Abstract
Objectives
To investigate the efficacy and safety of otilimab, an antigranulocyte-macrophage colony-stimulating factor antibody, in patients with active rheumatoid arthritis.
Methods
Two phase 3, double-blind randomised controlled trials including patients with inadequate responses to methotrexate (contRAst 1) or conventional synthetic/biologic disease-modifying antirheumatic drugs (cs/bDMARDs; contRAst 2). Patients received background csDMARDs. Through a testing hierarchy, subcutaneous otilimab (90/150 mg once weekly) was compared with placebo for week 12 endpoints (after which, patients receiving placebo switched to active interventions) or oral tofacitinib (5 mg two times per day) for week 24 endpoints. Primary endpoint: proportion of patients achieving an American College of Rheumatology response ≥20% (ACR20) at week 12.
Results
The intention-to-treat populations comprised 1537 (contRAst 1) and 1625 (contRAst 2) patients. Primary endpoint: proportions of ACR20 responders were statistically significantly greater with otilimab 90 mg and 150 mg vs placebo in contRAst 1 (54.7% (p=0.0023) and 50.9% (p=0.0362) vs 41.7%) and contRAst 2 (54.9% (p<0.0001) and 54.5% (p<0.0001) vs 32.5%). Secondary endpoints: in both trials, compared with placebo, otilimab increased the proportion of Clinical Disease Activity Index (CDAI) low disease activity (LDA) responders (not significant for otilimab 150 mg in contRAst 1), and reduced Health Assessment Questionnaire-Disability Index (HAQ-DI) scores. Benefits with tofacitinib were consistently greater than with otilimab across multiple endpoints. Safety outcomes were similar across treatment groups.
Conclusions
Although otilimab demonstrated superiority to placebo in ACR20, CDAI LDA and HAQ-DI, improved symptoms, and had an acceptable safety profile, it was inferior to tofacitinib.
Trial registration numbers
Keywords: rheumatoid arthritis, autoimmune diseases, biological therapy, cytokines, inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Not all patients with rheumatoid arthritis (RA) achieve effective disease control with current therapies. Therefore, there remains an unmet need for effective RA treatment.
Preclinical studies identified the granulocyte-macrophage colony-stimulating factor (GM-CSF) pathway as a promising target in RA and demonstrated that neutralisation of GM-CSF affects pain responses.
Phase 2 studies with monoclonal antibodies targeting GM-CSF or its receptor have shown mixed results in patients with RA and an inadequate response to conventional synthetic (cs) or biological (b) disease-modifying antirheumatic drugs (DMARDs).
WHAT THIS STUDY ADDS
Otilimab, a high-affinity anti-GM-CSF monoclonal antibody, demonstrated efficacy versus placebo in patients with RA and an inadequate response to cs or bDMARDs, with a statistically significant difference in the American College of Rheumatology 20 response.
Otilimab was not as effective as tofacitinib in achieving multiple clinical responses in this study.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
For many years, GM-CSF has been considered an attractive target in the treatment of RA, and a novel mechanism of action might have the potential to be effective in patients who fail to respond to currently approved therapies.
Although the primary endpoint was met in both trials, GM-CSF inhibition at the tested doses of otilimab does not confer advantage over the approved Janus kinase inhibitor, tofacitinib,
in the treatment of patients with moderate to severe, active RA and a prior inadequate response to cs/bDMARDs.
Introduction
Clinical outcomes have improved in the management of rheumatoid arthritis (RA).1 In patients with an inadequate response (IR) to methotrexate (MTX) or conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs), most treatment guidelines suggest addition of a biologic (b)DMARD, consideration of a Janus kinase inhibitor (JAKi), and recommend switching mechanisms of action (MoAs) where necessary, to achieve a target of remission or low disease activity (LDA).2 3 Many patients with RA refractory to current treatments have decreased health-related quality of life (HRQoL).1 4 There are also patients who meet the criteria for ‘clinical response’ but remain dissatisfied with their treatment due to the persistence of unacceptable subjective symptoms, notably pain and fatigue.5–7 Therefore, new treatment options and novel MoAs to help patients with IR to current therapy are needed.
Preclinical evidence suggests that granulocyte-macrophage colony-stimulating factor (GM-CSF) has a role in the pathogenesis of RA8–12 and is a key mediator of pain through a variety of mechanisms including neuroimmune interactions with sensory neurons.11 13 GM-CSF is produced by various cells including activated T-cells and B-cells, myeloid and stromal cells, including synovial fibroblasts, and both GM-CSF and its receptor (GM-CSFR) are elevated in RA synovial tissue and fluid.8 10 14–16 Owing to its ability to promote myeloid cell activation, differentiation, survival and priming (enhanced production of inflammatory cytokines including (interleukin) (IL)-6, IL-1 and tumour necrosis factor (TNF)-α), GM-CSF is hypothesised to promote of proinflammatory myeloid cell functions.8–11 17 This, theoretically, would lead to recruitment of myeloid cells into inflamed RA joints, driving further cytokine and chemokine production and generating a positive feedback loop, impacting T-cell activation, and resulting in chronic inflammation, tissue damage and pain.8–11 17 Therefore, GM-CSF is an attractive target for the treatment of the contemporary unmet need in RA.10–12 17 18
Otilimab is a high-affinity anti-GM-CSF monoclonal antibody19 that demonstrated clinical efficacy, including improvements in Disease Activity Score-28 joints (DAS28) and pain, and was generally well tolerated in combination with MTX in the phase 2b dose-ranging randomised controlled trial (RCT) in RA, BAROQUE.20 Here, we report results of two phase 3 RCTs that evaluated the efficacy and safety of otilimab compared with placebo, as well as with the JAKi, tofacitinib, with concomitant MTX (contRAst 1) or csDMARDs (contRAst 2), in adult patients with active RA and an IR to MTX (contRAst 1) or csDMARDs and/or bDMARDs (contRAst 2).
Methods
Trial design
ContRAst 1 and contRAst 2 were 52-week, phase 3, multicentre, double-blind RCTs. ContRAst 1 (study 201790; NCT03980483) was conducted at 227 sites across 19 countries, from 16 May 2019 to 16 August 2022. ContRAst 2 (study 201791; NCT03970837) was conducted at 303 sites across 19 countries, from 5 June 2019 to 14 September 2022 (online supplemental table 1). Recruitment continued in China and Japan to meet local regulatory requirements; however, these additional cohorts are outside the scope of the primary analysis and are not reported here. Both trials coincided with the COVID-19 pandemic.
ard-2023-224482supp001.pdf (11.5MB, pdf)
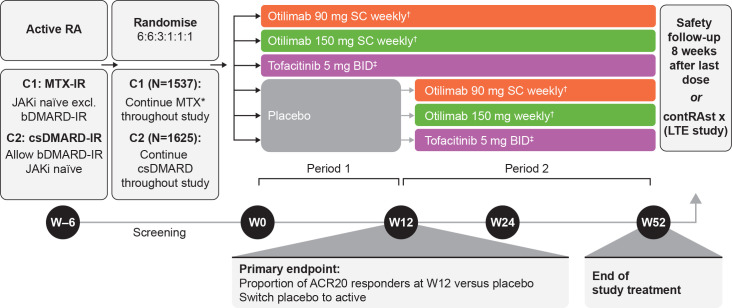
In both trials, patients were randomised 6:6:3:1:1:1 to receive otilimab 90 mg or 150 mg subcutaneously (SC) once weekly, tofacitinib 5 mg capsules orally two times per day, or placebo (1 placebo arm per active comparator) in combination with MTX (contRAst 1) or csDMARDs (contRAst 2) (figure 1). Otilimab doses were selected based on pharmacokinetic (PK), efficacy, safety, exposure–response and dose–response modelling of BAROQUE data (study 201755; NCT02504671).20 21 Patients initially treated with otilimab or tofacitinib continued treatment for 52 weeks. Patients allocated to placebo were treated to week 12 (time of primary endpoint; period 1), then switched to their respective active interventions (period 2) and continued treatment to week 52. At week 52, patients had the option to enter a long-term safety and efficacy extension study (contRAst X; study 209564; NCT04333147). Patients who did not enter contRAst X underwent a safety follow-up 8 weeks after their last blinded treatment administration.
Figure 1.
ContRAst 1 and 2 study design. *Stable oral dose of MTX and ≥5 mg/week folic acid as standard of care. †Otilimab solution in vial/pre-filled syringe. ‡Tofacitinib administered as 5 mg oral capsule. ACR20, 20% improvement in American College of Rheumatology criteria; b/csDMARD, biologic/conventional synthetic disease-modifying antirheumatic drug; C1, contRAst 1; C2, contRAst 2; IR, inadequate response; JAKi, Janus kinase inhibitor; LTE, long-term extension; MTX, methotrexate; RA, rheumatoid arthritis, SC, subcutaneous; W, week.
The trials were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation, Good Clinical Practice and applicable country-specific regulatory requirements. Protocols were approved by relevant Institutional Review Boards/Independent Ethics Committees (online supplemental table 1). Regulatory safety updates to the tofacitinib label to include a boxed warning for major adverse cardiovascular events (MACE) and updates to boxed warnings regarding mortality, malignancy and thrombosis led to protocol amendments after trial commencement. Permanent stopping criteria were updated to include venous thromboembolism, pulmonary embolism (PE) and deep vein thrombosis. All patients provided written informed consent.
Patients
Eligible patients were aged ≥18 years with a diagnosis of RA and fulfilling the American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR) 2010 Classification Criteria,22 a Global Functional Status in RA of class I, II or III per ACR 1991 Revised Criteria23 and a disease duration ≥6 months at screening. Active disease at screening and baseline, defined by tender joint count (TJC)≥6/68 and swollen joint count (SJC)≥6/66 were required, with a high sensitivity C reactive protein (hsCRP) ≥3 mg/L. Patients were required to have ≥1 bone erosion in hand, wrist or foot confirmed by central reading. In contRAst 1, patients had an IR despite current treatment with MTX (15–25 mg) for≥12 weeks prior to day 1 and currently receiving a stable dose for ≥8 weeks. In contRAst 2, patients had an IR despite current treatment with up to two of the permitted concomitant csDMARDs (online supplemental table 2), for at least 12 weeks prior to day 1. ContRAst 1 allowed prior bDMARD exposure in ≤20% of patients, provided they were not bDMARD-IR. ContRAst 2 allowed bDMARD-IR patients. In both trials, bDMARD therapy had to be discontinued prior to randomisation (online supplemental table 2).
Patients were excluded if they had any prior treatment with an anti-GM-CSF/GM-CSFR or JAKi, had active or recurrent infections, persistent cough or dyspnoea, or an abnormal chest radiograph within 12 weeks of study screening. Full inclusion/exclusion criteria are provided in online supplemental materials.
Randomisation and blinding
Patients were centrally randomised in a blinded manner using an interactive response technology system. In contRAst 2, randomisation was stratified by previously failed medication (csDMARD only, 1 bDMARD or >1 bDMARD). To maintain blinding, all patients received SC injections once weekly and oral capsules two times per day. Study treatments were dispensed by an unblinded pharmacist who ensured patients and study investigators remained blinded to the medication.
Trial treatments
Patients received either SC injection of otilimab 90 mg or 150 mg once weekly and tofacitinib placebo two times per day, or tofacitinib 5 mg capsules two times per day, and otilimab placebo once weekly; or both placebos and continued to receive their current stable dose of MTX (contRAst 1) or csDMARDs (contRAst 2). Oral corticosteroids at a stable dose of ≤10 mg/day prednisolone or equivalent were permitted prior to and throughout the study treatment period (dose adjustments permitted for safety reasons only). Analgesics, including acetaminophen (paracetamol) as rescue medication with limited dosage and timing for pain management, were permitted throughout the study (online supplemental table 2).
Endpoints and assessments
The primary endpoint was the proportion of patients achieving a ≥20% improvement in ACR criteria (ACR20 response)24 at week 12 vs placebo. Major multiplicity-controlled secondary efficacy endpoints included: proportion of patients achieving Clinical Disease Activity Index (CDAI) (LDA; ≤10); change from baseline (CFB) in Health Assessment Questionnaire-Disability Index (HAQ-DI) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue at week 12 vs placebo; proportion of patients achieving ACR20 responses at week 24 (superiority and non-inferiority vs tofacitinib); CFB in CDAI, pain Visual Analogue Scale (VAS) score, and FACIT-Fatigue at week 24 (superiority vs tofacitinib). Additional secondary endpoints included: CDAI remission (≤2.8), ACR50/70 responses, DAS28-CRP≤3.2/<2.6, CFB in van der Heijde modified total Sharp score (mTSS; see online supplemental methods), and Short Form (SF)-36 Physical and Mental component summary (PCS and MCS) scores. Safety endpoints included the incidence of adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESIs) and CFB in key laboratory parameters.
PK/pharmacodynamic and biomarker assessments
Blood samples for measurement of serum concentrations of otilimab, GM-CSF–otilimab complex, CC motif chemokine ligand (CCL17), IL-6 and matrix-metalloprotease-degraded Type I collagen (C1M) were collected on days 1, 8, 15, 29, 85 and 169 for pharmacodynamic (PD) and biomarker assessments, and on days 15, 29, 57, 85, 113, 169, 253 and 365 for PK assessments (post-day 85 data not reported).
Statistical analysis
For each trial, a minimum sample size of 1500 was required to provide >99% power to detect a 25% difference between otilimab and placebo in ACR20 response rates at week 12 based on a two-sided significance level of 0.05, using a pooled z-test. The primary endpoint was analysed using a logistic regression model, comparing otilimab with placebo at week 12, including fixed effects for treatment arm, baseline SJC66 and TJC68, and for previously failed medication (contRAst 2 only).
A step-down, graphical, multiple testing procedure was used to control for multiplicity of primary and major secondary endpoints. Statistical significance for a particular endpoint could be claimed only if the prior endpoints in the sequence met the requirements for significance (online supplemental figure 1). P values are only reported for endpoints that demonstrated statistical significance per the testing hierarchy. For the comparison of otilimab and tofacitinib, a non-inferiority margin of 12% was assumed. If patients agreed to continue to participate in the study, their data continued to be collected for use in the analysis. Missing data for this primary estimand were handled using multiple imputation (see online supplemental materials). Supplementary analysis using non-responder imputation, where patients who discontinued treatment were considered non-responders, was conducted.
Binary endpoints were analysed using logistic regression and continuous endpoints using analysis of covariance. The intention-to-treat (ITT) and safety populations were defined as all patients who were randomised and received ≥1 dose of study medication. In each trial, 15 patients randomised to the placebo-tofacitinib sequence were mistakenly allocated tofacitinib treatment from week 4 onwards. These patients were included in the placebo group (as randomised) for efficacy analyses, and in the tofacitinib group (as treated) for safety analyses. The PK population included all patients in the safety population who had at least one non-missing PK assessment.
Patient and public involvement
Patients were involved in patient advisory boards and in-person touchpoints in which the trial design and endpoints were discussed. There was no further patient or public involvement in the conduct or reporting of the trial.
Results
Trial population
In contRAst 1, of the 3051 patients screened, 1537 met the inclusion criteria, were randomised to receive study treatment and included in the ITT and safety populations; 118 patients completed the safety follow-up and 1171 entered contRAst X (online supplemental figure 2A). In contRAst 2, of 2888 patients screened, 1625 met the inclusion criteria, were randomised to receive study treatment and included in the ITT and safety populations; 123 patients completed the safety follow-up and 1223 entered contRAst X (online supplemental figure 2B). Withdrawal rates were similar across treatment arms in both trials, and the main reasons for screen failure were not meeting the inclusion/exclusion criteria (primarily low CRP levels and lack of bone erosions), and temporary suspension of randomisation from March to June 2020, due to COVID-19. Baseline demographics and clinical characteristics were generally balanced across treatment groups in both trials. In contRAst 2, 79% of patients had previously failed csDMARDs only, 14% had additionally failed 1 bDMARD and 7% had additionally failed >1 bDMARD (table 1).
Table 1.
Demographics and baseline characteristics
| ContRAst 1 | ContRAst 2 | |||||||||
| Placebo (N=256) | Otilimab 90 mg once weekly (N=513) | Otilimab 150 mg once weekly (N=510) | Tofacitinib 5 mg two times per day (N=258) | Total (N=1537) | Placebo (N=270) | Otilimab 90 mg once weekly (N=545) | Otilimab 150 mg once weekly (N=539) | Tofacitinib 5 mg two times per day (N=271) |
Total (N=1625) | |
| Baseline demographics | ||||||||||
| Sex, female, n (%) | 202 (79) | 401 (78) | 399 (78) | 209 (81) | 1211 (79) | 214 (79) | 431 (79) | 430 (80) | 229 (85) | 1304 (80) |
| Age* (years), mean (SD) | 52.4 (12.0) | 53.7 (12.1) | 54.2 (10.8) | 54.3 (11.7) | 53.8 (11.6) | 55.5 (11.9) | 55.2 (11.2) | 55.6 (11.4) | 55.4 (11.8) | 55.4 (11.5) |
| Race, n (%) | ||||||||||
| White | 218 (85) | 432 (84) | 434 (85) | 201 (78) | 1285 (84) | 203 (75) | 409 (75) | 393 (73) | 197 (73) | 1202 (74) |
| Asian | 16 (6) | 48 (9) | 39 (8) | 29 (11) | 132 (9) | 48 (18) | 96 (18) | 98 (18) | 49 (18) | 291 (18) |
| Black or African American | 7 (3) | 11 (2) | 11 (2) | 12 (5) | 41 (3) | 3 (1) | 10 (2) | 8 (1) | 3 (1) | 24 (1) |
| Baseline clinical characteristics | ||||||||||
| TJC68, mean (SD) | 24.6 (13.7) | 23.7 (12.5) | 24.0 (12.4) | 23.2 (12.2) | 23.8 (12.6) | 23.6 (13.2) | 23.0 (13.2) | 23.8 (14.0) | 24.2 (12.7) | 23.6 (13.4) |
| SJC66, mean (SD) | 15.1 (8.6) | 15.0 (8.1) | 14.8 (8.0) | 14.4 (7.4) | 14.8 (8.1) | 15.2 (8.6) | 14.7 (8.8) | 15.4 (8.5) | 15.3 (8.6) | 15.1 (8.6) |
| Pain VAS, mean (SD) | 66.2 (20.5) |
67.0 (18.3) | 65.3 (21.2) | 66.4 (18.9) | 66.2 (19.8) | 66.0 (20.8) | 65.2 (21.6) | 66.0 (20.8) | 65.2 (22.1) | 65.6 (21.3) |
| PtGA, mean (SD) | 67.3 (17.9) | 65.7 (18.5) | 65.3 (19.6) | 65.7 (19.2) |
65.9 (18.9) | 65.6 (18.9) | 64.5 (19.6) |
65.1 (20.3) |
67.0 (20.5) |
65.3 (19.9) |
| PhGA, mean (SD) | 68.0 (15.6) |
68.9 (15.8) |
68.8 (16.1) |
67.8 (15.9) |
68.5 (15.9) |
66.6 (16.6) |
65.8 (17.2) |
67.0 (18.1) |
67.3 (17.2) |
66.6 (17.4) |
| CRP (mg/L), mean (SD) | 18.9 (23.8) |
19.5 (28.7) |
16.4 (21.7) |
17.1 (21.2) |
17.9 (24.5) |
18.4 (27.1) |
20.0 (27.7) |
18.6 (25.3) |
20.4 (26.8) |
19.4 (26.7) |
| HAQ-DI, mean (SD) | 1.7 (0.6) |
1.6 (0.6) |
1.7 (0.6) |
1.6 (0.6) |
1.7 (0.6) |
1.6 (0.6) |
1.5 (0.6) |
1.5 (0.6) |
1.6 (0.6) |
1.6 (0.6) |
| CDAI, mean (SD) | 39.1 (11.6) |
39.3 (12.1) |
39.3 (11.6) |
38.2 (11.4) |
39.1 (11.7) |
38.5 (11.7) |
38.3 (11.9) |
39.0 (12.7) |
40.1 (12.2) |
38.9 (12.2) |
| DAS28-CRP, mean (SD) | 5.8 (0.9) |
5.8 (0.9) |
5.8 (0.9) |
5.7 (0.9) |
5.8 (0.9) |
5.7 (0.9) |
5.7 (0.9) |
5.7 (0.9) |
5.9 (1.0) |
5.7 (0.9) |
| FACIT-fatigue, mean (SD) | 26.6 (10.2) |
27.5 (10.1) |
26.5 (10.5) |
26.8 (9.9) |
26.9 (10.2) |
27.8 (10.6) |
28.5 (11.0) |
28.9 (10.5) |
28.1 (10.6) |
28.4 (10.7) |
| mTSS, mean (SD) | 26.5 (38.6) |
29.4 (43.1) |
30.0 (42.4) |
32.8 (42.6) |
29.7 (42.1) |
34.4 (45.1) |
29.2 (40.7) |
34.2 (47.5) |
33.8 (47.9) |
32.5 (45.0) |
| SF-36 MCS, mean (SD) | 45.1 (10.9) |
45.1 (10.5) | 44.9 (11.6) |
44.9 (10.3) |
45.0 (10.9) |
45.5 (11.0) |
45.8 (11.5) |
45.7 (10.7) |
45.1 (11.2) |
45.6 (11.1) |
| SF-36 PCS, mean (SD) | 32.8 (7.0) |
33.8 (7.1) |
33.1 (7.4) |
33.5 (6.9) |
33.3 (7.2) |
33.4 (7.0) |
34.2 (7.4) |
34.1 (7.4) |
33.7 (7.8) |
34.0 (7.4) |
| RA disease history | ||||||||||
| Time since RA diagnosis (years), mean (SD) | 8.66 (7.1) |
8.62 (7.4) |
8.97 (7.5) |
9.09 (7.5) |
8.82 (7.4) |
9.95 (8.4) |
10.14 (8.1) |
9.97 (8.2) |
10.58 (9.2) |
10.13 (8.4) |
| Stratum (previously failed medication) | ||||||||||
| csDMARD only, n (%) | N/A | N/A | N/A | N/A | N/A | 213 (79) |
431 (79) |
429 (80) |
213 (79) |
1286 (79) |
| 1 bDMARD | N/A | N/A | N/A | N/A | N/A | 38 (14) |
74 (14) |
70 (13) |
38 (14) |
220 (14) |
| >1 bDMARD | N/A | N/A | N/A | N/A | N/A | 19 (7) |
40 (7) |
40 (7) |
20 (7) |
119 (7) |
| RA medications taken at baseline (day 1) | ||||||||||
| MTX, n (%) | 256 (100) |
511 (>99) |
507 (>99) |
258 (100) |
1532 (>99) |
210 (78) |
450 (83) |
433 (80) |
216 (80) |
1309 (81) |
| MTX dose (mg/week), mean (SD) | 18.21 (4.6) |
17.63 (4.3) |
17.64 (4.2) |
18.09 (4.5) |
17.81 (4.3) |
17.08 (4.9) |
16.57 (4.9) |
16.29 (5.0) |
16.48 (4.6) |
16.55 (4.9) |
| csDMARDs>1, n (%)† | 0 | 0 | 2 (<1) | 2 (<1) | 4 (<1) | 37 (14) |
92 (17) |
94 (17) |
40 (15) |
263 (16) |
| Glucocorticoids, n (%)‡ | 127 (50) |
241 (47) |
259 (51) |
125 (48) |
752 (49) |
150 (56) |
270 (50) |
280 (52) |
139 (51) |
839 (52) |
| Glucocorticoids dose (mg/day)‡ | 6.77 (2.6) |
6.46 (2.7) |
6.25 (2.6) |
5.92 (2.4) |
6.35 (2.6) |
5.66 (2.4) |
5.53 (2.9) |
5.69 (2.7) |
5.44 (2.3) |
5.59 (2.6) |
*Age is imputed when full date of birth is not provided.
†csDMARDs other than MTX taken at baseline included hydroxychloroquine, sulfasalazine, azathioprine, chloroquine, leflunomide (contRAst 1); hydroxychloroquine, sulfasalazine, chloroquine, leflunomide, iguratimod, bucillamine and ciclosporin (contRAst 2).
‡Only includes patients who have taken oral corticosteroids for ≥4 weeks prior to baseline.
b/csDMARD, biologic/conventional synthetic disease-modifying antirheumatic drug; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; DAS28, Disease Activity Score-28 joints; FACIT, Functional Assessment of Chronic Illness Therapy; HAQ-DI, Health Assessment Questionnaire-Disability Index; MCS, mental component summary; mTSS, modified Total Sharp Scale; MTX, methotrexate; N/A, not available; PCS, physical component summary; PhGA, Physician’s Global Assessment; PtGA, Patient’s Global Assessment; RA, rheumatoid arthritis; SF-36, Short Form-36; SJC, swollen joint count; TJC, tender joint count; VAS, Visual Analogue Scale.
Primary endpoint
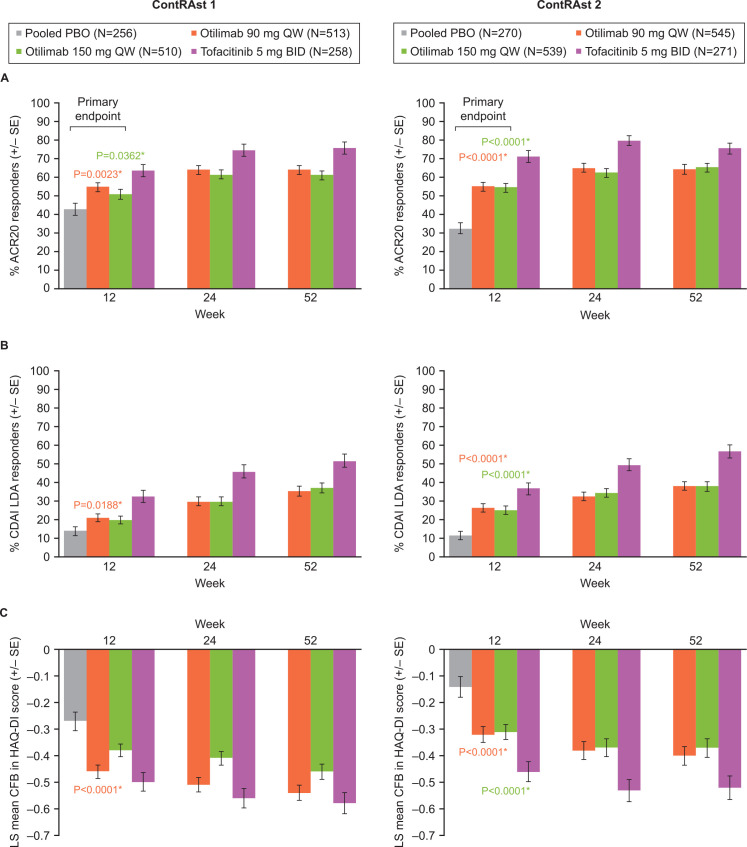
A significantly greater proportion of patients were ACR20 responders at week 12 with otilimab 90 and 150 mg compared with placebo in both contRAst 1 (54.7% (p=0.0023) and 50.9% (p=0.0362) vs 42.7%) and contRAst 2 (54.9% and 54.5% vs 32.5%, p<0.0001 for both) (figure 2, table 2, online supplemental figure 3).
Figure 2.
Proportion of patients achieving (A) ACR20 and (B) CDAI LDA at weeks 12, 24 and 52, and (C) LS mean CFB in HAQ-DI score at weeks 12, 24 and 52. *Otilimab versus placebo, per SAP. Comparison of tofacitinib versus placebo was not included in the SAP; however, statistical data are provided in the data tables. ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; CFB, change from baseline; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; LS, least squares; PBO, placebo; SAP, statistical analysis plan.
Table 2.
Primary and major secondary efficacy endpoints (week 12)
| ContRAst 1 | ContRAst 2 | |||||||
| Placebo (N=256) | Otilimab 90 mg once weekly (N=513) | Otilimab 150 mg once weekly (N=510) | Tofacitinib 5 mg two times per day (N=258) | Placebo (N=270) | Otilimab 90 mg once weekly (N=545) | Otilimab 150 mg once weekly (N=539) | Tofacitinib 5 mg two times per day (N=271) |
|
| ACR20 | ||||||||
| Responders, % | 42.7 | 54.7 | 50.9 | 63.6 | 32.5 | 54.9 | 54.5 | 71.1 |
| Difference versus placebo, % (95% CI) | 11.9 (4.3 to 19.5) | 8.2 (0.6 to 15.8) | 20.9 (12.2 to 29.5) | 22.4 (15.3 to 29.6) | 22.0 (14.8 to 29.2) | 38.6 (30.6 to 46.6) | ||
| OR versus placebo (95% CI) | 1.62 (1.19 to 2.21) | 1.39 (1.02 to 1.89) | 2.34 (1.62 to 3.37) | 2.57 (1.87 to 3.53) | 2.55 (1.85 to 3.50) | 5.38 (3.66 to 7.90) | ||
| CDAI LDA | ||||||||
| Responders, % | 13.9 | 20.9 | 19.8 | 32.5 | 11.4 | 26.5 | 25.1 | 36.8 |
| Difference versus placebo, % (95% CI) | 6.9 (1.3 to 12.6) | 5.9 (0.3 to 11.5) | 18.6 (11.3 to 25.9) | 15.1 (9.6 to 20.6) | 13.7 (8.3 to 19.2) | 25.4 (18.3 to 32.5) | ||
| OR vs placebo (95% CI) | 1.69 (1.09 to 2.63) | 1.60 (1.03 to 2.48) | 3.11 (1.94 to 4.97) | 2.96 (1.89 to 4.65) | 2.80 (1.78 to 4.40) | 5.43 (3.34 to 8.84) | ||
| HAQ–DI | ||||||||
| LS mean change (SE) | 0.27 (0.034) | 0.46 (0.025) | 0.38 (0.024) | 0.50 (0.034) | 0.14 (0.038) | 0.32 (0.029) | 0.31 (0.029) | 0.46 (0.037) |
| LS mean difference from placebo (95% CI) | 0.18 (–0.27 to –0.10) | 0.10 (–0.18 to –0.02) | 0.23 (–0.33 to –0.14) | 0.18 (–0.26 to –0.10) | 0.17 (–0.25 to –0.09) | 0.32 (–0.41 to –0.23) | ||
| Pain VAS | ||||||||
| LS mean change (SE) | 14.58 (1.466) | 22.00 (1.056) | 19.56 (1.033) | 27.26 (1.473) | 10.28 (1.657) | 18.06 (1.266) | 17.13 (1.256) | 27.17 (1.610) |
| LS mean difference from placebo (95% CI) | 7.43 (–10.92 to –3.93) | 4.98 (–8.47 to –1.49) | 12.69 (–16.73 to –8.65) | 7.78 (–11.24 to –4.31) | 6.85 (–10.32 to –3.37) | 16.89 (–20.89 to –12.88) | ||
| CDAI | ||||||||
| LS mean change (SE) | 13.01 (0.798) | 17.85 (0.547) | 17.15 (0.563) | 21.39 (0.801) | 9.56 (0.896) | 15.50 (0.680) | 16.31 (0.678) | 21.06 (0.878) |
| LS mean difference from placebo (95% CI) | 4.85 (–6.75 to –2.94) | 4.14 (–6.04 to –2.24) | 8.38 (–10.58 to –6.18) | 5.94 (–7.82 to –4.06) | 6.75 (–8.63 to –4.86) | 11.50 (–13.67 to –9.33) | ||
| FACIT-fatigue | ||||||||
| LS mean change (SE) | 4.72 (0.570) | 7.07 (0.410) | 6.30 (0.399) | 8.28 (0.577) | 3.68 (0.637) | 4.76 (0.482) | 4.91 (0.479) | 7.92 (0.615) |
| LS mean difference from placebo (95% CI) | 2.34 (0.99 to 3.70) | 1.58 (0.22 to 2.93) | 3.55 (1.98 to 5.12) | 1.08 (–0.25 to 2.41) | 1.24 (–0.09 to 2.56) | 4.25 (2.72 to 5.78) | ||
ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; CI, confidence interval; FACIT, Functional Assessment of Chronic Illness Therapy; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; LS, least squares; OR, odds ratio; SE, standard error; VAS, Visual Analogue Scale.
Secondary endpoints
In contRAst 1, otilimab 90 mg resulted in a significantly greater proportion of patients achieving CDAI LDA at week 12 vs placebo, but otilimab 150 mg did not (20.9% (p=0.0188) and 19.8% (p=0.0368) vs 13.9%). In contRAst 2, both doses resulted in a significant difference versus placebo (26.5% and 25.1%, respectively, vs 11.4%, p<0.0001 for both) (figure 2, table 2, online supplemental figure 3).
In contRAst 1, a greater reduction versus placebo in HAQ-DI at week 12 was reported with otilimab 90 mg (–0.46 vs –0.27, p<0.0001) and 150 mg (–0.38 vs –0.27); however, statistical significance could not be tested for the 150 mg arm according to the testing hierarchy. In contRAst 2, both doses resulted in a significantly greater CFB versus placebo (–0.32 and –0.31 vs –0.14, respectively, p<0.0001 for both) (figure 2, table 2, online supplemental figure 3).
Otilimab 150 mg failed to demonstrate non-inferiority versus tofacitinib based on ACR20 response at week 24. Tofacitinib resulted in a significantly greater proportion of ACR20 responders versus otilimab 90 mg and 150 mg in both contRAst 1 (74.4% vs 63.9% (p=0.0061) and 61.3% (p=0.0007)) and contRAst 2 (79.8% vs 65.0 and 62.5%, p<0.0001 for both) (figure 2; online supplemental table 4, online supplemental figure 3). Due to the step-down multiple testing approach, statistical significance could not be assessed for any of the subsequent endpoints in either trial.
Supplementary analyses using non-responder imputation yielded similar results to the primary analysis (online supplemental figure 4). The proportions of ACR20 responders at week 24 for patients who switched from placebo to otilimab or tofacitinib after week 12 were mostly similar to those who initiated active treatment at week 0 (online supplemental figure 5). No consistent trends in ACR20 response were observed across contRAst 1 and contRAst 2 in the analyses of subgroups stratified by sex, disease duration and serological status (positive for both rheumatoid factor and anticyclic citrullinated peptide antibody; online supplemental figures 6 and 7). Subgroup analysis of prior failed DMARD in contRAst 2 suggested a trend towards a greater benefit for patients with prior csDMARD failure only versus patients with ≥1 bDMARD failure; however, wide CIs were observed in the bDMARD failure subgroups (online supplemental figure 7).
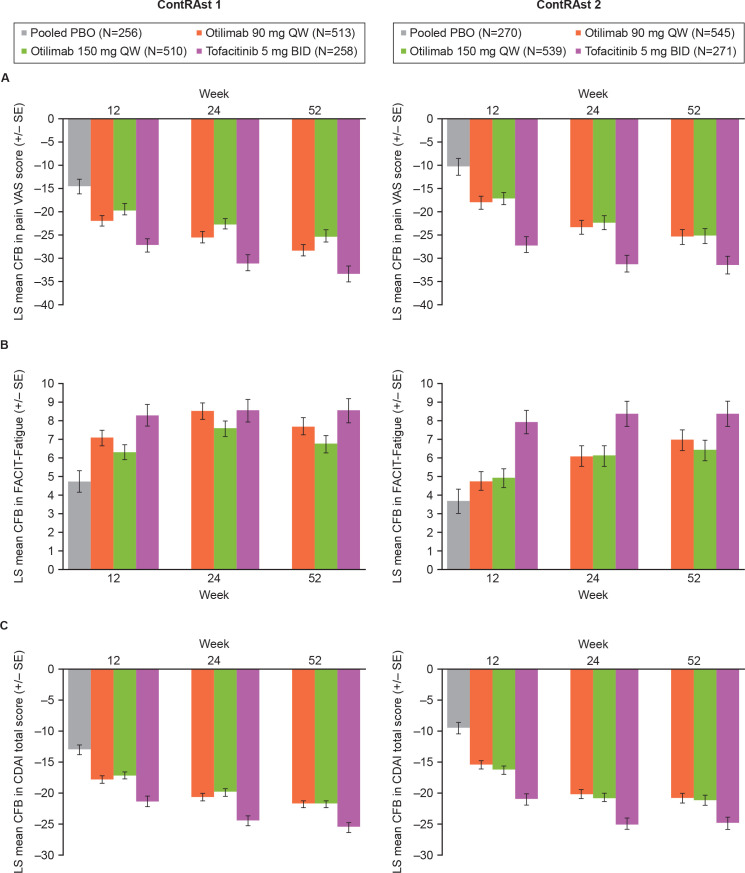
In both trials, otilimab demonstrated numerical improvements versus placebo in FACIT-Fatigue, pain VAS score and CDAI at week 12, which continued to week 52 (figure 3, table 2, online supplemental figure 8). There were no major differences in the proportions of patients achieving CDAI remission with either dose of otilimab versus placebo at week 12 in either trial (online supplemental figure 9 and online supplemental tables 4 and 5).
Figure 3.
LS mean CFB in (A) pain VAS, (B) FACIT-Fatigue and (C) CDAI Due to the step-down multiple testing approach, statistical significance was not assessed, or cannot be claimed, for otilimab versus placebo for these endpoints; however, statistical data are provided in the data tables. CDAI, Clinical Disease Activity Index; CFB, change from baseline; FACIT, Functional Assessment of Chronic Illness Therapy; LS, least squares; PBO, placebo; VAS, Visual Analogue Scale.
In both trials, otilimab treatment resulted in greater proportions of ACR50 and ACR70 responders at week 12 vs placebo, which increased up to week 52; the proportions of responders were similar for both otilimab doses in both trials (online supplemental figures 10 and 11, online supplemental table 4 and 5). Compared with placebo, both otilimab doses improved the additional ACR core data set measures of TJC and SJC, Patient and Physician Global Assessments and CRP to a similar extent (online supplemental table 4, online supplemental figure 12) and resulted in greater reductions from baseline in DAS28-CRP in both trials (online supplemental figure 13, online supplemental table 4 and 5). Additionally, there was an increase in the proportion of patients achieving DAS28-CRP ≤3.2 and <2.6 at week 12 with otilimab versus placebo.
In contRAst 1, both doses of otilimab reduced progression of joint damage as measured by a lower CFB in mTSS versus placebo at week 12; this was not observed in contRAst 2, where a relatively low mean progression was observed in the placebo arm. In both trials, patients who received tofacitinib showed the least progression (online supplemental figures 14–16, online supplemental tables 4 and 5). At week 12, the proportions of patients with mTSS changes ≤0.5 were 84% and 88% for otilimab 90 mg and 83% and 93% for otilimab 150 mg vs 77% and 86% for placebo, in contRAst 1 and contRAst 2, respectively (online supplemental figure 14).
Improvements versus placebo in SF-36 PCS scores were reported with both otilimab doses in both trials. No differences versus placebo in SF-36 MCS scores were reported with either otilimab dose in either trial (online supplemental table 5).
Greater improvements were reported with tofacitinib versus otilimab in most of the secondary endpoints including CDAI LDA response, HAQ-DI, pain VAS and FACIT-fatigue scores.
Safety
Unless otherwise indicated, week 52 safety data are reported for patients who were randomised to active treatments from baseline; a safety summary following the week 12 switch from placebo to active treatment is provided in online supplemental table 6. The incidence of AEs was similar across treatment groups in both trials (table 3); the most common (≥5%) AEs were COVID-19, RA, lymphopaenia, anaemia, injection site reaction, hypertension, increased alanine transaminase, urinary tract infection, upper respiratory tract infection, nasopharyngitis and headache (online supplemental tables 7 and 8). The incidence of SAEs was ≤8% in either otilimab group and ≤11% with tofacitinib (table 3, online supplemental table 9 and 10). The incidence of any AESI was 8%–14% (online supplemental table 11). In any treatment group, the incidence of serious infections was ≤4% (approximately half were COVID-19). The incidence of MACE was <1%; 1 patient in the tofacitinib arm reported an MACE in contRAst 2 (0 in contRAst 1), and 4 (<1%) and 3 (<1%) patients reported a MACE with otilimab 90 mg or 150 mg, respectively, in both trials. The incidence of malignancy in any treatment arm was ≤2% (table 3, online supplemental table 11). There were no events of active tuberculosis (TB) or reactivation of latent TB, no events of serious hypersensitivity reactions or pulmonary alveolar proteinosis (PAP) with otilimab. Herpes zoster was reported in 1 (<1%) patient in the otilimab 150 mg group, and in no patients in the tofacitinib group. Across both trials, 34 deaths occurred, including 4 in the placebo group (1 prior to the switch to active treatment and 3 after), and 5 occurring more than 56 days after the last treatment dose (online supplemental tables 6 and 12). None of the deaths were considered treatment-related (eight were COVID-19 related) and no patterns were observed in any of the treatment arms (online supplemental table 12). No clinically meaningful differences were observed with otilimab versus placebo in laboratory parameters (online supplemental tables 13 and 14) including neutropenia.
Table 3.
Safety summary
| Adverse event, no of patients (%) | ContRAst 1 | ContRAst 2 | ||||||
| Pooled placebo (N=241) | Otilimab 90 mg once weekly (N=513) |
Otilimab 150 mg once weekly (N=510) |
Tofacitinib 5 mg two times per day (N=273) |
Pooled placebo (N=255) | Otilimab 90 mg once weekly (N=545) |
Otilimab 150 mg once weekly (N=539) | Tofacitinib 5 mg two times per day (N=286) |
|
| Weeks 0–12 | ||||||||
| Any AE | 95 (39) | 222 (43) | 234 (46) | 122 (45) | 127 (50) | 267 (49) | 268 (50) | 143 (50) |
| Any SAE | 8 (3) | 8 (2) | 6 (1) | 9 (3) | 6 (2) | 12 (2) | 19 (4) | 6 (2) |
| Any AESI | 4 (2) | 25 (5) | 26 (5) | 5 (2) | 5 (2) | 27 (5) | 44 (8) | 5 (2) |
| Serious infection* | 3 (1) | 3 (<1) | 2 (<1) | 4 (1) | 1 (<1) | 2 (<1) | 7 (1) | 1 (<1) |
| Serious infection, excluding COVID-19* | 1 (<1) | 2 (<1) | 1 (<1) | 3 (1) | 1 (<1) | 2 (<1) | 3 (<1) | 1 (<1) |
| Active TB* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Latent TB* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TB reactivation* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PAP* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| COVID-19 diagnosis† | 8 (3) | 11 (2) | 12 (2) | 9 (3) | 3 (1) | 10 (2) | 13 (2) | 7 (2) |
| Any adjudicated CV event | 0 (0) | 1 (<1) | 1 (<1) | 0 (0) | 0 (0) | 1 (<1) | 2 (<1) | 0 (0) |
| Adjudicated MACE | 0 (0) | 1 (<1) | 1 (<1) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) | 0 (0) |
| VTE (DVT and/or PE) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 0 (0) |
| PE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 0 (0) |
| DVT | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Any malignancy | 2 (<1) | 1 (<1) | 1 (<1) | 0 (0) | 0 (0) | 0 (0) | 2 (<1) | 0 (0) |
| Any malignancy, excluding NMSC | 2 (<1) | 1 (<1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (<1) | 0 (0) |
| Fatal SAE | 1 (<1) | 1 (<1) | 1 (<1) | 2 (<1) | 0 (0) | 0 (0) | 4 (<1) | 0 (0) |
| Otilimab 90 mg once weekly (N=513) |
Otilimab 150 mg once weekly (N=510) |
Tofacitinib 5 mg two times per day (N=273) |
Otilimab 90 mg once weekly (N=545) |
Otilimab 150 mg once weekly (N=539) | Tofacitinib 5 mg two times per day (N=286) |
|
| Weeks 0–52‡ | ||||||
| Any AE | 367 (72) | 383 (75) | 207 (76) | 420 (77) | 408 (76) | 224 (78) |
| Any SAE | 33 (6) | 39 (8) | 23 (8) | 44 (8) | 43 (8) | 31 (11) |
| Any AESI | 65 (13) | 58 (11) | 22 (8) | 72 (13) | 75 (14) | 32 (11) |
| Serious infection* | 16 (3) | 18 (4) | 8 (3) | 12 (2) | 13 (2) | 12 (4) |
| Serious infection, excluding COVID-19* | 9 (2) | 8 (2) | 3 (1) | 6 (1) | 7 (1) | 6 (2) |
| Active TB* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Latent TB* | 22 (4) | 9 (2) | 11 (4) | 15 (3) | 10 (2) | 8 (3) |
| TB reactivation* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PAP* | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| COVID-19 diagnosis† | 58 (11) | 65 (13) | 39 (14) | 62 (11) | 46 (9) | 35 (12) |
| Any adjudicated CV event | 5 (<1) | 4 (<1) | 0 (0) | 4 (<1) | 7 (1) | 2 (<1) |
| Adjudicated MACE | 4 (<1) | 3 (<1) | 0 (0) | 4 (<1) | 3 (<1) | 1 (<1) |
| VTE (DVT and/or PE) | 1 (<1) | 1 (<1) | 0 (0) | 0 (0) | 4 (<1) | 1 (<1) |
| PE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (<1) | 1 (<1) |
| DVT | 1 (<1) | 1 (<1) | 0 (0) | 0 (0) | 2 (<1) | 0 (0) |
| Any malignancy | 1 (<1) | 3 (<1) | 2 (<1) | 1 (<1) | 4 (<1) | 6 (2) |
| Any malignancy, excluding NMSC | 1 (<1) | 2 (<1) | 2 (<1) | 1 (<1) | 4 (<1) | 6 (2) |
| Fatal SAE | 2 (<1) | 7 (1) | 3 (1) | 5 (<1) | 6 (1) | 2 (<1) |
*Only select AESIs with relevance to the MoA of otilimab or tofacitinib are reported. See online supplemental table 11 for all AESIs.
†Data reported for patients who were randomised to active treatments from baseline. See online supplemental table 5 for the safety summary for patients who switched from placebo to active treatment at week 12.
‡Total cases (either AEs or SAEs).
AE, adverse event; AESI, AE of special interest; CV, cardiovascular; DVT, deep vein thrombosis; MACE, major adverse cardiovascular event; MoA, mechanism of action; NMSC, non-melanoma skin cancer; PAP, pulmonary alveolar proteinosis; PE, pulmonary embolism; SAE, serious AE; TB, tuberculosis; VTE, venous thromboembolism.
PK/PD and biomarkers
In both trials, a steady-state mean serum otilimab concentration of ~2200 ng/mL was achieved with otilimab 90 mg by week 8; the 150 mg dose achieved a steady-state concentration of ~3700 ng/mL in contRAst 1 and ~4100 ng/mL in contRAst 2 by week 8 (online supplemental figure 17). Patients who switched from placebo to otilimab after week 12 showed similar serum otilimab concentrations from their first measurement at week 16 (4 weeks post-first otilimab dose), and serum otilimab concentrations remained stable for the duration of the trials. Similar baseline serum free GM-CSF levels of ~0.3 ng/L were reported across the treatment groups. Following otilimab treatment, GM-CSF–otilimab complex accumulation indicated target engagement, which stabilised from approximately week 8 and was maintained for the duration of the trial (online supplemental figure 18).
In both trials, both otilimab doses reduced CCL17 and CCL22 at week 12 compared with placebo and tofacitinib. Both otilimab doses and tofacitinib reduced VICM by week 12 vs placebo, with otilimab 150 mg and tofacitinib having a greater effect in contRAst 1 and contRAst 2, respectively. An early reduction of the inflammatory markers, IL-6 and C1M, was observed with both doses of otilimab compared with placebo (except for IL-6 in contRAst 2), which plateaued to week 12, while tofacitinib consistently showed greater reductions than otilimab in both (online supplemental figure 19).
Post hoc exposure–response analyses demonstrated trends for a higher otilimab exposure resulting in greater reductions in CCL17, CRP and CCL22 (only in contRAst 2 for CCL22), but not IL-6 (online supplemental figure 20).
Discussion
These two trials were conducted across a total of 30 countries across North and South America, Europe, Asia and Africa, with the COVID-19 pandemic spanning most of the trial duration. In patients with active RA, a statistical improvement in ACR20 response (primary endpoint) was demonstrated with both doses of otilimab versus placebo; however, differences in response were modest. In both trials, a significantly greater proportion of patients achieved CDAI-LDA responses and a significant reduction from baseline HAQ-DI with otilimab 90 mg vs placebo. The same was observed for otilimab 150 mg in contRAst 2; however, in contRAst 1, otilimab 150 mg failed to result in a significant difference versus placebo in CDAI LDA, therefore, HAQ-DI could not be tested for significance. As the most recent MoA to be added to the therapeutic armamentarium for RA, the JAKi, tofacitinib, was considered an appropriate active comparator for these trials. Otilimab was consistently inferior to tofacitinib across multiple endpoints.
A higher placebo response was noted in contRAst 1, compared with contRAst 2, which may account for the generally greater otilimab treatment effect over placebo observed across multiple endpoints in contRAst 2, despite the patient population having a slightly longer disease duration and a subgroup of patients having failed multiple DMARDs. However, otilimab was associated with inhibition of structural joint damage in contRAst 1, but not in contRAst 2. Indeed, contRAst 2 reported a greater CFB in mTSS with otilimab 90 mg vs placebo, although this may reflect the relatively low mean radiographic progression in the placebo arm, possibly due to outliers with high negative scores. Nevertheless, while both doses of otilimab resulted in reductions versus placebo in pain VAS, FACIT-Fatigue, CDAI, DAS28-CRP as well as increases in ACR50, DAS28-CRP≤3.2 and <2.6 responses, the benefit with tofacitinib was consistently greater than that of either otilimab dose and was comparable to that reported in previously published tofacitinib trials conducted in similar populations, such as ORAL Standard, Strategy and Scan.25–27
While otilimab met the primary endpoint versus placebo, the limited efficacy versus tofacitinib in these trials does not support the use of otilimab in a broad RA patient population. As expected, a greater proportion of ACR20 responses was reported in the subgroup of patients with csDMARD failure only, compared with patients with 1 bDMARD and >1 bDMARD failures.
The steady-state serum otilimab concentrations achieved in these trials were higher than predicted for these dosing regimens,21 and higher than those achieved in BAROQUE.20 At week 12 in the contRAst trials, serum otilimab concentrations with otilimab 90 mg and 150 mg weekly were ≥2.1 fold and ≥3.8 fold higher than those achieved with otilimab 180 mg every 2 weeks in BAROQUE. Despite achieving higher steady-state concentrations for the 150 mg vs 90 mg dose, similar efficacy was observed, suggesting a lack of dose-response. Otilimab treatment resulted in ~30%–40% reduction in serum concentrations of CCL17 (the putative PD biomarker for otilimab activity), which is similar to the reduction observed with otilimab 180 mg once weekly in the phase 2a mechanistic study, RENAISSANCE,28 suggesting that both otilimab doses were pharmacologically active.
Residual pain is a key unmet need in patients with RA.29 The clinically meaningful improvements in pain VAS scores with otilimab in the BAROQUE trial, despite non-significant DAS28-CRP<2.6 responses at week 24,20 were a key consideration of the rationale for this phase 3 programme. In contRAst 1 and 2, both otilimab and tofacitinib improved pain; however, the benefit with otilimab was lower than that with tofacitinib, conceivably due to the greater anti-inflammatory effects of tofacitinib versus otilimab. Additionally, the use of the weekly, rather than biweekly, otilimab dosing strategy in contRAst 1 and contRAst 2 compared with BAROQUE did not improve on previous pain responses, nor did it increase ACR20/50/70 responses as previously predicted,21 despite achieving higher steady-state serum concentrations. The current results are similar to those of other anti-GM-CSF therapies such as namilumab and mavrilimumab which reduced DAS28-CRP scores versus placebo in RA clinical trials.30 31 However, a more pronounced inhibition of the inflammatory biomarker, IL-6, was reported with mavrilimumab.32
Previously, there have been theoretical safety concerns regarding anti-GM-CSF therapies due to the potential impairment of myeloid cell production and function that could result in PAP,33 and the potential impact on immunological responses34 that could result in increased infections or reactivation of latent infections such as TB, as observed with anti-TNFs.35 36 However, there were no reports of PAP, the incidence of neutropenia or serious infections was ≤4% with either otilimab dose (COVID-19 infection was balanced across treatment groups and accounted for approximately half of serious infections) and there were no reports of active TB or reactivation of latent TB in these trials. Moreover, the incidence of MACE was <1% across treatment arms. These safety results add to those of prior anti-GM-CSF phase 2 RCTs.20 30 31 37–41
A strength of both trials was the consistency of responses to tofacitinib with those of previous phase 3 RCTs,42–44 indicating that both trials were well conducted, despite difficulties imposed by the COVID-19 pandemic, and providing confidence in the results observed. Ultimately, apart from pausing trial recruitment for 3 months to accommodate local restrictions, the overall impact of COVID-19 was minimal, with a low number of protocol deviations and missing data points. Additional strengths include the global reach of the trials, which captured various regional populations, and the reporting of positive outcomes considered important to patients such as pain and its impacts, physical function, fatigue, sleep disturbance and HRQoL.45–47 Study limitations include the differing geographic locations between contRAst 1 and contRAst 2, and the low subgroup numbers in contRAst 2, making it difficult to interpret whether the number of prior failed DMARDs impacted the responses to otilimab.
Conclusions
Otilimab met the primary endpoint of ACR20 response versus placebo at week 12 in both contRAst 1 and contRAst 2. Despite the lack of statistical significance for a number of endpoints, otilimab treatment reduced measures of RA disease activity, reduced inflammatory markers, improved symptoms such as pain and fatigue, and demonstrated an acceptable safety profile. However, despite a plausible evidence base, the limited efficacy of otilimab compared with tofacitinib in these RCTs, or compared with sarilumab in contRAst 3 (published separately), does not support a suitable benefit/risk profile for its use in the treatment of RA in these patient populations.
Acknowledgments
The authors would like to thank Katherine Davy, Mark Layton and Jatin Patel for their support in the design of the trial. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Clare Cunningham, PhD and Chrystelle Rasamison of Fishawack Indicia, UK, part of Fishawack Health, and was funded by GSK.
Footnotes
Handling editor: Josef S Smolen
Contributors: RMF contributed to the conception/design of the study and the acquisition and analysis of data and is acting as guarantor. MEW, PCT, VS, DvdH, MW, DS, SW, LAS, DB, JES and AG contributed to the conception/design of the study and the analysis of data. TA contributed to the acquisition and analysis of data. IBM, TT, RW, JD, MB, CS, CO’S, CG and SM contributed to the analysis of data. All authors contributed to data interpretation, drafting, or critically revising the article. All authors provided final approval and agreement of accountability.
Funding: GSK (NCT03980483/NCT03970837).
Competing interests: RMF has received research support from AbbVie, Amgen, Arthrosi, AstraZeneca, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Galvani, Genentech/Roche, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung, Sanofi-Genzyme, Selecta and UCB; consulting fees from AbbVie, Amgen, Arthrosi, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Galapagos, Galvani, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Samsung and UCB; has received honoraria from AbbVie, GSK and Pfizer and is an Annals of the Rheumatic Diseases Editorial Board Member. DvdH has received consulting fees from AbbVie, Bayer, Bristol Myers Squibb, Galapagos, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, UCB Pharma, is Director of Imaging Rheumatology BV and is an Annals of the Rheumatic Diseases Editorial Board Member. VS has received consulting fees from AbbVie, Alpine, Alumis, Amgen, Aria, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Ermium, Genentech/Roche, GSK, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Merck, MiMedx, Novartis, Omeros, Pfizer, R-Pharm, RAPT, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix, Tonix and Urica. TA has accepted research grants and/or honoraria for meetings from AbbVie, Alexion, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, GSK, Lilly Japan, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Takeda Pharmaceutical, and UCB Japan. IBM has received consultancy and research support from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Causeway, Compugen, Gilead Sciences, GSK, Lilly, Novartis, Pfizer, and UCB and holds a leadership role in Evelo, University of Glasgow, Versus Arthritis, and is an NHS GGC Board Member and an Annals of the Rheumatic Diseases Editorial Board Member. TT received payment or honoraria from AbbVie, Asahi Kasei, Astellas, AstraZeneca K.K., Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Gilead Sciences, Janssen K.K, Lilly Japan, Mitsubishi-Tanabe, Pfizer Japan and is an Annals of the Rheumatic Diseases Editorial Board Member. PCT has received consulting fees from AbbVie, Biogen, Bristol Myers Squibb, Fresenius, Galapagos, Gilead Sciences, GSK, Janssen, Lilly, Nordic Pharma, Pfizer, Roche, Sanofi, and UCB, and research support from Galapagos. MB, DB, JD, CG, AG, SM, CO’S, DS, LAS, CS, JES, MW, RW and SW are employees of GSK and hold GSK stock/shares. MEW receives research support from AbbVie, Aqtual, Bristol Myers Squibb and Lilly, and consultation fees from AbbVie, Aclaris, Amgen, Bayer, Bristol Myers Squibb, Corvitas, Genosco, Gilead Sciences, GSK, Horizon, Johnson & Johnson, Lilly, Novartis, Pfizer, Rami Therapeutics, R Pharma, Roche, Sanofi, Scipher, Sci Rhom, Set Point and Tremeau. He holds stock/stock options of CanFite, Inmedix, and Scipher.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Anonymised individual participant data and study documents can be requested for further research from https://www.gsk-studyregister.com/en/.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and protocols were approved by relevant country-specific institutional review boards/independent ethics committees (provided in online supplemental materials). Participants gave informed consent to participate in the study before taking part.
References
- 1. Buch MH. Defining refractory rheumatoid arthritis. Ann Rheum Dis 2018;77:966–9. 10.1136/annrheumdis-2017-212862 [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 3. Fraenkel L, Bathon JM, England BR, et al. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. 10.1002/art.41752 [DOI] [PubMed] [Google Scholar]
- 4. Gerhold K, Richter A, Schneider M, et al. Health-related quality of life in patients with long-standing rheumatoid arthritis in the era of biologics: data from the German Biologics register RABBIT. Rheumatology (Oxford) 2015;54:1858–66. 10.1093/rheumatology/kev194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Radawski C, Genovese MC, Hauber B, et al. Patient perceptions of unmet medical need in rheumatoid arthritis: a cross-sectional survey in the USA. Rheumatol Ther 2019;6:461–71. 10.1007/s40744-019-00168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michaud K, Pope J, van de Laar M, et al. Systematic literature review of residual symptoms and an unmet need in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021;73:1606–16. 10.1002/acr.24369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McWilliams DF, Walsh DA. Factors predicting pain and early discontinuation of tumour necrosis factor-alpha-inhibitors in people with rheumatoid arthritis: results from the British Society for Rheumatology Biologics register. BMC Musculoskelet Disord 2016;17:337. 10.1186/s12891-016-1192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lotfi N, Thome R, Rezaei N, et al. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front Immunol 2019;10:1265. 10.3389/fimmu.2019.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wicks IP, Roberts AW. Targeting GM-CSF in inflammatory diseases. Nat Rev Rheumatol 2016;12:37–48. 10.1038/nrrheum.2015.161 [DOI] [PubMed] [Google Scholar]
- 10. Greven DEA, Cohen ES, Gerlag DM, et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann Rheum Dis 2015;74:1924–30. 10.1136/annrheumdis-2014-205234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamilton JA. GM-CSF in inflammation. J Exp Med 2020;217:e20190945. 10.1084/jem.20190945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell IK, Rich MJ, Bischof RJ, et al. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol 1998;161:3639–44. 10.4049/jimmunol.161.7.3639 [DOI] [PubMed] [Google Scholar]
- 13. Tewari D, Cook AD, Lee M-C, et al. Granulocyte-macrophage colony stimulating factor as an indirect mediator of Nociceptor activation and pain. J Neurosci 2020;40:2189–99. 10.1523/JNEUROSCI.2268-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu WD, Firestein GS, Taetle R, et al. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest 1989;83:876–82. 10.1172/JCI113971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu CQ, Field M, Allard S, et al. Detection of cytokines at the cartilage/Pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol 1992;31:653–61. 10.1093/rheumatology/31.10.653 [DOI] [PubMed] [Google Scholar]
- 16. Bell AL, Magill MK, McKane WR, et al. Measurement of colony-stimulating factors in synovial fluid: potential clinical value. Rheumatol Int 1995;14:177–82. 10.1007/BF00262295 [DOI] [PubMed] [Google Scholar]
- 17. Avci AB, Feist E, Burmester G-R. Targeting GM-CSF in rheumatoid arthritis. Clin Exp Rheumatol 2016;34:39–44. [PubMed] [Google Scholar]
- 18. Cook AD, Braine EL, Campbell IK, et al. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the Effector phase of disease. Arthritis Res 2001;3:293–8. 10.1186/ar318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eylenstein R, Weinfurtner D, Härtle S, et al. Molecular basis of in vitro affinity maturation and functional evolution of a neutralizing anti-human GM-CSF antibody. MAbs 2016;8:176–86. 10.1080/19420862.2015.1099774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckley CD, Simón-Campos JA, Zhdan V, et al. Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: a randomised, phase 2B, dose-ranging study. The Lancet Rheumatology 2020;2:e677–88. 10.1016/S2665-9913(20)30229-0 [DOI] [PubMed] [Google Scholar]
- 21. Gupta A, Zecchin C, Watts S, et al. Model informed selection of phase III dosing regimens not fully tested in phase II: anti-GM-CSF mAb, GSK3196165 (Otilimab) in moderate to severe rheumatoid arthritis. American Conference on Pharmacometrics; Orlando, FL, 2019. [Google Scholar]
- 22. Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 23. Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992;35:498–502. 10.1002/art.1780350502 [DOI] [PubMed] [Google Scholar]
- 24. Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 25. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL strategy): a phase 3B/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. 10.1016/S0140-6736(17)31618-5 [DOI] [PubMed] [Google Scholar]
- 26. van der Heijde D, Strand V, Tanaka Y, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: clinical efficacy, radiographic, and safety outcomes from a twenty-four-month, phase III study. Arthritis Rheumatol 2019;71:878–91. 10.1002/art.40803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or Adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. 10.1056/NEJMoa1112072 [DOI] [PubMed] [Google Scholar]
- 28. Genovese MC, Berkowitz M, Conaghan PG, et al. MRI of the joint and evaluation of the granulocyte–macrophage colony-stimulating factor–Ccl17 axis in patients with rheumatoid arthritis receiving Otilimab: a phase 2A randomised mechanistic study. The Lancet Rheumatology 2020;2:e666–76. 10.1016/S2665-9913(20)30224-1 [DOI] [PubMed] [Google Scholar]
- 29. Lee YC, Cui J, Lu B, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 2011;13:R83. 10.1186/ar3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burmester GR, McInnes IB, Kremer J, et al. A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis. Ann Rheum Dis 2017;76:1020–30. 10.1136/annrheumdis-2016-210624 [DOI] [PubMed] [Google Scholar]
- 31. Taylor PC, Saurigny D, Vencovsky J, et al. Efficacy and safety of namilumab, a human Monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: a randomized, controlled trial. Arthritis Res Ther 2019;21:101. 10.1186/s13075-019-1879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo X, Higgs BW, Bay-Jensen A-C, et al. Blockade of GM-CSF pathway induced sustained suppression of myeloid and T cell activities in rheumatoid arthritis. Rheumatology (Oxford) 2018;57:175–84. 10.1093/rheumatology/kex383 [DOI] [PubMed] [Google Scholar]
- 33. Rooney L, Veale DJ, Orr C, et al. Targeting GM-CSF in rheumatological conditions: risk of PAP. The Lancet Rheumatology 2021;3:e473. 10.1016/S2665-9913(21)00145-4 [DOI] [PubMed] [Google Scholar]
- 34. Bykerk VP. The efficacy and safety of targeting GM-CSF in arthritis. Lancet Rheumatol 2020;2:e648–50. 10.1016/S2665-9913(20)30352-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with Infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001;345:1098–104. 10.1056/NEJMoa011110 [DOI] [PubMed] [Google Scholar]
- 36. Benmerzoug S, Marinho FV, Rose S, et al. GM-CSF targeted Immunomodulation affects host response to M. Tuberculosis infection. Sci Rep 2018;8:8652. 10.1038/s41598-018-26984-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo X, Wang S, Godwood A, et al. Pharmacodynamic biomarkers and differential effects of TNF- and GM-CSF-targeting biologics in rheumatoid arthritis. Int J Rheum Dis 2019;22:646–53. 10.1111/1756-185X.13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel J, Bass D, Beishuizen A, et al. A randomised trial of anti-GM-CSF otilimab in severe COVID-19 pneumonia (OSCAR). Eur Respir J 2023;61:2101870. 10.1183/13993003.01870-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burmester GR, Weinblatt ME, McInnes IB, et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis 2013;72:1445–52. 10.1136/annrheumdis-2012-202450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinblatt ME, McInnes IB, Kremer JM, et al. A randomized phase IIb study of mavrilimumab and golimumab in rheumatoid arthritis. Arthritis Rheumatol 2018;70:49–59. 10.1002/art.40323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takeuchi T, Tanaka Y, Close D, et al. Efficacy and safety of mavrilimumab in Japanese subjects with rheumatoid arthritis: findings from a phase IIa study. Mod Rheumatol 2015;25:21–30. 10.3109/14397595.2014.896448 [DOI] [PubMed] [Google Scholar]
- 42. Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. 10.1056/NEJMoa1109071 [DOI] [PubMed] [Google Scholar]
- 43. Kremer J, Li Z-G, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. 10.7326/0003-4819-159-4-201308200-00006 [DOI] [PubMed] [Google Scholar]
- 44. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. 10.1016/S0140-6736(12)61424-X [DOI] [PubMed] [Google Scholar]
- 45. Alten R, van de Laar M, De Leonardis F, et al. Physical and emotional burden of rheumatoid arthritis: data from RA matters, a web-based survey of patients and healthcare professionals. Rheumatol Ther 2019;6:587–97. 10.1007/s40744-019-00179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Irwin MR, Olmstead R, Carrillo C, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep 2012;35:537–43. 10.5665/sleep.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Becker B, Raymond K, Hawkes C, et al. Qualitative and Psychometric approaches to evaluate the PROMIS pain interference and sleep disturbance item banks for use in patients with rheumatoid arthritis. J Patient Rep Outcomes 2021;5:52. 10.1186/s41687-021-00318-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2023-224482supp001.pdf (11.5MB, pdf)
Data Availability Statement
Data are available on reasonable request. Anonymised individual participant data and study documents can be requested for further research from https://www.gsk-studyregister.com/en/.