Abstract
Adrenal insufficiency (AI) is characterised by lack of cortisol production from the adrenal glands. This can be a primary adrenal disorder or secondary to adrenocorticotropic hormone deficiency or suppression from exogenous glucocorticoids. Symptoms of AI in children may initially be non-specific and include growth faltering, lethargy, poor feeding, weight loss, abdominal pain, vomiting and lingering illnesses. AI is treated with replacement doses of hydrocortisone. At times of physiological stress such as illness, trauma or surgery, there is an increased requirement for exogenous glucocorticoids, which if untreated can lead to an adrenal crisis and death. There are no unified guidelines for those <18 years old in the UK, leading to substantial variation in the management of AI. This paper sets out guidance for intercurrent illness, medical, dental and surgical procedures to allow timely and appropriate recognition and treatment of AI and adrenal crisis for children and young people.
Keywords: Endocrinology; Emergency Service, Hospital; Paediatrics
What is already known?
Patients with adrenal insufficiency (AI) are at risk of an adrenal crisis and death if they are not treated with additional glucocorticoids during illness or surgery.
There is variation in the management of paediatric AI with differing recommendations for glucocorticoid doses for sick days and surgery
What this study adds?
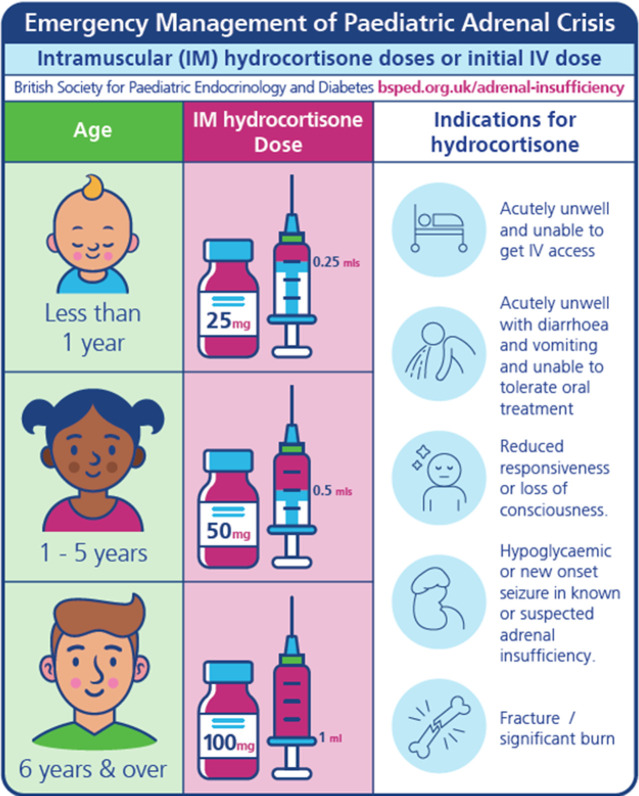
An adrenal crisis in children should be treated promptly with intramuscular (or intravenous) hydrocortisone: 25 mg in less than 1 year, 50 mg in 1–5 years, 100 mg 6 years and over.
For surgery under a general anaesthetic, hydrocortisone at a dose of 2 mg/kg intravenously (max 100 mg) is recommended at induction, followed by 1 mg/kg intravenously (max 50 mg) every 6 hours (for neonates the doses are 4 mg/kg intravenously at induction followed by 2 mg/kg intravenously every 6 hours). Alternatively, a hydrocortisone infusion can be used following the induction dose of hydrocortisone.
Our recommended approach of oral glucocorticoid for sick day episodes is a total daily hydrocortisone dose of around 30mg/m2/day given as four equally divided doses.
Background
Most forms of adrenal insufficiency (AI) are characterised by lack of cortisol production from the adrenal glands. Children with AI have a daily requirement for glucocorticoid at a replacement dose, but at times of physiological stress require additional exogenous glucocorticoids, usually given as hydrocortisone. Clinical features at presentation can vary with age and the doses required for a neonate with AI are different to a teenager. The diagnosis of AI in children is often delayed because symptoms can be non-specific and overlap with other common childhood conditions. Failure to recognise and instigate prompt treatment of AI can result in life-threatening adrenal crises. There are three scenarios requiring bespoke management: adrenal crisis (AC), sick day episodes and perioperative management.
In August 2020, there was a national patient safety alert to support early recognition and treatment of an AC in adults highlighting that untreated AI can be fatal. Guidelines for emergency management of adults (over 18 years) with AI have recently been published by Simpson et al. 1 2
Currently, there are no unified guidelines for patients younger than 18 years in the UK. This can lead to variation in the management of AI in both an emergency and perioperative situation, resulting in the potential for overtreatment and undertreatment and a lack of consistency when providing education and training to families and health professionals.
Adrenal insufficiency and adrenal crisis
Primary AI occurs due to pathologies of the adrenal glands (such as autoimmune Addison’s disease or congenital adrenal hyperplasia (CAH)) and is usually associated with both glucocorticoid and mineralocorticoid deficiency. Hyperpigmentation from ACTH excess may occur in primary AI. Secondary AI is due to pathologies of the pituitary gland where a lack of ACTH leads to cortisol deficiency (eg, hypopituitarism). Suppression of ACTH leading to AI may also result from exogenous high dose glucocorticoid use. Symptoms of AI may initially be non-specific and include growth faltering, lethargy, poor feeding, abdominal pain, vomiting and weight loss.3 Infants may develop hypoglycaemia irrespective of the cause of AI. A recent review provides a comprehensive summary of causes of AI in children and adults.4
An acute AC is a life-threatening deterioration due to glucocorticoid insufficiency which may result in cardiovascular instability, abnormalities of plasma electrolytes and seizures. The features are not attributable to any other illness and show significant improvement following parenteral glucocorticoids.5 Most estimates of AC are derived from adult populations where approximately 1 in 12 individuals with AI will experience a life-threatening AC within the next year, of who 1 in 200 will die from an AC.6 In children, the literature is limited but the main risk factors for AC are younger age at diagnosis, primary AI, requirement for mineralocorticoid treatment and recurrent hospital admissions.7 Children presenting with diarrhoea and vomiting are at particular risk of AC, which may be refractory to standard oral stress dosing, due to lack of absorption of glucocorticoid treatment.8 Other risk factors include new onset seizures, trauma and fractures.
Paediatric patients who should be considered to be at risk of adrenal crisis
NHS England operates the National Reporting and Learning System. Incident level patient safety data on reporting harm occurring in children with AI/AC over a 3-year period from September 2019 to August 2022 were obtained. During this period, there were 83 reports of individuals who were at risk from issues related to timely treatment and management of AI. Of these, one incident caused severe harm and two caused moderate harm. As these incidents were self-reported events by staff, it is likely that the true number of incidents was higher; however, three common themes emerged from these reports, (1) glucocorticoids not administered in the perioperative period (2) glucocorticoids not given as prescribed (included delayed or missed doses) and (3) combinations of issues related training, recognition or access to treatments for AI from caregivers in the community.9
Glucocorticoids are used for multiple different indications and administration by any route can cause AI. Defining the paediatric patients at risk of AC is outside the scope of this guidance. There is published guidance for adults and children.2 10–13 The Neonatal and Paediatric Pharmacy Group recommends when a steroid treatment card stating the child is at risk of AI should be issued.11 This is the responsibility of the prescribing clinician.11 Those children with confirmed AI should be provided with a British Society for Paediatric Endocrinology and Diabetes (BSPED) AI card and a plan for sick day doses of hydrocortisone along with training of the patient and caregivers in how to administer emergency hydrocortisone injections.14
Methods
In 2021, the Paediatric AI Group was convened under the auspices of the BSPED with the aim to standardise the management of paediatric AI across the UK and Northern Ireland (12 paediatric endocrinologists, 1 paediatric endocrinology trainee, 2 paediatric endocrinology clinical nurse specialists, 1 paediatric pharmacist). An initial literature search was performed and these, along with existing UK hospital guidelines for management of paediatric AI, were reviewed. Subgroups reviewed the management for oral sick day episodes and separately the doses required for intravenous and intramuscular treatment. The recommendations were reviewed by the whole group prior to obtaining draft consensus. The final draft consensus was circulated to relevant stakeholders including the BSPED clinical and executive committees, the BSPED membership, Society for Endocrinology (SFE) clinical committee and patient organisations (CAH support group, Addison’s Disease Self-Help Group and The Pituitary Foundation). This is a consensus document of the BSPED Paediatric AI group which incorporates other stakeholders’ views.
Current evidence base for hydrocortisone doses used for treatment of childhood adrenal insufficiency
Many recommendations of glucocorticoid doses are based on expert consensus and established practice, with data often extrapolated from adult studies. A recent comprehensive Cochrane review encapsulates the lack of good quality evidence on which to base recommendations for treatment specifically in CAH; in particular, there are a limited number of trials comparing efficacy of different glucocorticoid regimens and no trials included long-term outcomes such as prevention of AC.15
Children on daily glucocorticoid replacement for AI have hydrocortisone doses calculated for body surface area (BSA) with doses of around 8 mg/m2/day for physiological replacement16 and higher doses around 10–15 mg/m2/day to optimise growth in children with CAH. These are administered as three or four times a day dosing regimens.17
During sick day episodes, there are several approaches to steroid sick day management which include doubling or tripling hydrocortisone doses for mild and moderate illness and giving them either three or four times a day. However, definitions for severity of illness are difficult to quantify and practice varies widely on when sick day hydrocortisone doses are advocated for specific situations.18
A summary of hydrocortisone recommendations is provided in table 1. These use a combination of either age, BSA or weight-based hydrocortisone doses. Each approach has merits and downsides. Age-based doses are easy to remember but will cause significant variations depending on the individual weight and BSA. BSA-based doses are potentially more accurate, particularly in infants who have a relatively larger BSA, but are less easy to calculate in urgent situations. Weight-based doses are very commonly used in paediatrics, but presently there is no suggested maximum recommended daily hydrocortisone dose, in contrast to adult guidance which advocates 200 mg a day.2 The adult recommendations are derived from the paper by Prete et al 19 who compared cortisol levels in patients undergoing major stress such as sepsis, trauma and elective surgery to patients with AI who were given different hydrocortisone dose schedules by different routes of administration (table 1).19
Table 1.
Summary of hydrocortisone dose recommendations
| Publication | Indication | Hydrocortisone dose |
| Shulman et al (2006)24
Rushworth et al (2019)25 Nowotny et al (2021)26 Nisticò et al (2021)27 Bornstein et al (2016)28 BNFc10 |
Adrenal crisis | 50–100 mg/m2 intravenously or intramuscularly initially Followed by 50–100 mg/m2 per day either as a continuous infusion or in four divided doses given every 6 hours intravenously (or intramuscularly)24–26 0–2 years 25 mg, 2–6 years 50 mg, >6 years 100 mg26 4 mg/kg bolus intravenously, followed by continuous infusion 2 mg/kg/day until stabilisation. If weight unknown: 25 mg (<1 year), 50 mg (2–5 years), 100 mg (if >5 years)27 Intravenous hydrocortisone 50 mg/m2 or estimate; infants, 25 mg; school-age children, 50 mg; adolescents, 100 mg28 Neonates: 10 mg, then 100 mg/m2/day (infusion or divided doses) 1 month to 11 years: 2–4 mg/kg every 6 hours 12–17 years: 100 mg every 6–8 hours10 |
| Shulman et al (2006)24
Nowotny et al (2021)26 Bornstein et al (2016)28 Nisticò et al (2021)27 Woodcock et al (2020)29 |
Surgery | 50–100 mg/m2 intravenously or intramuscularly initially Followed by 50–100 mg/m2 per day either as a continuous infusion or in four divided doses given every 6 hours intravenously (or intramuscularly)24 26 28 Major surgery: 2 mg/kg intravenously at induction, then 2 mg/kg intravenously every 4 hours or by infusion based on weight ranges:
Postoperative dose: 2 mg/kg every 4 hours intravenously27 29 Minor surgery: |
| Shulman et al (2006)24
Nowotny et al (2021)26 Nisticò D et al (2021)27 Bornstein et al (2016)29 |
Oral sick day doses | 30–50 mg/m2/day divided into three or four doses. Gradually reduce to maintenance level24 26
Doubling or tripling oral glucocorticoid doses27 29 |
| Simpson et al (2020)2
(Society for Endocrinology (adult guidelines)) |
Adrenal crisis and surgery | Hydrocortisone 100 mg intravenously for acute adrenal crisis or for induction for surgical procedures Followed by infusion of hydrocortisone 200 mg in 24 hours or alternatively can be given as 50 mg intravenously every 6 hours. In severe obesity, 100 mg of hydrocortisone can be substituted for the 50 mg dose of hydrocortisone |
BNFc, British National Formulary for Children.
Therefore, several principles were considered in the development of the paediatric AI guidance. These included harmonising the recommended maximum dose of 200 mg in 24 hours in adults with older teenagers and developing recommendations for children that provided hydrocortisone doses of 50–100 mg/m2/day for surgical procedures and hydrocortisone doses of around 30 mg/m2/day for oral sick day episodes. Neonates require higher weight-based doses due to a relatively larger BSA. Oral maintenance doses for hydrocortisone are based on the BSA of the child. We recommend the BSA approach is used for the oral sick day hydrocortisone recommendations for children undergoing regular follow-up in the endocrine clinic. For emergencies, age-based intramuscular or intravenous doses are recommended. For perioperative management, weight-based intravenous hydrocortisone infusion or bolus doses are recommended.
BSPED consensus recommendations for oral hydrocortisone doses for sick day episodes
Our recommended option for management of sick day episodes is a dose-based strategy aiming for a total daily hydrocortisone dose of around 30 mg/m2/day given as four equally divided doses. This avoids long gaps between doses and overcomes the relatively short half-life of oral hydrocortisone,20 thus maintaining plasma levels during times of stress. Increased doses should be used for the duration of the illness. However, with improvement of the underlying illness, the frequency and duration of the hydrocortisone treatment can be adjusted to suit the individual circumstance. The advantage of this approach (compared with a doubling or tripling approach) is that children with AI of any cause will all broadly have the same sick day hydrocortisone dose. Therefore, there is no need to discern between those on relatively low doses of physiological glucocorticoid replacement to those on somewhat higher doses as used in CAH.
Table 2 outlines the common situations when additional sick day episode oral and emergency hydrocortisone is recommended. This has been incorporated into the BSPED AI card which is provided to families at time of diagnosis.14
Table 2.
Patient information: sick days: when to give additional steroids
| British Society for Paediatric Endocrinology and Diabetes Adrenal Insufficiency Guidance (link) | |||
| Situation | Change to usual steroid dose | Length of change | When to get help? |
| Minor Illness | |||
| Mild cold/runny nose with no fever. Minor playground bumps and bruises | No change | ||
| Moderate or severe illness | |||
| Fever, influenza, infection, childhood illnesses (usually not well not enough to go to school) | Sick day doses required | For as long as the illness lasts | Contact GP or medical team if not improving after 24–48 hours |
| Vomiting or diarrhoea | Sick day doses required | ||
| If sick day dose tolerated (kept down for at least 30 min with no frequent diarrhoea or vomiting), then continue oral sick day dosing | |||
| If sick day dose not tolerated, give intramuscular hydrocortisone injection | Dial 999 and inform them that the patient is having an adrenal crisis | ||
| Drowsy and unresponsive | Give intramuscular hydrocortisone injection | ||
| Major trauma or severe shock (eg, suspected fracture, road traffic accident, head injury with loss of consciousness). | Give intramuscular hydrocortisone injection | ||
| Other (discuss with medical team) | |||
| Routine or travel vaccinations | Consider 1 or 2 doses of sick day steroids. | Continue if unwell | |
| Long haul flights | Give usual morning dose at 6 to 8 hourly intervals | ||
| Child or centre specific recommendations | |||
| Surgical and dental procedures | |||
| Minor surgery (eg, tooth extraction under local anaesthetic) |
Sick day dose prior to procedure. Return to usual dose immediately afterwards. | Continue sick day doses for up to 24 hours if in pain or unwell | Inform medical staff including dentist and anaesthetist that you/your child have adrenal insufficiency and takes steroids |
| Major surgery (eg, operation requiring general anaesthetic) |
Sick day oral steroids on day of procedure even when fasting. Intravenous hydrocortisone will be given a little before putting your child to sleep and during surgery, and after surgery if needed |
As per local policy or contact treatment centre for advice | |
GP, general practictioner.
Fludrocortisone is an oral mineralocorticoid used in primary AI. The dose does not need adjustment in the event of a sick day episode or an AC. If the child is unable to take oral medication, then intravenous fluids may be required to maintain the salt and water balance depending on the clinical situation. However, the mineralocorticoid effect of hydrocortisone at stress doses is often sufficient to cover the mineralocorticoid requirement. Oral fludrocortisone should be restarted when tolerated.
BSPED consensus recommendations for oral hydrocortisone dose for sick day episodes: Specific situations
In patients on supraphysiological glucocorticoid therapy such as prednisolone, deflazacort or dexamethasone for an existing chronic condition, a simple and safe approach is to consider additional hydrocortisone, at sick day doses to ensure adequate plasma cortisol levels throughout the day and night. Alternatively, if the child is taking a glucocorticoid at over 30 mg/m2/day of a hydrocortisone equivalent dose, then further sick day doses with additional hydrocortisone may not be necessary in principle but they should have a bespoke plan for the management of AI. For those on regular prednisolone, our recommendation is for sick day dosing with hydrocortisone. If this is not practical, then prednisolone 7.5/mg/m2/day given in two divided doses can be used. If the existing prednisolone dose is greater than the required sick day dose, then the prescribed prednisolone should be split into two doses given at 12-hourly intervals. The relatively short half-life of Deflazacort necessitates the use of sick day dosing with hydrocortisone.
For patients prescribed Alkindi, increase doses as per sick day guidance using either Alkindi granules in capsules for opening or oral hydrocortisone (immediate release preparation).
If the child is on two times a day Efmody (modified release hydrocortisone), they should be switched to immediate release oral hydrocortisone as per sick day episode guidance in four divided doses while unwell. Alternatively, Efmody may be continued at the usual dose and additional immediate release hydrocortisone added in four divided doses to provide a combined (Efmody and immediate release oral hydrocortisone) glucocorticoid dose of 30 mg/m2/day.
BSPED consensus recommendations for treatment of adrenal crisis
Management of AC includes urgent administration of hydrocortisone, along with assessment and treatment of hypoglycaemia, hypotension and dehydration (table 3). Young people (16–18 years) admitted under the care of adult services should be managed as per the SFE guidelines.21 For a new presentation of AI or AC, diagnostic investigations should be taken prior to the administration of hydrocortisone where possible (urea and electrolytes, glucose, ACTH, cortisol, renin, aldosterone, 17OHP, urine steroid profile).
Table 3.
Emergency management of paediatric adrenal crisis
| British Society for Paediatric Endocrinology and Diabetes Adrenal Insufficiency Guidance: (link) | ||
| Paediatric adrenal crisis management | ||
 * *
| ||
| Paediatric adrenal crisis | ||
| Hydrocortisone dose (age-based) | Or weight-based hydrocortisone dose and frequency | |
| Age-based doses given intramuscularly or intravenously less than 1 year: 25 mg 1–5 years: 50 mg 6 years and over: 100 mg |
Children (>28 days CGA)
2 mg/kg (max 100 mg) intravenous bolus initially then bolus dose 6 hourly Neonates (<28 days CGA ) 4 mg/kg intravenous bolus initially then bolus dose 6 hourly |
|
| Can consider giving 4 hourly hydrocortisone or an infusion (table 4) if needed. | ||
 † †
| ||
| Blood glucose. Fluid type and volume | ||
| Blood glucose <3 mmol/L | Shock or moderate to severe dehydration | Maintenance fluids type and amount |
| 2 mL/kg of 10% glucose as intravenous bolus Recheck blood glucose after 15 min and repeat bolus if necessary. | Give 10 mL/kg of 0.9% sodium chloride as a bolus Repeat if necessary Check electrolytes immediately at presentation to inform fluid usage |
0.9% sodium chloride/5% glucose is usually an appropriate starting point: 100 mL/kg/day for first 10 kg, 50 mL/kg/day for second 10 kg, 20 mL/kg/day>20 kg |

| ||
| Stable and improving | ||
| Stable and improving | 1 mg/kg (max 50 mg) intravenously 6 hourly. Neonates: 2 mg/kg intravenously 6 hourly (can consider giving 4 hourly or an infusion (table 4) if needed) | |
| Stable and tolerating drinks/diet | Oral sick day hydrocortisone: 30mg/m2/day in four equally divided doses Restart fludrocortisone if indicated |
|
Treatment should not be delayed. A safe approach is to administer emergency hydrocortisone in any unwell child with AI.
The child must be observed until they are tolerating oral hydrocortisone at sick day doses.
Young people (16–18 years) admitted under the care of adult services should be managed as per the Society for Endocrinology guidelines: https://www.endocrinology.org/adrenal-crisis.21
See BSPED website for more detailed guidance about fluid and electrolyte management in AI and different considerations for primary and secondary AI.15
*If a new presentation, take biochemical investigations BEFORE giving hydrocortisone but do not unduly delay treatment (renal profile, cortisol, renin, aldosterone (lithium heparin tubes), ACTH (EDTA tube), glucose (blood gas or fluoride oxalate tube).
†Consider paediatriic high dependency unit/paediatric intensive care unit admission, monitor blood gas, electrolytes and glucose regularly depending on severity.
‡It would seem prudent to use the neonatal dosing for infants who are significantly small for gestational age or failing to thrive and as such, while not neonates, are a neonatal size.
ACTH, adrenocorticotropic hormone; AI, adrenal insufficiency; BSPED, British Society for Paediatric Endocrinology and Diabetes; CGA, corrected gestational age.
Glucocorticoids
Hydrocortisone 100 mg for injection is available as a 1 mL premixed solution (hydrocortisone sodium phosphate) or as powder for reconstitution with water for injection (hydrocortisone sodium succinate). As the first dose is often given in the community, our recommendations are for intramuscular hydrocortisone based on age (figure 1, table 3). For children presenting acutely unwell to hospital or any other emergency situation, the same age-based hydrocortisone doses can be used for initial management given either by the intramuscular or intravenous route. Alternatively, weight-based doses can be used for immediate and ongoing care (table 3). As premature infants and neonates have a relatively larger an initial dose of 4 mg/kg is recommended compared with 2 mg/kg (max 100mg) for older infants and children.
Figure 1.
Emergency hydrocortisone doses based on age of child.
Hypoglycaemia
Blood glucose should be checked, and hypoglycaemia treated with 2 mL/kg of 10% glucose as an intravenous bolus (table 3).
Shock or dehydration
Give 10 mL/kg of 0.9% sodium chloride as a bolus and repeat if necessary (table 3).
Hyperkalaemia
Children with primary AI can be hyperkalaemic at presentation or in an AC because of mineralocorticoid deficiency. Emergency management of AC with intravenous glucocorticoids and intravenous fluids (0.9% sodium chloride) will reduce potassium levels. Hyperkalaemia is potentially life-threatening and can lead to cardiac arrhythmias so additional measures such as the use of intravenous calcium gluconate, nebulised salbutamol, intravenous insulin and glucose or intravenous bicarbonate and cation exchange resins should also be considered.22
Hyponatraemia and fluids
Sodium chloride 0.9%/5% glucose is usually a good starting point for initial fluid management if the clinical and biochemical pictures suggest that the low sodium has arisen primarily because of salt wasting. In primary AI insufficiency, mineralocorticoid deficiency will cause hyponatraemia due to renal losses. In secondary AI, cortisol deficiency can lead to a lack of free water clearance which can contribute to hyponatraemia. In this latter scenario (those with secondary AI), a degree of fluid restriction may be more appropriate.
BSPED consensus recommendation for the management of dental, medical and surgical procedures
It should usually be possible for the child to continue with their usual oral glucocorticoid pre-operatively even if fasting. If this is not possible, the usual dose should be given intravenously. They should ideally be first on the operating list. The period of fasting should not be more than 6 hours without intravenous fluid replacement containing sodium chloride and glucose.
The recommended hydrocortisone doses for major surgery are given in table 4; this includes an initial bolus of hydrocortisone of 2 mg/kg (max 100 mg) in children or 4 mg/kg in neonates (less than 28 days corrected gestational age), followed by either a continuous infusion or using intermittent bolus doses. We advise the use of neonatal dosing for infants who are significantly small for gestational age or failing to thrive. The minimum hydrocortisone dose using this schedule would be 84 mg/m2/day in the first 24 hours. The initial higher bolus dose can be given 4–6 hourly for prolonged major surgical procedures or if the patient is unstable. Children are at particular risk of hypoglycaemia and if not on intravenous fluids should have regular capillary blood glucose every 2 hours until oral feeds or fluids are commenced. Our recommendations for minor procedures are provided in table 5.
Table 4.
Recommended doses for preoperative, perioperative and postoperative hydrocortisone cover in children with AI undergoing major surgery using either a continuous infusion (4.1) or intermittent bolus in children (4.2a) and neonates (4.2b)
| British Society for Paediatric Endocrinology & Diabetes adrenal insufficiency guidance (link) | |||
| 4. Major surgery: Hydrocortisone doses for infusion or bolus | |||
| 4.1 Major surgery: IVI hydrocortisone doses | |||
| Induction | Intravenous bolus of hydrocortisone 2 mg/kg (max 100 mg) (premature infants and neonates <28 days corrected gestational age: 4 mg/kg) |
||
| Intraoperative | Intravenous hydrocortisone infusion as below | ||
| Weight | Total dose in 24 hours |
Infusion rate
(50 mg hydrocortisone in 50 mL 0.9% sodium chloride) |
Additional considerations |
| ≤10 kg | 25 mg | 1 mL/hour |
|
| 10.1–20 kg | 50 mg | 2 mL/hour | |
| 20.1–40 kg | 100 mg | 4 mL/hour | |
| 40.1–70 kg | 150 mg | 6 mL/hour | |
| Over 70 kg | 200 mg | 8 mL/hour | |
| Postoperative | Continue hydrocortisone infusion Change to oral sick day hydrocortisone when stable and tolerating oral fluids/diet |
||
| 4.2 a) Major surgery: Child (over 28 days corrected gestational age) Intravenous hydrocortisone bolus doses | |||
| Hydrocortisone bolus dose | Frequency | Additional considerations | |
| Induction | 2 mg/kg (max 100 mg) |
|
|
| Intraoperative | 2 mg/kg (max 100 mg) |
Given at 6 hours intravenously |
|
| Postoperative | 1 mg/kg (max 50 mg) |
Every 6 hours intravenously Change to oral sick day hydrocortisone when stable and tolerating oral fluids/diet |
|
| 4.2 b) Major Surgery: Premature infants and neonates (less than 28 days corrected gestational age) Intravenous hydrocortisone bolus doses | |||
| Hydrocortisone bolus dose | Frequency | Additional considerations | |
| Induction | 4 mg/kg | ||
| Intraoperative | 2 mg/kg | Given at 6 hours intravenously |
|
| Postoperative | 2 mg/kg | Every 6 hours intravenously Change to oral sick day steroids when stable and tolerating oral feeds |
|
Major surgery is defined as surgery lasting more than 90 min with variable recovery periods and expected delay in restarting oral intake. Management includes an initial bolus of hydrocortisone followed by either a hydrocortisone infusion or regular bolus doses.
AI, adrenal insufficiency; IVI, intravenous infusion.
Table 5.
Recommended glucocorticoid cover in children with AI undergoing minor procedures requiring (5.1) or not requiring (5.2) a general anaesthetic
| British Society for Paediatric Endocrinology and Diabetes Adrenal Insufficiency guidance (link) | ||
| 5.1. Minor procedures requiring general anaesthesia: Hydrocortisone dose | ||
| Hydrocortisone bolus dose | Postoperative | |
| Induction | 2 mg/kg (max 100 mg)* (4 mg/kg in neonates) |
Oral sick day steroid doses for 24 hours |
| 5.2. Minor procedures NOT requiring general anaesthesia: Hydrocortisone advice | ||
| Medical procedures (local anaesthetic or sedation) | Oral hydrocortisone dose | |
| Local anaesthetic/ sedation | Minor procedure—eg, skin biopsy Minor dental procedures—eg, filling, tooth extraction |
Give oral sick day steroid dose prior to procedure. Continue for up to 24 hours if in pain or unwell |
| MRI scans (using sedation) Non-anaesthetic sedation (eg, chloral hydrate) does not merit use of intravenous hydrocortisone. Sick day dosing with oral hydrocortisone is sufficient |
Give oral sick day steroid dose prior to procedure and continue for the day | |
Minor surgery is defined as a procedure lasting less than 90 min and the patient is expected to be eating and drinking by the next meal. This may include procedures such as MRI scans, endoscopy, dental extractions under general anaesthetic or other day case procedures. If the procedure exceeds 4 hours or if the child is unstable, a further bolus of intravenous hydrocortisone as outlined in the major surgery guidance (table 4) is required.
*Consider neonatal doses (4.2b) for infants who are significantly small for gestational age or with growth faltering.
AI, adrenal insufficiency.
For planned elective surgical procedures, an assessment of the patient’s hydration status and intravenous fluids need to be considered depending on the type and length of procedure. Sodium chloride 0.9%/5% glucose at a maintenance rate is usually appropriate as in table 3. A more detailed fluid assessment is required if intravenous fluids are necessary for more than 48 hours or in patients with primary AI on fludrocortisone.
Education, information sharing and discharge planning for children with AI
Every child with confirmed AI requires a comprehensive package of information and education along with a management plan for urgent care as in box 1. Following diagnosis, education should be initiated as soon as possible to ensure that the child or young person and their family are confident in managing AI on discharge. Hospital records should have an alert in place to indicate that the child has AI (box 1).23 A BSPED AI card with their maintenance and sick day doses of hydrocortisone along with details of the clinical team should be provided and periodically reviewed.14
Box 1. Information, education and training required prior to initial discharge/at time of diagnosis.
Written information about adrenal insufficiency (eg, BSPED website (link), European Society for Paediatric Endocrinology, Scottish Paediatric Endocrine Group)
-
Recommend use of BSPED AI card website hyperlink (or equivalent) which covers the information below:
Current steroid treatment plan and doses (and mineralocorticoid if relevant).
Sick day episode oral steroid dose.
Emergency intramuscular hydrocortisone dose.
Emergency hydrocortisone injection kits (for home, nursery/school and any other regular residence (grandparents, parents/carers not cohabiting)
Training to administer hydrocortisone injection (in person, virtual, YouTube) (https://www.pituitary.org.uk/news/2020/08/hydrocortisone-injection-video) (Solu-cortef; hydrocortisone sodium succinate—powder for dilution).
https://www.youtube.com/watch?v=R5_BScN6HwE (Glass vial: hydrocortisone sodium phosphate).
Open access to local paediatric medical ward/paediatric emergency department.
Provide information for appropriate support group if applicable. livingwithCAH.com, addisonsdisease.org.uk, pituitary.org.uk.
Emergency contact numbers for local paediatric team and regional paediatric endocrinology team if applicable.
Registered with the ambulance service (Red alert category).
Medical alert added to notes/electronic hospital systems to flag that child has adrenal insufficiency.
Advice to wear a medical alert bracelet/necklace.
Inform GP of diagnosis, glucocorticoid doses (including sick day episodes) and request glucocorticoids put on repeat a prescription.
Care plan for school/nursery.
Ensure clinic appointment in place with medical staff.
Recommended actions for family
Download MyCortisol app.
Inform nursery/school.
Ensure adequate supplies of hydrocortisone for sick days and travel.
Medical alert bracelets/necklace/mobile phone medical ID.
Contact appropriate patient support group.
Regularly check that hydrocortisone injections are in date.
Subsequent visits (frequency dependent upon patient’s needs)
Review child/young person and family understanding of condition and sick day rules.
Review BSPED AI leaflet and update daily replacement, sick day episode and emergency intramuscular hydrocortisone doses based on up to date measurements.
Ensure appropriate supply of medication/equipment and training up to date.
Ensure alerts remain in place (Ambulance/patient notes/personal alert for, eg, jewellery or phone).
Check care plans for school/nursery are up to date.
Review how any sick days were managed. Check if emergency hydrocortisone was required.
AI, adrenal insufficiency; BSPED, British Society for Paediatric Endocrinology and Diabetes.
Summary and conclusion
Prompt recognition and treatment of AI is essential to prevent a life-threatening AC. Glucocorticoid doses should never be omitted and emergency treatment should not be delayed, especially when presenting to acute medical teams. The risks of AI and AC can be reduced by effective patient education, training for medical staff and AI alerts on hospital records. This guidance should serve as a foundation to improve the care and treatment of children with AI.
Acknowledgments
The BSPED Paediatric AI group would like to acknowledge the contributions of the BSPED clinical committee, BSPED executive committee, SFE clinical committee, CAH support group, Addison’s Disease Self-Help Group, Pituitary Foundation. We are also very grateful to the Patient Safety Team at NHS England for providing the incident level patient safety data.
Footnotes
Twitter: @Nabil_Boulos, @charlottejelder, @hwganendodoc
Contributors: All authors provided valuable input to different sections of the manuscript. TM and FR led the project on behalf of the British Society for Paediatric Endocrinology and Diabetes.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. NHS England Safety Alert: 13/8/2020: Reference no: NatPSA/2020/005/NHSPS. n.d. Available: www.england.nhs.uk/wp-content/uploads/2020/08/NPSA-Emergency-Steroid-Card-FINAL-2.3.pdf
- 2. Simpson H, Tomlinson J, Wass J, et al. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin Med (Lond) 2020;20:371–8. 10.7861/clinmed.2019-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worth C, Vyas A, Banerjee I, et al. Acute illness and death in children with adrenal insufficiency. Front Endocrinol (Lausanne) 2021;12:757566. 10.3389/fendo.2021.757566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet 2021;397:613–29. 10.1016/S0140-6736(21)00136-7 [DOI] [PubMed] [Google Scholar]
- 5. Rushworth RL, Torpy DJ, Stratakis CA, et al. Adrenal crises in children: perspectives and research directions. Horm Res Paediatr 2018;89:341–51. 10.1159/000481660 [DOI] [PubMed] [Google Scholar]
- 6. Allolio B. Extensive expertise in endocrinology. adrenal crisis. Eur J Endocrinol 2015;172:R115–24. 10.1530/EJE-14-0824 [DOI] [PubMed] [Google Scholar]
- 7. Eyal O, Levin Y, Oren A, et al. Adrenal crises in children with adrenal insufficiency: epidemiology and risk factors. Eur J Pediatr 2019;178:731–8. 10.1007/s00431-019-03348-1 [DOI] [PubMed] [Google Scholar]
- 8. Rezai M, Fullwood C, Hird B, et al. Cortisol levels during acute illnesses in children and adolescents: a systematic review. JAMA Netw Open 2022;5:e2217812. 10.1001/jamanetworkopen.2022.17812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National reporting and learning system (NRLS). n.d. Available: www.england.nhs.uk/patient-safety/monthly-data-patient-safety-incident-reports/
- 10. BNFc . Available: https://bnfc.nice.org.uk/drugs/hydrocortisone/
- 11. Neonatal and paediatric pharmacy group (NPPG) . Available: http://nppg.org.uk/wp-content/uploads/2021/12/Position-Statement-Steroid-Cards-V1.pdf
- 12. SIGN . Available: www.sign.ac.uk/media/1773/sign158-updated.pdf
- 13. National Institute of Clinical Excellence (NICE) . Available: www.nice.org.uk/guidance/ng80/evidence/chronic-asthma-management-pdf-7079863934
- 14. British Society for Paediatric Endocrinology and Diabetes . Adrenal insufficiency card. n.d. Available: https://www.bsped.org.uk/media/ewaphps5/bsped-adrenal-insufficiency-card.pdf
- 15. Ng SM, Stepien KM, Krishan A. Glucocorticoid replacement regimens for treating congenital adrenal hyperplasia. Cochrane Database Syst Rev 2020;3:CD012517. 10.1002/14651858.CD012517.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters CJ, Hill N, Dattani MT, et al. Deconvolution analysis of 24-h serum cortisol profiles informs the amount and distribution of hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 2013;78:347–51. 10.1111/j.1365-2265.2012.04502.x [DOI] [PubMed] [Google Scholar]
- 17. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, et al. Congenital adrenal hyperplasia-current insights in pathophysiology, diagnostics, and management. Endocr Rev 2022;43:91–159. 10.1210/endrev/bnab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ali SR, Bryce J, Krone NP, et al. Management of acute adrenal insufficiency-related adverse events in children with congenital adrenal hyperplasia: results of an international survey of specialist centres. Horm Res Paediatr 2022;95:363–73. 10.1159/000525075 [DOI] [PubMed] [Google Scholar]
- 19. Prete A, Taylor AE, Bancos I, et al. Prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J Clin Endocrinol Metab 2020;105:2262–74. 10.1210/clinem/dgaa133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hindmarsh PC, Charmandari E. Variation in absorption and half-life of hydrocortisone influence plasma cortisol concentrations. Clin Endocrinol (Oxf) 2015;82:557–61. 10.1111/cen.12653 [DOI] [PubMed] [Google Scholar]
- 21. Society for Endocrinology . Available: www.endocrinology.org/adrenal-crisis
- 22. Rubens M, Kanaris C. Fifteen-minute consultation: emergency management of children presenting with hyperkalaemia. Arch Dis Child Educ Pract Ed 2022;107:344–50. 10.1136/archdischild-2021-322080 [DOI] [PubMed] [Google Scholar]
- 23. British society for paediatric endocrinology and diabetes adrenal insufficiency guidance. n.d. Available: www.bsped.org.uk/adrenal-insufficiency
- 24. Shulman DI, Palmert MR, Kemp SF, et al. Adrenal insufficiency: still a cause of morbidity and death in childhood. Pediatrics 2007;119:e484–94. 10.1542/peds.2006-1612 [DOI] [PubMed] [Google Scholar]
- 25. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med 2019;381:852–61. 10.1056/NEJMra1807486 [DOI] [PubMed] [Google Scholar]
- 26. Nowotny H, Ahmed SF, Bensing S, et al. Therapy options for adrenal insufficiency and recommendations for the management of adrenal crisis. Endocrine 2021;71:586–94. 10.1007/s12020-021-02649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nisticò D, Bossini B, Benvenuto S, et al. Pediatric adrenal insufficiency: challenges and solutions. Ther Clin Risk Manag 2022;18:47–60. 10.2147/TCRM.S294065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016;101:364–89. 10.1210/jc.2015-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodcock T, Barker P, Daniel S, et al. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: guidelines from the association of anaesthetists, the Royal College of physicians and the Society for endocrinology UK. Anaesthesia 2020;75:654–63. 10.1111/anae.14963 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study.