- Sosin P, Dutka J, Stabach M. A comparison of kinesitherapy with and without continuous passive motion (CPM) after the entire allograft surgery of the knee. Chirurgia Narzadow Ruchu I Ortopedia Polska 2000;65:47‐53. [PubMed] [Google Scholar]
Additional references
Cates 2008 [Computer program]
- Cates C. Visual Rx 2.0 NNT Calculator. www.nntonline.net. Dr Chris Cates' EBM website, 2000.
Dickersin 1993
- Dickersin K, Min YI. NIH clinical trials and publication bias. The Online Journal of Current Clinical Trials 1993;April 28:Doc No 50. [PubMed] [Google Scholar]
Du Plessis 2011
- Du Plessis M, Eksteen E, Jenneker A, Kriel E, Mentoor C, Stucky T, et al. The effectiveness of continuous passive motion on range of motion, pain and muscle strength following rotator cuff repair: a systematic review. Clinical Rehabilitation 2011;25:291‐302. [DOI] [PubMed] [Google Scholar]
Egger 1998
- Egger M, Smith GD. Meta‐analysis. Bias in location and selection of studies. BMJ 1998;316:61‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fazalare 2010
- Fazalare JA, Griesser MJ, Siston RA, Flanigan DC. The use of continuous passive motion following knee cartilage defect surgery: a systematic review. Orthopedics (Online) 2010;33:878. [DOI] [PubMed] [Google Scholar]
Fisher 1985
- Fisher RL, Kloter K, Bzdyra B, Cooper JA. Continuous passive motion (C.P.M.) following total knee replacement. Connecticut Medicine 1985;49:498‐501. [PubMed] [Google Scholar]
Fox 1981
- Fox JL, Poss R. The role of manipulation following total knee replacement. Journal of Bone and Joint Surgery 1981;63‐A(3):357‐62. [PubMed] [Google Scholar]
Gluud 2006
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163:493‐501. [DOI] [PubMed] [Google Scholar]
Grella 2008
- Grella, RJ. Continuous passive motion following total knee arthroplasty: a useful adjunct to early mobilisation?. Physical Therapy Reviews 2008;13:269‐79. [Google Scholar]
Hemmerich 2006
- Hemmerich A, Brown H, Smith S, Marthandam SS, Wyss UP. Hip, knee, and ankle kinematics of high range of motion activities of daily living. Journal of Orthopaedic Research 2006;24(4):770‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lenssen 2003b
- Lenssen AF, Koke AJ, Ade Bie RA, Geesink RGT. Continuous passive motion following primary total knee arthroplasty: short‐ and long‐term effects on range of motion. Physical Therapy Reviews 2003;8:113‐21. [Google Scholar]
Lobb 2012
- Lobb R, Tumilty S, Claydon LS. A review of systematic reviews on anterior cruciate ligament reconstruction rehabilitation. Physical Therapy in Sport 2012;13:270‐8. [DOI] [PubMed] [Google Scholar]
McCarthy 1992
- McCarthy MR, O'Donoghue PC, Yates CK, Yates‐McCarthy JL. The clinical use of continuous passive motion in physical therapy. Journal of Orthopaedic and Sports Physical Therapy 1992;15(3):132‐40. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rowe 2000
- Rowe PJ, Myles CM, Walker C, Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life?. Gait and Posture 2000;12(2):143‐55. [DOI] [PubMed] [Google Scholar]
Salter 1989
- Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clinical Orthopaedics and Related Research 1989;May(242):12‐25. [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16:1‐82. [DOI] [PubMed] [Google Scholar]
Schnebel 1989
- Schnebel BE, Evans JP, Flinn D. The use of a passive motion machine. American Journal of Knee Surgery 1989;2:131‐6. [Google Scholar]
Sheppard 1995
- Sheppard MS, Westlake SM, McQuarrie A. Continuous passive motion ‐ where are we now?. Physiotherapy Canada 1995;47(1):36‐9. [Google Scholar]
Smith 2007
- Smith TO, Davies L. The efficacy of continuous passive motion after anterior cruciate ligament reconstruction: a systematic review. Physical Therapy in Sport 2012;13:270‐8. [DOI] [PubMed] [Google Scholar]
van Dijk 2007
- Dijk H, Elvers J, Oostendorp R. Effect of continuous passive motion after total knee arthroplasty: a systematic review. Physiotherapy Singapore 2007;10:9‐19. [Google Scholar]
Videman 1987
- Videman T. Connective tissue and immobilization: key factors in musculoskeletal degeneration. Clinical Orthopaedics and Related Research 1987;221:26‐32. [PubMed] [Google Scholar]
Viswanathan 2010
- Viswanathan P, Kidd M. Effect of continuous passive motion following total knee arthroplasty on knee range of motion and function: a systematic review. New Zealand Journal of Physiotherapy 2010;38:14‐22. [Google Scholar]
References to other published versions of this review
Harvey 2010
- Harvey LA, Brosseau L, Herbert RD. Continuous passive motion following total knee arthroplasty in people with arthritis. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004260.pub2] [DOI] [PubMed] [Google Scholar]
Milne 2003
- Milne S, Brosseau L, Robinson V, Noel MJ, Davis J, Drouin H, et al. Continuous passive motion following total knee arthroplasty. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004260] [DOI] [PubMed] [Google Scholar]


