Abstract
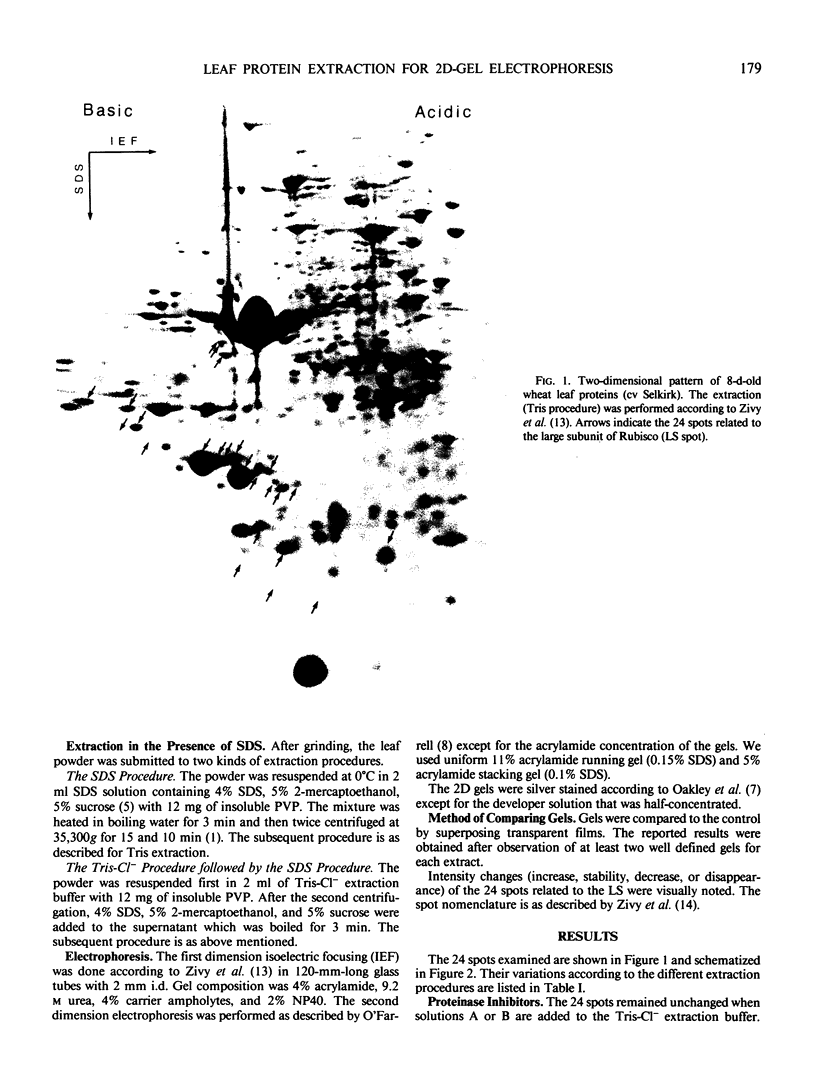
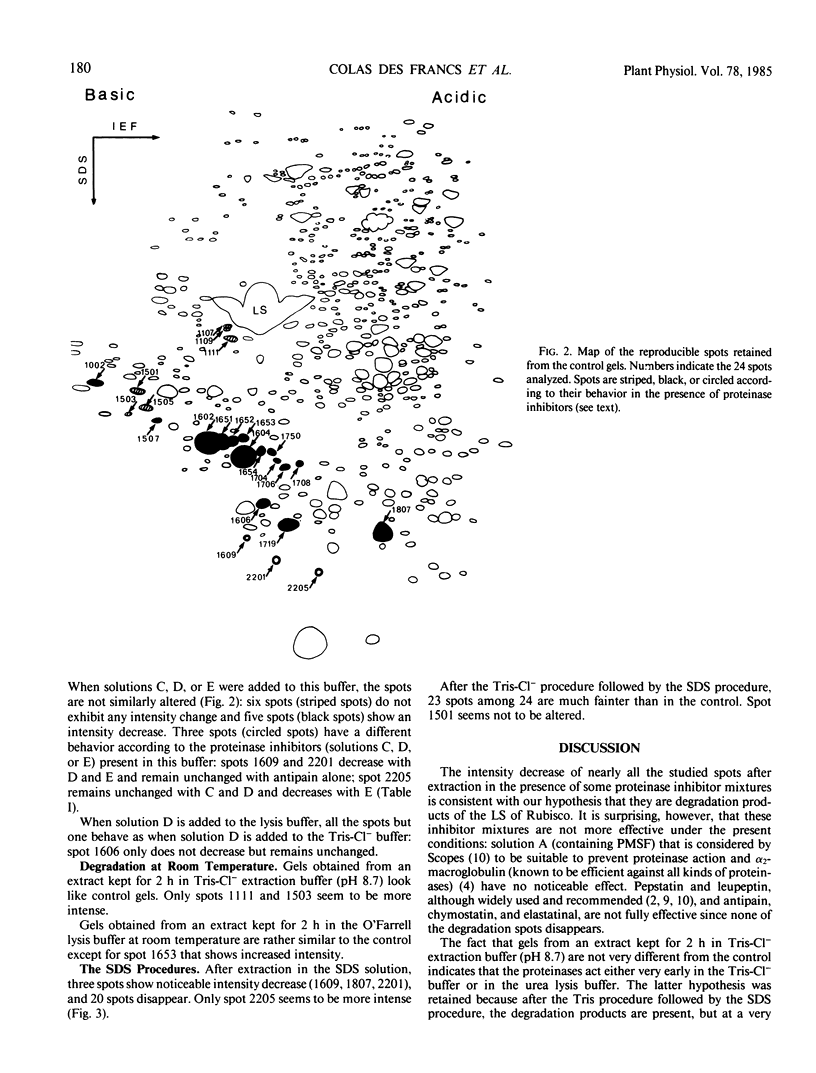
A previous genetic analysis by two-dimensional electrophoresis of wheat leaf proteins led us to hypothesize that several polypeptides were degradation products of the large subunit of ribulose bisphosphate carboxylase/oxygenase (EC 4.1.1.39).
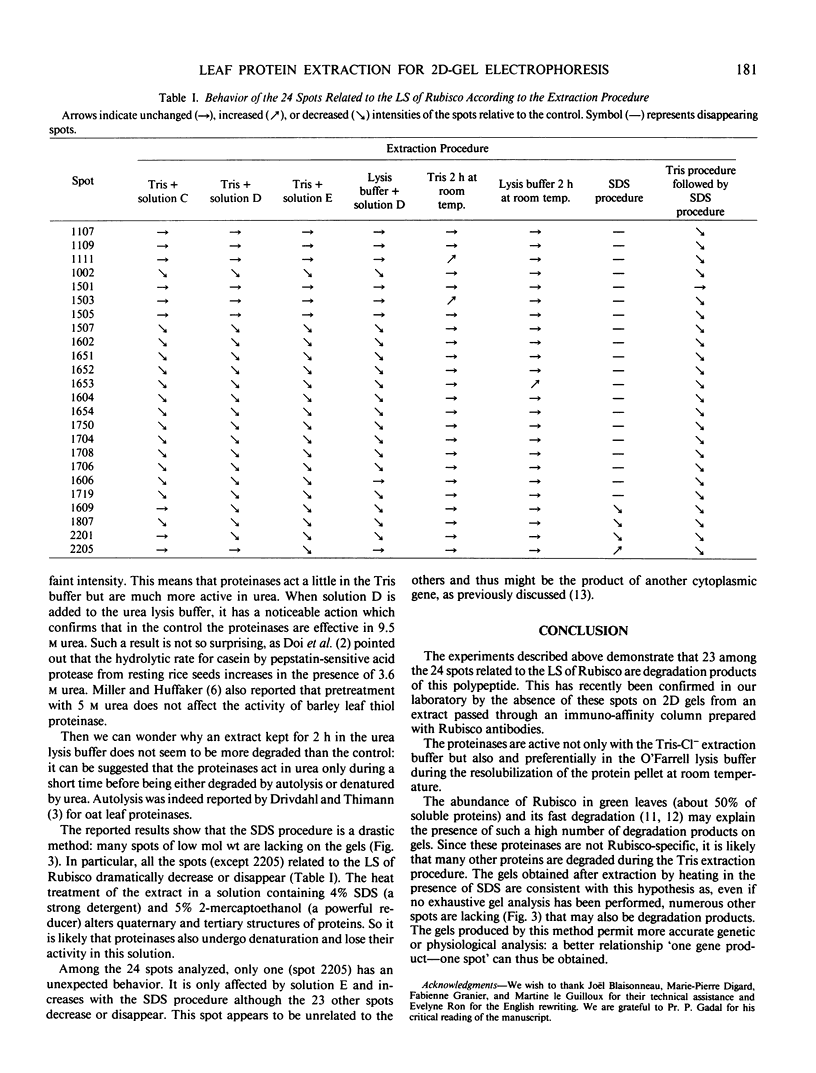
Various extraction procedures including the use of proteinase inhibitors allowed us to: (a) confirm that the suspected polypeptides are indeed degradation products; (b) find out under which conditions the proteinases act; (c) find a method which prevents degradation, by boiling the extract in the presence of sodium dodecyl sulfate and 2-mercaptoethanol.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drivdahl R. H., Thimann K. V. Proteases of Senescing Oat Leaves: II. Reaction to Substrates and Inhibitors. Plant Physiol. 1978 Apr;61(4):501–505. doi: 10.1104/pp.61.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. A., Black C. C. Two-Dimensional Electrophoretic Mapping of Proteins of Bundle Sheath and Mesophyll Cells of the C(4) Grass Digitaria sanguinalis (L.) Scop. (Crabgrass). Plant Physiol. 1982 Nov;70(5):1359–1366. doi: 10.1104/pp.70.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. L., Huffaker R. C. Partial purification and characterization of endoproteinases from senescing barley leaves. Plant Physiol. 1981 Oct;68(4):930–936. doi: 10.1104/pp.68.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rosichan J. L., Huffaker R. C. Source of endoproteolytic activity associated with purified ribulose bisphosphate carboxylase. Plant Physiol. 1984 May;75(1):74–77. doi: 10.1104/pp.75.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Ribulose Bisphosphate Carboxylase and Proteolytic Activity in Wheat Leaves from Anthesis through Senescence. Plant Physiol. 1979 Nov;64(5):884–887. doi: 10.1104/pp.64.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]