Abstract
The effects of salinity and growth temperature on the accumulation of intracellular organic solutes were examined by nuclear magnetic resonance spectroscopy (NMR) in Thermococcus litoralis, Thermococcus celer, Thermococcus stetteri, and Thermococcus zilligii (strain AN1). In addition, the effects of growth stage and composition of the medium were studied in T. litoralis. A novel compound identified as β-galactopyranosyl-5-hydroxylysine was detected in T. litoralis grown on peptone-containing medium. Besides this newly discovered compound, T. litoralis accumulated mannosylglycerate, aspartate, α-glutamate, di-myo-inositol-1,1′(3,3′)-phosphate, hydroxyproline, and trehalose. The hydroxyproline and β-galactopyranosyl-5-hydroxylysine were probably derived from peptone, while the trehalose was derived from yeast extract; none of these three compounds was detected in the other Thermococcus strains examined. Di-myo-inositol-1,1′(3,3′)-phosphate, aspartate, and mannosylglycerate were detected in T. celer and T. stetteri, and the latter organism also accumulated α-glutamate. The only nonmarine species studied, T. zilligii, accumulated very low levels of α-glutamate and aspartate. The levels of mannosylglycerate and aspartate increased in T. litoralis, T. celer, and T. stetteri in response to salt stress, while di-myo-inositol-1,1′(3,3′)-phosphate was the major intracellular solute at supraoptimal growth temperatures. The phase of growth had a strong influence on the types and levels of compatible solutes in T. litoralis; mannosylglycerate and aspartate were the major solutes during exponential growth, while di-myo-inositol-1,1′(3,3′)-phosphate was the predominant organic solute during the stationary phase of growth. This work revealed an unexpected ability of T. litoralis to scavenge suitable components from the medium and to use them as compatible solutes.
Most microorganisms subjected to water stress accumulate organic solutes to control their internal water activity, maintain the appropriate cell volume and turgor pressure, and protect intracellular macromolecules (14). The compatible solutes, also called osmolytes, include sugars, amino acids and their derivatives, polyols and their derivatives, betaines, and ectoines (11, 12, 14). The term compatible solute is generally used for low-molecular-weight organic compounds that accumulate to high intracellular levels under osmotic stress and that are compatible with the metabolism of the cell. Compatible solutes can be synthesized de novo or, if present in the medium, can be taken up by the organisms. The latter strategy is preferred when appropriate substances are present in the environment or in the growth medium (14). Glycine betaine, for example, is a very common compatible solute in mesophilic bacteria and archaea that is not synthesized by most microorganisms but is taken up from the medium and used for osmoadaptation (11, 14).
Recent investigations of water and temperature stress in thermophilic and hyperthermophilic microorganisms led to the identification of several new compatible solutes, some of which (mannosylglycerate [MG], di-glycerol-phosphate, and β-glutamate) accumulate primarily in response to salt stress. Others, such as di-myo-inositol-1,1′(3,3′)-phosphate (DIP) and cyclic-2,3-bisphosphoglycerate, accumulate primarily in response to growth at supraoptimal temperatures (9, 15, 20–22, 26, 31). A growing body of evidence also suggests that some solutes may stabilize macromolecules facing potential thermal denaturation; for example, DIP, cyclic-2,3-bisphosphoglycerate, and MG have been shown to stabilize enzymes (15, 27, 31). These results suggest that these solutes play an important role in protecting cellular components in vivo that may account, in part, for the thermophilic nature of the organisms. Other solutes, such as di-myo-inositol-1,3′-phosphate and 2-O-β-di-mannosyl-di-myo-inositol-1,1′(3,3′)-phosphate, whose levels increase in response to high temperatures in some hyperthermophilic organisms, may also have similar properties (21, 22). Therefore, in this paper, the term compatible solute is used for major intracellular organic solutes that accumulate in response to osmotic or temperature stress.
Recently, we examined several thermophiles and hyperthermophiles for the accumulation of organic solutes (20–22, 26); in particular, we observed that Pyrococcus furiosus accumulates only the following two compatible solutes: MG, whose concentration increases as the salinity of the medium increases; and DIP, whose concentration increases sharply at supraoptimum growth temperatures (20). Moreover, the same solutes were also observed in Pyrococcus woesei (31). Given the close phylogenetic relationship between the genera Pyrococcus and Thermococcus (30), we decided to compare the compatible solutes and strategies of thermoadaptation and osmoadaptation in species of these two genera. In this work, we examined the effects of salinity and growth temperature on the intracellular solute pools of four Thermococcus species. A new solute, β-galactopyranosyl-5-hydroxylysine (GalHL), was identified in Thermococcus litoralis. Furthermore, we found that the composition of the medium had a major effect on the type of organic solutes that accumulated during salt stress in this species and that the proportions of several solutes changed significantly with the growth stage.
MATERIALS AND METHODS
Strains and culture conditions.
T. litoralis DSM 5474T, Thermococcus celer DSM 2470T, Thermococcus stetteri DSM 5262T, and Thermococcus sp. strain AN1T (= DSM 2770T), recently classified as Thermococcus zilligii (30), were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany. Unless indicated otherwise, T. litoralis, T. celer, and T. stetteri were grown in Bacto Marine Broth (Difco). For growth of T. celer and T. stetteri this medium was supplemented with sodium sulfide (final concentration, 2 g · liter−1). T. zilligii was grown in the medium described by Lanzotti et al. (18) supplemented with S0.
Cultures of T. litoralis were routinely grown in a 2-liter fermentation vessel with continuous gassing with pure N2 and stirring at 100 rpm. T. stetteri was grown under the same conditions except that the gas mixture contained N2 and CO2 (80:20). T. zilligii and T. celer were grown in static 2-liter flasks that were flushed with N2 for 1 h before the medium was autoclaved. Cell growth was monitored by measuring turbidity at 600 nm. Most experimental cultures were grown until the late exponential phase, harvested by centrifugation (8000 × g, 30°C, 10 min), and washed once with a solution lacking organic nutrients but otherwise identical to the medium in which the cells were grown. The effect of salinity on the levels of intracellular solutes was examined by changing the NaCl concentration while the same concentrations of the other medium components were maintained. The effect of growth phase on the accumulation of solutes was examined in T. litoralis by growing the cultures in a 5-liter fermentation vessel with medium containing 4% NaCl. Samples (500 ml) were collected at appropriate culture times and treated as described above.
The effects of organic components on the accumulation of compatible solutes by T. litoralis were examined by replacing peptone (5.0 g · liter−1) with Casitone (5.0 g · liter−1) or tryptone (5.0 g · liter−1) or by replacing yeast extract (1.0 g · liter−1) with maltose (2.0 g · liter−1) in a medium containing 3% NaCl and having the same basal salts composition as Bacto Marine Broth.
Extraction of intracellular solutes, cell protein determination, partial purification and hydrolysis of β-galactosyl-hydroxylysine, and quantification of organic solutes.
Cells were extracted twice with boiling 80% ethanol by the method of Reed et al. (28), modified as previously described by Martins and Santos (20). Freeze-dried extracts were dissolved in D2O for nuclear magnetic resonance (NMR) analysis. Quantification was performed by 1H NMR by using formate as an internal concentration standard. Glutamate content was determined by the enzymatic assay of Lund (19), and hydroxyproline was determined by using a Pico-Tag amino acid analysis system (Waters, Milford, Mass.).
The protein content of cells was determined by the Bradford assay (6) after treatment of the cells with 1 M NaOH (100°C, 10 min) and neutralization with 1 M HCl.
The novel compound GalHL was purified by ion-exchange chromatography on an SP-Sepharose C-25 (Pharmacia, Uppsala, Sweden) column (19 by 2.6 cm). The extract was eluted with a sodium phosphate buffer (pH 7.0) gradient ranging from 5 mM to 1 M. Fractions were eluted and examined by NMR for the presence of the compound. Fractions containing the compound were pooled and freeze-dried. The residue was dissolved in a small volume of water, salt was removed by passage through a Sephadex G-25 column (type PD-10; Pharmacia), and the eluted sample was concentrated by freeze-drying. The residue was dissolved in D2O for NMR analysis. Part of the sample was hydrolyzed with 3.0 M HCl at 100°C for 3 h under an N2 atmosphere in a sealed ampoule to liberate the sugar moiety from the amino acid.
NMR spectroscopy.
All spectra were obtained with a Bruker model AMX500 spectrometer. 13C NMR spectra were obtained at 125.77 MHz by using a 5-mm carbon selective probe head. Typically, spectra were acquired with a repetition delay of 1.5 s and a pulse width of 7 μs corresponding to a 90° flip angle. Proton decoupling was applied during the acquisition time only by using the wide-band alternating-phase low-power technique for zero-residue splitting sequence. Chemical shifts were referenced to the resonance of external methanol at 49.3 ppm.
1H NMR spectra were acquired with water presaturation, a 6-μs pulse width corresponding to a 60° flip angle, and a repetition delay of 15 s. Chemical shifts were determined relative to 3-(trimethylsilyl)propanesulfonic acid (sodium salt). Formate was added as an internal concentration standard. Two-dimensional spectra were obtained by using standard Bruker pulse programs. Phase-sensitive nuclear Overhauser effect spectroscopy (NOESY), proton-homonuclear shift correlation spectroscopy (COSY), and total-correlation spectroscopy (TOCSY) were performed by collecting 4,096 (t2) × 512 (t1) data points; in 1H-13C heteronuclear multiple quantum coherence (HMQC) spectra (3), a delay of 3.5 ms was used for evolution of 1JCH. The heteronuclear multiple bond connectivity (HMBC) spectrum was obtained by collecting 4,096 (t2) × 256 (t1) data points; a delay of 73.5 ms was used for evolution of long-range couplings.
RESULTS
Identification of organic solutes in Thermococcus spp.
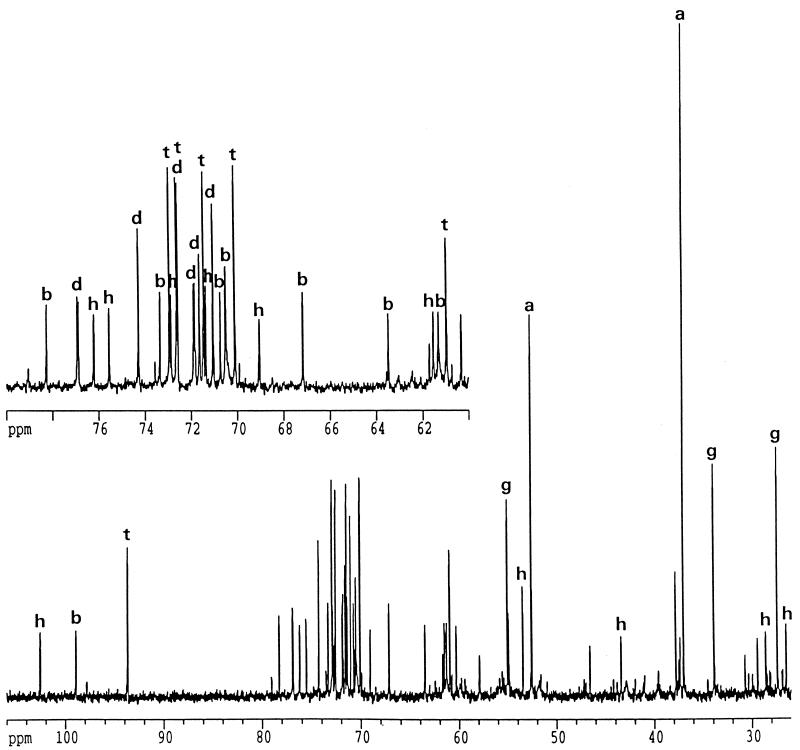
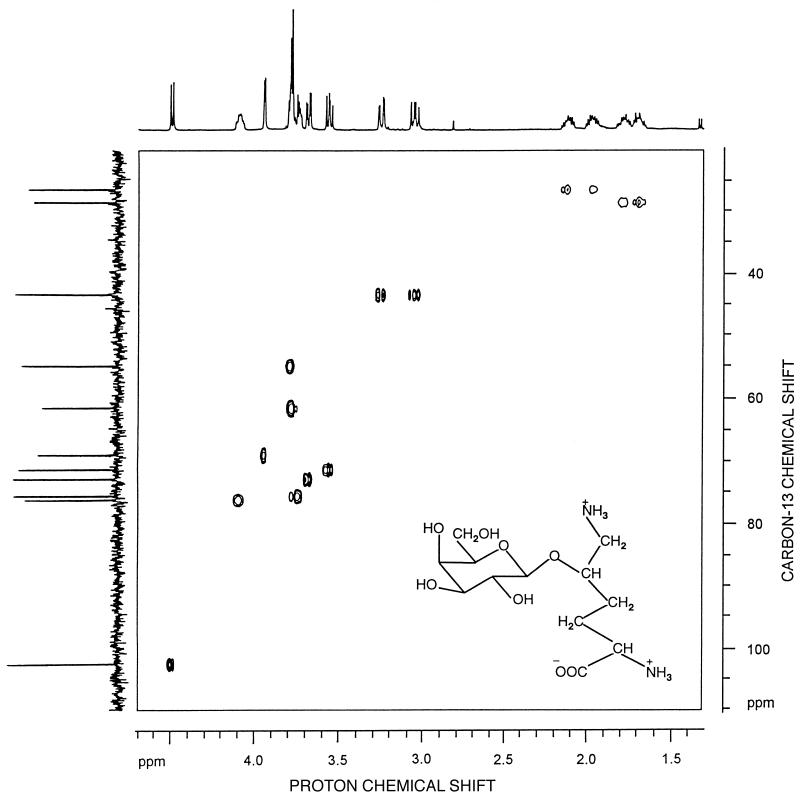
The 13C-NMR spectra of ethanol extracts of the thermococci grown in Bacto Marine Broth contained several sets of resonances that were assigned to DIP, MG, trehalose, α-glutamate, hydroxyproline, and aspartate (Fig. 1). Resonances were assigned by comparison with the carbon chemical shift values reported previously (7, 26, 31). The identities of the compounds were confirmed by 1H NMR, and assignment of the resonances due to DIP was further confirmed by spiking a T. litoralis extract with an aliquot of a P. woesei extract, in which DIP was initially identified (31). Initially, the remaining resonances in the 13C- NMR spectrum of T. litoralis (176.0, 102.5, 75.5, 75.5, 72.8, 71.2, 68.9, 61.4, 55.0, 43.4, 28.4, and 27.1 ppm) could not be assigned to a known compound, but identification was significantly facilitated by partial purification of the extract. The resonances at 176.0 and 102.5 ppm were assigned to a carboxylic group and an anomeric CH group, respectively. The HMQC spectrum showed that the latter resonance was connected to a proton resonance at 4.49 ppm (Fig. 2). The resonances at 27.1, 28.4, 43.4, and 61.4 ppm were due to methylene groups, while the remaining resonances were due to methyne groups. The final structure of the molecule was established by using the results of a set of COSY, NOESY, TOCSY, and HMBC experiments. The COSY and TOCSY spectra showed that the anomeric carbon at 102.5 ppm belonged to a network of a six-carbon monosaccharide (resonances at 102.5, 75.5, 72.8, 71.2, 68.9, and 61.4 ppm). The HMBC experiments showed that there was a connection between the anomeric carbon of the hexose and the methyne group of the nonsugar moiety at 76.5 ppm. Further analysis of the COSY and TOCSY spectra led to identification of this moiety as 5-hydroxylysine. The hexose and the amino acid fragments were firmly identified as galactose and 5-hydroxylysine after hydrolysis of the intact compound and spiking of the hydrolysate with pure galactose and 5-hydroxylysine. The carbon chemical shift values of the original compound indicated that the galactose moiety was in the pyranose configuration rather than the furanose configuration (5). The presence of connectivities between H1 and H2 and between H3 and H5 but not between H1 and H4 in the NOESY spectra led to the conclusion that galactose was in the β-pyranosyl configuration. This conclusion was supported by the low chemical shift value for galactose H1 (4.49 ppm) and the magnitude of the coupling constants (3JH1,H2 =7.7 Hz, 1JC1,H1 =160 Hz), which unambiguously indicated that the configuration was a β-pyranosyl configuration (2, 4, 23). There was also a clear connectivity between H1 of galactose and H5 of 5-hydroxylysine in the NOESY spectrum, which showed that the location of the linkage was between C-1 of galactose and the hydroxyl group of the amino acid. The position of this linkage was also confirmed by the HMBC spectrum. On the basis of these results, the unknown compound was identified as GalHL (Fig. 2, inset). The corresponding carbon and proton chemical shift values are shown in Table 1.
FIG. 1.
Proton decoupled 13C-NMR spectrum of an ethanol extract of T. litoralis grown in Bacto Marine Broth containing 4% NaCl at 85°C. Resonances due to aspartate (peaks a), MG (peaks b), DIP (peaks d), glutamate (peaks g), GalHL (peaks h), and trehalose (peaks t) are indicated.
FIG. 2.
13C-1H correlation spectrum through one bond coupling (heteronuclear multiple quantum correlation) of the novel compound GalHL. (Inset) Schematic representation of GalHL.
TABLE 1.
NMR parameters of the novel compound GalHL
| Galactosyl moiety |
13C
|
1H
|
Hydroxylysine moiety |
13C
|
1H
|
|||
|---|---|---|---|---|---|---|---|---|
| δ (ppm) | 1JCH (Hz) | δ (ppm) | 3JHH (Hz) | δ (ppm) | 1JCH (Hz) | δ (ppm) | ||
| C1 | 102.5 | 160 | 4.49 | 7.7 | C1 | 176.0 | ||
| C2 | 71.2 | 148 | 3.55 | 9.8 | C2 | 55.0 | 146 | 3.60 |
| C3 | 72.8 | 142 | 3.65 | 3.3 | C3 | 27.1 | 126 | 2.00, 1.90 |
| C4 | 68.9 | 145 | 3.95 | NDa | C4 | 28.4 | 122 | 1.75, 1.60 |
| C5 | 75.5 | 139 | 3.75 | ND | C5 | 76.5 | 150 | 4.05 |
| C6 | 61.4 | 144 | 3.80 | ND | C6 | 43.4 | 144 | 3.00, 3.20 |
ND, not determined.
Effects of temperature and salt stress on the accumulation of compatible solutes by Thermococcus spp.
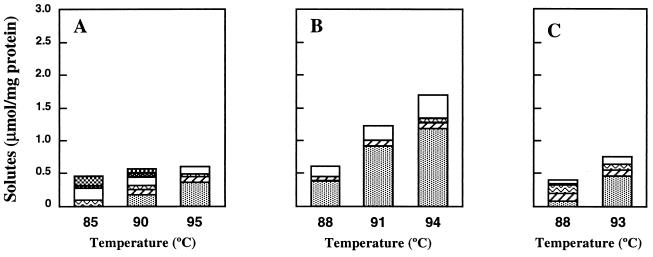
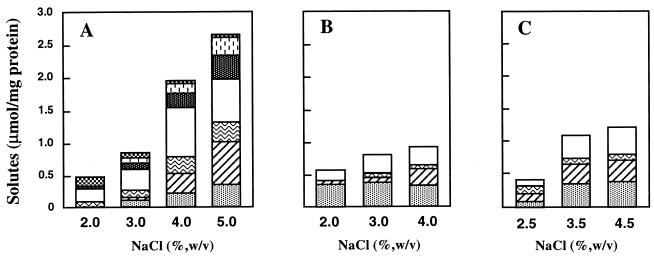
The optimum growth temperatures of T. litoralis, T. celer, T. stetteri, and T. zilligii are 85, 88, 88, and 75°C, respectively. All of these organisms except T. zilligii, which is not halophilic, have optimum salinities for growth of about 2.0 to 2.5% NaCl (17, 24, 25, 37). Under optimal growth conditions in Bacto Marine Broth, the total solute pools of these organisms were small; the highest levels of organic solutes were found in T. celer, in which they did not exceed 0.6 μmol · mg of protein−1 (Table 2). As the growth temperature or the salinity of the medium was increased above the optimal level for growth, the total solute pool also increased due to differential accumulation of some solutes (Table 2, and Fig. 3 and 4). There was, for example, a positive correlation in T. litoralis between growth in medium containing higher concentrations of NaCl and pronounced increases in the levels of aspartate, MG, and (to a lesser extent) GalHL. On the other hand, the accumulation of compatible solutes was less pronounced when the temperature was raised above the optimum temperature for growth. However, DIP became the major solute during growth at supraoptimum temperatures.
TABLE 2.
Quantification of organic solutes as determined by 1H NMR in species of the genus Thermococcusa
| Species | Conditions
|
Solute concn
(μmol/mg of protein)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) | NaCl concn (%) | DIP | MG | Glutamateb | Aspartate | Trehalose | GalHL | Hydroxyprolinec | |
| T. litoralis | 85 | 2.0 | NDd | ND | 0.088 (19)e | 0.21 (47) | 0.02 (4) | ND | 0.148 (33) |
| 85 | 3.0 | 0.11 (24) | 0.04 (9) | 0.105 (24) | 0.33 (73) | 0.10 (22) | 0.07 (16) | 0.093 (21) | |
| 85 | 4.0 | 0.20 (44) | 0.31 (69) | 0.260 (58) | 0.75 (167) | 0.23 (51) | 0.14 (31) | 0.052 (12) | |
| 85 | 5.0 | 0.34 (76) | 0.66 (147) | 0.307 (68) | 0.65 (144) | 0.37 (82) | 0.28 (62) | 0.050 (11) | |
| 90 | 2.0 | 0.18 (40) | 0.07 (16) | 0.070 (15) | 0.13 (29) | 0.06 (13) | ND | 0.058 (13) | |
| 95 | 2.0 | 0.37 (82) | 0.09 (20) | 0.030 (7) | 0.11 (24) | ND | ND | ND | |
| T. celer | 88 | 2.0 | 0.38 (84) | 0.02 (4) | 0.006 (1) | 0.15 (33) | ND | ND | ND |
| 88 | 3.0 | 0.35 (78) | 0.08 (18) | 0.007 (2) | 0.27 (60) | ND | ND | ND | |
| 88 | 4.0 | 0.31 (69) | 0.25 (56) | 0.017 (4) | 0.27 (60) | ND | ND | ND | |
| 91 | 2.0 | 0.95 (211) | 0.09 (20) | 0.004 (1) | 0.22 (49) | ND | ND | ND | |
| 94 | 2.0 | 1.20 (267) | 0.11 (24) | 0.009 (2) | 0.37 (82) | ND | ND | ND | |
| T. stetteri | 88 | 2.5 | 0.09 (20) | 0.12 (27) | 0.136 (30) | 0.07 (16) | ND | ND | ND |
| 88 | 3.5 | 0.35 (78) | 0.31 (69) | 0.078 (17) | 0.36 (80) | ND | ND | ND | |
| 88 | 4.5 | 0.39 (87) | 0.34 (76) | 0.080 (18) | 0.41 (91) | ND | ND | ND | |
| 93 | 2.5 | 0.45 (100) | 0.09 (20) | 0.090 (20) | 0.10 (22) | ND | ND | ND | |
| T. zilligii | 75 | 0.2 | ND | ND | 0.004 (1) | 0.02 (4) | ND | ND | ND |
| 80 | 0.2 | ND | ND | 0.006 (1) | 0.03 (7) | ND | ND | ND | |
| 84 | 0.2 | ND | ND | 0.013 (4) | 0.05 (11) | ND | ND | ND | |
The majority of the values are the means from two to four replicate determinations, but in some cases determinations were performed only once. The values obtained in replicate determinations never varied more than 15%.
Glutamate concentrations were determined by an enzymatic assay.
Hydroxyproline concentrations were determined by a Pico-Tag amino acid analysis.
ND, not detected by 1H NMR.
The values in parentheses are crude estimates of the intracellular concentrations of solutes (millimolar) based on a cell volume of 4.5 μl · mg of protein−1 for P. furiosus (20).
FIG. 3.
Effect of the growth temperature, at the optimum salinity, on the accumulation of the following compatible solutes by T. litoralis (A), T. celer (B), and T. stetteri (C): DIP (░⃞), MG (▨), glutamate ( ), aspartate (□), trehalose(▩), GalHL ( ), and hydroxyproline ( ).
FIG. 4.
Effect of the NaCl concentration of the medium, at the optimum growth temperature, on the accumulation of the following compatible solutes by T. litoralis (A), T. celer (B), and T. stetteri (C): DIP (░⃞), MG (▨), glutamate ( ), aspartate (□), trehalose (▩), GalHL ( ), and hydroxyproline ( ).
Trehalose, GalHL, and hydroxyproline were not detected in T. celer or T. stetteri, which at elevated salinities accumulated significant levels of MG and aspartate. The levels of MG also increased at supraoptimum temperatures, but the most pronounced alterations of the organic solute pools were due to increases in the DIP concentrations from about 0.4 to 1.2 μmol · mg of protein−1 in T. celer and from 0.1 to 0.4 μmol · mg of protein−1 in T. stetteri.
T. zilligii was the only nonhalophilic and nonhalotolerant species examined in this study, and, unlike the other species of the genus Thermococcus, T. zilligii had very low intracellular solute concentrations (Table 2). No solutes other than aspartate and glutamate were detected in this organism, and no clear correlation between the levels of these solutes and a high growth temperature was evident.
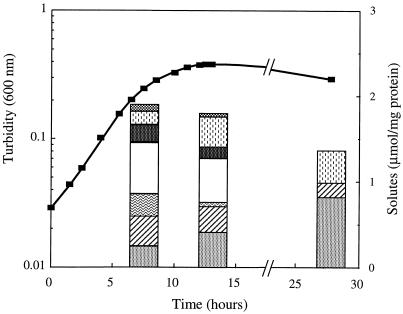
Effect of the growth phase on the accumulation of solutes in T. litoralis.
Alterations in the levels of the organic solutes during the growth cycle of T. litoralis were determined in Bacto Marine Broth containing 4% NaCl at 85°C (Fig. 5). During the exponential phase of growth, aspartate was the most abundant solute, but trehalose, MG, DIP, glutamate, and GalHL were also detected in moderate amounts, while hydroxyproline was present at very low levels. At the onset of the stationary phase, increases in the concentrations of DIP and GalHL were observed concomitant with slight decreases in the concentrations of all other solutes except glutamate, whose level decreased notably. The total solute pool decreased significantly after prolonged incubation of the culture; only DIP, MG, and GalHL were detected, and DIP was the predominant organic solute during this stage of growth.
FIG. 5.
Correlation between growth phase and accumulation of organic solutes by T. litoralis. The culture was grown in medium containing 4.0% NaCl at 85°C (■). The intracellular concentrations of the following solutes were determined at different phases of growth: DIP (░⃞), MG (▨), glutamate ( ), aspartate (□), trehalose (▩), GalHL ( ), and hydroxyproline ( ).
Dramatic alterations in the relative amounts of the compatible solutes with the growth stage were observed in cells grown at 85°C in Bacto Marine Broth containing 3% NaCl when peptone was replaced by tryptone. In particular, the levels of MG changed drastically; the concentrations of MG ranged from 0.37 μmol · mg of protein−1 when cells were harvested during the mid-exponential phase to vestigial during the early stationary phase. On the other hand, the levels of DIP remained almost constant. These results show that it is critical to control the cell growth stage if meaningful compatible solute accumulation data are going to be obtained.
Effect of the growth medium on the accumulation of organic solutes in T. litoralis.
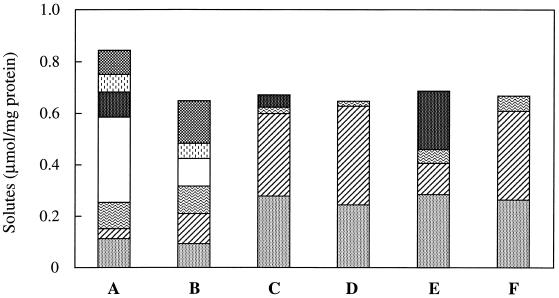
Due to the uniqueness of GalLH and the rarity of hydroxyproline in prokaryotes, we though that it was important to ascertain whether these solutes were synthesized de novo or taken up from medium containing peptone or yeast extract. For this reason, T. litoralis was grown in media that were identical to Bacto Marine Broth except that they contained Casitone or tryptone instead of peptone and in media in which maltose replaced yeast extract. T. litoralis accumulated DIP, MG, trehalose, and glutamate in medium containing yeast extract plus Casitone or tryptone, but, unlike growth in medium containing peptone, aspartate, GalHL, and hydroxyproline were not detected (Fig. 6). In these media there were also pronounced increases in the levels of DIP and MG compared to the levels accumulated by the organism in peptone-containing medium. Substitution of yeast extract for maltose in medium containing peptone, Casitone, or tryptone led to an absence of trehalose.
FIG. 6.
Effect of medium composition on the accumulation of organic solutes by T. litoralis grown at 85°C with 3.0% NaCl. The media contained peptone plus yeast extract (bar A), peptone plus maltose (bar B), Casitone plus yeast extract (bar C), Casitone plus maltose (bar D), tryptone plus yeast extract (bar E), and tryptone plus maltose (bar F). The intracellular concentrations of the following solutes were determined: DIP (░⃞), MG (▨), glutamate ( ), aspartate (□), trehalose (▩), GalHL ( ), and hydroxyproline ( ).
DISCUSSION
The results presented in this paper show that T. celer, T. stetteri, and T. litoralis accumulate different solutes in response to two different types of stress. The levels of MG, aspartate, and GalHL increased in response to salt stress, while the levels of DIP responded primarily to temperature stress. In T. litoralis, however, the effect of the salinity of the medium on the accumulation of compatible solutes was much more pronounced than the effect of the growth temperature. The reverse was true in T. celer; in this organism the growth temperature had a clear effect on the accumulation of compatible solutes, primarily the levels of DIP, while there were only slight increases in the concentrations of solutes as the salinity of the medium was increased (Fig. 3 and 4). In P. furiosus, on the other hand, both temperature and salinity had very pronounced effects on the accumulation of compatible solutes (20). Accumulation of MG and DIP in the species of the genus Thermococcus was expected because these organisms are closely related to Pyrococcus species, in which these solutes are also encountered (20). However, we did not expect to find that aspartate was a major compatible solute in the halophilic Thermococcus species grown in peptone-containing medium, while glutamate played a lesser role in the osmoadaptation of these organisms. Glutamate has been shown to be a common compatible solute during low-level osmoadaptation in many bacteria and archaea (11, 12, 14), while aspartate is generally found at very low intracellular concentrations and contributes only slightly to the compatible solute pool (34). Aspartate accumulates to higher levels in Methanococcus thermolithotrophicus and Methanosarcina thermophila, but there is only a slight positive correlation between the level of aspartate and salinity and this amino acid is not thought to behave like a compatible solute (29, 33). However, under salt stress conditions aspartate is an important compatible solute of halophilic thermococci grown in medium containing peptone, in which it reaches levels comparable to those of MG.
The newly identified solute GalHL was detected only in peptone-derived T. litoralis cells, in which it accumulated in response to increasing levels of salinity. Aside from choline and acetylcholine, which are taken up from the medium by Lactobacillus plantarum (16), no other known compatible solute has a net positive charge (12, 14). However, two neutral lysine derivatives are known to serve as compatible solutes; Nɛ-acetyl-β-lysine accumulates in several methanogens, such as Methanosarcina thermophila and Methanogenium cariaci (33), and Nɛ-acetyl-lysine behaves like an osmolyte in a few moderately halophilic bacteria (13). It is also important to note the resemblance between GalHL and the α-glucosyl-β-galactosyl-5-hydroxylysine moiety found in mammalian collagen. In fact, GalHL is probably derived from collagen, since this protein is present in peptone (13a); it may be formed from the diglycosyl-hydroxylysine component after removal of the terminal glucose residue by the microorganisms or during the production of peptone. It should also be noted that hydroxyproline is a major amino acid in collagen and that low levels of this amino acid also accumulate in T. litoralis. Accumulation of hydroxyproline was detected previously in Listeria monocytogenes under water stress conditions in peptone-containing media or when hydroxyproline or prolyl-hydroxyproline was added to the medium (1). The observation that GalHL and hydroxyproline were detected only in T. litoralis cells grown in peptone-containing media leads us to believe that these solutes are derived from peptone. It is, however, difficult to explain why aspartate was not accumulated from Casitone or tryptone by this organism, since this amino acid is present in these casein-based preparations. It is also not known why GalHL and hydroxyproline were not detected in the other Thermococcus species or in the closely related species P. furiosus grown in media that also contain peptone (22).
The intracellular levels of compatible solutes in the thermococci never balanced the levels of osmotically active substances in the medium. This indicates that inorganic ions (namely, K+ and Cl−) may significantly contribute to the osmolyte pools of these organisms. Large contributions by inorganic ions to the osmolyte pools have been found in some methanogens and have been inferred for other hyperthermophilic archaea (9, 20, 21), and such contributions may also occur in the thermococci. However, it should be pointed out that the values obtained were calculated by assuming that the cell volume was independent of the salinity of the medium, which is unlikely.
Trehalose is a common disaccharide in bacteria and archaea and may play a role in osmotic adaptation in several organisms (11, 14). Yeast extract, in which the trehalose level is about 11% (wt/wt) (36), is a common source of this disaccharide for many organisms. Our results show that trehalose accumulated in T. litoralis only when yeast extract was added to the medium; therefore, we concluded that this disaccharide was not synthesized de novo under the conditions examined in this study. Rather, trehalose was taken up from yeast extract by the recently described high-affinity maltose-trehalose transport system, which was shown to be induced by trehalose (36). Our results reinforce the importance of solute uptake by organisms under water stress conditions and extend previous results showing that unusual compatible solutes, such as carnitine, dimethylsulfoniopropionate, 3-morpholino-1-propanesulfonic acid (MOPS), and arsenobetaine (10, 14, 16, 32) are taken up.
This work revealed the an unexpected ability of T. litoralis to scavenge suitable components from the medium and to use them as compatible solutes, thus bypassing de novo synthesis and saving energy. It is noteworthy that neither the other Thermococcus species examined nor the closely related Pyrococcus species were able to derive such a variety of solutes from the medium.
The differential accumulation of osmolytes observed during the growth cycle of T. litoralis corroborates previous results obtained with hyperthermophilic archaea, mesophilic bacteria, and yeasts (8, 20, 32, 35). For example, it is often observed that the total solute pool decreases during the stationary phase, although no explanation for this observation has been given. Perhaps some compatible solutes replace others that accumulate during the exponential phase because they are not as easily lost to the external environment. Some compatible solutes that accumulate during the exponential phase of growth may also be preferentially consumed when the concentrations of nutrients in the medium decrease below critical levels.
Unlike the other organisms examined here, T. zilligii AN1T was isolated from a low-salinity continental hot spring and does not tolerate the levels of NaCl required by the other species of the genus Thermococcus for growth (17, 30). This organism does not accumulate appreciable levels of compatible solutes at the optimum growth temperature, nor does it accumulate compatible solutes as the growth temperature is raised. Other nonhalophilic thermophilic and hyperthermophilic organisms, such as Thermotoga thermarum, Pyrobaculum islandicum, and Fervidobacterium islandicum, unlike slightly halophilic members of the same genera, do not accumulate compatible solutes at the optimum growth temperature (21, 22). The observation that only halophilic strains accumulate compatible solutes at supraoptimum growth temperatures suggests that the role played by these solutes may be something other than a role in thermoadaptation. However, it is possible that solutes such as DIP, cyclic-2,3-bisphosphoglycerate, and (to some extent) MG may play a role as thermoprotectants primarily when two stresses (namely, water stress and temperature stress) are applied simultaneously. In fact, it is difficult to explain the consistent accumulation of specific solutes by most hyperthermophilic organisms at supraoptimal temperatures unless a role in thermoprotection is envisioned. Intrinsic properties of cell components are very important for growth at high temperatures, but with the present state of knowledge we cannot discount the contribution of extrinsic factors, such as the thermostabilizing attributes of some compatible solutes, to the growth of microorganisms at high temperatures.
ACKNOWLEDGMENTS
This work was supported by grant BIO4-CT96-0488 from the European Community Biotech Programme (Extremophiles as Cell Factories) and by Praxis XXI and FEDER Programme grants PRAXIS 2/2.1/BIO/20/94 (to H.S. and M.S.D.) and PRAXIS/2/2.1/BIO/1109/95 (to H.S.).
REFERENCES
- 1.Amezaga M-R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenesATCC 23074 at high osmolarity. Microbiology. 1995;141:41–49. doi: 10.1099/00221287-141-1-41. [DOI] [PubMed] [Google Scholar]
- 2.Baumann H, Tzianabos A O, Brisson J-R, Kasper D L, Jennings H J. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilisusing high-resolution NMR spectroscopy. Biochemistry. 1992;31:4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- 3.Bax A, Summers M F. 1H and 13C assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J Am Chem Soc. 1986;108:2093–2094. [Google Scholar]
- 4.Bock K, Pedersen C. A study of 13CH coupling constants in hexopyranoses. J Chem Soc Perkin Trans II. 1974;1974:293–297. [Google Scholar]
- 5.Bock K, Pedersen C. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv Carbohydr Chem Biochem. 1983;41:27–66. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Breitmaier E, Voelter W. Carbon-13 NMR spectroscopy. New York, N.Y: VCH Verlagsgesellschaft mbH; 1990. [Google Scholar]
- 8.Brown A D. Compatible solutes and extreme water stress in eukaryotic micro-organisms. Adv Microb Physiol. 1978;17:181–242. doi: 10.1016/s0065-2911(08)60058-2. [DOI] [PubMed] [Google Scholar]
- 9.Ciulla R A, Burggraf S, Stetter K O, Roberts M F. Occurrence and role of di-myo-inositol-1,1′-phosphate in Methanococcus igneus. Appl Environ Microbiol. 1994;60:3660–3664. doi: 10.1128/aem.60.10.3660-3664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciulla R A, Diaz M R, Taylor B F, Roberts M F. Organic osmolytes in aerobic bacteria from Mono Lake, an alkaline, moderately hypersaline environment. Appl Environ Microbiol. 1997;63:220–226. doi: 10.1128/aem.63.1.220-226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Costa M S, Santos H, Galinski E A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv Biochem Eng Biotechnol. 1998;61:117–153. doi: 10.1007/BFb0102291. [DOI] [PubMed] [Google Scholar]
- 13.del Moral A, Severin J, Ramos-Cormenzana A, Trüper H G, Galinski E A. Compatible solutes in new moderately halophilic isolates. FEMS Microbiol Lett. 1994;122:165–172. [Google Scholar]
- 13a.Difco Laboratories. Personal communication.
- 14.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 15.Hensel R, König H. Thermoadaptation of methanogenic bacteria by intracellular ion concentration. FEMS Microbiol Lett. 1988;49:75–79. [Google Scholar]
- 16.Kets E P W, Galinski E A, de Bont J A M. Carnitine: a novel compatible solute in Lactobacillus plantarum. Arch Microbiol. 1994;162:243–248. [Google Scholar]
- 17.Klages K U, Morgan H W. Characterisation of an extremely thermophilic sulphur-metabolizing archaebacterium belonging to the Thermococcales. Arch Microbiol. 1994;162:261–266. [Google Scholar]
- 18.Lanzotti V, Trincone A, Nicolaus B, Zillig W, de Rosa M, Gambacorta A. Complex lipids of Pyrococcus and AN1, thermophilic members of archaebacteria belonging to Thermococcales. Biochim Biophys Acta. 1989;1004:44–48. [Google Scholar]
- 19.Lund P. UV method with glutaminase and glutamate dehydrogenase. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. VIII. Weinheim, Germany: VCH; 1987. pp. 357–363. [Google Scholar]
- 20.Martins L O, Santos H. Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus furiosusin response to salinity and temperature. Appl Environ Microbiol. 1995;61:3299–3303. doi: 10.1128/aem.61.9.3299-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins L O, Carreto L S, da Costa M S, Santos H. Novel compatible solutes related to di-myo-inositol-phosphate in the order Thermotogales. J Bacteriol. 1996;178:5644–5651. doi: 10.1128/jb.178.19.5644-5651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins L O, Huber R, Huber H, Stetter K O, da Costa M S, Santos H. Organic solutes in hyperthermophilic archaea. Appl Environ Microbiol. 1997;63:896–902. doi: 10.1128/aem.63.3.896-902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehlert A, Richardson J M, Ferguson M A J. Structure of the glycosylphosphatidylinositol membrane anchor glycan of a class-2 variant surface glycoprotein from Trypanossoma brucei. J Mol Biol. 1998;277:379–392. doi: 10.1006/jmbi.1997.1600. [DOI] [PubMed] [Google Scholar]
- 24.Miroshnichenko M L, Bonch-Osmolovskaya E A, Neuner A, Kostrikina N A, Chernych N A, Alekseev V A. Thermococcus stetterisp. nov., a new extremely thermophilic marine sulphur-metabolizing archaebacterium. Syst Appl Microbiol. 1989;12:257–262. [Google Scholar]
- 25.Neuner A, Jannasch H W, Belkin S, Stetter K O. Thermococcus litoralissp. nov.: a new species of extremely thermophilic marine archaebacteria. Arch Microbiol. 1990;153:205–207. [Google Scholar]
- 26.Nunes O C, Manaia C M, da Costa M S, Santos H. Compatible solutes in the thermophilic bacteria Rhodothermus marinus and “Thermus thermophilus.”. Appl Environ Microbiol. 1995;61:2351–2357. doi: 10.1128/aem.61.6.2351-2357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos A, Raven N D H, Sharp R J, Bartolucci S, Rossi M, Cannio R, Lebbink J, van der Oost J, de Vos W M, Santos H. Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microbiol. 1997;63:4020–4025. doi: 10.1128/aem.63.10.4020-4025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed R H, Richardson D L, Warr S R C, Stewart W D P. Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol. 1984;130:1–4. [Google Scholar]
- 29.Robertson D E, Roberts M F, Belay N, Stetter K O, Boone D R. Occurrence of β-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl Environ Microbiol. 1990;56:1504–1508. doi: 10.1128/aem.56.5.1504-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronimus R S, Reysenbach A L, Musgrave D R, Morgan H W. The phylogenetic position of the Thermococcus isolate AN1 based on 16S rRNA gene sequence analysis: a proposal that AN1 represents a new species, Thermococcus zilligiisp. nov. Arch Microbiol. 1997;168:245–248. doi: 10.1007/s002030050495. [DOI] [PubMed] [Google Scholar]
- 31.Scholz S, Sonnenbichler J, Schäfer W, Hensel R. Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett. 1992;306:239–242. doi: 10.1016/0014-5793(92)81008-a. [DOI] [PubMed] [Google Scholar]
- 32.Smith L T. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenesgrown in liquid media and on processed meat surfaces. Appl Environ Microbiol. 1996;62:3088–3093. doi: 10.1128/aem.62.9.3088-3093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowers K R, Robertson D E, Noll D, Gunsalus R P, Roberts M F. Nɛ-acetyl-β-lysine: an osmolyte synthesized by methanogenic archaebacteria. Proc Natl Acad Sci USA. 1990;87:9083–9087. doi: 10.1073/pnas.87.23.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tempest D W, Meers J L, Brown C M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970;64:171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- 35.Whatmore A M, Chudek J A, Reed R H. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J Gen Microbiol. 1990;136:2527–2535. doi: 10.1099/00221287-136-12-2527. [DOI] [PubMed] [Google Scholar]
- 36.Xavier K B, Martins L O, Peist R, Kossmann M, Boos W, Santos H. High-affinity maltose/trehalose transport system in the hyperthermophilic archaeon Thermococcus litoralis. J Bacteriol. 1996;178:4773–4777. doi: 10.1128/jb.178.16.4773-4777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zillig W, Holtz I, Janekovic D, Schäfer W, Reiter W D. The archaebacterium Thermococcus celerrepresents a novel genus within the thermophilic branch of the archaebacteria. Syst Appl Microbiol. 1983;4:88–94. doi: 10.1016/S0723-2020(83)80036-8. [DOI] [PubMed] [Google Scholar]