Abstract
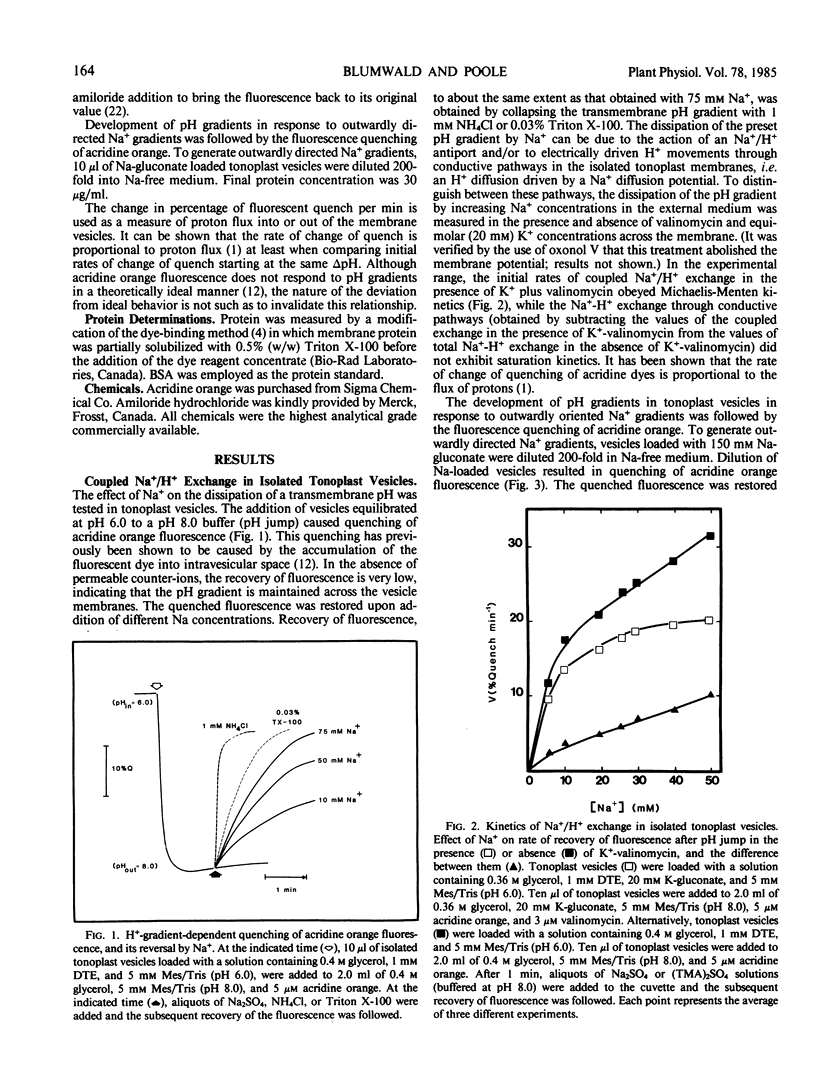
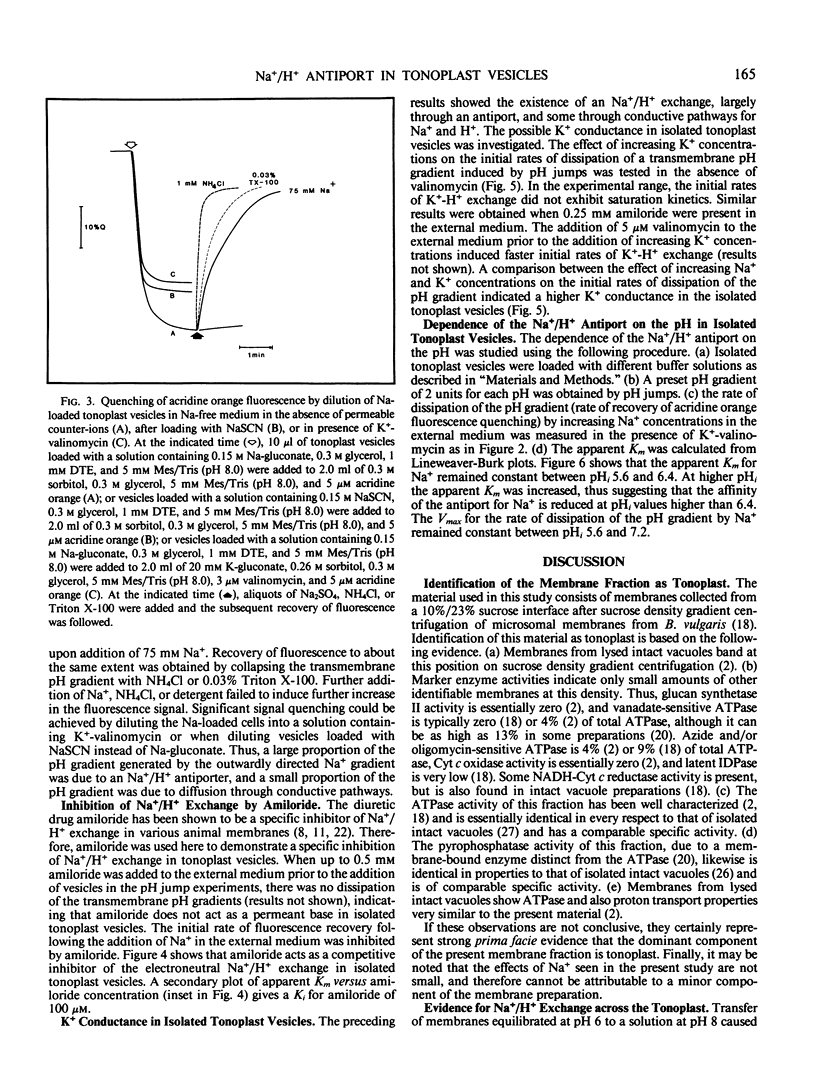
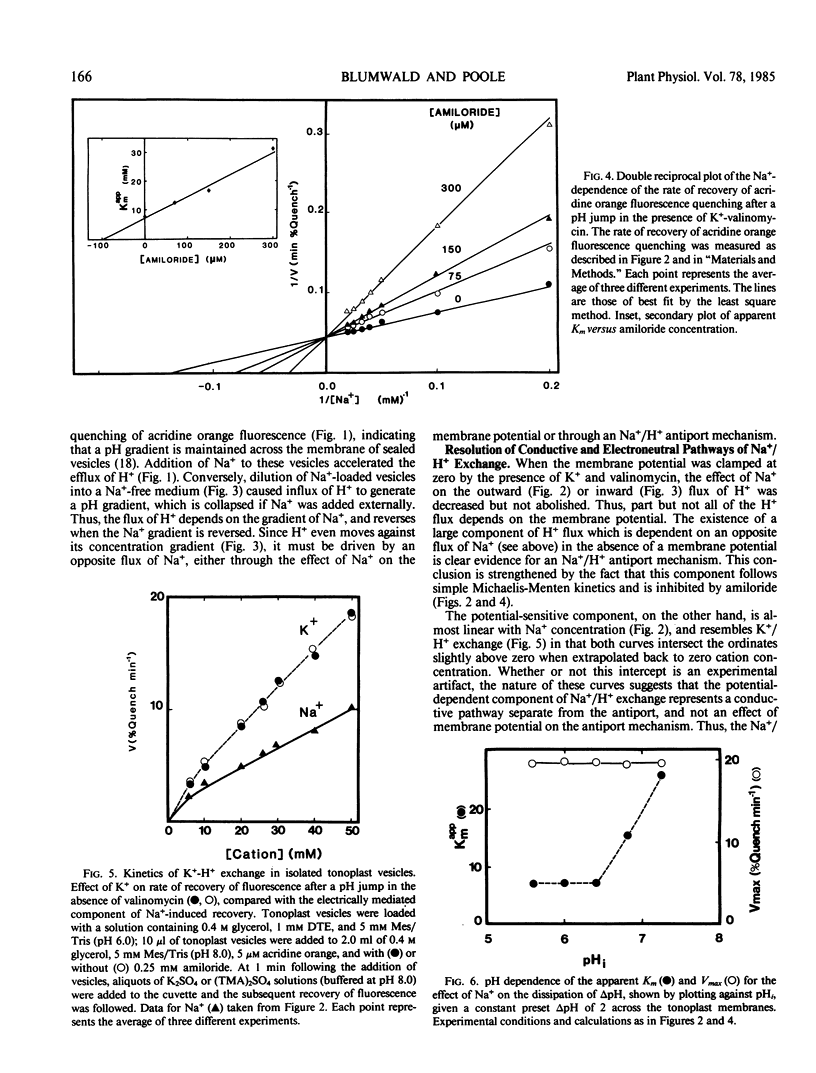
The pH-dependent fluorescence quenching of acridine orange was used to study the Na+- and K+-dependent H+ fluxes in tonoplast vesicles isolated from storage tissue of red beet and sugar beet (Beta vulgaris L.). The Na+-dependent H+ flux across the tonoplast membrane could be resolved into two components: (a) a membrane potential-mediated flux through conductive pathways; and (b) an electroneutral flux which showed Michaelis-Menten kinetics relationship to Na+ concentration and was competitively inhibited by amiloride (Ki = 0.1 millimolar). The potential-dependent component of H+ flux showed an approximately linear dependence on Na+ concentration. In contrast, the K+-dependent H+ flux apparently consisted of a single component which showed an approximately linear dependence on K+ concentration, and was insensitive to amiloride. Based on the Na+- and K+-dependent H+ fluxes, the passive permeability of the vesicle preparation to Na+ was about half of that to K+.
The apparent Km for Na+ of the electroneutral Na+/H+ exchange varied by more than 3-fold (7.5-26.5 millimolar) when the internal and external pH values were changed in parallel. The results suggest a simple kinetic model for the operation of the Na+/H+ antiport which can account for the estimated in vivo accumulation ratio for Na+ into the vacuole.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Wolosin J. M., Packer L. Na+/H+ exchange in the cyanobacterium Synechococcus 6311. Biochem Biophys Res Commun. 1984 Jul 18;122(1):452–459. doi: 10.1016/0006-291x(84)90497-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky W. P., Jr, Frizzell R. A. A novel effect of amiloride on H+-dependent Na+ transport. Am J Physiol. 1983 Jul;245(1):C157–C159. doi: 10.1152/ajpcell.1983.245.1.C157. [DOI] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. The role of the Na+/H+ exchange system in cardiac cells in relation to the control of the internal Na+ concentration. A molecular basis for the antagonistic effect of ouabain and amiloride on the heart. J Biol Chem. 1984 Jul 25;259(14):8880–8885. [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Forte J. G. A study of H+ transport in gastric microsomal vesicles using fluorescent probes. Biochim Biophys Acta. 1978 Apr 4;508(2):339–356. doi: 10.1016/0005-2736(78)90336-x. [DOI] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J. Development and Characteristics of Sodium-selective Transport in Red Beet. Plant Physiol. 1971 Jun;47(6):735–739. doi: 10.1104/pp.47.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Apparent inhibition of Na+/H+ exchange by amiloride and harmaline in acridine orange studies. Biochim Biophys Acta. 1983 Jun 10;731(2):354–360. doi: 10.1016/0005-2736(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Effect of the preparation method on Na+-H+ exchange and ion permeabilities in rat renal brush-border membranes. Biochim Biophys Acta. 1984 May 16;772(2):140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]


