Abstract
Background
Keratoconus remains difficult to diagnose, especially in the early stages. It is a progressive disorder of the cornea that starts at a young age. Diagnosis is based on clinical examination and corneal imaging; though in the early stages, when there are no clinical signs, diagnosis depends on the interpretation of corneal imaging (e.g. topography and tomography) by trained cornea specialists. Using artificial intelligence (AI) to analyse the corneal images and detect cases of keratoconus could help prevent visual acuity loss and even corneal transplantation. However, a missed diagnosis in people seeking refractive surgery could lead to weakening of the cornea and keratoconus‐like ectasia. There is a need for a reliable overview of the accuracy of AI for detecting keratoconus and the applicability of this automated method to the clinical setting.
Objectives
To assess the diagnostic accuracy of artificial intelligence (AI) algorithms for detecting keratoconus in people presenting with refractive errors, especially those whose vision can no longer be fully corrected with glasses, those seeking corneal refractive surgery, and those suspected of having keratoconus. AI could help ophthalmologists, optometrists, and other eye care professionals to make decisions on referral to cornea specialists.
Secondary objectives
To assess the following potential causes of heterogeneity in diagnostic performance across studies.
• Different AI algorithms (e.g. neural networks, decision trees, support vector machines) • Index test methodology (preprocessing techniques, core AI method, and postprocessing techniques) • Sources of input to train algorithms (topography and tomography images from Placido disc system, Scheimpflug system, slit‐scanning system, or optical coherence tomography (OCT); number of training and testing cases/images; label/endpoint variable used for training) • Study setting • Study design • Ethnicity, or geographic area as its proxy • Different index test positivity criteria provided by the topography or tomography device • Reference standard, topography or tomography, one or two cornea specialists • Definition of keratoconus • Mean age of participants • Recruitment of participants • Severity of keratoconus (clinically manifest or subclinical)
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register), Ovid MEDLINE, Ovid Embase, OpenGrey, the ISRCTN registry, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). There were no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 29 November 2022.
Selection criteria
We included cross‐sectional and diagnostic case‐control studies that investigated AI for the diagnosis of keratoconus using topography, tomography, or both. We included studies that diagnosed manifest keratoconus, subclinical keratoconus, or both. The reference standard was the interpretation of topography or tomography images by at least two cornea specialists.
Data collection and analysis
Two review authors independently extracted the study data and assessed the quality of studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool. When an article contained multiple AI algorithms, we selected the algorithm with the highest Youden's index. We assessed the certainty of evidence using the GRADE approach.
Main results
We included 63 studies, published between 1994 and 2022, that developed and investigated the accuracy of AI for the diagnosis of keratoconus. There were three different units of analysis in the studies: eyes, participants, and images. Forty‐four studies analysed 23,771 eyes, four studies analysed 3843 participants, and 15 studies analysed 38,832 images.
Fifty‐four articles evaluated the detection of manifest keratoconus, defined as a cornea that showed any clinical sign of keratoconus. The accuracy of AI seems almost perfect, with a summary sensitivity of 98.6% (95% confidence interval (CI) 97.6% to 99.1%) and a summary specificity of 98.3% (95% CI 97.4% to 98.9%). However, accuracy varied across studies and the certainty of the evidence was low.
Twenty‐eight articles evaluated the detection of subclinical keratoconus, although the definition of subclinical varied. We grouped subclinical keratoconus, forme fruste, and very asymmetrical eyes together. The tests showed good accuracy, with a summary sensitivity of 90.0% (95% CI 84.5% to 93.8%) and a summary specificity of 95.5% (95% CI 91.9% to 97.5%). However, the certainty of the evidence was very low for sensitivity and low for specificity.
In both groups, we graded most studies at high risk of bias, with high applicability concerns, in the domain of patient selection, since most were case‐control studies. Moreover, we graded the certainty of evidence as low to very low due to selection bias, inconsistency, and imprecision.
We could not explain the heterogeneity between the studies. The sensitivity analyses based on study design, AI algorithm, imaging technique (topography versus tomography), and data source (parameters versus images) showed no differences in the results.
Authors' conclusions
AI appears to be a promising triage tool in ophthalmologic practice for diagnosing keratoconus. Test accuracy was very high for manifest keratoconus and slightly lower for subclinical keratoconus, indicating a higher chance of missing a diagnosis in people without clinical signs. This could lead to progression of keratoconus or an erroneous indication for refractive surgery, which would worsen the disease.
We are unable to draw clear and reliable conclusions due to the high risk of bias, the unexplained heterogeneity of the results, and high applicability concerns, all of which reduced our confidence in the evidence.
Greater standardization in future research would increase the quality of studies and improve comparability between studies.
Keywords: Humans, Artificial Intelligence, Case-Control Studies, Cross-Sectional Studies, Keratoconus, Keratoconus/diagnostic imaging, Physical Examination
Plain language summary
How accurate is artificial intelligence for diagnosing keratoconus?
Key messages
• The included studies suggest that artificial intelligence (AI) can identify keratoconus. This may lead to early detection and prevention of vision loss. • Estimates were similar for different types of AI algorithms. • We have little confidence in the evidence; there is a need for more research on this topic.
What is keratoconus and why is (early) diagnosis so important?
Keratoconus is a disease of the cornea (the clear window at the front of the eye) that affects people between the ages of 10 and 40 years. In those affected, the cornea weakens and thins over the years, gradually bulging into the typical cone‐like shape, which leads to reduced vision. Glasses can resolve this problem in the early stages of keratoconus, but no longer offer a satisfying solution as the disease becomes more severe. Early diagnosis is imperative to ensure follow‐up and treatment and thus prevent loss of vision.
The diagnosis of keratoconus is based on an eye exam (measuring the eye and evaluating the cornea with a vertical beam of light and a microscope) and imaging (computer‐assisted techniques that create three‐dimensional pictures or maps of the cornea). Interpreting the images can be challenging, especially in primary eye care settings and in the early stages of the disease. Not recognizing keratoconus could lead to worsening of the disease and worsening of vision. For example, people at risk of developing keratoconus who undergo refractive surgery (surgery to correct their vision) could end up with worse vision.
What is artificial intelligence and how can it help detect keratoconus?
Detecting keratoconus based on images is challenging, especially for untrained clinicians. AI gives machines the ability to adapt, reason, and find solutions. Algorithms can be developed and trained to analyse images of the cornea and recognize keratoconus. These tests could help ophthalmologists, optometrists, and other eye care professionals to make a diagnosis and refer people with keratoconus to cornea specialists in time to preserve their vision. There are many different types of algorithms, but they all distinguish between healthy eyes and keratoconus based on images of the cornea.
What did we want to find out?
The aim of the review was to find out whether AI can correctly diagnose keratoconus in people seeking refractive surgery and people whose vision can no longer be corrected fully with glasses.
What did we do?
We searched for studies that investigated the accuracy of AI for diagnosing keratoconus, preferably in people seeking refractive surgery or people whose vision can no longer be corrected fully with glasses. We compared and summarized the results of the studies to calculate two measures of accuracy: sensitivity (the ability of AI to correctly identify keratoconus) and specificity (the ability of AI to correctly rule out keratoconus). The closer sensitivity and specificity were to 100%, the better the algorithm.
What did we find?
We found 63 studies that used three different units (eyes, participants, and images) to analyse the accuracy of AI for detecting keratoconus: 44 studies analysed 23,771 eyes, four studies analysed 3843 participants, and 15 studies analysed 38,832 images.
The accuracy of AI for detecting manifest keratoconus (keratoconus that can be detected through a clinical examination) was high. If 1000 people were tested, 30 people with keratoconus would be correctly referred to a cornea specialist, and none would be missed. Of the remaining 970 people (without keratoconus), only 17 would be wrongly referred. These people would receive additional non‐invasive tests to verify whether they had keratoconus.
The accuracy of AI for detecting early keratoconus was lower. If 1000 people were tested, nine people with keratoconus would be correctly referred to a cornea specialist and one would be missed. If this person received refractive surgery, it would aggravate the disease and worsen their vision. Of the remaining 990 people (without keratoconus), 941 would be reassured that they did not have the disease and would receive refractive surgery or glasses; 49 people would be wrongly referred.
The evidence suggests that AI may be good at detecting manifest keratoconus but may not be ideal for screening early keratoconus.
What are the limitations of the evidence?
We have little confidence in the evidence on the accuracy of AI for detecting manifest keratoconus, and we have little to no confidence in the evidence related to early keratoconus. There were problems with how the studies were conducted, which may result in AI appearing more accurate than it really is.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to 29 November 2022.
Summary of findings
Summary of findings 1. Summary of findings: artificial intelligence for the detection of keratoconus in refractive surgery candidates and people with refractive errors.
| Review Question | What is the diagnostic accuracy of AI algorithms in the detection of keratoconus in people presenting with refractive errors, people seeking corneal refractive surgery, or people suspected of having keratoconus? | |||||
| Population | People presenting with refractive errors, especially those whose vision can no longer be corrected fully with glasses, people seeking corneal refractive surgery, or people suspected of having keratoconus | |||||
| Index test | AI algorithms e.g. neural network, logistic regression, support vector machine, etc. analysing topography and tomography images | |||||
| Target condition | Keratoconus | |||||
| Reference standard | Topography and tomography images interpreted by at least two cornea specialists | |||||
| Action | (Early) referral of people suspected of having keratoconus to a cornea specialist by ophthalmologists, optometrists, and other eye care professionals. | |||||
| Quantity of evidence | 63 studies | |||||
| Outcome | Effect (95% CI) | Number of participants (studies) | Test result | Number of results per 1000 participants tested | Certainty of evidence | |
| Real clinical setting* | Included studies** | |||||
| Manifest keratoconus |
Summary sensitivity 98.6% (97.6% to 99.1%) |
21,330 (54) | True positive | 30 (29 to 30) |
493 (488 to 496) |
⊕⊕⊝⊝ Lowa |
| False negative | 0 (0 to 1) |
7 (5 to 12) |
||||
|
Summary specificity 98.3% (97.4% to 98.9%) |
29,189 (54) | True negative | 954 (945 to 959) |
492 (487 to 495) |
⊕⊕⊝⊝ Lowa |
|
| False positive | 16 (11 to 27) |
9 (6 to 13) |
||||
| Subclinical keratoconus |
Summary sensitivity 90.0% (84.5% to 93.8%) |
2758 (28) | True positive | 9 (8 to 9) |
225 (211 to 235) |
⊕⊝⊝⊝ Very lowb |
| False negative | 1 (1 to 2) |
25 (16 to 39) |
||||
|
Summary specificity 95.5% (91.9% to 97.5%) |
6750 (28) | True negative | 945 (911 to 970) |
716 (687 to 731) |
⊕⊕⊝⊝ Lowa |
|
| False positive | 45 (20 to 79) |
34 (19 to 63) |
||||
| *Estimated prevalence in the real clinical setting was 3% for manifest keratoconus and 1% for subclinical keratoconus.
**Prevalence calculated from the included studies was 50% for manifest keratoconus and 25% for subclinical keratoconus. AI: artificial intelligence; CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for risk of bias (high risk of selection bias and other biases due to case‐control design with indirectness) and one level for inconsistency (sensitivity varies widely across studies). b Downgraded one level for risk of bias (high risk of selection bias and other biases due to case‐control design with indirectness), one level for inconsistency (sensitivity varies widely across studies), and one level for imprecision (wide CIs for sensitivity).
Background
Target condition being diagnosed
Keratoconus is an ectatic degenerative disorder of the cornea, usually affecting both eyes. Ultra‐structural examination of the human cornea ex vivo has revealed disruption and loss of the native collagen network, leading to biomechanical instability and severe corneal thinning (Hayes 2012; Meek 2005). The disease is generally progressive in nature, resulting in the cornea taking a typical cone shape. This causes myopia and irregular astigmatism, impairing visual acuity.
Normally, keratoconus begins during puberty and gradually progresses until the person affected is in their 30s. It usually progresses more rapidly in people younger than 17 years and stabilizes with age (Ferdi 2019). The reported prevalence of keratoconus varies among studies (Hashemi 2020). This may be due to several reasons, such as different diagnostic criteria, different diagnostic methods, change in testing rates over time, genetic variation, or environmental differences.
The pathophysiology of keratoconus is not well understood; however, both environmental and genetic factors seem to play a role (Rabinowitz 2021). One risk factor that has been investigated extensively is eye rubbing; others include the wearing of contact lenses and allergic disease. Research on the genetic contribution to keratoconus suggests a possible association (Rabinowitz 2021). However, diagnostic genetic testing for keratoconus is not currently available.
Some people who undergo refractive surgery may be at risk of developing iatrogenic keratoectasia (i.e. weakening of the biomechanical stability of the cornea due to the surgery), which leads to a keratoconus‐like ectasia. Although this is not a frequent occurrence (Giri 2017), the consequences can be sight‐threatening, so it is crucial to detect corneas at risk of developing the condition. Possible risk factors are irregular topography and thin corneal pachymetry (Giri 2017).
The treatment for keratoconus depends on the severity of the disease. In the initial stage, the aim of treatment is usually to correct visual acuity with glasses or specialized contact lenses. However, these treatments do not cure keratoconus. As the disease progresses, visual acuity can worsen to the point that glasses no longer offer a satisfactory solution. Corneal cross‐linking has been used since 2003 to stop the progression of keratoconus, but this treatment cannot reverse visual impairment (Sykakis 2015). Before the introduction of corneal cross‐linking, the only treatment to cure keratoconus was corneal transplantation. Despite the development of cross‐linking, keratoconus is still one of the most common reasons for corneal transplantation (Kelly 2011; Röck 2018). Thus, the diagnosis of keratoconus may help to avoid poor visual outcomes and possible corneal transplantation, especially if the diagnosis is made early.
Keratoconus diagnosis is based primarily on corneal topographic and tomographic analysis in people presenting with refractive errors, especially those whose vision can no longer be fully corrected with glasses, and those seeking corneal refractive surgery. A global consensus committee of ophthalmology experts concluded that "abnormal posterior ectasia, abnormal corneal thickness distribution, and clinical non‐inflammatory corneal thinning are mandatory findings to diagnose keratoconus" (Gomes 2015). However, applying this definition in practice is not straightforward: because the consensus mentions no cut‐offs or parameters, the definition is open to the interpretation of the specialist. Ocular findings that may point to early keratoconus include abnormal keratometry readings and a distorted red reflex when using an ophthalmoscope, both of which indicate an irregular cornea. Detecting keratoconus at an early stage may be challenging, as people affected are often asymptomatic, and there are few or no clinical signs. In later stages of the disease, clinical signs are visible during slit‐lamp examination and include stromal thinning, conical protrusion of the cornea at the apex, Fleischer's ring, and Vogt's striae (Zadnik 1996). Another challenge in the diagnosis of keratoconus is detecting an at‐risk cornea or subclinical keratoconus in people seeking corneal refractive surgery. Iatrogenic keratoectasia due to biomechanical decompensation may occur in these people if the disease is not detected (Giri 2017).
Currently, there is no accurate and objective method to detect keratoconus. An artificial intelligence (AI)‐based tool for keratoconus detection could help ophthalmologists, optometrists, and other eye care professionals to make decisions on referral to cornea specialists.
AI is a growing field within ophthalmology, and is expected to play an important role in the diagnosis and characterization of eye diseases in the future. There has been an increasing interest in the application of AI methods for diseases of the anterior segment (Ting 2020). This review will seek to determine if AI is a valid tool for diagnosing keratoconus as an aid for ophthalmologists.
Index test(s)
This review will evaluate the application of AI in the diagnosis of keratoconus. AI methods are already contributing to many aspects of human life and society, ranging from home automation, smart assistants (e.g. 'Siri', 'Google Assistant'), and self‐driving cars to facial recognition and automatic detection of 'fake news' on social media. There has been notable progress with the use of AI in the field of medical image analysis, including applications in ophthalmology (Ting 2019).
AI provides machines with the capability to adapt, reason, and find solutions. Machine learning is a subdiscipline of AI that enables machines to learn from data and experience through algorithms. Examples of machine learning algorithms are support vector machine, random forest, and decision tree. Deep learning is a subdiscipline of machine learning that uses neural networks, much like the human brain. It teaches machines to learn through pattern recognition and even to improve themselves (LeCun 2015).
Initially, most AI research in ophthalmology focused on the posterior segment. Studies have investigated multiple deep learning applications for several common ophthalmic diseases, including diabetic retinopathy (Abràmoff 2016; Gargeya 2017; Gulshan 2016; Ting 2017), age‐related macular degeneration (Grassmann 2018; Ting 2017), glaucoma (Shibata 2018), and retinopathy of prematurity (Brown 2018). More recent research has concentrated on the development of deep learning applications for the anterior segment, in particular keratoconus (Ting 2020).
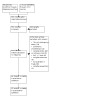
In keratoconus, the AI algorithm analyses images of the cornea using a computer to determine whether the disease is present (see Figure 1). There are different devices for producing these non‐invasive corneal images, which are called topography or tomography images. Most devices (e.g. Scheimpflug‐based devices, optical coherence tomography (OCT)) take both tomography and topography images. However, some devices (e.g. Placido disc devices) only take topography images. The image is uploaded to a computer, where the algorithm performs a series of analyses to come to a decision on whether keratoconus is present.
1.

Clinical pathway.
The first step in developing an algorithm is collecting a representative data set for keratoconus, which includes topographic or tomographic images of both keratoconus and healthy eyes. The data set is then divided into training, validation, and test sets. The training set is used to determine the parameters or features of keratoconus via an optimization procedure. The validation set is used for model selection (e.g. determining the best neural network architecture) and monitoring for overfitting (i.e. the algorithm is only applicable to the data on which it was trained). The independent test set is used for evaluation of the model (i.e. determining the performance of the model on unseen data). In principle, the test set should only be used once, after the model is developed and trained. When these three phases are completed, the algorithm will theoretically be able to differentiate keratoconus eyes from healthy eyes.
Each AI algorithm has its own grading system to classify keratoconus and healthy eyes. Depending on the goal of the AI tool (screening or diagnosis), the thresholds of sensitivity and specificity will differ.
Topography was previously considered the best method for diagnosing keratoconus, but according to current guidelines, corneal tomography is now the gold standard (Gomes 2015). Topography only analyses the anterior corneal surface, whereas tomography analyses both anterior and posterior corneal surfaces and can create three‐dimensional images, resulting in improved accuracy. In clinical practice, diagnosis of keratoconus involves both tomography and topography parameters, including maximum keratometry, minimal pachymetry, astigmatism, and asphericity. These show only a moderate correlation with keratoconus (Kanellopoulos 2013a; Kanellopoulos 2013b; Lopes 2012; Sedghipour 2012). Most devices also provide objective indices to aid diagnosis, including the keratoconus index, the index of surface variance, and the inferior‐superior index. However, these parameters and indices individually do not provide sufficient information, but must be combined and interpreted together (Martínez‐Abad 2017). Unfortunately, not all ophthalmologists, optometrists, or eye care professionals have these diagnostic skills. A second issue is the intra‐ and interobserver variability in the diagnosis of keratoconus (Brunner 2018; Flynn 2016). AI could be a solution to both problems, as it can easily combine tomography and topography parameters and indices based on an enormous amount of data, and it is not affected by diagnostic variability. It could help young ophthalmologists, ophthalmologists in non‐academic centres, optometrists, and other eye care specialists to diagnose the disease early and refer affected people to a cornea specialist. In this way, follow‐up can start earlier and specialists can detect any progression before visual loss.
Clinical pathway
The clinical pathway to diagnosing keratoconus is based on clinical examination, which includes visual acuity testing, slit‐lamp examination of the anterior segment, and corneal imaging (all non‐invasive tests). Corneal imaging is performed in people presenting with refractive errors, especially those whose vision can no longer be corrected fully with glasses, those seeking corneal refractive surgery, and those referred by ophthalmologists, optometrists, or other eye care professionals because of suspected keratoconus.
The different methods of corneal imaging include Placido topography, Scheimpflug tomography, and slit‐scanning tomography. Interpretation of the images can be challenging, and the signs of keratoconus can be subtle for general ophthalmologists, optometrists, and other eye care professionals. In current practice, the ophthalmologist will analyse the corneal images, looking for patterns and evaluating device‐dependent parameters such as keratometry, elevation, and pachymetry parameters (see Appendix 1). As the global consensus mentions no cut‐offs in the definition of keratoconus, specialists need to rely on their knowledge and experience, which means the diagnosis is subjective.
After being diagnosed with keratoconus, the person affected will need regular follow‐up visits to check if the disease progresses. The global consensus document states that treatment is essential when there is documented clinical progression, defined as a consistent change in at least two of the following parameters where the magnitude of the change is above the normal noise of the testing system (Gomes 2015).
Steepening of the anterior corneal surface
Steepening of the posterior corneal surface
Thinning or an increase in the rate of corneal thickness change from the periphery to the thinnest point
As with the definition of keratoconus, these criteria are open to interpretation. The consensus document mentions no cut‐offs, time intervals, or specific parameters.
A missed diagnosis of keratoconus could lead to delayed treatment, poor visual outcome, and a greater risk for corneal transplantation, all of which impact on patients' quality of life, especially because the disease normally affects young people who are active and in their primary income‐earning years.
The same corneal images that are analysed by clinicians can be uploaded to a computer and analysed by an AI algorithm. AI based on a large ophthalmic data set can achieve high accuracy in distinguishing a normal cornea from a keratoconus cornea by analysing the topography or tomography images (Lin 2019; Lopes 2019). Since the global consensus does not give an accurate definition of keratoconus or keratoconus progression, AI could be helpful in making this decision. It could help with early diagnosis of keratoconus so that affected people can be monitored and any progression can be detected sooner. Once progression is detected and confirmed by a cornea specialist, the patient would receive corneal cross‐linking to halt the deterioration of the disease, which in turn would lead to a better visual prognosis and lower risk of corneal transplantation. Since the cornea specialist is still responsible for the diagnosis, the first role of AI would be as triage to make decisions on referral.
To implement an AI algorithm in clinical practice, it needs to be efficient and able to analyse images in a few seconds. It should give one clear indication of whether keratoconus is present.
Devices that measure biomechanical properties, such as the Corvis ST or the Ocular Response Analyzer (ORA), were not included in this review.
Rationale
AI is a rapidly growing field in ophthalmology, with numerous new developments in the detection of keratoconus (Ting 2020). It is important to have reliable evidence regarding the accuracy of these developments. This review will give a clear overview of the different AI detection tools and their accuracy.
Corneal imaging devices are becoming increasingly sophisticated, and with the help of AI algorithms, they can detect keratoconus earlier. AI uses a vast amount of data to learn characteristic features of keratoconus. It can process thousands of images in a short time to learn how to detect the disease, whereas an ophthalmologist needs years of practice. AI will help ophthalmologists, optometrists, and eye care professionals in the diagnosis of keratoconus, potentially leading to earlier diagnoses. This is beneficial for patients because they may have a better visual outcome, which would improve their quality of life. There are also important financial consequences, in terms of reduced healthcare costs and personal costs.
Nevertheless, AI has its limitations. The accuracy of the algorithms relies on the generalizability of the training sets. If training sets do not contain sufficient data or sufficiently varied data, the algorithms could miss diagnoses due to inadequate learning (LeCun 2015).
One narrative review published in 2019 suggested that AI may be a reliable tool (Lin 2019). Another review discussed AI in the anterior segment and mentioned the detection of keratoconus (Ting 2020). However, neither of these previous reviews determined the reliability of the included studies.
There is a need for a reliable overview of current knowledge on the different existing AI algorithms and an analysis of their accuracy.
Objectives
To assess the diagnostic accuracy of artificial intelligence (AI) algorithms for detecting keratoconus in people presenting with refractive errors, especially those whose vision can no longer be fully corrected with glasses, those seeking corneal refractive surgery, and those suspected of having keratoconus. AI could help ophthalmologists, optometrists, and other eye care professionals to make decisions on referral to cornea specialists.
Secondary objectives
To assess the following potential causes of heterogeneity in diagnostic performance across studies.
Different AI algorithms (e.g. neural networks, decision trees, support vector machines)
Index test methodology (preprocessing techniques, core AI method, and postprocessing techniques)
Sources of input to train algorithms (topography and tomography images from Placido disc system, Scheimpflug system, slit‐scanning system, or optical coherence tomography (OCT); number of training and testing cases/images; label/endpoint variable used for training)
Study setting
Study design
Ethnicity, or geographic area as its proxy
Different index test positivity criteria provided by the topography or tomography device
Reference standard, topography or tomography, one or two cornea specialists
Definition of keratoconus
Mean age of participants
Recruitment of participants
Severity of keratoconus (clinically manifest or subclinical)
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional studies and diagnostic case‐control studies (either prospective or retrospective).
We organized the included studies based on the main characteristics of the AI methodology (preprocessing techniques, core AI method, and postprocessing techniques), data that were used to train the model (patient inclusion criteria, number of training and testing cases/images, label/endpoint variable used for training), and evaluation (evaluation metric, reported performance on the independent test set).
Participants
We aimed to include people with refractive errors:
whose vision could not be fully corrected with glasses; or
who were seeking refractive surgery; or
who had suspected keratoconus (for whom a decision was to be made on referral to cornea specialists).
However, research in this field is still in its early stages, and we accepted studies that did not satisfy this optimal definition of participants, such as case‐control studies that included people with keratoconus and healthy controls based on different sets of criteria.
As keratoconus can progress until the fourth decade of life, we included participants up to the age of 50 years.
Index tests
We included studies reporting accuracy data for automated diagnostic tests. All AI algorithms developed to analyse corneal topography or tomography for detecting keratoconus were eligible.
Target conditions
The target condition was keratoconus of any stage. When studies reported accuracy for multiple severity levels, we prioritized data from participants with at least mild severity. In fact, fruste keratoconus is generally non‐progressive, or very slowly progressive.
Reference standards
The reference standard for keratoconus is topography or tomography. These non‐invasive examinations are routinely performed on people who are seeking refractive surgery or people referred to an ophthalmologist for suspected keratoconus. Two or more cornea specialists should independently analyse and interpret the corneal images. We accepted studies that used only one cornea specialist for diagnosis as a low‐quality reference standard.
Topography examines the anterior corneal surface. The Placido disc system is a device that uses topography. Concentric rings of light are projected on the cornea. Thousands of points along these concentric rings are analysed, and these data are translated to the curvature of the anterior corneal surface (Fan 2018). The main parameters measured by Placido systems are maximum keratometry, steep keratometry, flat keratometry, and astigmatism (see Appendix 1).
Tomography examines both the anterior and posterior corneal surfaces. The Scheimpflug system uses a single rotating Scheimpflug camera (e.g. Pentacam (Oculus GmbH, Wetzlar, Germany)), a single rotating Scheimpflug camera combined with Placido disc topography (Sirius, CSO, Italy), or a dual‐Scheimpflug camera with Placido disc technology incorporated to improve curvature information on the central cornea (e.g. the Galilei (Ziemer, Biel, Switzerland)). Another device that examines both anterior and posterior corneal surfaces is the slit‐scanning system; this is an elevation‐based method for the assessment of topography and tomography (e.g. the Orbscan IIz (Bausch & Lomb, Rochester, NY)). Multiple complimentary slits are used to perform an assessment of the corneal surface. In addition to keratometry (which is also measured by the Placido systems), the Scheimpflug system and slit‐scanning system measure corneal elevation and pachymetry (see Appendix 1).
OCT also examines both the anterior and posterior corneal surfaces. Anterior segment OCT (AS‐OCT) uses low‐coherence interferometry to assess the cornea and the anterior segment. The low‐coherent light is emitted and split by an interferometer into a reference beam and a probe beam (Wojtkowski 2010). The probe beam is backscattered from the different corneal layers. The echo time delay is measured and transformed into two‐ or three‐dimensional images by the OCT (Subhash 2013). A new instrument called the MS‐39 (CSO, Italy) combines Placido disc corneal topography with high‐resolution OCT‐based anterior segment tomography. It measures keratometry, elevation, pachymetry, and other parameters.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases and trials registries on 29 November 2022. There were no restrictions on language or date of publication.
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 11), which contains the Cochrane Eyes and Vision Trials Register, in the Cochrane Library (searched 29 November 2022; Appendix 2)
MEDLINE Ovid (1946 to 29 November 2022; Appendix 3)
Embase Ovid (1980 to 29 November 2022; Appendix 4)
System for Information on Grey Literature in Europe (OpenGrey; 1995 to 29 November 2022; Appendix 5)
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 29 November 2022; Appendix 6)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 29 November 2022; Appendix 7)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp; searched 29 November 2022; Appendix 8)
Searching other resources
We searched the reference lists of the review's included studies.
Data collection and analysis
Selection of studies
Two review authors (MV and EF) independently evaluated the records retrieved by the searches using the online review management software Covidence. They screened the titles of the records and eliminated those that were clearly ineligible. The same two review authors then assessed the full‐text articles of the remaining records against our inclusion criteria. They resolved any disagreements by discussion or, if necessary, by involving the other review authors.
Data extraction and management
The two review authors independently extracted the following data from the selected articles using a standardized data collection form.
Study design
Study population
Definition of keratoconus
Reference standard
Index tests
Description of architecture and training mechanisms
The ground truth (one observer versus multiple observers)
Size of data sets used
Data required to fill in a 2 × 2 diagnostic contingency table for each index test
We compared the data collected independently by the two review authors, and resolved any discrepancies through discussion and consensus. If we needed to obtain further data from a paper, or if there were missing data, we tried to contact the study author for further information.
When an article reported multiple AI algorithms, we selected the algorithm with the highest Youden's index. We are aware that this selection could inflate accuracy, especially in smaller studies, and we highlighted this as a limitation. However, we considered this decision acceptable in this early stage of research as it could also reduce redundancy. Examples of algorithms are random forest, support vector machine, decision tree, and neural network.
We used GRADEpro GDT to create a summary of findings table (GRADEpro GDT).
Assessment of methodological quality
Two authors (MV and EF) independently assessed the included studies for bias using the revised Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS‐2), as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Reitsma 2009). The QUADAS‐2 tool has four assessment domains: patient selection, index test, reference test, and flow and timing. Each domain has signalling questions to assess the risk of bias. The tool also assesses applicability for the first three domains.
During the quality assessment process, we decided to add an item that is specific to AI studies (Appendix 9). In the domain 'Index test', we added the question 'Was the model designed in an appropriate manner?'. We considered a study at low risk of bias if data from a single participant were reserved to only one data partition, parameters were tuned, and the optimal model was selected. We considered a study at high risk of bias if data from a single participant were not reserved to only one data partition, parameters were not tuned, and the optimal model was not selected. When the design of the model was unclear, and we could not determine the above‐mentioned properties, we considered the study at unclear risk. In the protocol for this review, we stated that the 'Concerns regarding applicability' question in the domain 'Reference standard' ('Are there concerns that the target condition as defined by the reference standard does not match the review question?') was not applicable to this review (Vandevenne 2021); however, we corrected this during quality assessment. Additionally, in the domain 'Flow and timing', we removed the question 'Was there an appropriate interval between index test(s) and reference standard?', as it was not applicable to this review. The reference test and index test were performed on the same corneal images or parameters, so the interval between the index and reference test is irrelevant.
Statistical analysis and data synthesis
We conducted all statistical analysis and data synthesis in accordance with Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010).
Initially, we presented data in a 2 × 2 table, showing cross‐classification of the index test result versus the reference standard outcome. For each index test, in all studies, we calculated the sensitivity and specificity with a 95% confidence interval (CI). To visually evaluate the variation in calculations of sensitivity and specificity, we used Review Manager 5 (RevMan 5) to generate coupled forest plots and present studies in receiver operating characteristic (ROC) plots (Review Manager 2020).
Since AI studies were unlikely to give a definite threshold that would be comparable across studies, we had planned to use a hierarchical summary ROC (HSROC) model and estimate the average sensitivity at fixed specificity values according to cut‐offs for terciles of specificity (Macaskill 2010). However, we found accuracy was nearly perfect in the vast majority of studies, which clustered close to the upper‐left corner of the ROC space. Therefore, we pooled data using a bivariate model, which is equivalent to an HSROC model in absence of covariates (Harbord 2007). We conducted analyses using the 'metadas' user‐written command in SAS software, as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Takwoingi 2022).
We had planned to conduct direct comparisons between the index tests (different types or data sources for AI) if sufficient data were available. However, few studies presented paired data for this comparison, so we decided to explore heterogeneity between studies in subgroup analyses. We conducted these analyses with a test covariate in the bivariate model as suggested in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Takwoingi 2022).
Investigations of heterogeneity
To investigate heterogeneity, where data were available, we added covariates in a meta‐regression, using the sources presented in the Objectives. We used all covariates as categorical variables.
Sensitivity analyses
We conducted a sensitivity analysis by excluding studies that used images as the unit of analysis, since sample sizes could exceed the number of participants by several times.
Certainty of the evidence assessment
We graded the certainty of evidence for each outcome using the GRADE approach and following Chapter 12 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Leeflang 2022). GRADE considers five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. We explained all decisions to downgrade the certainty of evidence using footnotes to the summary of findings tables. We decided post hoc to adopt a threshold of 0.95 as desirable sensitivity to assess imprecision in GRADE, given a triage test role. We set the threshold for specificity at 0.90, considering the workload generated at low prevalence.
Assessment of reporting bias
We assessed reporting biases when a study protocol was available. We attempted to maximize data collection by using comprehensive search methods and contacting study authors when we needed further information to reach a decision on study eligibility.
Results
Results of the search
The searches yielded a total of 707 records (Figure 2). After deduplication, we assessed the titles and abstracts of 553 records, of which we considered 436 to be clearly irrelevant. We retrieved and read the full‐text articles of the remaining 117 records, excluding 54 articles for different reasons (see Figure 2). We included 63 studies (63 reports) in the final qualitative synthesis.
2.
Flow diagram illustrating study selection process.
Included studies
Twelve studies had a prospective design. Fifty‐eight were case‐control studies; the remaining five were cross‐sectional studies. Only one study had randomized allocation; the rest were non‐randomized or had an unclear sampling. In the protocol, we described the study population as "patients with refractive errors, whose vision cannot be fully corrected with glasses, patients seeking refractive surgery or patients suspected of keratoconus, for whom a decision is to be made on referral to cornea specialists" (Vandevenne 2021). Of the 63 studies, only 17 included refractive surgery candidates, and only one included referred patients. The remaining studies included people with diagnosed keratoconus and healthy controls. More extensive details on these articles are available in the Characteristics of included studies table.
Excluded studies
Of the 54 articles excluded during full‐text analysis, 11 were study protocols and 11 were conference proceedings. We contacted the study authors, but no additional data were available. Eight articles had an ineligible index test or reference test (e.g. devices that measure the biomechanical properties of the cornea). Eight studies had no 2 × 2 data available to compute sensitivity and specificity. We only included studies published in English, so we excluded six articles published in a different language. Four studies reported ineligible outcomes, and four studies included ineligible populations (e.g. allergic eye disease).
Methodological quality of included studies
Figure 3 and Figure 4 give an overview of the methodological quality of the included studies.
3.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
4.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Regarding the first domain, 'Patient selection', we considered most studies at very high risk of bias due to their case‐control design, as the participants were already diagnosed with keratoconus and did not fit the definition stated in our protocol (Vandevenne 2021). Kalin 1996 was the only study that we judged at low risk of bias in this domain, as it included consecutive people seeking refractive surgery. Six studies were at unclear risk of bias because they provided insufficient information on sampling. With respect to applicability, we considered concern was low in Kalin 1996 only. There was unclear concern for three studies because they provided an insufficient description of the population. We considered concern was high for the remaining 59 studies because they all included people attending cornea services for known disease, were population‐based studies, or were registry‐based studies.
When evaluating the second domain, 'Index test', we judged Kojima 2020 at high risk of bias because it did not provide an appropriate description of the AI algorithm. We judged 18 studies at unclear risk of bias, mainly because they provided an unclear description of the study design. We considered the remaining 45 studies at low risk of bias.
In the third domain, 'Reference standard', we judged 17 studies at high risk of bias: although almost all reference standard diagnoses preceded the index test diagnoses, none of the 17 studies had two or more cornea specialists interpreting the images. We considered 18 studies at low risk of bias because at least two cornea specialists interpreted the images to diagnose keratoconus. The remaining 28 studies were at unclear risk of bias because they did not clearly state whether the results were interpreted independently. Regarding applicability, we considered there was unclear concern for 13 studies and low concern for the other 50 studies.
Regarding the fourth domain, 'Flow and timing', we judged 23 studies at unclear risk of bias because it was unclear whether all participants had received the same reference standard. In the studies that used a pre‐existing database of people with keratoconus, it was unclear how the diagnosis was established. The remaining 43 studies were at low risk of bias.
In the last domain, 'Comparative', we judged risk of bias and applicability concerns in the 12 studies that developed and compared multiple AI algorithms. We considered all 12 studies at unclear risk of bias because none indicated whether the results of the different algorithms were interpreted independently. Only a few mentioned the size of the data set used to validate the different tests. We could not use directly comparative data as these were sparse and difficult to group. Thus, we conducted indirect comparisons between AI algorithms.
Findings
We included 63 studies that developed and investigated the accuracy of an AI algorithm for the diagnosis of keratoconus. The studies were published between 1994 and 2022. The median prevalence of keratoconus across all studies was 45% (interquartile range 28% to 54%, range 6% to 94%).
Characteristics of included studies
Table 2 shows the main characteristics of all the included studies, such as study design, population, sample size, country, instrument, index test, and reference test. Most studies (58) had a case‐control design, and the other six studies had a cross‐sectional design. Most studies had a single‐ or multicentre data set of people with keratoconus and controls. The controls were healthy people or refractive surgery candidates in most cases, though some studies included people with other ocular diseases (for details see Table 2). The sample size was often large, which is necessary for the development and training of an AI algorithm. Only five studies included fewer than 100 people (Cao 2020; Carvalho 2005; Castro‐Luna 2020; Consejo 2020; Xu 2022a). The studies included data from 21 different countries and represented all continents.
1. Study characteristics.
| Study ID | Study design | Study population | Sample size | Country | Instrument | Index test | Reference standard |
| Abdelmotaal 2020 | Retrospective, single‐centre, case‐control | Refractive surgery candidates, subclinical KC, and manifest KC | 3218 images (3218 eyes of 1669 participants) | Egypt | Pentacam | Convolutional neural network | 2 cornea specialists |
| Accardo 2002 | Retrospective, single‐centre, case‐control | Healthy controls, KC, and other ocular diseases | 396 images (396 eyes of 198 participants) | Italy | Eyesys | Neural network | Unclear |
| Almeida 2022 | Retrospective, multicentre, case‐control | Healthy controls (who underwent PRK or LASIK), subclinical KC, manifest KC | 2893 eyes (2893 participants) | Brazil | Pentacam | Multiple logistic regression | 1 cornea specialist |
| Al‐Timemy 2021 | Retrospective, multicentre, case‐control | Healthy controls, subclinical KC, and manifest KC | 1050 images (150 eyes of 85 participants) | Brazil | Pentacam | Convolutional neural network | 3 cornea specialists |
| Arbelaez 2012 | Retrospective, multicentre, case series | Healthy controls, subclinical KC, and manifest KC | 3502 eyes | Oman, Italy | Sirius | Support vector machine | Unclear |
| Bessho 2006 | Retrospective, multicentre, case‐control | Healthy controls and KC | 165 eyes (120 participants) | Japan | Orbscan IIz | Logistic regression | Unclear |
| Cao 2020 | Retrospective, case‐control | Healthy controls and subclinical KC | 88 participants | Australia | Pentacam | Random forest | Unclear |
| Cao 2021a | Retrospective, case‐control | Healthy controls and subclinical KC | 267 eyes (226 participants): 186 training, 81 test | Australia | Pentacam | Random forest | 2 cornea specialists |
| Carvalho 2005 | Retrospective, single‐centre, case‐control | Instrument database | 80 eyes: 40 training, 40 test set | Brazil | Eyesys | Neural network | 2 cornea specialists |
| Castro‐Luna 2020 | Retrospective, single‐centre, case‐control | Control group and KC | 60 eyes (60 participants) | Spain | CSO topography system | Bayesian network | Unclear |
| Cavas‐Martinez 2017 | Retrospective, single‐centre, case‐control | Control group and KC | 464 eyes (464 participants) | Spain | Sirius | Logistic regression | Unclear |
| Chan 2015 | Retrospective, single‐centre, case‐control | Database of KC of the Singapore National Eye Center | 128 images (128 participants) | Singapore | Orbsan II | Discriminant analysis | 1 cornea specialist |
| Chandapura 2019 | Retrospective, multicentre, case‐control | Healthy controls, subclinical KC, and manifest KC | 439 eyes | India, Brazil | OCT RTVue + Pentacam | Random forest | 1 cornea specialist |
| Chastang 2000 | Retrospective, single‐centre, case‐control | Control group (e.g. healthy, regular astigmatism, radial keratotomy) and KC | 208 eyes: 104 training, 104 validation set | France | Eyesys | Binary decision tree | 2 cornea specialists |
| Chen 2021 | Retrospective, multicentre, case‐control | Healthy controls and KC | 1926 images | UK, Iran, New Zealand | Pentacam | Convolutional neural network | Unclear |
| Cohen 2022 | Retrospective, single‐centre, case‐control | Healthy controls, KC, and subclinical KC | 8526 corneal tomography examinations (2525 participants) | Israel | Galilei Dual Scheimpflug Analyzer | Random forest | 1 cornea specialist |
| Consejo 2020 | Prospective, single‐centre, case‐control | Control group and KC | 50 eyes | Belgium | Corvis‐ST | Support vector machine | 1 ophthalmologist |
| De Almeida Jr 2021 | Prospective, single‐centre, case‐control | LASIK or PRK candidates and KC | Training 777 eyes, validation 237 eyes | Brazil | Pentacam | Support vector machine | 1 cornea specialist |
| Elsawy 2021 | Prospective, single‐centre, case‐control | Control group (healthy, dry eye, Fuchs' endothelial dystrophy) and KC | 158,220 images (879 eyes, 478 participants): training 134,460, validation 23,760 | USA | Envisu R2210 (AS‐OCT) | Neural network | 6 cornea specialists |
| Feizi 2016 | Prospective, single‐centre, case‐control | Refractive surgery candidates, subclinical KC, and manifest KC | 210 eyes (207 participants) | Iran | Galilei Dual Scheimpflug Analyzer | Logistic regression | Unclear |
| Gairola 2022 | Retrospective, single‐centre, case‐control | Healthy controls and KC | 2224 images | India | Topography (Keratron and KC‐smart device) | Convolutional neural network | 1 ophthalmologist |
| Gao 2022 | Retrospective, single‐centre, case‐control | Healthy controls, KC, and subclinical KC | 1040 images (208 participants) | China | Pentacam | Neural network | Unclear |
| Ghaderi 2021 | Retrospective, single‐centre, case‐control | Healthy controls and KC (single‐centre database) | 450 eyes (separated into training, validation, and test sets) | Iran | Pentacam | Ensemble learning system | Unclear |
| Issarti 2019 | Retrospective, single‐centre, case‐control | Healthy controls and KC (single‐centre database) | 851 eyes | Belgium | Pentacam | Feedforward neural network | 1 ophthalmologist, 1 optometrist |
| Issarti 2020 | Retrospective, multicentre, case‐control | Healthy controls and KC (multicentre database) | 812 eyes | Belgium | Pentacam | Feedforward neural network | 1 ophthalmologist, 1 optometrist |
| Kalin 1996 | Prospective, consecutive, cross‐sectional study | Refractive surgery candidates and KC | 106 eyes (53 participants) | USA | TMS‐1 | Binary decision tree | 1 ophthalmologist |
| Kamiya 2019 | Retrospective, single‐centre, case‐control | Refractive surgery candidates, contact lens fitting candidates, and KC | 543 eyes | Japan | CASIA SS‐1000 | Convolutional neural network | Cornea specialists |
| Kamiya 2021 | Retrospective, single‐centre, case‐control | Refractive surgery candidates, contact lens fitting candidates, and KC | 349 eyes | Japan | TMS‐4 topographer | Convolutional neural network | Cornea specialists |
| Kojima 2020 | Retrospective, multicentre, case‐control | Healthy controls and KC | 329 eyes | Japan | Auto‐keratometer | Logistic regression | 2 cornea specialists |
| Kojima 2021 | Retrospective, single‐centre, case‐control | Healthy controls, KC, and subclinical KC | 647 eyes (335 participants) | Japan | Auto‐keratometer (ARK‐1) | Regression algorithm | 2 cornea specialists |
| Kovacs 2016 | Retrospective, single‐centre, case‐control | Refractive surgery candidates, normal eye of unilateral KC, and KC | 135 eyes: training 70%, test set 30% | Hungary | Pentacam | Neural network | Unclear |
| Kuo 2020 | Retrospective, single‐centre, case‐control | Refractive surgery candidates and KC | 354 images (206 participants) | Taiwan | TMS‐4 | Convolutional neural network | 4 cornea specialists |
| Lavric 2021 | Retrospective, case‐control | Controls and KC | 5881 eyes (2800 participants) | Brazil | Pentacam | Support vector machine | Unclear |
| Lopes 2018 | Retrospective, multicentre, case‐control | LASIK cases, post‐LASIK ectasia, and KC | 3648 eyes | USA, Brazil, UK, Italy | Pentacam | Random forest | 1 cornea specialist |
| Lucena 2021 | Retrospective, case‐control | Control group and KC | 1172 images: training 960, test set 212 | Brazil | Topographers | Convolutional neural network | 1 cornea specialist |
| Maeda 1994 | Single‐centre, case‐control | Control group and KC | 200 eyes: training 100, test 100 | USA | TMS‐1 | Combined discriminant analysis and classification tree | 3 cornea specialists |
| Maeda 1995a | Single‐centre, case‐control | Control group and KC | 176 eyes (125 participants) | USA | TMS‐1 | Combined discriminant analysis and classification tree | Unclear |
| Maeda 1995b | Single‐centre, case‐control | Control group and KC | 183 eyes: training 108, test set 75 | USA | TMS‐1 | Neural network | Unclear |
| Mohammadpour 2022 | Prospective, diagnostic test accuracy study | Healthy controls, subclinical KC, and manifest KC | 217 eyes (212 participants) | Iran | Sirius | Neural network | 2 cornea specialists |
| Mahmoud 2013 | Retrospective, multicentre, case‐control | Healthy controls and KC | 407 eyes | Colombia, Switzerland, USA | Galilei Dual Scheimpflug‐Placido tomographer | Logistic regression | Unclear |
| Mahmoud 2021 | Case‐control | Healthy controls and KC | 250 eyes | Unclear | CASIA SS‐1000 | Convolutional neural network | 1 ophthalmologist |
| Pavlatos 2020 | Prospective, multicentre, case‐control | Healthy controls, subclinical KCT, and manifest KC | 215 eyes | USA, China | OCT RTVue or Avanti | CTN index | Unclear |
| Rabinowitz 1999 | Retrospective, single‐centre, case‐control | Healthy controls and KC | 281 participants | USA | TMS‐1 | KISA% index | Unclear |
| Ruiz 2016 | Retrospective, single‐centre, case‐control | Healthy controls, refractive surgery candidates, irregular astigmatism, subclinical KC, and manifest KC | 860 eyes | Belgium | Pentacam | Support vector machine | 1 cornea specialist, 1 optometrist |
| Ruiz 2017 | Retrospective, multicentre, case‐control | Healthy controls, post‐refractive surgery candidates, subclinical KC, and manifest KC | 131 eyes (102 participants) | Belgium, France | Topographers | Support vector machine | Unclear |
| Saad 2014 | Prospective, single‐centre, case‐control | Refractive surgery candidates, subclinical KC, and manifest KC | 166 eyes | France | Orbscan IIz | Discriminant analysis | 1 cornea specialist |
| Saad 2016 | Prospective, single‐centre, case‐control | Refractive surgery candidates, subclinical KC, and manifest KC | 119 eyes (176 participants) | France | Placido disk topographer | Discriminant analysis | Unclear |
| Saika 2013 | Single‐centre case‐control | Healthy controls, LASIK candidates, subclinical KC, and manifest KC | 212 eyes | Japan | Placido disk topographer | Linear discriminant analysis | Unclear |
| Shetty 2015 | Retrospective, single‐centre, case‐control | Healthy controls and KC | 128 eyes | India | Pentacam | Logistic regression | Unclear |
| Shi 2020 | Prospective, single‐centre, case‐control | Healthy controls and KC | 121 eyes (121 participants) | China | Scheimpflug and UHR‐OCT | Neural network | 2 cornea specialists |
| Sideroudi 2017 | Prospective, cross‐sectional, non‐randomized observational study | Refractive surgery candidates, subclinical KC, and manifest KC | 185 eyes (185 participants) | Greece | Pentacam | Logistic regression | Unclear |
| Smadja 2013 | Retrospective, single‐centre, case‐control | Refractive surgery or routine ophthalmic examination, referrals, subclinical KC, and manifest KC | 372 eyes (197 participants) | France | Galilei rotating Scheimpflug tomography | Tree classification | Unclear |
| Smolek 1997 | Retrospective, single‐centre, case‐control | Normal, with‐the‐rule astigmatism, KC, subclinical KC, contact lens‐induced corneal warpage, pellucid marginal degeneration, PRK, radial keratotomy, and keratoplasty | 300 examinations (150 training, 150 test) | USA | TMS‐1 | Neural network | Unclear |
| Souza 2010 | Retrospective, single‐centre, case‐control | Healthy controls, astigmatism, photorefractive keratectomy, and KC | 318 participants | Brazil | Orbscan IIz | Support vector machine | Unclear |
| Subramaniam 2022 | Case‐control study | Healthy controls, subclinical KC, and manifest KC | 1500 images | India | Topography images synthesized with SyntEye | Convolutional neural network | Unclear |
| Twa 2005 | Retrospective, single‐centre, case‐control | Refractive surgery candidates and KC | 224 eyes | USA | Topography | Decision tree | Unclear |
| Xie 2020 | Retrospective, observational | Refractive surgery candidates, KC | 6465 images (1385 participants) | China | Pentacam | Convolutional neural network | 3 ophthalmologists |
| Xu 2017 | Prospective, single‐centre, cross‐sectional | Healthy controls, subclinical KC, and manifest KC | 363 eyes (363 participants) | China | Pentacam | Discriminant analysis | 2 ophthalmologists |
| Xu 2022a | Retrospective, single‐centre, case‐control | Healthy controls and subclinical KC | 92 eyes (80 participants) | China | Sirius | Logistic regression | Unclear |
| Yang 2021 | Cross‐sectional, observational | Healthy controls, refractive surgery candidates, subclinical KC, and manifest KC | 176 eyes (124 participants) | USA | OCT | Decision tree (2‐step) | Unclear |
| Yousefi 2018 | Retrospective, multicentre, case‐control | Healthy controls and KC | 3156 participants | Japan, USA | CASIA OCT | Density‐based clustering | Unclear |
| Zeboulon 2020a | Retrospective, case‐control | Healthy controls, refractive surgery candidates, subclinical KC, and manifest KC | 3000 examinations | France | Orbscan | Convolutional neural network | 1 ophthalmology resident, 1 corneal tomography expert |
| Zeboulon 2020b | Retrospective, case‐control | Healthy controls, history of myopic refractive surgery, Fuchs' corneal dystrophy, and KC | 6979 participants | France | Orbscan | Convolutional neural network | 1 ophthalmology resident, 1 corneal tomography expert |
AS‐OCT: anterior segment optical coherence tomography; CTN index: Coincident Thinning Index; KC: keratoconus; KISA% index: keratoconus percentage index, derived from central keratometry, the inferior‐superior value, the astigmatism index, and the SRAX index, an expression of irregular astigmatism occurring in keratoconus; LASIK: laser‐assisted in situ keratomileusis; OCT: optical coherence tomography; PRK: photorefractive keratectomy; TMS: Topographic Modeling System; UHR‐OCT: ultrahigh‐resolution optical coherence tomography.
The instruments used were Pentacam, Eyesys, Sirius, Orbscan IIz, CSO, RTVue, Envisu R2210, Galilei, Topographic Modeling System (TMS), and CASIA. All devices belonged to one of the groups described in the Reference standards section.Consejo 2020 used the Corvis‐ST. In the protocol for this review, we specified that we would not include any devices measuring biomechanical properties (Vandevenne 2021); however, only the first image of each measurement was used for analysis in Consejo 2020. This image is taken before the air stimulus and is the same as a Scheimpflug‐based measurement. The studies described 12 different AI algorithms.
The most frequently used algorithm was the neural network; 11 studies used a simple neural network, and 13 studies used the convolutional neural network. The second most common algorithm was logistic regression (N = 10). Seven studies each used decision tree, discriminant analysis, and support vector machine. The right‐hand column of Table 2 shows the number of cornea specialists. Thirty studies did not provide information about who made the keratoconus diagnosis or how they made it.
Detection of manifest keratoconus
Figure 5 shows the summary ROC (SROC) plot of sensitivity and specificity of the AI algorithms for detecting manifest keratoconus (54 studies, 50,519 eyes/images). Sensitivities range from 76% to 100%, and the summary sensitivity is 98.6% (95% CI 97.6% to 99.1%). Specificities range from 82% to 100%, and the summary specificity is 98.3% (95% CI 97.4% to 98.9%). Most studies are clustered in the upper‐left corner of the graph, indicating a high accuracy of the tested algorithms to diagnose keratoconus. There appears to be little heterogeneity between the studies. The confidence ellipse of the summary point lies close to the upper‐left angle of the ROC plane and is hidden behind the symbols of most studies. The prediction interval is larger, which seems to be attributable mainly to three large studies with lower accuracy.
5.

Summary receiver operating characteristics (SROC) plot of accuracy of AI for detecting manifest keratoconus.
Detection of subclinical keratoconus
Figure 6 shows the SROC plot of sensitivity and specificity of the AI algorithms for detecting subclinical keratoconus (28 studies, 9508 eyes/images). Sensitivities range from 47% to 100%, and the summary sensitivity is 90.0% (95% CI 84.5% to 93.8%). Specificities range from 54% to 100%, and the summary specificity is 95.5% (95% CI 91.9% to 97.5%). More than half of the studies are near the y‐axis of the SROC plot, indicating high specificity. A few studies are located around the upper left corner. However, the distribution of the dots is fairly wide, indicating high heterogeneity between the studies, particularly for sensitivity.
6.

Summary receiver operating characteristics (SROC) plot of accuracy of AI for detecting subclinical keratoconus.
Detection of mixed keratoconus
We analysed the studies that developed and trained algorithms to diagnose and distinguish both manifest and subclinical keratoconus. The corresponding SROC plot includes 11 studies (Figure 7). Sensitivities range from 75% to 100%, and the summary sensitivity is 96.2% (95% CI 92.5% to 98.1%). Specificities range from 51% to 100%, and the summary specificity is 98.0% (95% CI 92.6% to 99.5%). There is a wide distribution of the studies in the curve. Accuracy is almost perfect in five studies, and sensitivity or specificity is high in two other studies.
7.

Summary receiver operating characteristics (SROC) plot of accuracy of AI for detecting subclinical and manifest keratoconus (mixed).
Subgroup analyses
We conducted subgroup analyses, restricted to manifest keratoconus with adequate numerosity, by study design (clinical series versus registry), AI algorithm (as listed in the secondary objectives), imaging technique (tomography, topography, OCT), and data source (parameters, images). None of these covariates were associated with accuracy (Table 3).
2. Subgroup analyses.
| Subgroups |
No. of studies (participants) |
Sensitivity (95% CI) |
P‐value for relative sensitivity |
Specificity (95% CI) |
P‐value for relative specificity | |
| Study design | Clinical series | 46 (38,788) | 0.987 (0.977, 0.993) |
Reference | 0.984 (0.975, 0.993) |
Reference |
| Registries | 8 (11,731) | 0.975 (0.919, 0.993) |
0.458 | 0.975 (0.936, 0.990) |
0.464 | |
| AI algorithm | Logistic regression | 8 (2,889) | 0.983 (0.957, 0.993) |
Reference | 0.992 (0.974, 0.997) |
Reference |
| Bayesian network | 3 (788) | 0.994 (0.972‐0.999) |
0.260 | 0.982 (0.834, 0.998) |
0.666 | |
| Convolutional neural network | 13 (13,452) | 0.979 (0.945‐0.991) |
0.734 | 0.978 (0.960, 0.988) |
0.110 | |
| Discriminant analysis | 3 (462) | 0.977 (0.945, 0.990) |
0.628 | 1.000 (0.814, 1.000) |
0.093 | |
| Decision tree | 5 (8,96) | 0.976 (0.895, 0.995) |
0.731 | 0.978 (0.935, 0.993) |
0.299 | |
| Neural network | 10 (16,296) | 0.973 (0.920, 0.991) |
0.561 | 0.968 (0.931, 0.986) |
0.093 | |
| Other | 6 (4,338) | 0.990 (0.892, 0.999) |
0.629 | 0.968 (0.931, 0.987) |
0.068 | |
| Random forest | 2 (3,487) | 1.000 (0, 1.000) | 0.038 | 0.997 (0.994, 0.999) |
0.270 | |
| SVM | 4 (7,911) | 0.994 (0.982, 0.998) |
0.203 | 0.993 (0.928, 0.999) |
0.916 | |
| Imaging technique | OCT | 6 (19,585) | 0.971 (0.941, 0.985) |
Reference | 0.984 (0.885, 0.998) |
Reference |
| Tomography | 26 (27,267) | 0.993 (0.985, 0.996) |
0.042 | 0.986 (0.976, 0.992) |
0.910 | |
| Topography | 21 (3,579) | 0.965 (0.931, 0.983) |
0.744 | 0.978 (0.958, 0.989) |
0.756 | |
| Data type | Images | 13 (27,532) | 0.980 (0.950, 0.992) |
Reference | 0.975 (0.947, 0.988) |
Reference |
| Parameters | 39 (22,792) | 0.987 (0.976, 0.947) |
0.461 | 0.984 (0.975, 0.990) |
0.342 | |
CI: confidence interval.
Sensitivity analyses
We conducted sensitivity analyses by excluding the 14 studies that used images as the unit of analyses (as this inflated the number of observations several times), and we found that sensitivity and specificity remained very high for both manifest keratoconus (98.5%, 95% CI 97.4% to 99.1%) and subclinical keratoconus (98.5%, 95% CI 97.5% to 99.1%). As most studies were unclear on the unit of analysis, and only 16 of the remaining 49 studies clearly stated that they analysed one eye per participant, we did not run additional sensitivity analyses.
Discussion
Summary of main results
In this DTA review, we investigated the accuracy of AI algorithms for diagnosing keratoconus. These automated tools could help ophthalmologists, optometrists, and other eye care professionals to recognize the disease and refer people in time. An early diagnosis of keratoconus is beneficial for the patient as it leads to regular follow‐up and thus detection and treatment of progression before visual acuity has decreased. AI could be used in a primary or secondary eye care setting as a triage test for people seeking refractive surgery or people whose visual acuity cannot be fully corrected with glasses. Studies investigating these tools should aim for high sensitivity so the AI algorithm can correctly detect as many people with keratoconus as possible.
We included al large number of studies (63), although 58 had a case‐control design, and the population of most was not as described in our protocol (Vandevenne 2021), leading to high risk of bias and low applicability in the domain of 'Patient selection'.
We found the diagnostic accuracy achieved with AI algorithms was almost perfect for detecting manifest keratoconus; however, the certainty of evidence was low for sensitivity and specificity. The main issue was the case‐control design for all but five studies, which may have led to an overestimation of accuracy. Moreover, while the confidence region is very narrow for detection of manifest keratoconus, the prediction area in the SROC curve is rather large because some studies (including two large studies) had lower sensitivity and specificity (Elsawy 2021, Zeboulon 2020b). The methodological quality of these studies was low, and we could not investigate whether they differed in the population or index test due to poor reporting. However, a possible explanation for the lower sensitivity and specificity in Elsawy 2021 could be that it developed a multidisease algorithm.
The diagnostic accuracy of AI algorithms for subclinical keratoconus may be suboptimal. The summary sensitivity of the 28 studies was 90.0% (95% CI 84.5% to 93.8%). The evidence was of very low certainty due to indirectness (the population did not match our definition) and high risk of selection and other biases (case‐control design with poor generalizability). Because a missed diagnosis of subclinical keratoconus in people seeking refractive surgery could lead to iatrogenic ectasia, future studies should maximize sensitivity rather than specificity.
Few studies tested AI algorithms for detection of mixed (both subclinical and manifest) keratoconus, and the distribution of accuracy measures in the SROC plot was very heterogeneous. We were unable to draw any firm conclusions due to the low number of studies.
Subgroup analyses
As stated in our protocol, we aimed to investigate different causes of heterogeneity (Vandevenne 2021); however, it was not feasible to examine all the predefined factors because of poor reporting in the included studies and because the subgroups were too small. We were only able to perform the following subgroup analysis of manifest keratoconus.
Study design (clinical series and registries): there were no differences between the two subgroups.
Different AI algorithms (of which there were 10): we found no evidence of a difference in accuracy between the different AI algorithms. This could indicate that different algorithms are suitable for detecting keratoconus based on cornea imaging. However, four subgroups included fewer than five studies, and the number of participants in two subgroups was small.
Imaging technique: we found no differences between topography‐, tomography‐, or OCT‐based AI algorithms.
Data source (images versus parameters): there were no subgroup differences.
Strengths and weaknesses of the review
We conducted a comprehensive search; because AI in keratoconus is a very focused topic, it is unlikely that we missed any studies. However, we only included articles published in English. We did not include ongoing studies because it was unclear whether they were still in process. When the two review authors (MV and EF) did not agree on the eligibility of an article, they asked the opinion of the rest of the review team.
The extracted data were sometimes limited due to poor reporting in the articles, especially with regard to the reference standard and comparison between AI algorithms within a study. Almost half of the studies were at unclear risk of bias with regard to who made the initial diagnosis and how they made it.
Methods for quality assessment of directly comparative test accuracy studies have been developed, but this type of analysis was not feasible in our review (Yang 2021). Few articles presented accuracy estimates for multiple AI algorithms, and we selected the algorithm with the highest Youden's index. We are aware that this selection may inflate accuracy, especially in smaller studies. The fact that all AI algorithms yielded very high and similar accuracy in subgroup analyses may support our approach.
We could not manage unit of analysis issues appropriately because data for participants (e.g. only one eye) were rarely available, and many studies analysed both eyes or multiple images of the same participant, or the unit of analysis was unclear. However, accuracy remained high even after the exclusion of studies with important unit of analysis issues (i.e. those that analysed multiple images per eye instead of eyes or participants).
The sensitivity analyses suggested that the covariates listed in Secondary objectives had no effect on accuracy. Unfortunately, we were unable to determine the sources of heterogeneity. Subgroup analyses showed no differences between the studies based on study design, AI algorithm, imaging technique, or data source. We were unable to carry out HSROC models to assess specificity at high sensitivity (as necessary for a triage role) due to clustering of the data at the upper left corner of the SROC plot.
Finally, a few studies had zero counts, especially for false positives and false negatives, and this may have introduced estimation bias.
Comparison with previous research
There are published reviews on AI in ophthalmology, AI in anterior segment diseases, and AI in keratoconus. Hogarty 2019 provides a general overview of relevant AI algorithms and new developments in ophthalmology. Lin 2019 specifically investigates AI for keratoconus and refractive surgery screening, but only provides an overview of the literature. These two reviews concluded that AI is a promising technique to process corneal imaging data and detect keratoconus, but as they were narrative reviews with no meta‐analysis, their results are not comparable with ours. Cao 2022 evaluated the accuracy of different AI algorithms in a meta‐analysis. It found 30 articles detecting manifest keratoconus with a summary sensitivity of 97% (95% CI 94.9% to 98.2%) and a summary specificity of 98.5% (95% CI 97.1% to 99.3%). Our results were very similar; however, our summary sensitivity was slightly higher. Cao 2022 found 15 articles detecting subclinical keratoconus with a summary sensitivity of 88.2% (95% CI 82.2% to 92.3%) and a summary specificity of 94.7% (95% CI 91.4% to 96.7%). The summary sensitivity in our review was slightly higher, but the sensitivity was similar. Based on their results, the authors of Cao 2022 concluded that AI algorithms are not yet applicable in a clinical setting, and more research is needed.
Applicability of findings to the review question
We defined the eligible population as people with refractive errors:
whose vision could not be fully corrected with glasses; or
who were seeking refractive surgery, or
who had suspected keratoconus (for whom a decision was to be made on referral to cornea specialists).
Figure 3 shows that 'Patient selection' is the most problematic domain for applicability. This is due to the case‐control design of most studies, which included people already diagnosed with keratoconus. According to the clinical pathway described in our protocol, the AI algorithm could serve as a triage test for eye care professionals, such as ophthalmologists, optometrists, and others, to detect keratoconus and refer patients to a cornea specialist for appropriate diagnosis, follow‐up, and treatment (Vandevenne 2021). However, the applicability of AI is questionable in this population; further research is needed to confirm the reliability of this approach.
Authors' conclusions
Implications for practice.
We found that artificial intelligence (AI) may be very accurate for detecting keratoconus, but there was some heterogeneity. A local assessment of AI accuracy is advisable wherever this technique is implemented, particularly for detection of subclinical keratoconus. It is important to interpret the results of this review with caution: there is a high risk for overestimation of accuracy due to the case‐control design of most included studies.
The goal of AI algorithms is to help eye care professionals to make a medical decision for individuals. If found to be accurate, AI algorithms could be used in a triage setting to detect subclinical keratoconus before refractive surgery. When subclinical keratoconus is detected by the AI algorithm based on corneal imaging, the patient can be referred to a cornea specialist to receive proper follow‐up and treatment. If a diagnosis is missed and the patient receives refractive surgery, there is a high chance they will develop iatrogenic ectasia. People whose vision cannot be fully corrected with glasses should receive corneal imaging. The AI algorithm could help the eye care professional to detect whether keratoconus is present. Positive cases would be referred to a cornea specialist. If the diagnosis is missed, there is a chance of progression and eventually the need for corneal transplantation. The impact of a missed diagnosis is much higher than that of a false diagnosis. While a missed diagnosis would lead to visual impairment, a false diagnosis would entail referral to a cornea specialist, where the patient would undergo some additional non‐invasive examinations.
Implications for research.
AI could be a solution for consistent and unbiased diagnoses; however, the accuracy of the tests for detecting keratoconus – especially subclinical keratoconus – needs to be evaluated in higher‐quality studies. The ideal study would include a consecutive cohort of people seeking ophthalmic care or refractive surgery. The following changes would improve the evidence in this area.
Future studies should have access to larger and more diverse data sets that provide a more representative picture of clinical practice, to validate their results. This is a hurdle every researcher encounters when developing an AI‐based algorithm in medicine. An important problem is patient privacy: there is a need for a large, international, anonymized database with corneal imaging data.
Researchers should establish standardized methodology to develop and validate AI algorithms for detecting keratoconus (or any other disease). This should make it easier to compare studies and draw clear conclusions about the accuracy and applicability of the AI algorithms.
AI algorithms should be platform‐independent so they can analyse data from different imaging devices.
A clear global consensus on the definition of (subclinical) keratoconus and progression would facilitate test development and comparison between studies.
History
Protocol first published: Issue 12, 2021
Acknowledgements
We would like to thank:
Cochrane Eyes and Vision (CEV) for creating and running the electronic searches;
Roy Schwartz for his comments on this protocol;
Vito Romano for his comments on the protocol and review;
the Diagnostic Test Accuracy team for their comments on the protocol and review;
Anupa Shah, Managing Editor of Cochrane Eyes and Vision, for her assistance throughout the review process;
the Cochrane Central Editorial Service for completing the editorial processing upon transfer from the Eyes and Visions review group; and
Julia Turner, copy editor, Cochrane Central Production Service, for her comments and editing of this review.
Appendices
Appendix 1. Glossary of terms
Asphericity: a measure of corneal shape and how it affects the refraction of light Astigmatism: refractive error due to an abnormal shape of the cornea BAD‐D: Belin‐Ambrósio Enhanced Ectasia Display total deviation Curvature: the rate of change of direction of a curve with respect to distance along the curve Dioptre (D): a unit of measurement for the strength of a lens (i.e. the light breaking ability of a lens) Iatrogenic ectasia: weakening of the biomechanical stability of the cornea due to surgery, which leads to the development of a keratoconus‐like ectasia Keratometry: the measurement of the corneal radius of curvature Kmax: maximum keratometry expressed in dioptres Pachymetry: corneal thickness Tomography: imaging by sections, able to describe the anterior and posterior surface of an object Topography: imaging and description of the features of a surface
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Keratoconus] this term only #2 keratoconus* #3 cornea* near/5 ectatic* #4 cornea* near/5 ectasia #5 conical near/2 cornea* #6 cornea* near/2 thinning #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 #8 MeSH descriptor: [Artificial Intelligence] this term only #9 MeSH descriptor: [Deep Learning] this term only #10 MeSH descriptor: [Machine Learning] explode all trees #11 MeSH descriptor: [Neural Networks, Computer] this term only #12 MeSH descriptor: [Algorithms] this term only #13 MeSH descriptor: [Decision Trees] this term only #14 MeSH descriptor: [Automation] this term only #15 MeSH descriptor: [Databases, Factual] this term only #16 MeSH descriptor: [Electronic Data Processing] this term only #17 artificial NEAR/1 intelligence #18 (deep or machine) NEAR/2 learning #19 vector NEAR/3 machine #20 AI or DL or DLS #21 (deep or convolutional or neural) NEAR/3 network* #22 automat* NEAR/2 (screen* or detect* or diagnos* or algorithm* or identif* or grading or graded or method*) #23 Bagging #24 Naive NEAR/1 Bayes #25 Multilayer NEAR/1 Perceptron #26 (multi‐layer NEAR/1 perceptron) or MLP #27 Radial NEAR/1 Basis NEAR/1 Function #28 Random NEAR/1 Forest #29 Ensemble NEAR/1 Selection #30 (Ada or gradient) NEAR/1 boost* #31 LASSO #32 Elastic NEAR/1 Net #33 genetic NEAR/1 algorithm* #34 (decision or classification or regression or probability or model*) NEAR/3 tree* #35 logistic* NEAR/2 regression NEAR/15 learn* #36 augment* NEAR/1 clinical NEAR/1 decision* NEAR/1 mak* #37 nearest NEAR/1 (neighbor or neighbour) #38 fuzzy NEAR/3 (logit or logic or logistic) #39 kernel #40 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 #41 #7 AND #40
Appendix 3. MEDLINE Ovid search strategy
1. Keratoconus/ 2. keratoconus$.tw. 3. (cornea$ adj5 ectatic$).tw. 4. (cornea$ adj5 ectasia).tw. 5. (conical adj2 cornea$).tw. 6. (cornea$ adj2 thinning).tw. 7. or/1‐6 8. artificial intelligence/ 9. deep learning/ 10. exp machine learning/ 11. "neural networks (computer)"/ 12. fuzzy logic/ 13. algorithms/ 14. decision tree/ 15. automation/ 16. databases, factual/ 17. information processing/ 18. (artificial adj1 intelligence).tw. 19. ((deep or machine) adj2 learning).tw. 20. (vector adj3 machine).tw. 21. (AI or DL or DLS).tw. 22. ((deep or convolutional or neural) adj3 network$).tw. 23. (automat$ adj2 (screen$ or detect$ or diagnos$ or algorithm$ or identif$ or grading or graded or method$)).tw. 24. Bagging.tw. 25. (Naive adj1 Bayes).tw. 26. (Multilayer adj1 Perceptron).tw. 27. ((multi‐layer adj1 perceptron) or MLP).tw. 28. (Radial adj1 Basis adj1 Function).tw. 29. (Random adj1 Forest).tw. 30. (Ensemble adj1 Selection).tw. 31. ((Ada or gradient) adj1 boost$).tw. 32. LASSO.tw. 33. (Elastic adj1 Net).tw. 34. (genetic adj1 algorithm$).tw. 35. ((decision or classification or regression or probability or model$) adj3 tree$).tw. 36. (logistic$ adj2 regression adj15 learn$).tw. 37. (augment$ adj1 clinical adj1 decision$ adj1 mak$).tw. 38. (nearest adj1 (neighbor or neighbour)).tw. 39. (fuzzy adj3 (logit or logic or logistic)).tw. 40. kernel.tw. 41. or/8‐40 42. 7 and 41
Appendix 4. Embase Ovid search strategy
1. keratoconus/ 2. keratoconus$.tw. 3. (cornea$ adj5 ectatic$).tw. 4. (cornea$ adj5 ectasia).tw. 5. (conical adj2 cornea$).tw. 6. (cornea$ adj2 thinning).tw. 7. or/1‐6 8. artificial intelligence/ 9. deep learning/ 10. machine learning/ 11. supervised machine learning/ or support vector machine/ or unsupervised machine learning/ 12. perceptron/ 13. artificial neural network/ 14. convolutional neural network/ 15. deep neural network/ 16. automated pattern recognition/ 17. decision tree/ 18. detection algorithm/ 19. learning algorithm/ 20. classification algorithm/ 21. data classification/ 22. disease classification/ 23. disease simulation/ 24. automation/ 25. information processing/ 26. feature extraction/ 27. bayesian learning/ 28. fuzzy system/ 29. k nearest neighbor/ 30. kernel method/ 31. random forest/ 32. (artificial adj1 intelligence).tw. 33. ((deep or machine) adj2 learning).tw. 34. (vector adj3 machine).tw. 35. (AI or DL or DLS).tw. 36. ((deep or convolutional or neural) adj3 network$).tw. 37. (automat$ adj2 (screen$ or detect$ or diagnos$ or algorithm$ or identif$ or grading or graded or method$)).tw. 38. Bagging.tw. 39. (Naive adj1 Bayes).tw. 40. (Multilayer adj1 Perceptron).tw. 41. ((multi‐layer adj1 perceptron) or MLP).tw. 42. (Radial adj1 Basis adj1 Function).tw. 43. (Random adj1 Forest).tw. 44. (Ensemble adj1 Selection).tw. 45. ((Ada or gradient) adj1 boost$).tw. 46. LASSO.tw. 47. (Elastic adj1 Net).tw. 48. (genetic adj1 algorithm$).tw. 49. ((decision or classification or regression or probability or model$) adj3 tree$).tw. 50. (logistic$ adj2 regression adj15 learn$).tw. 51. (augment$ adj1 clinical adj1 decision$ adj1 mak$).tw. 52. (nearest adj1 (neighbor or neighbour)).tw. 53. (fuzzy adj3 (logit or logic or logistic)).tw. 54. kernel.tw. 55. or/8‐54 56. 7 and 55
Appendix 5. OpenGrey search strategy
keratoconus AND (Artificial intelligence OR deep learning OR machine learning)
Appendix 6. ISRCTN search strategy
keratoconus AND (Artificial intelligence OR deep learning OR machine learning)
Appendix 7. ClinicalTrials.gov search strategy
keratoconus AND (Artificial intelligence OR deep learning OR machine learning)
Appendix 8. WHO ICTRP search strategy
keratoconus AND Artificial intelligence OR keratoconus AND deep learning OR keratoconus AND machine learning
Appendix 9. QUADAS 2 guidance
| DOMAIN | Low risk/concern | Unclear | High risk/concern |
| PATIENT SELECTION | Describe methods of patient selection; describe included patients (prior testing, presentation, intended use of index test and setting): | ||
| Was a consecutive or random sample of patients enroled? | Consecutive sampling or random sampling seeking refractive error correction or refractive surgery in eye services. | Unclear whether consecutive or random sampling used. | Selection of non‐consecutive patients. |
| Was a case‐control design avoided? | No selective recruitment of people with or without keratoconus. | Unclear selection mechanism. | Selection of either cases or control in a predetermined, non‐random fashion; or enrichment of the cases from a selected population. |
| Did the study avoid inappropriate exclusions? | Exclusions are detailed and felt to be appropriate (e.g. people already diagnosed with keratoconus or with other corneal diseases). | Exclusions are not detailed (pending contact with study authors). | Inappropriate exclusions are reported (e.g. of people with borderline index test results). |
| Risk of bias: could the selection of patients have introduced bias? | 'No' for any of the above | ||
| Concerns regarding applicability: are there concerns that the included patients do not match the review question? | Inclusion of patients seeking refractive error correction or refractive surgery in primary or secondary care eye services. | Unclear inclusion criteria. | Inclusion of patients attending cornea services for known disease, population‐based studies, registry‐based studies. |
| INDEX TEST | Describe the index test and how it was conducted and interpreted: | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Test performed "blind" or "independently and without knowledge of" reference standard results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding. | Unclear whether results are interpreted independently. | Reference standard results available to those who conducted or interpreted the index test. |
| If a threshold was used, was it prespecified? | The study authors declare that the selected cut‐off used to dichotomize data was specified a priori, or a protocol is available with this information. | No information on preselection of index test cut‐off values. | A study is classified at higher risk of bias if the authors define the optimal cut‐off post hoc based on their own study data. |
| Risk of bias: could the conduct or interpretation of the index test have introduced bias? | 'No' for any of the above. | ||
| Concerns regarding applicability: are there concerns that the index test, its conduct, or interpretation differ from the review question? | Tests used and testing procedure clearly reported and tests executed by personnel with sufficient training. | Unclear execution of the tests or unclear study personnel profile, background, and training. | Tests used are not validated, or study personnel is insufficiently trained. |
| REFERENCE STANDARD | Describe the reference standard and how it was conducted and interpreted: | ||
| Is the reference standard likely to correctly classify the target condition? | Topography and/or tomography interpreted independently by 2 or more cornea specialists. | Topography and/or tomography interpreted by cornea specialists, but not enough details to adjudicate 'yes' or 'no'. | Topography and/or tomography interpreted by only one cornea specialist. |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Reference standard performed "blind" or "independently and without knowledge of" index test results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding. | Unclear whether results are interpreted independently. | Index test results available to those who conducted the reference standard. |
| Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias? | 'No' for any of the above. | ||
| Concerns regarding applicability: are there concerns that the target condition as defined by the reference standard does not match the review question? | Same or similar definition of the target condition as described in the protocol. | Unclear definition of the target disease diagnosed by the reference standard. | Different definition of the target condition as defined in the protocol. |
| FLOW AND TIMING | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard. | ||
| Was there an appropriate interval between index test(s) and reference standard? | No more than three months between index and reference test execution. | — | More than three months between index and reference test execution. |
| Did all patients receive a reference standard? | All participants receiving the index test are verified with the reference standard. | — | Not all participants receiving the index test are verified with the reference standard. |
| Did all patients receive the same reference standard? | Not applicable for this review. | ||
| Were all patients included in the analysis? | The number of participants included in the study matches the number in analyses, or participants with undefined or borderline test results are excluded. | — | The number of participants included in the study does not match the number in analyses, or participants with undefined or borderline test results are excluded. |
| Risk of bias: could the patient flow have introduced bias? | 'No' for any of the above, | ||
| ADDITIONAL QUESTIONS | These questions concern the direct comparisons between AI tests, | ||
| Were different AI tests developed and interpreted without knowledge of each other? | Different AI tests were developed and interpreted "blind" or "independently and without knowledge of" the results of each other. | — | Different AI tests were developed or their results interpreted with knowledge of the results of each other. |
| Are the proportions and reasons for missing data similar for all index tests? | Missing data and their causes were similar for each AI test. | — | The amount of missing data or their causes differed between AI tests. |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Artificial intelligence (all studies) | 63 | 56364 |
| 2 Artificial intelligence (manifest keratoconus) | 54 | 50519 |
| 3 Artificial intelligence (subclinical keratoconus) | 28 | 9508 |
| 4 Artificial intelligence (mixed) | 11 | 11644 |
1. Test.

Artificial intelligence (all studies)
2. Test.

Artificial intelligence (manifest keratoconus)
3. Test.

Artificial intelligence (subclinical keratoconus)
4. Test.

Artificial intelligence (mixed)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelmotaal 2020.
| Study characteristics | |||
| Patient Sampling | Single‐centre, retrospective, case‐control study. Scheimpflug tomographic (Pentacam (Oculus GmbH, Wetzlar, Germany)) images obtained from non‐consecutive refractive surgery candidates, people with unilateral or bilateral keratoconus, or subclinical keratoconus in Egypt (3218 images from 3218 eyes of 1669 people). | ||
| Patient characteristics and setting |
|
||
| Index tests | Convolutional neural network (CNN) for 4‐map selectable display images. The CNN was trained with corneal colour‐coded maps of whole Scheimpflug images. | ||
| Target condition and reference standard(s) | The keratoconus class included those with a clinical diagnosis of keratoconus or an irregular cornea (as determined by distorted keratometry mires or distortion of retinoscopic red reflex, or both) and the following topographic findings.
The subclinical keratoconus class included subtle corneal topographic changes in the aforementioned keratoconus abnormalities in the absence of slit‐lamp or visual acuity changes typical of keratoconus. The cases were labelled before the analysis with the algorithm by 2 experienced corneal specialists and any disagreements were reviewed by a 3rdspecialist. |
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Accardo 2002.
| Study characteristics | |||
| Patient Sampling | Single‐centre, retrospective, case‐control study including 396 corneal topographic maps (396 eyes, 198 participants), obtained with a videokeratoscope (EyeSys, EyeSys Vision, Houston, Texas), selected from the cases of keratoconus or other conditions recorded over a 3‐year period at the centre, in Italy. | ||
| Patient characteristics and setting |
|
||
| Index tests | A neural network using as input the parameters of both eyes of the same subject and as output the 3 categories of clinical classification (normal, keratoconus, other alterations) for each subject, a low number of neurons in the hidden layer (< 10), and a learning rate of 0.1. | ||
| Target condition and reference standard(s) | Keratoconus group comprised cases of mild and moderate severity with a sagittal cone apex power < 53 D, with no clinical sign (early keratoconus) or only the Vogt's striae, and keratoconus suspects that met one of the following criteria.
The maps were classified before the analysis with the algorithm, the number of observers is unclear. |
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable. | ||
| Notes | This work was partially supported by the University of Trieste (MURST60%) and by Burlo Garofolo Hospital in Trieste, Italy. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Al‐Timemy 2021.
| Study characteristics | |||
| Patient Sampling | The study design is unclear, it seems to be case‐control study. 3794 Pentacam (Oculus GmbH, Wetzlar, Germany) corneal images from University of Sao Paulo were included and an independent validation subset with 1050 images was collected from 150 eyes of 85 subjects from a separate centre in Brazil. | ||
| Patient characteristics and setting | The criteria for keratoconus diagnosis are unclear. People with manifest keratoconus, suspected keratoconus and normal eyes were included. | ||
| Index tests | A hybrid deep learning model which integrates multiple convolutional neural network (CNN) models for detecting keratoconus based on corneal topographic maps. | ||
| Target condition and reference standard(s) | The criteria for keratoconus diagnosis are unclear. Eyes were labelled as keratoconus suspects if corneal topography included atypical, localized steepening or an asymmetrical bowtie pattern; the keratometric curvature was > 47.00 D, the oblique cylinder was > 1.50 D, the central corneal thickness was < 500 μm, BAD‐D was between 1.6 and 3.0. 3 corneal specialists performed the eye classification, before the analysis with the algorithm. | ||
| Flow and timing | All cases were included in reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported by National Institute of Health (NIH), National Eye Institute (NEI), and Bright Focus Foundation. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Almeida 2022.
| Study characteristics | |||
| Patient Sampling | Multicentre, case‐control study. All participants were examined at the Visum Eye Center and Rio Claro Eye Institute between January 2012 and January 2019. Exclusion criteria were a history of ocular trauma, corneal scarring, and neurotrophic keratopathy.
|
||
| Patient characteristics and setting |
|
||
| Index tests | Multiple logistic regression analysis (MLRA) is based on the logistic function that bounds its output within the range of 0 to 1. To build the algorithm extracted from MLRA, 22 variables were used. | ||
| Target condition and reference standard(s) | All eyes were examined by rotating Scheimpflug corneal and anterior segment tomography (Pentacam HR, Oculus Optikgerate GmbH). Image quality was checked to ensure that only cases with acceptable‐quality images were included. All cases were reviewed by an experienced fellowship‐trained corneal specialist (G.C.A.J.) for correct classification into keratoconus and VAE‐NT groups. Objective criteria for considering normal topography included objective front surface curvature metrics derived from Pentacam. Normal topography criteria were rigorously considered based on the objective criteria of a maximum keratometry curvature (Kmax; steepest front keratometry) of < 47.2 D, a paracentral I‐S asymmetry value at 6 mm (3 mm radii) of < 1.45, and a keratoconus percentage index score of < 60. An objective criterion for normal tomography criteria was adopted for the control group and the VAE‐NT group, and the maximum values were 3.8 mm for anterior chamber depth, 4 mm for front apical elevation, 5 mm for front corneal elevation at the thinnest point, and 12 mm for front corneal elevation in the central 4.0 mm. The corresponding posterior elevation values were 7 mm, 13 mm, and 25 mm. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This work was supported by the Sao Paulo State Research Support Foundation (FAPESP, grant nos: 2015/17226‐7 and 2019/04475‐0) and the National Council for Scientific and Technological Development (CNPq, grant no: 306808/2018‐8.). The funding organizations had no role in design or conduct of this research, and they have no related commercial interests. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Arbelaez 2012.
| Study characteristics | |||
| Patient Sampling | Retrospective case series. Clinical data and corneal examinations were retrieved from clinical records from 2 centres (Oman and Italy). 3502 eyes were enroled. | ||
| Patient characteristics and setting | According to the clinical diagnosis, participants were classified into the following 4 groups.
Each group was divided into a training set (including 200 eyes) to be used to develop the keratoconus detection program and a validation set (including the remaining eyes). Participants were excluded if tomography scans had poor quality. |
||
| Index tests | Classification algorithm based on support vector machine (SVM), a supervised learning technique that can be used for pattern classification. It analysed symmetry index of front and back corneal curvature, best fit radius of the front corneal surface, Baiocchi Calossi Versaci front index (BCVf) and BCV back index (BCVb), root‐mean‐square of front and back corneal surface higher order aberrations, and thinnest corneal point provided by a Scheimpflug camera combined with Placido corneal topography (Sirius, CSO, Italy). | ||
| Target condition and reference standard(s) | Unclear who performed the classification of the eyes, which was done before the analysis with the algorithm. | ||
| Flow and timing | It is unclear if all cases received the same reference standard. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Bessho 2006.
| Study characteristics | |||
| Patient Sampling | Retrospective, multicentre, case‐control study. 165 eyes of 120 subjects were included at 2 centres in Japan. | ||
| Patient characteristics and setting |
|
||
| Index tests | Fourier‐incorporated keratoconus detection Index (FKI) created performing a logistic regression analysis with a training set to differentiate the keratoconus group from the non‐keratoconus group. The index is based on information obtained by Fourier analysis from not only the anterior corneal surface but also from the posterior corneal surface and corneal thickness. | ||
| Target condition and reference standard(s) | Corneal topographic analysis was performed with a slit‐scanning corneal topographer (Orbscan II, Bausch & Lomb). It is unclear how the diagnosis was made; however, cases were classified before the inclusion. | ||
| Flow and timing | It is unclear if all cases received the same reference standard. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This study was supported in part by Grant‐in‐Aid No.15591854 for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (N. Maeda), and by a research grant from the Osaka Eye Bank Foundation, Suita, Japan (N. Maeda). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Cao 2020.
| Study characteristics | |||
| Patient Sampling | Retrospective, case‐control study including the following groups.
|
||
| Patient characteristics and setting |
|
||
| Index tests | Random forest method using 11 tomographic parameters (Pentacam) for the diagnosis of subclinical keratoconus. | ||
| Target condition and reference standard(s) | Subclinical keratoconus was defined as those eyes with abnormal corneal topography, including I‐S localized steepening or asymmetric bowtie pattern and no detectable clinical signs. It is unclear how the diagnosis was made; however, cases were classified before the inclusion. | ||
| Flow and timing | All cases were included in reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | It is unclear whether different AI tests were developed and interpreted blind or independently and without knowledge of the results of each other, and whether missing data and their causes were similar for each AI test. | ||
| Notes | This study was supported by the Australian National Health and Medical Research Council (NHMRC) project Ideas grant APP1187763 and Senior Research Fellowship (1138585 to PNB), the Louisa Jean De Bretteville Bequest Trust Account, University of Melbourne, the Angior Family Foundation, Keratoconus Australia, Perpetual Impact Philanthropy grant (SS), and a Lions Eye Foundation Fellowship (SS). The Centre for Eye Research Australia (CERA) receives Operational Infrastructure Support from the Victorian Government. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Unclear | ||
| Unclear risk | |||
Cao 2021a.
| Study characteristics | |||
| Patient Sampling | Single‐centre, retrospective, case‐control study. The data collection was conducted at the Royal Victorian Eye and Ear Hospital in Australia from between 2007 and 2019. | ||
| Patient characteristics and setting |
|
||
| Index tests | Random forest‐based model trained using a modest number (15) of components derived from a reduced dimensionality representation of complete Pentacam system parameters. | ||
| Target condition and reference standard(s) | The diagnosis was made by an experienced optometrist together with > 1 cornea specialist, using Pentacam corneal tomography system; cases were classified before the inclusion. | ||
| Flow and timing | All cases were included in reference standard and index. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This study was supported by the Australian National Health and Medical Research Council (NHMRC) project Ideas grant APP1187763 and Senior Research Fellowship (1138585 to PNB), Lions Eye Donation Service (SS), Angior Family Foundation (SS), Perpetual Impact Philanthropy grant (SS), and Keratoconus Australia Funding (SS). The Centre for Eye Research Australia (CERA) receives Operational Infrastructure Support from the Victorian Government. The sponsor or funding organizations had no role in the design or conduct of this research. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Carvalho 2005.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre case‐control study. 80 corneal maps were selected from the database of the EyeSys System 2000 (EyeSys Vision, Houston, TX) topographer in Brazil. | ||
| Patient characteristics and setting | 80 corneal maps of different people were selected according to the following 5 categories (16 corneas for each category).
Criteria for diagnosis of keratoconus are unclear. Corneal maps had few or no nose or eyelid shadows; only the right eye of each person was allowed. Right and left eyes of the same person were not used. The investigators excluded corneas with incipient keratoconus, keratoconus with high degrees of symmetrical astigmatism, and other cases for which a single prevailing diagnosis could not be issued. In the case of regular profiles, given that even the most symmetrical corneas have some degree of with‐the‐rule astigmatism, only corneas with simulated keratometry 0.25 D were considered "regular" or "normal." |
||
| Index tests | A neural network which used the first 15 Zernike coefficients | ||
| Target condition and reference standard(s) | The selection and classification were performed by 2 eye care specialists, with unclear criteria. However, cases were classified before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported in part by FAPESP (São Paulo Research Foundation) process #03132–8. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Castro‐Luna 2020.
| Study characteristics | |||
| Patient Sampling | Retrospective single‐centre case‐control study in 60 eyes from 60 people from the Department of keratoconus of INVISION Ophthalmology clinic in Almería, Spain | ||
| Patient characteristics and setting | Participants were divided into the following 2 groups depending on their preliminary diagnosis based on the classical topographic criteria.
Grade 4 keratoconus with excessively distorted corneal topography was excluded. All cases were examined using the CSO topography system (CSO, Firenze, Italy). |
||
| Index tests | Bayesian network classifier for keratoconus identification that uses previously developed topographic indices, calculated directly from the digital analysis of the Placido ring images. | ||
| Target condition and reference standard(s) | It is unclear who performed the selection and classification. However, cases were classified before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This research was partially supported by the Andalucian regional government (grant PIN‐0530‐2017). Research of A.M.‐F. and D.R.‐L. was also supported in part by the Spanish Government – European Regional Development Fund (grant MTM2017‐89941‐P), the Andalucian regional government (research group FQM‐229), and the University of Almería (Campus de Excelencia Internacional del Mar CEIMAR). A.M.‐F. acknowledges an additional support from the Carlos I Institute of Theoretical and Computational Physics, while D.R.‐L. thanks the support from CDTIME (Center for Development and Transfer of Mathematical Research to Industry, University of Almería). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Cavas‐Martinez 2017.
| Study characteristics | |||
| Patient Sampling | Single‐centre case‐control study in Spain, including 464 eyes of 464 participants | ||
| Patient characteristics and setting | Participants were divided into the following 2 groups.
|
||
| Index tests | A model of detection of early keratoconus (only grade 1) obtained by logistic regression considering the new parameters defined according to a new geometric approach | ||
| Target condition and reference standard(s) | The standard criteria for keratoconus diagnosis were the presence of an asymmetric bowtie pattern in corneal topography, KISA% index ≥ 100%, a central keratometry with different cut‐off values to keratoconus suspect (> 47.2 D), an I‐S asymmetry with a cut‐off value of 1.4 D difference between average inferior and superior corneal powers at 3 mm from the centre of the cornea, as well as other topographic indices and ≥ 1 keratoconus sign on slit‐lamp examination, such as stromal thinning, conical protrusion on the cornea at the apex, Fleischer's ring, Vogt's striae, or anterior stromal scar. Corneal analysis was performed by the Sirius system (CSO, Italy). It seems that a single experienced examiner was involved in selection and classification. However, cases were classified before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | The study was carried out in the framework of the Thematic Network for Co‐Operative Research in Health (RETICS) reference number RD12/0034/0007 and RD16/0008/0012, financed by the Carlos III Health Institute ± General Subdirection of Networks and Cooperative Investigation Centers (R&D&I National Plan 2008±2011) and the European Regional Development Fund (FEDER). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Chan 2015.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study; 128 topographic images of 128 people were selected at the Singapore National Eye Center | ||
| Patient characteristics and setting |
|
||
| Index tests | The SCORE Analyzer is based on a linear regression analysis that constructs a set of linear functions of variables known as discriminant functions. It combines 12 Placido and tomographic indices in a weighted fashion to classify corneas as suspicious for keratoconus or normal. | ||
| Target condition and reference standard(s) | Clinically evident keratoconus was defined by evidence of ≥ 1 slit‐lamp biomicroscopic findings including conical protrusion of the cornea at the apex, Fleischer's ring, Vogt's striae, and corneal stromal thinning. The classification was performed by 1 corneal specialist. Cases were classified before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Chandapura 2019.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study, involving 439 eyes from 2 centres (India and Brazil). Comparison between 4 AI models. | ||
| Patient characteristics and setting |
|
||
| Index tests | Random forest models based on Pentacam (Oculus GmbH, Wetzlar, Germany) or OCT parameters (OCT topography of the Bowman's layer) | ||
| Target condition and reference standard(s) |
Examination of topographies of the anterior surface was performed by only 1 experienced refractive surgeon, who was masked to the information (disease present in 1 or both eyes) about the participants and the eyes. Classification was performed before the index tests. |
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | It is unclear whether different AI tests were developed and interpreted blind or independently and without knowledge of the results of each other. Missing data and their causes were similar for each AI test. | ||
| Notes | Indo‐German Science and Technology Center, Grant/Award Number: SIBAC | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Chastang 2000.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study, single‐centre (France) involving 208 corneal topographies (EyeSys System 2000) of 208 corneas from 8 groups of participants. | ||
| Patient characteristics and setting | Participants were classified by the following diagnoses.
|
||
| Index tests | Binary decision tree. In the first step, the distribution of keratoconic and non‐keratoconic patterns was studied based on the value of each index in the training set. For each index, corneas with an index value higher than the threshold (or cut‐off value) were classified as keratoconic corneas (positive test), whereas corneas with an index value less than the threshold were classified as non‐keratoconic (negative test). In the second step, binary decision trees were built by combining 2 indices to improve the classification method. The 6 indices with the highest sensitivity and specificity were used in these models. The first index was used to divide the training set into 2 populations (i.e. population with a positive test and population with a negative test) based on the previously calculated optimum threshold. In each of these populations, the distribution of keratoconic and non‐keratoconic patterns was studied based on the value of the second index. In each population, sensitivity and specificity curves as a function of the second index threshold were generated to evaluate the optimum cut‐off value. This resulted in 2 thresholds according to the response to the first test. In fact, the second index's most efficient threshold (i.e. the threshold with maximum sensitivity and specificity) in the population with a positive test was different from that in the population with a negative test. A cornea was classified as keratoconic when the second test was positive. |
||
| Target condition and reference standard(s) | Maps were classified by 2 cornea specialists based on clinical records and topographic appearances, before the index test. | ||
| Flow and timing | All cases were included in reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Unclear whether different AI tests were developed and interpreted blind or independently and without knowledge of the results of each other, and whether missing data and their causes were similar for each AI test. | ||
| Notes | Supported in part by the Fondation Claude Bernard, Paris, France. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Unclear | ||
| Unclear risk | |||
Chen 2021.
| Study characteristics | |||
| Patient Sampling | Multicentre retrospective case‐control study comparing 4 convolutional neural network methods. It included 1926 images from the Pentacam (Oculus GmbH, Wetzlar, Germany) of keratoconic and healthy volunteers' eyes provided by 3 centres (UK, Iran, New Zealand). | ||
| Patient characteristics and setting |
|
||
| Index tests | Convolutional neural network method that uses 4 colour‐coded corneal maps obtained by a Scheimpflug camera (Pentacam) | ||
| Target condition and reference standard(s) | The definition of keratoconus is unclear. Unclear who performed the classification. However, cases were classified before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Unclear whether different AI tests were developed and interpreted blind or independently and without knowledge of the results of each other. Missing data and their causes were similar for each AI test. | ||
| Notes | The study authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not‐for‐profit sectors. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Cohen 2022.
| Study characteristics | |||
| Patient Sampling | Single centre, retrospective, case‐control study that evaluated 8526 corneal tomography examinations of 2525 participants obtained between November 2010 and July 2017 with the Galilei dual Scheimpflug/Placido disc analyzer system (software version 5.2.1; Ziemer Ophthalmic Systems, Port, Switzerland). Low‐quality samples were excluded. | ||
| Patient characteristics and setting | Of the 7104 included samples:
Label distribution was similar in train and test sets. |
||
| Index tests | Random forest; the model integrated keratoconus prediction indexes of the device in addition to the 94 instrument‐derived output parameters. The model was first trained and tested, then validated with a separate validation set. | ||
| Target condition and reference standard(s) | All images were graded by a single cornea specialist (D.V.). A normal cornea would have a regular spherical or spherocylindrical curvature, thinning toward the centre without epicentral posterior or anterior elevation, with relatively normal numerical values. A suspected irregular cornea describes an at‐risk cornea. Such a cornea may have subtle inconclusive signs like I‐S values outside the reference range or aberrant C‐shaped or mild posterior surface elevations. Alternatively, a suspected irregular cornea may have an unusual corneal thinning. | ||
| Flow and timing | All cases were included in reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | All study authors declared that they received no grant support or research funding for the study. All study authors certified that they had no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or non‐financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in the manuscript. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Consejo 2020.
| Study characteristics | |||
| Patient Sampling | Prospective case‐control study, involving 50 eyes selected in a single centre in Belgium | ||
| Patient characteristics and setting | Scheimpflug single‐image snapshots obtained with Corvis‐ST (Oculus, Germany) were analysed and grouped as follows.
|
||
| Index tests | The combination of central corneal thickness with microscopic parameters extracted from statistical modelling of light intensity distribution | ||
| Target condition and reference standard(s) | The definition of keratoconus is unclear. The classification was performed by only 1 experienced ophthalmologist, before the index test. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable. | ||
| Notes | This project received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 779960 and support from the Statutory Funds of Wroclaw University of Science and Technology. MW received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 666295 and from the financial resources for science in the years 2016 to 2019 awarded by the Polish Ministry of Science and Higher Education for the implementation of an international co‐financed project. JJR received a grant from the Flemish Fund for Scientific Research (FWO‐TBM T000416N). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
De Almeida Jr 2021.
| Study characteristics | |||
| Patient Sampling | Prospective, single‐centre, case‐control study, including a data set of 777 images of 777 participants | ||
| Patient characteristics and setting | Data set included data from a 777 people, distributed as follows.
|
||
| Index tests | AI model based on Paraconsistent Feature Engineering (PFE) and Support Vector Machine (SVM), that received subsets of the 52 original Pentacam tomographic descriptors as input and produced, as output, the scalar value called Corneal Tomography Multivariate Index (CTMVI) | ||
| Target condition and reference standard(s) | Classification was performed by only 1 cornea specialist, before the index test. | ||
| Flow and timing | All cases were included in the reference standard and index. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Funding: CAPES (Coordination for Improvement of Higher‐education Personnel), FAPESP (São Paulo Research Foundation) process no. 2015/17226‐7 and process no. 2019/04475‐0, and CNPq (National Council for Scientific and Technological Development) process no. 306808/20018‐8. The study was supported by FAPESP [2015/17226‐ 7] to GCAJr and [2019/04475‐0] to RCG; CNPq (National Council for Scientific and Technological Development) [306808/20018‐8] to RCG; PIBIC‐CNPq to JSN. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Elsawy 2021.
| Study characteristics | |||
| Patient Sampling | Prospective, single‐centre, case‐control study. People with keratoconus, dry eye, Fuchs' dystrophy, or normal corneas were consecutively recruited from a single centre in the USA. | ||
| Patient characteristics and setting | The investigators prospectively recruited 478 participants (875 eyes, 158,220 AS‐OCT images). The images were grouped as follows according to the clinical diagnosis.
|
||
| Index tests | A multidisease deep learning diagnostic network of common corneal diseases, using AS‐OCT images (Envisu R2210, LEICA, USA) | ||
| Target condition and reference standard(s) | Classification was performed by 1 cornea specialist, who was masked to the automated diagnosis given by the algorithm. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | The study was supported by an NEI K23 award (K23EY026118), NEI core center grant to the University of Miami (P30 EY014801), and Research to Prevent Blindness (RPB). Financial Disclosures: United States Non‐Provisional Patent (application no. 14/247903) and United States Provisional Patent (application no. 62/445,106) (to M.A.); United States Non‐Provisional Patents (application no. 8992023 and 61809518), and PCT/US2018/013409 (to M.A. and A.E.). The patents and Patent Cooperation Treaty are owned by University of Miami and licenced to Resolve Ophthalmics, LLC. M.A. is an equity holder and sits on the Board of Directors for Resolve Ophthalmics, LLC. The funding organization had no role in the design or conduct of the research. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Feizi 2016.
| Study characteristics | |||
| Patient Sampling | Prospective single‐centre case‐control study. 210 eyes of 210 people were included in 1 centre in Iran. | ||
| Patient characteristics and setting |
|
||
| Index tests | Logistic regression analysis of sets of parameters obtained with Galilei dual Scheimpflug system (Ziemer Ophthalmic System AG, Port, Switzerland) | ||
| Target condition and reference standard(s) | The diagnosis of subclinical keratoconus and keratoconus was based on clinical slit‐lamp findings (stromal thinning, conical protrusion, Fleischer's ring, and Vogt's striae) and characteristic patterns based on Placido disc corneal topography (Tomey, EM‑3000, version 4.20, Nagoya, Japan). Participants who had 1 abnormal biomicroscopic finding and 1 major or 2 minor criteria were diagnosed with keratoconus. Participants with a normal appearing cornea and 1 major or 2 minor topographic criteria were diagnosed with subclinical keratoconus. It seems that a single experienced examiner was involved in classification. However, cases were classified before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funds, grants, or other support were received. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Gairola 2022.
| Study characteristics | |||
| Patient Sampling | Single‐centre, retrospective, case‐control study. First data set (64 participants, 114 eye samples) obtained from topography device, SmartKC at the Sankara Eye Hospital in Bengaluru, India. Second data set (2110 samples; 1637 non‐keratoconus and 473 keratoconus) consisted of downloaded anonymized data from the Keratron device database for all the people who took the corneal topography examination at the hospital from April 2008 to May 2010. | ||
| Patient characteristics and setting |
|
||
| Index tests | Convolutional neural network. The network is organized into 2 branches – 1 each for axial and tangential heatmaps – with a shared convolutional backbone, followed by 2 distinct feed‐forward classifiers, 1 for each branch. The shared backbone comprises the convolutional layers from a ResNet34 model. The model has a 2‐class (keratoconus versus non‐keratoconus) classification task. The article provides a clear explanation of the model and training procedure. |
||
| Target condition and reference standard(s) | The first data set was diagnosed by 1 senior ophthalmologist at the hospital. The diagnoses in the second data set were obtained based on the PPK‐based classification. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Gao 2022.
| Study characteristics | |||
| Patient Sampling | Single‐centre, retrospective, case‐control study, in the Affiliated Eye Hospital of Wenzhou Medical University, China. A total of 208 participants (1040 corneal topography images) were evaluated and divided into the following 3 groups.
Data were collected between 2012 and 2018 using the Pentacam system and analysed from February 2019 to December 2021. The corneal data of pachymetry and elevation were exported from the Pentacam HR system (Oculus, Optikgeräte GmbH, Wetzlar, Germany). |
||
| Patient characteristics and setting | Each image was previously assigned to the following 3 groups.
|
||
| Index tests | Neural network, called Keratoscreen. The preprocessing model separately established Zernike coefficients (ZC) data sets of 5 corneal surface maps: the anterior corneal curvature (DA), posterior corneal curvature (DP), anterior corneal elevation (DAE), posterior corneal elevation (DPE), and corneal thickness data (pachymetry DPAC). The first N (N #15) ZC terms (5 orders, when N = 15) obtained from each map were used to form ZC data sets. KeratoScreen, with L = 4 layers (1 input layer, 2 hidden layers, and 1 output layer), was used in this study. The neurons on the input layer have N nodes that take an input vector ZC and outputs through 2 hidden layers. To train the algorithm they used 20 repeats with the randomly generated training and test sets, and the results were averaged. However, it is unclear whether they reused the same data for training and testing. | ||
| Target condition and reference standard(s) |
|
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | The research received funding through 21NDJC309YBM from the Zhejiang Philosophy and Social Science Planning Project (China), LZ21F020008 from the Natural Science Foundation of Zhejiang Province (China), 2019C03045 from the National Key Project of Research and Development Program of Zhejiang Province (China), and LY21A040001 from the Natural Science Foundation of Zhejiang Province (China). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Ghaderi 2021.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre (Iran), case‐control study. A data set of 450 people with keratoconus and healthy controls was analysed. | ||
| Patient characteristics and setting | Data set of people with keratoconus and healthy controls. Scheimpflug tomographic images (Pentacam HR (Oculus GmbH, Wetzlar, Germany)) were analysed. | ||
| Index tests | Ensemble learning system, based on combining multiple initial classifiers as experts for primary classification and a combination rule for combining results of classifiers. | ||
| Target condition and reference standard(s) | Unclear definition of keratoconus. Unclear who performed the classification; it seems 1 cornea specialist. Unclear if reference standard results were interpreted without knowledge of the results of the index test. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funds, grants, or other support were received. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Issarti 2019.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre, case‐control study that used a previously collected database containing the Pentacam Scheimpflug measurements of 851 eyes (Pentacam (Oculus GmbH, Wetzlar, Germany)). | ||
| Patient characteristics and setting |
|
||
| Index tests | A CAD (computer‐aided diagnosis) system, which combines a feedforward neural network (FFN) and a Grossberg‐Runge Kutta architecture to detect clinical and suspect keratoconus. | ||
| Target condition and reference standard(s) |
An ophthalmologist and an optometrist performed the classification. Cases were classified before inclusion. |
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | |||
| Notes | This work was supported in part by a research grant from the Flemish government agency for Innovation by Science and Technology (grant no. TBM‐T000416N). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Issarti 2020.
| Study characteristics | |||
| Patient Sampling | Retrospective, multicentre, case‐control study. Scheimpflug Pentacam measurements (Oculus GmbH, Wetzlar, Germany) of 812 eyes were retrospectively collected from 2 centres in Belgium. | ||
| Patient characteristics and setting |
|
||
| Index tests | A score‐based machine learning system based on feedforward neural network (Logistic Index for Keratoconus, Logik), capable of classifying keratoconus according to its severity, to objectively discriminate suspect keratoconus from healthy eyes, and to provide a consistent, time‐continuous scoring system for keratoconus progression | ||
| Target condition and reference standard(s) |
An ophthalmologist and an optometrist performed the classification. Cases were classified before inclusion. |
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This work was supported in part by a research grant from the Flemish government agency for Innovation by Science and Technology (grant no. TBM‐T000416N). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Kalin 1996.
| Study characteristics | |||
| Patient Sampling | Prospective, consecutive, cross‐sectional study, including 106 eyes of 53 consecutive refractive surgery candidates in a single centre (USA) | ||
| Patient characteristics and setting | 53 consecutive refractive surgery candidates for myopia correction with no history of ophthalmic diseases or ocular surgery were enroled during a 2‐year interval. | ||
| Index tests | Expert system classification: an algorithm that incorporates 8 indices. Discriminant analysis was used to produce keratoconus prediction index (KPI) and in a binary decision tree. | ||
| Target condition and reference standard(s) | Keratoconus was diagnosed if clinical signs were present and the topography performed with TMS‐1 (Computer anatomy 1, New York) was abnormal (irregular astigmatism, loss of radial symmetry, or absence of the normal progressive flattening from the centre to the periphery). The classification was performed by an experienced ophthalmologist, before the index test. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported in part by US Public Health Service grants EY10056 and EY0311 from the National Eye Institute, National Institutes of Health, Bethesda Maryland. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Kamiya 2019.
| Study characteristics | |||
| Patient Sampling | Single‐centre (Japan), retrospective case‐control study, which included a total of 304 keratoconic eyes and 239 healthy eyes (refractive surgery candidates and contact lens fitting candidates) | ||
| Patient characteristics and setting | The data of people with keratoconus who underwent corneal tomography obtained by a swept‐source anterior segment OCT (CASIA SS‐1000, Tomey, Aichi, Japan) between March 2013 and April 2018 at Miyata Eye Hospital were retrospectively reviewed. 304 keratoconic eyes with good quality scans of corneal tomography were enroled and divided according to the Amsler‐Krumeich classification, as follows.
The control group comprised 239 eyes in subjects with normal corneal and ocular findings applying for a contact lens fitting or a refractive surgery consultation. |
||
| Index tests | Deep learning (convolutional neural network) of the arithmetical mean output data of 6 colour‐coded maps of an anterior segment OCT. | ||
| Target condition and reference standard(s) | Diagnosis of keratoconus was performed based on evident findings characteristic of keratoconus (e.g. corneal tomography with asymmetric bowtie pattern with or without skewed axes), and ≥ 1 keratoconus sign (e.g. stromal thinning, conical protrusion of the cornea at the apex, Fleischer's ring, Vogt's striae, or anterior stromal scar) on slit‐lamp examination. It is unclear how many corneal specialists classified the cases. However, classification was performed before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | The study authors declared no specific grant for this research from any funding agency in the public, commercial or not‐for‐profit sectors. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Kamiya 2021.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre (Japan), case‐control study involving 349 keratoconus eyes and 170 normal eyes (refractive surgery candidates, contact lens fitting candidates). | ||
| Patient characteristics and setting | A total of 349 eyes with good‐quality images of corneal topography measured with a Placido disc corneal topographer (TMS‐4 TM, Tomey, Aichi, Japan) were included. The disease was graded according to the Amsler‐Krumeich classification, as follows.
Control group: 170 eyes in people with normal ocular findings applying for a contact lens fitting or for a refractive surgery consultation, who had a refractive error of < 6 D as well as astigmatism of < 3 D. |
||
| Index tests | Deep learning (convolutional neural network) of a single colour‐coded topography map. | ||
| Target condition and reference standard(s) | Multiple corneal specialists diagnosed keratoconus with distinctive features (e.g. corneal colour‐coded map with asymmetric bowtie pattern with or without skewed axes), and ≥ 1 keratoconus sign (e.g. stromal thinning, conical bulging, Fleischer's ring, Vogt's striae, or apical scar). It is unclear how many corneal specialists classified the cases; however, classification was performed before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This work was in part supported by Grants‐in‐Aid for Scientific Research (Grant Number 21K09706). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Kojima 2020.
| Study characteristics | |||
| Patient Sampling | Multicentre (Japan), retrospective, case‐control study which included 329 eyes (healthy controls and people with keratoconus). | ||
| Patient characteristics and setting |
|
||
| Index tests | Multivariate logistic regression analysis to create an equation that predicts early keratoconus (keratometer keratoconus index) using auto‐keratometer parameters. | ||
| Target condition and reference standard(s) | Keratoconus diagnosis was based on corneal topography or tomography results (corneal steepening and asymmetric astigmatism, protrusion of the posterior cornea, and thinning of the cornea at the area of protrusion) and slit‐lamp findings. 2 corneal specialists classified the cases before inclusion. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Kojima 2021.
| Study characteristics | |||
| Patient Sampling | Single centre, retrospective, case‐control study that included 179 eyes of 99 consecutive people with keratoconus and suspected keratoconus (68 males and 31 females, mean age 33.48 (SD 15.41) years), who visited the Nagoya Eye Clinic from January 2019 to December 2020 and were tested with an auto‐keratometer (ARK‐1s, Gamagori, Japan, NIDEK). During the same period, 468 eyes of 235 consecutive people (125 men and 110 women; mean age 37.55 (SD 22.70) years) examined for refractive correction were included as normal controls. The control group included people who had no abnormalities on slit‐lamp biomicroscopy examination and corneal topography. | ||
| Patient characteristics and setting | People already diagnosed with keratoconus or suspected keratoconus were included. The exact reason why healthy controls visited the centre is unclear. | ||
| Index tests | Regression algorithm, modified keratoconus keratometer index (KKI) using 3 variables: steep K‐value, flat K‐value, and astigmatism. logit = 1.284 × steep K (dioptre) − 0.618 × flat K (dioptre) − 3.163 × (0: non‐with‐the‐rule astigmatism; 1: with‐the‐rule astigmatism) − 28.662, KKI = exp(logit)/(1 + exp[logit]). The cut‐off value of 0.461 had been determined previously. | ||
| Target condition and reference standard(s) | 2 cornea specialists diagnosed keratoconus through slit‐lamp microscopy and corneal topography. Keratoconus signs found in both slit‐lamp microscopy and corneal topography were classified as keratoconus, while signs found only in corneal topography were classified as suspected keratoconus. Forme fruste keratoconus was defined as an eye with normal corneal topography in the contralateral eye of the keratoconus. The severity of keratoconus was based on the Amsler–Krumeich classification. |
||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This research received no external funding. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Kovacs 2016.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre (Hungary), case‐control study, which involved 135 eyes of people with bilateral keratoconus (keratoconus group), normal fellow eyes of people with unilateral keratoconus (fellow‐eye group), and eyes of refractive surgery candidates (control group). | ||
| Patient characteristics and setting |
|
||
| Index tests | Multilayer perception classifier (neural network) trained on bilateral data of index of height decentration. | ||
| Target condition and reference standard(s) | Keratoconus was diagnosed according to classic corneal biomicroscopic and topographic findings using the criteria of Rabinowitz: the existence of central protrusion of the cornea with Fleischer's ring, Vogt's striae, or both, by slit‐lamp examination in addition to the following topographic findings: a central keratometry (K) value > 47.2 D or an I‐S value > 1.4 D, or KISA% > 100%. Both eyes in the keratoconus group and the affected eye in the unilateral keratoconus group had abnormal keratoconus indices measured by a Scheimpflug camera (Pentacam HR). It is unclear who classified the cases. Classification was performed before the index test. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Unclear whether different AI tests were developed and interpreted blind or independently and without knowledge of the results of each other. However, missing data and their causes were similar for each AI test. | ||
| Notes | Supported by OTKA NN106649 from the Hungarian Scientific Research Fund. The funder had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Kuo 2020.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre (Taiwan), case‐control study. The investigators retrospectively collected corneal topographies (TMS‐4; Tomey Corporation, Nagoya, Japan) of the study group with clinically manifested keratoconus, the subclinical keratoconus group, and the control group with regular astigmatism (354 images of 206 participants). | ||
| Patient characteristics and setting |
|
||
| Index tests | 3 convolutional neural network models | ||
| Target condition and reference standard(s) | The diagnosis of keratoconus was based on clinical signs (the existence of central protrusion of the cornea, Fleischer's ring, Vogt's striae, and focal corneal thinning on slit‐lamp examination) and topographic criteria (central K value > 47 D, I‐S value > 1.4 D, KISA% >100%, and asymmetric bowtie presentation). 4 corneas specialists classified the cases before the index test. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Unclear whether different AI tests were developed and interpreted blind or independently and without knowledge of the results of each other. However, missing data and their causes were similar for each AI test. | ||
| Notes | Supported by Grants 107L891002 and 108L891002 from National Taiwan University. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Lavric 2021.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study. Pentacam data (Oculus GmbH, Wetzlar, Germany) obtained from people screened for keratoconus disease in Brazil. Elevation, topography, and pachymetry parameters were obtained from 5881 eyes of 2800 participants. | ||
| Patient characteristics and setting | Participant characteristics and setting are not clearly described in the study. Data seems to originate from a larger data set which included people with keratoconus and healthy controls. |
||
| Index tests | Support vector machine that uses elevation, topography or pachymetry parameters obtained from the raw data of the Pentacam to detect keratoconus. The workflow for the development of the algorithm was as follows: splitting the initial data set in elevation, topography and pachymetry data sets; data cleaning and elimination; feature selection; machine learning validation; and performance evaluation. It is unclear whether different data were used for testing and validating the model. |
||
| Target condition and reference standard(s) | The target condition was keratoconus; however, the article provided no definition. Tomography images of the Pentacam were used in this study. It is unclear who interpreted the images and made the diagnosis; however, the diagnosis was made before the algorithms analysed the images. | ||
| Flow and timing | The article did not describe the reference standard, nor did it describe whether all participants received the same reference standard. All data were included in a 2 × 2 table. |
||
| Comparative | In total, 6 algorithms were developed, tested, and compared: decision tree, discriminant naïve Bayes, support vector machine, k‐nearest neighbour, and ensemble. | ||
| Notes | This work was supported in part by a grant from the Romanian Ministry of Research and Innovation, CCCDI‐UEFISCDI, within PNCDI III, under Project PN‐III‐P2‐2.1‐PTE‐2019‐0642, and in part by the Romania National Council for Higher Education Funding, CNFIS, under Project CNFIS‐FDI‐2021‐0357. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Lopes 2018.
| Study characteristics | |||
| Patient Sampling | Retrospective, multicentre, case‐control study. A total of 3693 people were enroled from 5 different clinics. Participants were divided into 2 data sets: 1 training set and 1 validation set. The training set included the preoperative data of the following 3 groups.
The algorithm was independently tested in a different set of stable LASIK cases and people with very asymmetric ectasia; these people had clinically diagnosed ectasia in 1 eye and normal topography in the fellow eye. |
||
| Patient characteristics and setting | The participants were grouped as follows.
|
||
| Index tests | Random forest: multiple decision trees were built and merged to improve accuracy of the prediction. 2 steps of validation were used to assess the generalization and clinical validity of the models and their ability to correctly classify new data. The first was a holdout validation: the training set was randomly split into 2 data sets: the first comprised 70% of the total data set and was used to actually train the models; the other 30% was used to test the model accuracy. The second validation step was an independent test with cases that were not part of the training set. The algorithm analysed the raw tomographic data to identify the different patterns and detect keratoconus. | ||
| Target condition and reference standard(s) | All eyes were examined by rotating Scheimpflug corneal and anterior segment tomography (Pentacam HR; Oculus GmbH, Wetzlar, Germany). Image quality was checked, so that only cases with acceptable‐quality images were included in the study. 1 experienced fellowship‐trained corneal specialist reviewed all the cases so that they were correctly classified in the keratoconus and very asymmetric ectasia groups. All cases were diagnosed before the algorithm analysed the images. | ||
| Flow and timing | All eyes received the reference standard and were included in the 2 × 2 table of the index test. | ||
| Comparative | 5 models were developed and compared: regularized discriminant analysis (RDA), support vector machine (SVM), naïve Bayes (NB), neural networks (NN), and random forest (RF). It is unclear if all tests were developed and interpreted without knowledge of each other and if all data were used for each test. | ||
| Notes | No funding or grant support. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Unclear | ||
| Unclear risk | |||
Lucena 2021.
| Study characteristics | |||
| Patient Sampling | Retrospective, case‐control study. A diagnostic pattern bank generated by a specialist physician. The database consisted of 1172 examples of corneal topography, divided into 275 spherical patterns, 302 regular symmetrical astigmatism patterns, 295 regular asymmetrical astigmatism patterns, and 300 irregular astigmatism patterns (keratoconus). | ||
| Patient characteristics and setting | This study is a registry‐based study; the registry contained healthy controls and people with keratoconus. | ||
| Index tests | Convolutional neural network. The algorithm is a semi‐automatic, manual interference model. It uses a hierarchical system that tries to represent the structure in relation to the recognition of an image, where pixels form edges, edges form patterns, patterns form objects, which in turn describe the scenes. The algorithm analyses the topographic images and decides whether it is regular or irregular astigmatism (keratoconus). The algorithm was developed with a training phase and a validation phase. | ||
| Target condition and reference standard(s) | A specialist physician developed the diagnostic pattern bank of images made by topographers. He divided the included topographies into the following 4 groups.
The cases were divided before the algorithm analysed the images. |
||
| Flow and timing | All topographies included in the diagnostic pattern bank were judged by the specialist physician and all images were included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | This study was supported by Government of the State of Ceará (Foundation for the Support to Scientific and Technological Development of Ceará), and Ophthalmology School of Ceará. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Maeda 1994.
| Study characteristics | |||
| Patient Sampling | Single‐centre, case‐control study. Videokeratographs were drawn from the Louisiana State University Eye Center patient population and were divided randomly by category into 2 sets. Each set comprised 8 categories: normal, keratoconus, keratoplasty, epikeratophakia, excimer laser photorefractive keratectomy, radial keratotomy, contact lens‐induced warpage, and other. | ||
| Patient characteristics and setting | The study included the following categories of participants.
The study did not include people seeking refractive surgery. |
||
| Index tests | Combined discriminant analysis and classification tree analysing images from the TMS‐1. The keratoconus detection programme was developed using a training set of 100 corneas and evaluated with a validation set of an additional 100 corneas. Maps were first classified as either keratoconus, borderline, or non‐keratoconus. The borderline maps were then divided into keratoconus or non‐keratoconus by certain indices. Next, all keratoconus patterns were classified into either peripheral or central keratoconus using a threshold combination of these indices. Final output of the system was the display of the certainty of keratoconus. |
||
| Target condition and reference standard(s) | The included cases were diagnosed by 3 corneal topography researchers based on clinical records and topography. All images were made by a TMS‐1. The diagnosis was made before the algorithm analysed the images. | ||
| Flow and timing | All participants received the index and reference test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported in part by National Institutes of Health grants EY03311 and EYO2377 and by Computed Anatomy, Inc. and Menicon, Co., Ltd. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Maeda 1995a.
| Study characteristics | |||
| Patient Sampling | Single‐centre, case‐control study. Corneal topographic maps of the TMS‐1 were drawn from the Louisiana State University Eye Center patient population. | ||
| Patient characteristics and setting | Maps from 176 eyes of 125 people were selected and grouped as follows.
Maps of eyes with keratoconus were selected from the charts of people previously diagnosed as having keratoconus in our clinic. |
||
| Index tests | Combined discriminant analysis and classification tree, based on topographic images. The algorithm determined whether a keratoconus‐like pattern was seen in a particular map in the binary classification tree and, if so, reported a value between 5% and 95% in proportion to the linear discriminant function to quantify the severity of the keratoconus pattern. | ||
| Target condition and reference standard(s) | Topography images were made with the TMS‐1. It is unclear who diagnosed the cases; however, the diagnosis was made before the algorithm analysed the images. | ||
| Flow and timing | It is unclear whether all eyes were diagnosed with the same reference standard. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This study was supported in part by US Public Health Service grants EY03311 and 02377 from the National Eye Institute, National Institutes of Health, Bethesda, Md; an unrestricted departmental grant from Research to Prevent Blindness Ine, New York, NY; and funds from Computed Anatomy Ine, New York, NY, and Menicon Co, Ltd, Nagoya, Japan. The Conecare data analysis software used in this study was provided courtesy of Yaron S. Rabinowitz, MD. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Maeda 1995b.
| Study characteristics | |||
| Patient Sampling | Single‐centre, case‐control study. Corneal topographic maps of the TMS‐1 were drawn from the Louisiana State University Eye Center patient population. | ||
| Patient characteristics and setting | Participants were divided into the following 7 categories.
The study excluded maps in which focusing or alignment were not properly achieved or that contained atypical topographic appearances. For this project, 183 eyes were selected. The maps were randomly divided into a training set (108 maps) and a test set (75 maps). |
||
| Index tests | Neural network constructed in 3 layers. The input layer consisted of 11 neurons equal to 11 topographic indices. There was 1 hidden layer of 18 neurons. The output layer consisted of 7 neurons, 1 for each topographic category. | ||
| Target condition and reference standard(s) | Topography images were made with the TMS‐1. All eyes were diagnosed by the 3 study authors and classified according to the topographic image and clinical records. The diagnosis was made before the images were analysed by the algorithm. | ||
| Flow and timing | All eyes received the same reference standard, and all data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported in part by US Public Health Service grant EY03311 and EY01377 from the National Eye Institute, National Institutes of Health (Bethesda, MD); Computed Anatomy (New York, NY); Menicon Co. Ltd. (Nagoya, Japan). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Mahmoud 2013.
| Study characteristics | |||
| Patient Sampling | Participants were selected from 4 different hospitals: Department of Ophthalmology of the Ohio State University, Columbus, Ohio; Clinica de Oftalmologia de Cali, Pontificia Universidad Javeriana, Cali, Colombia; Cullen Eye Institute, Department of Ophthalmology, Baylor College of Medicine, Houston, Texas; and Department of Ophthalmology, University Hospital of Bern, Inpelspital, Bern, Switzerland. | ||
| Patient characteristics and setting |
|
||
| Index tests | Logistic regression. The algorithm included both anterior and posterior curvature maps; results were divided into 3 categories: normal (0–0.25), suspect (0.25–0.8), and keratoconus (0.8–1.0). | ||
| Target condition and reference standard(s) | Tomography images from the Galilei Dual Scheimpflug‐Placido tomographer were used. It was unclear how the reference standard made the diagnosis; however, all cases were diagnosed before the index test analysed them. | ||
| Flow and timing | It was unclear whether all participants received the same reference standard, but all cases were diagnosed before inclusion. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Mahmoud 2021.
| Study characteristics | |||
| Patient Sampling | Case‐control study. 250 cases were extracted and a physician made the medical diagnoses. Medical evaluations were found in the data set Dataverse. | ||
| Patient characteristics and setting | The study included people with keratoconus and healthy controls. Further participant characteristics are described in Dataverse. | ||
| Index tests | Convolutional neural network using a 3D reconstruction of corneal images as input. The output was healthy or keratoconus. The algorithm also defined severity of keratoconus. | ||
| Target condition and reference standard(s) | The OCT SS‐1000 (CASIA) was used to make the corneal images. The diagnosis was made by a physician. All cases were diagnosed before the algorithm analysed the images. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast‐track Research Funding Program. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Mohammadpour 2022.
| Study characteristics | |||
| Patient Sampling | Prospective, diagnostic test accuracy study including 217 eyes of 212 people aged 17–49 years who were referred to the Keratoconus Clinic or were refractive surgery candidates at the Refractive Surgery Unit. Exclusion criteria: a history of ocular surgery, corneal cross‐linking, or ring implantation; corneal hydrops or scarring; signs and symptoms of dry eye or ocular diseases other than keratoconus; connective tissue diseases; systemic diseases affecting the eyes; corneal haze; pregnancy; and contact lens use in the previous month. |
||
| Patient characteristics and setting | The study included people already diagnosed with keratoconus or suspected keratoconus. | ||
| Index tests | The algorithm combines Placido and Scheimpflug technologies to provide complete information on the anterior and posterior corneal surfaces. Sirius (Costruzione Strumenti Oftalmici, Florence, Italy) takes 25 Scheimpflug images and 1 Placido image in < 1 second. Height, slope, and curvature data are then calculated with an arc‐step method. This system provides comprehensive information on the entire cornea and classifies keratoconus via the Phoenix software through a neural network process. The study performed a comparison of existing algorithms, which are already validated. |
||
| Target condition and reference standard(s) | Participants were grouped based on the clinical diagnosis of 2 independent experienced corneal specialists (M. Mohammadpour, K. Amanzadeh), through slit‐lamp biomicroscopy, retinoscopy, corrected distance visual acuity (CDVA) measurement with a Snellen chart, and evaluation of the Pentacam Refractive 4 Maps. The specialists were blinded to classification reports. Diagnostic discrepancies were resolved by a third expert examiner (A. Moghaddasi) for a definitive diagnosis. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | The study authors received no financial support for the research, authorship, or publication of the article. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Pavlatos 2020.
| Study characteristics | |||
| Patient Sampling | Case‐control study that recruited participants from the Casey Eye Institute at Oregon Health & Science University (Portland, Oregon) and the Affiliated Eye Hospital of Wenzhou Medical College (Wenzhou, China) | ||
| Patient characteristics and setting | The study grouped eyes as follows.
|
||
| Index tests | Custom‐made MATLAB algorithms generated pattern deviation maps of pachymetry and epithelial thickness, captured using Fourier‐domain OCT images of the cornea. The co‐localized thinning of the 2 maps was quantified using a novel coincident thinning index, which was calculated from Gaussian fits of the regions of maximum relative thinning. | ||
| Target condition and reference standard(s) | OCT scans were obtained using commercial Fourier‐domain OCT systems (RTVue or Avanti; Optovue, Inc) with a corneal adaptor module for imaging of the anterior eye. It is unclear how the diagnosis was made. It seems the cases were divided into normal and keratoconus groups before they were analysed with the algorithm. | ||
| Flow and timing | It is unclear if all cases received the same reference standard. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported by the National Institutes of Health, Bethesda, MD (Grant Nos. R01EY028755, R01EY029023, T32EY023211, and P30EY010572); a research grant and equipment support from Optovue, Inc, Fremont, California; and unrestricted grants to Casey Eye Institute and Bascom Palmer Eye Institute from Research to Prevent Blindness, Inc, New York, New York. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Rabinowitz 1999.
| Study characteristics | |||
| Patient Sampling | Case‐control study. The cases were selected from a database of more than 400 people with keratoconus recruited for longitudinal videokeratography and genetic studies of keratoconus at the Cedars‐Sinai Medical Center (CSMC), Los Angeles, California. | ||
| Patient characteristics and setting |
|
||
| Index tests | Regression algorithm. The KISA% index quantifies the topographic features seen in people with clinical keratoconus. | ||
| Target condition and reference standard(s) | Topography images were taken of both eyes of each study participant with the TMS‐1. It was unclear how the diagnosis was made. The cases were labelled before the analysis with the algorithm. | ||
| Flow and timing | It is unclear if all cases received the same reference standard. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported by NIH Grant EY09052 and The Eye Birth Defects Research Foundation, Inc. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Ruiz 2016.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study; unclear how the data were collected. | ||
| Patient characteristics and setting | Participants were grouped as follows.
Exclusion criteria: any systemic disease, a history of ocular surgery (except for laser refractive surgery in the refractive surgery group), cross‐linking, ametropia > 610 D (for the normal group), and very advanced keratoconus with corneal scarring. Note that keratoconus suspect cases (i.e. eyes that the investigators could not reliably categorize as either normal or keratoconus based on Pentacam or slit‐lamp examination) were removed from the analysis, as they could not be properly categorized for training purposes. |
||
| Index tests | Support vector machine First, the data were preprocessed to ensure there were no impossible values or missing data. The second step was a correlation‐based hierarchical clustering. The output of this analysis is dendrogram, in which variables are organized along branches according to their degree of correlation with each other. Next, the dendrogram was revised to select only 1 variable from each branch, which was typically the variable with the highest clinical relevance. This procedure led to a data set of 22 variables. All classifications were performed using a linear kernel support vector machine; this method provides a linear decision boundary for binary classification problems. |
||
| Target condition and reference standard(s) | An experienced keratoconus specialist and an experienced optometrist classified the eyes into 5 groups using criteria based on patient history and corneal tomography maps from the Pentacam. | ||
| Flow and timing | All participants received the same reference standard and were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Study supported by an Agency for Innovation by Science and Technology grant. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Ruiz 2017.
| Study characteristics | |||
| Patient Sampling | Retrospective, case‐control study including 131 eyes from 102 people during routine clinical practice at the Rothschild Foundation in the period between October 2015 and January 2016. | ||
| Patient characteristics and setting | Participants were grouped as follows.
All participants had a complete ophthalmic evaluation that consisted of manifest refraction, slit‐lamp examination, and corneal tomography evaluation by both Pentacam HR and Orbscan IIz. Exclusion criteria: a history of ocular surgery (apart from laser correction in the refractive surgery group), corneal cross‐linking, and very advanced keratoconus with corneal scarring. Rigid contact lens wearers were asked to remove their lenses at least 1 week before testing. |
||
| Index tests | The support vector machine uses 25 topography and tomography parameters of the anterior and posterior corneal surfaces from the Pentacam. The algorithm automatically classifies corneal patterns and shows the probability that the cornea has keratoconus, forme fruste or suspect keratoconus, photorefractive surgery, normal, or regular astigmatism. | ||
| Target condition and reference standard(s) | Participants were clinically diagnosed at the Rothschild Foundation based on slit‐lamp examination and corneal topography and classified into 4 groups. Topography and tomography were measured with the Pentacam HR and the Orbscan IIz. Participants were diagnosed before the corneal images were analysed with the algorithm. | ||
| Flow and timing | It was unclear whether all participants received the same reference standard. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | Supported by a research grant of the Flemish government agency for Innovation by Science and Technology (grant nr. IWT/110684). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Saad 2014.
| Study characteristics | |||
| Patient Sampling | Case‐control study; part of a prospective study evaluating clinical, topographic, tomographic, and biomechanical characteristics of people with keratoconus and keratoconus suspect at the Rothschild Foundation, Paris, France. | ||
| Patient characteristics and setting | Participants were grouped as follows.
Exclusion criteria: corneal scarring in the anterior or posterior segment. |
||
| Index tests | Discriminant analysis composed of 3 variables: the difference between steep and flat keratometry, the 3‐mm irregularity, and the anterior elevation of the thinnest point. The algorithm was developed to discriminate keratoconus eyes from normal eyes. | ||
| Target condition and reference standard(s) | All keratoconus eyes were diagnosed by 1 corneal specialist based on clinical and topographic signs. | ||
| Flow and timing | All participants received the same reference and index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Saad 2016.
| Study characteristics | |||
| Patient Sampling | Case‐control study; part of a prospective study evaluating clinical, topographic, tomographic, and biomechanical characteristics of people with keratoconus and keratoconus suspect at the Rothschild Foundation, Paris, France. The study included healthy people and people with keratoconus suspect. | ||
| Patient characteristics and setting | 119 eyes of 176 people from the Department of Ophthalmology of the Rothschild Foundation were included and separated into 2 groups: normal and keratoconus suspect. The 2 groups were divided based on the results of the Nidek Corneal Navigator (NCN) automated corneal classification software in the OPD‐Scan (Nidek Co. Ltd., Gamagori, Japan).
|
||
| Index tests | Discriminant analysis, combining Placido (topography) and corneal wavefront data to detect early forms of keratoconus and to classify corneas as healthy, keratoconus suspect, or keratoconus. | ||
| Target condition and reference standard(s) | The article did not mention who made the diagnosis of keratoconus or keratoconus suspect; however, all cases were separated in the 2 groups before the discriminant analysis. | ||
| Flow and timing | It was unclear whether all participants received the same reference standard. All cases did receive the same index test, and all data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Saika 2013.
| Study characteristics | |||
| Patient Sampling | Single‐centre case‐control study including 212 eyes: 51 eyes of 37 people (24 men, 13 women) with keratoconus; 46 eyes of 35 people (20 men, 15 women) with keratoconus suspect; 50 eyes of 28 people (13 men, 15 women) who had undergone LASIK for myopia; and 65 healthy control eyes of 65 people (38 men, 27 women). The corneal surface of each was measured with a Placido‐based corneal topographer. | ||
| Patient characteristics and setting | Included eyes were divided into the following groups.
|
||
| Index tests | Linear discriminant analysis is a statistical method of finding a linear combination of several explanatory variables that characterizes or separates categories of objects. The variables used were: Zernike expansion coefficients of the 2nd to 4th terms of the 4th‐order approximation for a 4‐mm‐diameter pupil; the 2nd to 6th terms of the 6th‐order approximation for a 6‐mm‐diameter pupil; and the Simulated Keratometry. The categories were: keratoconus, keratoconus suspect, LASIK, and healthy control. The model was trained with 2 different sets of participants. |
||
| Target condition and reference standard(s) | It was unclear how the diagnosis of keratoconus was made by the reference standard. All cases were labelled before analysis with the AI algorithm. | ||
| Flow and timing | It is unclear whether all participants received the same reference standard. All participants were included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | Supported in part by the Japan Ministry of Education, Science, Sports, and Culture, Tokyo, Japan (No. 24592669). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Shetty 2015.
| Study characteristics | |||
| Patient Sampling | Retrospective single‐centre case‐control study in people with clinically diagnosed keratoconus before they received corneal crosslinking (85 eyes) and normal controls before they received an ablation (43 eyes). | ||
| Patient characteristics and setting |
Exclusion criteria were ocular hypertension, corneal inflammation, prior eye surgery, and current topical medication use. |
||
| Index tests | Logistic regression using Zernike coefficients, curvature, corneal volume, and corneal anterior wavefront analyses to calculate if keratoconus is present. | ||
| Target condition and reference standard(s) | The diagnosis of keratoconus was based on evidence of stromal thinning, curvature asymmetry leading to abnormal corneal astigmatism, or increase in corneal curvature measured by the Pentacam, and clinical signs (e.g. Fleischer ring's, Vogt's striae, scissoring of the red reflex, an abnormal retinoscopy, and focal protrusion). However, it was unclear who made the diagnosis. | ||
| Flow and timing | It was unclear whether all participants received the same reference standard. All participants were included in the analysis. | ||
| Comparative | Multiple logistic regression equations were compared; however, it was unclear if the development and interpretation was done without the knowledge of each other and if all cases were analysed by all the equations. | ||
| Notes | Dr Dupps is a recipient of National Eye Institute, USA R01 grant (# NIH R01 EY023381). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Unclear | ||
| Unclear risk | |||
Shi 2020.
| Study characteristics | |||
| Patient Sampling | Prospective, single‐centre, case‐control study. People with keratoconus and subclinical keratoconus were recruited from Affiliated Eye Hospital of Wenzhou Medical University. Normal subjects were recruited from the hospital's working staff and students. |
||
| Patient characteristics and setting |
|
||
| Index tests | An automated classification system using a machine learning classifier to distinguish clinically unaffected eyes in people with keratoconus from a normal control population based on a combination of Scheimpflug camera images and ultra‐high‐resolution optical coherence tomography (UHR‐OCT) imaging data. A neural network was used. | ||
| Target condition and reference standard(s) | 2 experienced doctors (YY and JJ) performed a comprehensive ocular exam, including a review of family and medical history, corrected‐distance visual acuity, slit‐lamp biomicroscope examination, fundus examination, and corneal topography. | ||
| Flow and timing | All participants received the same reference standard and were included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | This study was supported by research grants from Key R&D Program Projects in Zhejiang Province (2019C03045), the National Major Equipment Program of China (2012YQ12008004), the National Key Research and Development Program of China (2016YFE0107000, the National Nature Science Foundation of China (Grant No. 81570880). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Sideroudi 2017.
| Study characteristics | |||
| Patient Sampling | A cross‐sectional, observational study. Study participants were recruited from the cornea outpatient service in a consecutive if eligible basis; controls were refractive surgery candidates. 80 eyes formed the keratoconus group, 55 eyes formed the subclinical keratoconus group, and 50 normal eyes populated the control group. |
||
| Patient characteristics and setting |
Exclusion criteria: previous incisional eye surgery, corneal scars and opacities, history of herpetic keratitis, severe eye dryness, current corneal infection, glaucoma, suspicion of glaucoma, intraocular pressure‐lowering treatment, pregnancy or nursing, contact lens use, and underlying autoimmune disease. |
||
| Index tests | A self‐developed algorithm based on logistic regression in Visual Basic for Microsoft Excel performed a Fourier series harmonic analysis for the posterior corneal sagittal curvature data and evaluated the derived parameters in the diagnosis of Subclinical Keratoconus and Keratoconus. | ||
| Target condition and reference standard(s) | The diagnosis of keratoconus was based on the Amsler‐Krumeich classification and tomography images; however, it was unclear who made the diagnosis. The diagnosis was made before the analysis with the AI algorithm. | ||
| Flow and timing | It was unclear whether all included participants were diagnosed by the same person(s). All participants were included in the analyses. | ||
| Comparative | Different algorithms were developed; it was unclear whether the results were interpreted separately. All data were included in the different analyses. | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Smadja 2013.
| Study characteristics | |||
| Patient Sampling | Retrospective, single centre, case‐control study. Normal eyes were selected from suitable candidates undergoing a screening examination for refractive surgery and among the general population undergoing a routine ophthalmologic examination. |
||
| Patient characteristics and setting |
|
||
| Index tests | A new screening program for the detection of forme fruste keratoconus using the GALILEI Dual Scheimpflug Analyzer (Ziemer Ophthalmic Systems AG, Port, Switzerland). The method is based on an automated decision tree classification that helps to discriminate between normal corneas, forme fruste keratoconus eyes, and keratoconus eyes. | ||
| Target condition and reference standard(s) | Participants were divided into 3 groups, but it was unclear who made the diagnosis. The diagnoses were made before the analysis with the classification tree. |
||
| Flow and timing | It was unclear if the diagnoses were made by the same person(s). All participants were included in the analyses. | ||
| Comparative | Different algorithms were developed. It was unclear whether the results were interpreted separately. All data were included in the different analyses. | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Unclear | ||
| Are the proportions and reasons for missing data similar for all index tests? | Yes | ||
| Unclear risk | |||
Smolek 1997.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre, case‐control study. 300 TMS‐1 examinations (Tomey USA, Cambridge, MA) were collected from medical records at the LSU Eye Center |
||
| Patient characteristics and setting |
Exclusion criteria were unclear. |
||
| Index tests | The study reports the development of a pair of neural networks. One network detects and classifies clinical keratoconus and keratoconus suspects from among a variety of potentially confounding topographic patterns. A second network quantifies the severity of any conelike feature that matches the topographic pattern of clinical keratoconus or keratoconus suspect. | ||
| Target condition and reference standard(s) | Unclear who made the diagnosis. All cases were diagnosed before the analyses. | ||
| Flow and timing | It was unclear if the diagnoses were made by the same person(s). All participants were included in the analyses. | ||
| Comparative | Not applicable | ||
| Notes | Supported by National Eye Institute grants EY03311 and EY02377. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Souza 2010.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre, case‐control study, in which data was collected from medical records: normal (n = 172), astigmatism (n = 89), keratoconus (n = 46), and photorefractive keratectomy (n = 11). | ||
| Patient characteristics and setting | There were 4 groups: normal, astigmatism, keratoconus, and photorefractive keratectomy.
Exclusion criteria: Orbscan II maps with poor corneal coverage, missing data points, poor fixation, or lid artefacts |
||
| Index tests | The performance of support vector machine was evaluated to detect keratoconus apart from all other corneal patterns, using Orbscan II data. | ||
| Target condition and reference standard(s) | It was unclear if the diagnoses were made by the same person(s). All participants were included in the analyses. | ||
| Flow and timing | Participants were divided into 4 groups, but it is unclear who made the diagnosis. The diagnoses were made before the analysis with the classification tree. |
||
| Comparative | Different algorithms were developed; it is unclear whether the results were interpreted separately. All data were included in the different analyses. | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | Yes | ||
| Are the proportions and reasons for missing data similar for all index tests? | Unclear | ||
| Unclear risk | |||
Subramaniam 2022.
| Study characteristics | |||
| Patient Sampling | The SyntEye KTC model (Rozema et al.) has been used to generate the data set that is to be used for the training of the convolutional neural network. The data set consists of topography images of healthy normal eyes, developing keratoconus eyes, and keratoconus eyes. | ||
| Patient characteristics and setting | Data set consists of subclinical keratoconus and keratoconus eyes. | ||
| Index tests | Convolutional neural network. It analyses topography images and classifies them into 3 categories: normal, subclinical and keratoconus. The article provides a clear explanation of the model and training procedure. |
||
| Target condition and reference standard(s) | The topography images were artificially synthesized by a program called Synteye; it made 300 images of each classification: normal, subclinical keratoconus, and keratoconus. No human observation is mentioned. | ||
| Flow and timing | All cases were included in the reference standard and index test. All data were presented in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Twa 2005.
| Study characteristics | |||
| Patient Sampling | Retrospective, single‐centre, case‐control study that included eyes diagnosed with keratoconus and a reference group of normal eyes of corneal refractive surgery candidates | ||
| Patient characteristics and setting |
|
||
| Index tests | An automated decision tree analysis that analyses videokeratography and classifies the cases into normal or keratoconus. | ||
| Target condition and reference standard(s) | Unclear if the diagnoses were made by the same person(s). All participants were included in the analyses. | ||
| Flow and timing | Participants were divided into 2 groups, but it was unclear who made the diagnosis. The diagnoses were made before the analysis with the classification tree. |
||
| Comparative | Not applicable | ||
| Notes | This study was supported by National Institutes of Health grants EY16225 and EY13359 (MDT), American Optometric Foundation Ocular Sciences Ezell Fellowship, Ameritech faculty fellowship (SP), and NIH‐EY12952 (MAB). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Xie 2020.
| Study characteristics | |||
| Patient Sampling | Retrospective, case‐control study, including people throughout China who wanted to undergo refractive surgery, had a primary diagnosis of keratoconus, and had stable postoperative refractive states. In total, 6465 corneal tomographic images from 1385 people were collected to develop the AI model. |
||
| Patient characteristics and setting | The following groups were included: normal cornea, suspected irregular cornea, early‐stage keratoconus, keratoconus, and myopic postoperative cornea.
|
||
| Index tests | InceptionResNetV2 architecture in a convolutional neural network on the TensorFlow platform to create the AI model with transfer learning technique. The algorithm uses a deep learning algorithm with corneal tomographic imaging and divides the images into the previously mentioned groups. This model may aid in identifying at‐risk corneas and determining which people are unsuited to corneal refractive surgery, thereby assisting in surgery decision‐making. | ||
| Target condition and reference standard(s) | The expert team included 3 senior ophthalmologists with at least 5 years of practical experience in the refractive surgery centre of the study clinic. Each image was independently labelled by the 3 experts, none of whom knew the labels selected by the others. When the labels differed, that chosen by 2 of the 3 experts was selected as the standard. |
||
| Flow and timing | The data from all participants were checked by 3 ophthalmologists. The diagnosis was made before the analysis with the AI algorithm. | ||
| Comparative | Not applicable | ||
| Notes | The research received funding through grants 2018YFC0116500 from the National Key R&D Program of China, 31671000 from the Natural Science Foundation of China, 201804020007 from the Guangzhou Science and Technology Planning Project, 81822010 from the National Natural Science Foundation of China, 2018B010109008 from the Science and Technology Planning Projects of Guangdong Province, and 2017TX04R031 from the Guangdong Science and Technology Innovation Leading Talents. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Xu 2017.
| Study characteristics | |||
| Patient Sampling | Prospective, single‐centre, cross‐sectional study. People with keratoconus were enroled at the Affiliated Eye Hospital of Wenzhou Medical University in China. Complete ocular examinations were performed by 2 experienced doctors, including a review of medical and family history, corrected distance visual acuity, slit‐lamp biomicroscopy, fundus examination, and corneal topography using Medmont E300 (Medmont, Inc., Nunawading Melbourne, Australia). |
||
| Patient characteristics and setting | Participants were divided into the following 3 groups.
|
||
| Index tests | Participants were divided into a training set (normal, subclinical keratoconus group, and keratoconus group) used to build the discrimination function, and a validation set (normal and subclinical group) used to test the diagnostic power. The goal of the present study was to apply the Zernike fitting method to describe the 3D varying complexity of corneal shapes and the 3D distribution of corneal thickness, and to characterize the entire corneal topography and tomography data in subclinical eyes, keratoconus eyes, and normal eyes using Pentacam tomography. Furthermore, the metrics constructed from Zernike polynomials were compared to improve the diagnostic sensitivity and specificity for the detection of subclinical keratoconus corneas. |
||
| Target condition and reference standard(s) | 2 experienced doctors performed complete ocular examinations, including a review of medical and family history, corrected distance visual acuity, slit‐lamp biomicroscopy, fundus examination, and corneal topography using Medmont E300 (Medmont, Inc., Nunawading Melbourne, Australia). | ||
| Flow and timing | The data from all participants were checked by 3 ophthalmologists. The diagnosis was made before the analysis with the AI algorithm. | ||
| Comparative | Not applicable | ||
| Notes | This study was supported by the National Natural Science Foundation of China (81400441 to Shen), the National Key Research and Development Program of China (2016YFC0102500 to Wang), and the Zhejiang provincial Natural Science Foundation of China (LQ17H120008 to Xu). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Xu 2022a.
| Study characteristics | |||
| Patient Sampling | Single centre, retrospective, case‐control study. The control group consisted of refractive surgery candidates with normal clinical and topographic features. Early keratoconus was defined as local corneal steepening and asymmetric astigmatism, or the contralateral eye was diagnosed with keratoconus. It also included people with keratoconus. | ||
| Patient characteristics and setting | Data set consisted of subclinical keratoconus and keratoconus eyes. | ||
| Index tests | A predictive index, Sirius Keratoconus Index (SKI), was constructed using LASSO and Logistic regression analyses based on topographic, pachymetric, and aberrometry variables of the Sirius. The cut‐off value of the SKI was set at 0.44. | ||
| Target condition and reference standard(s) | Unclear how the cases were diagnosed. | ||
| Flow and timing | Unclear whether all cases received the same reference standard. All cases were included in the index test. All data were included in a 2 × 2 table. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
| Were different AI tests were developed and interpreted without knowledge of each other. | |||
| Are the proportions and reasons for missing data similar for all index tests? | |||
Yang 2021.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, observational study that recruited participants at the Casey Eye Institute at Oregon Health and Science University (OHSU), Portland, Oregon. Age‐matched normal participants were recruited from volunteers and people seeking refractive surgery consultation. All participants were aged 18 years or older. | ||
| Patient characteristics and setting | A clinical diagnosis of keratoconus was established using a combination of corrected distance visual acuity (CDVA), slit‐lamp physical findings, topographic patterns, and the quantitative topography KISA%. Each keratoconic eye was assigned to 1 of the 3 keratoconic subgroups according to following classification scheme.
Age‐matched normal participants were recruited from volunteers and people seeking refractive surgery consultation. All normal eyes had CDVA ≥ 20/20, no signs of keratoconus on slit‐lamp examination, regular axial power map topography pattern (round, oval, symmetric bowtie, etc.), KISA% < 100%, and no ocular pathology other than myopia or hyperopia. Exclusion criteria: previous corneal surgeries, recent contact lens usage (soft contact lens within 1 week or rigid gas‐permeable lens within 3 weeks), inability to give informed consent, or inability to maintain stable fixation for imaging. Severe keratoconus with corneal scarring has unpredictable corneal and epithelial thickness patterns and does not pose a challenge for clinical diagnosis. |
||
| Index tests | A 2‐step decision tree. Step 1 uses quantitative OCT pachymetric and epithelial thickness map parameters. If any of the 4 parameters listed in the previous section exceeds the cut‐off, the eye is suspicious for keratoconus and proceeds to step 2. If none of the 4 parameters exceeds the cut‐off, then the eye is considered normal and does not require step 2 examination. Step 2 requires a human grader to visually inspect the corneal and epithelial thickness maps and search for characteristic keratoconic map patterns of coincident thinning and concentric epithelial thinning. | ||
| Target condition and reference standard(s) | Unclear who made the diagnosis. All cases were diagnosed before the analysis with the 2‐step decision tree. |
||
| Flow and timing | Unclear if all cases were diagnosed by the same cornea specialists. All cases were included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | Supported by the National Institutes of Health, Bethesda, Maryland, USA (R01EY028755, R01EY029023, T32EY023211, and P30EY010572; E. Pavlatos, D. Huang, and Y. Li); a research grant and equipment support from OptoVue, Inc., Fremont, California (D. Huang and Y. Li); unrestricted grants to Casey Eye Institute from Research to Prevent Blindness, Inc., New York, New York (E. Pavlatos, W. Chamberlain, D. Huang, and Y. Li); National Natural Science Foundation of China, Beijing, China (81900830; Y. Yang). The sponsors did not participate in the data collection, data management, or data analysis in the study. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Yousefi 2018.
| Study characteristics | |||
| Patient Sampling | Multicentre, retrospective, case‐control study. Corneal OCT images were collected from 12,242 eyes of 3162 people using SS‐1000 CASIA OCT Imaging Systems (Tomey, Japan) and other parameters from the electronic health record (EHR) system. | ||
| Patient characteristics and setting | All available data were collected without any preconditions. The investigators then selected a single visit from each eye and excluded eyes with missing ectasia status index (ESI). Eyes were grouped as follows according to ESI.
Using Casia labels, the data set included 1970 healthy eyes, 796 eyes with forme fruste keratoconus, and 390 eyes with keratoconus. |
||
| Index tests | The algorithm included 3 major steps, as follows.
|
||
| Target condition and reference standard(s) | The article does not state who made the diagnosis. All cases were diagnosed before the analysis. |
||
| Flow and timing | Unclear whether all participants were diagnosed by the same cornea specialists. Unclear if all cases included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | The study authors were funded by an unrestricted grant from Research to Prevent Blindness (RPB), New York, NY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
| DOMAIN 5: Comparative | |||
Zeboulon 2020a.
| Study characteristics | |||
| Patient Sampling | Retrospective, machine‐learning, experimental study. The Orbscan (Bausch & Lomb, Bridgewater, NJ) database was exported using the batch export functionality both as image files and the underlying numeric data matrixes represented by each colour map. They selected 3000 examinations in total, 1000 per class (normal, keratoconus, history of refractive surgery). All 3000 examinations were obtained from different people, and only 1 eye per person was selected. They balanced the examinations to have exactly 500 left eyes and 500 right eyes in each class. The selection process was as follows: consecutive examinations were preselected by a resident and reviewed by a corneal tomography expert. |
||
| Patient characteristics and setting |
|
||
| Index tests | The possibility of using numeric data matrixes instead of colour maps to train a convolutional neural network (CNN) for a classification task. Specifically, the investigators used 4 maps that are frequently used in clinical practice, stacked together as if they were 4 colour channels of a single image to classify examinations into 3 categories: normal, keratoconus, and history of refractive surgery. The training set was trained during 15 epochs with a learning rate of 0.0001 and a batch size of 2. |
||
| Target condition and reference standard(s) | The diagnosis was made by a resident and corneal tomography specialist with at least 5 years of experience. The diagnosis was made before the convolutional neural network analysis. |
||
| Flow and timing | All participants were diagnosed by 2 cornea specialists. All cases were included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | The study authors received no funds or support for the study. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
Zeboulon 2020b.
| Study characteristics | |||
| Patient Sampling | Retrospective, machine‐learning, experimental study. 22,066 Orbscan (Bausch&Lomb, USA) examinations were randomly extracted from the Orbscan database using the batch export functionality. The last examination of the first visit for each eye of each person was selected. This process reduced the number of examinations to 13,705. The cases were divided into the following groups: normal, keratoconus, history of myopic refractive surgery, Fuchs' corneal dystrophy, and other. |
||
| Patient characteristics and setting |
|
||
| Index tests | The efficiency of unsupervised algorithms was tested to extract and sort usable examinations from a large unlabelled corneal topography database into different diagnostic clusters, with little human intervention, data cleaning or feature selection. Convolutional neural network (CNN) was used. |
||
| Target condition and reference standard(s) | All 13,705 examinations were manually labelled and checked by 2 corneal topography experts (with at least 5 years of practice in a corneal and refractive surgery department) in a random order. The cases were diagnosed before the convolutional neural network analysis. |
||
| Flow and timing | All participants were diagnosed by 2 cornea specialists. All cases were included in the analysis. | ||
| Comparative | Not applicable | ||
| Notes | No funding source mentioned. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the model designed in an appropriate manner? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference standard | |||
| Is the reference standard likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
| DOMAIN 5: Comparative | |||
AI: artificial intelligence; AS‐OCT: anterior segment optical coherence tomography; AST: astigmatism index; BAD‐D: Belin‐Ambrósio Enhanced Ectasia Display total deviation; D: dioptre; I‐S: inferior‐superior; KISA% index: keratoconus percentage index, derived from central keratometry, the inferior‐superior value, the astigmatism index, and the SRAX index, an expression of irregular astigmatism occurring in keratoconus; LASIK: laser‐assisted in situ keratomileusis; OCT: optical coherence tomography; PPK: percent probability of keratoconus; SD: standard deviation; TMS: Topographic Modeling System.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aatila 2021 | No 2 × 2 data available. |
| Al‐Timemy 2022 | No article available, conference proceedings. |
| Buehren 2018 | Conference proceedings. |
| Cao 2021b | Conference proceedings. |
| Castro‐Luna 2021 | Ineligible index test/reference test. |
| ChiCTR2000037484 | Clinical trial protocol/ongoing study. |
| ChiCTR2000039070 | Clinical trial protocol/ongoing study. |
| DosSantos 2019 | Ineligible outcomes. |
| Elsawy 2022 | Ineligible outcomes. |
| Feng 2021 | No 2 × 2 data available. |
| Hazarbessanov 2022 | No article available; conference proceedings. |
| Hernandez 2020 | Conference proceedings. |
| Hidalgo 2014 | Conference proceedings. |
| Hjordtal 1995 | Ineligible population. |
| Issarti 2018 | Conference proceedings. |
| JPRN‐UMIN000034587 | Clinical trial protocol. |
| JPRN‐UMIN000040128 | Clinical trial protocol. |
| JPRN‐UMIN000040308 | Clinical trial protocol. |
| JPRN‐UMIN000040321 | Clinical trial protocol. |
| JPRN‐UMIN000043831 | Clinical trial protocol/ongoing study. |
| Kleinhans 2019 | Language. |
| Klyce 2005 | Ineligible outcomes. |
| Kundu 2021 | No 2 × 2 data available. |
| Lavric 2019 | Ineligible population. |
| Li 2009 | Ineligible outcomes. |
| Li 2021 | Language. |
| Liu 2021 | Conference proceedings. |
| Malyugin 2021 | No 2 × 2 data available. |
| Matalia 2020 | Ineligible population. |
| Nasrin 2018 | Clinical trial protocol/ongoing study. |
| NCT01746823 | Clinical trial protocol. |
| NCT04313387 | Clinical trial protocol/ongoing study. |
| NCT04763785 | Clinical trial protocol/ongoing study. |
| Omidi 2022 | Ineligible outcomes. |
| Pavlatos 2022 | No article available, conference proceeding. |
| Ramos‐Lopez 2011 | No 2 × 2 data available. |
| Rozema 2017 | Ineligible outcomes. |
| Saad 2010 | Ineligible index test/reference test. |
| Saad 2012 | Ineligible index test/reference test. |
| Schatteburg 2022 | No article available, clinical trial protocol. |
| Souza 2008 | Language. |
| Steinberg 2015a | Wrong index test/reference test. |
| Steinberg 2015b | Wrong index test/reference test. |
| Takahashi 2021 | Conference proceedings. |
| Tan 2019 | Language. |
| Tas 2021 | Conference proceedings. |
| Toprak 2021 | Ineligible index test/reference test. |
| Velazquez‐Blazquez 2020 | No 2 × 2 data available. |
| Vieira de Carvalho 2008 | No 2 × 2 data available. |
| Wang 2022 | Ineligible population. |
| Xu 2022b | No 2 × 2 data available. |
| Yucekul 2022 | Wrong index test. |
| Zghal 1997 | Language. |
| Zou 2019 | Language. |
Differences between protocol and review
During the quality assessment process, we noticed that the QUADAS‐2 tool was not entirely fitted to our review. In the domain 'Index test', we added the question 'Was the model designed in an appropriate manner?'. We considered a study at low risk of bias if data from a single participant were reserved to only one data partition, parameters were tuned, and the optimal model was selected. We considered a study at high risk of bias if data from a single participant were not reserved to only one data partition, parameters were not tuned, and the optimal model was not selected. When the design of the model was unclear, and we could not determine the above‐mentioned properties, we considered the study at unclear risk. In the protocol for this review, we stated that the 'Concerns regarding applicability' question in the domain 'Reference standard' ('Are there concerns that the target condition as defined by the reference standard does not match the review question?') was not applicable to this review (Vandevenne 2021); however, we corrected this during quality assessment. Additionally, in the domain 'Flow and timing', we removed the question 'Was there an appropriate interval between index test(s) and reference standard?', as it was not applicable to this review. The reference test and index test were performed on the same corneal images or parameters, so an interval between index and reference test is irrelevant.
We had planned to use a hierarchical summary receiver operating characteristic (HSROC) model and estimate the average sensitivity at fixed specificity values according to cut‐offs for terciles of specificity (Macaskill 2010). However, we found accuracy was nearly maximal in the vast majority of studies, which clustered close to the upper‐left corner of the ROC plane. Thus, we pooled data using a bivariate model, which is equivalent to an HSROC model in absence of covariates (Harbord 2007).
We had planned to conduct direct comparisons between the index tests, (different types or data sources for AI) if sufficient data were available. We conducted these analyses with a test covariate in the bivariate model as suggested in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Takwoingi 2022).
We had planned to conduct analyses using the 'metadas' user‐written command in SAS software (SAS software) and to make predictions at fixed specificities using NLMIXED procedure postestimation commands. Since we fitted bivariate models, we used Stata softwaremetandi and melogit commands, as recommended in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Takwoingi 2022).
The search line (logistic$ adj2 regression$).tw. in the protocol was amended to (logistic$ adj2 regression adj15 learn$).tw. in the review (Vandevenne 2021). The original line retrieved all reports of logistic regression being used as a statistical method in studies relating to keratoconus. The search phrase was retrieving too many false hits and was edited to identify reports where logistic regression was used in conjunction with some form of machine learning.
Contributions of authors
MMSV: conception of the review, design of the review, search and selection of studies for inclusion in the review, collection of data for the review, assessment of the risk of bias in the included studies, assessment of the certainty in the body of evidence, interpretation of data, writing of the review EF: search and selection of studies for inclusion in the review, collection of data for the review, assessment of the risk of bias in the included studies MV: critical revision of artificial intelligence sections of the review, providing advice on artificial intelligence EL: analysis of data, critical revision of statistical section TB: critical revision of the review RM: critical revision of clinical sections of the review RMMAN: critical revision of clinical sections of the review GV: design of the review, analysis of data, assessment of the certainty in the body of evidence, critical revision of all review sections MMD: conception of the review, critical revision of all review sections
Sources of support
Internal sources
-
University Eye Clinic Maastricht, Maastricht University Medical Center (MUMC+), Maastricht, Netherlands
Authors' place of employment (MMSV, TB, RMMAN, MMD)
-
Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA), University of Florence, Florence, Italy
Authors' place of employment (RM, GV)
-
Department of Neurosciences, Psychology, Pharmacology and Child Health, University of Florence, Florence, Italy
Authors' place of employment (EF)
-
Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Authors' place of employment (MV)
-
Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
Authors' place of employment (EL)
-
Department of Neuroscience, School for Mental Health and Neuroscience (MHeNS), Maastricht University, Maastricht, Netherlands
Authors' place of employment (MMSV)
-
Centre for Public Health, Queen's University Belfast, UK
Authors' place of employment (GV)
External sources
-
Public Health Agency, UK
This review was supported by the HSC Research and Development (R&D) Division of the Public Health Agency which funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast.
Declarations of interest
MMSV, EF, MV, EL, TB, RM: have no conflicts of interest. RMMAN: Carl Zeiss Meditec AG (Independent Contractor ‐ Consultant), Alcon Laboratories Inc (Independent Contractor ‐ Consultant), Johnson & Johnson Health Care Systems Inc. (Independent Contractor ‐ Consultant) GV: former Cochrane Editor, has not been involved in the editorial process of this review MMD: Maastricht University (Employment), Maastricht Universitair Medisch Centrum (MUMC+) (Employment)
New
References
References to studies included in this review
Abdelmotaal 2020 {published data only}
- Abdelmotaal H, Mostafa MM, Mostafa AN, Mohamed AA, Abdelazeem K. Classification of color-coded scheimpflug camera corneal tomography images using deep learning. Translational Vision Science and Technology 2020;9(13):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Accardo 2002 {published data only}
- Accardo PA, Pensiero S. Neural network-based system for early keratoconus detection from corneal topography. Journal of Biomedical Informatics 2002;35(3):151-9. [DOI] [PubMed] [Google Scholar]
Almeida 2022 {published data only}
- Almeida GC, Guido R, Silva H, Brandão C, Mattos LC, Lopes BT, et al. New artificial intelligence index based on Scheimpflug corneal tomography to distinguish subclinical keratoconus from healthy corneas. Journal of Cataract & Refractive Surgery 2022;48(10):1168-74. [DOI] [PubMed] [Google Scholar]
Al‐Timemy 2021 {published data only}
- Al-Timemy AH, Mosa ZM, Alyasseri Z, Lavric A, Lui MM, Hazarbassanov RM, et al. A hybrid deep learning construct for detecting keratoconus from corneal maps. Translational Vision Science and Technology 2021;10(14):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arbelaez 2012 {published data only}
- Arbelaez MC, Versaci F, Vestri G, Barboni P, Savini G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology 2012;119(11):2231-8. [DOI] [PubMed] [Google Scholar]
Bessho 2006 {published data only}
- Bessho K, Maeda N, Kuroda T, Fujikado T, Tano Y, Oshika T. Automated keratoconus detection using height data of anterior and posterior corneal surfaces. Japanese Ophthalmological Society 2006;50(5):409-16. [DOI] [PubMed] [Google Scholar]
Cao 2020 {published data only}
- Cao K, Verspoor K, Sahebjada S, Baird PN. Evaluating the performance of various machine learning algorithms to detect subclinical keratoconus. Translational Vision Science and Technology 2020;9(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2021a {published data only}
- Cao K, Verspoor K, Chan E, Daniell M, Sahebjada S, Baird PN. Machine learning with a reduced dimensionality representation of comprehensive Pentacam tomography parameters to identify subclinical keratoconus. Computer in Biology and Medicine 2021;138:104884. [DOI] [PubMed] [Google Scholar]
Carvalho 2005 {published data only}
- Carvalho LA. Preliminary results of neural networks and zernike polynomials for classification of videokeratography maps. Optometry and Vision Science 2005;82(2):151-8. [DOI] [PubMed] [Google Scholar]
Castro‐Luna 2020 {published data only}
- Castro-Luna GM, Martínez-Finkelshtein A, Ramos-López D. Robust keratoconus detection with Bayesian network classifier for Placido-based corneal indices. Contact Lens and Anterior Eye 2020;43(4):366-72. [DOI] [PubMed] [Google Scholar]
Cavas‐Martinez 2017 {published data only}
- Cavas-Martinez F, Bataille L, Fernandez-Pacheco DG, Cañavate FJF, Alio JL. A new approach to keratoconus detection based on corneal morphogeometric analysis. PLoS One 2017;12(9):e0184569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2015 {published data only}
- Chan C, Ang M, Saad A, Chua D, Mejia M, Lim L, et al. Validation of an objective scoring system for forme fruste keratoconus detection and post-LASIK ectasia risk assessment in Asian eyes. Cornea 2015;34(9):996-1004. [DOI] [PubMed] [Google Scholar]
Chandapura 2019 {published data only}
- Chandapura R, Salomão MQ, Ambrósio Jr R, Swarup R, Shetty R, Sinha Roy A. Bowman's topography for improved detection of early ectasia. Journal of Biophotonics 2019;12(10):e201900126. [DOI] [PubMed] [Google Scholar]
Chastang 2000 {published data only}
- Chastang PJ, Borderie VM, Carvajal-Gonzalez S, Rostene W, Laroche L. Automated keratoconus detection using the EyeSys videokeratoscope. Journal of Refractive Surgery and Cataract 2000;26(5):675-83. [DOI] [PubMed] [Google Scholar]
Chen 2021 {published data only}
- Chen X, Zhao J, Iselin KC, Borroni D, Romano D, Gokul A, et al. Keratoconus detection of changes using deep learning of colour- coded maps. BMJ Open Ophthalmology 2021;6(1):e000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2022 {published data only}
- Cohen E, Bank D, Sorkin N, Giryes R, Varssano D. Use of machine learning to achieve keratoconus detection skills of a corneal expert. International Ophthalmology 2022;42(12):3837-47. [DOI] [PubMed] [Google Scholar]
Consejo 2020 {published data only}
- Consejo A, Solarski J, Karnowski K, Rozema JJ, Wojtkowski M, Iskander DR. Keratoconus detection based on a single scheimpflug image. Translational Vision Science and Technology 2020;9(7):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Almeida Jr 2021 {published data only}
- Almeida Jr GC, Capobianco Guidod R, Netod JS, Rosad JM, Castiglionia L, Mattosa LC, et al. Corneal Tomography Multivariate Index (CTMVI) effectively distinguishes healthy corneas from those susceptible to ectasia. Biomedical Signal Processing and Control 2021;10:102995. [Google Scholar]
Elsawy 2021 {published data only}
- Elsawy A, Eleiwa T, Chase C, Ozcan E, Tolba M, Feuer W, et al. Multidisease deep learning neural network for the diagnosis of corneal diseases. American Journal of Ophthalmology 2021;226:252-61. [DOI] [PubMed] [Google Scholar]
Feizi 2016 {published data only}
- Feizi S, Yaseri M, Kheiri B. Predictive ability of galilei to distinguish subclinical keratoconus and keratoconus from normal corneas. Journal of Ophthalmic and Vision Research 2016;11(1):8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gairola 2022 {published data only}
- Gairola S, Joshi P, Balasubramaniam A, Murali K, Kwatra N, Jain M. Keratoconus classifier for smartphone-based corneal topographer. International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 2022;2022:1875-78. [DOI] [PubMed] [Google Scholar]
Gao 2022 {published data only}
- Gao HB, Pan ZG, Shen MX, Lu F, Li H, Zhang XQ. KeratoScreen: early keratoconus classification with zernike polynomial using deep learning. Cornea 2022;41(9):1158-65. [DOI] [PubMed] [Google Scholar]
Ghaderi 2021 {published data only}
- Ghaderi M, Sharifi A, Jafarzadeh Pour E. Proposing an ensemble learning model based on neural network and fuzzy system for keratoconus diagnosis based on Pentacam measurements. International Ophthalmology 2021;41(12):3935-48. [DOI] [PubMed] [Google Scholar]
Issarti 2019 {published data only}
- Issarti I, Consejo A, Jimenez-Garcia M, Hershko S, Koppen C, Rozema JJ. Computer aided diagnosis for suspect keratoconus detection. Computers in Biology and Medicine 2019;109:33-42. [DOI] [PubMed] [Google Scholar]
Issarti 2020 {published data only}
- Issarti I, Consejo A, Jimenez-Garcia M, Kreps EO, Koppen C, Rozema JJ. Logistic index for keratoconus detection and severity scoring (Logik). Computers in Biology and Medicine 2020;122:103809. [DOI] [PubMed] [Google Scholar]
Kalin 1996 {published data only}
- Kalin NS, Maeda N, Klyce SD, Hargrave S, Wilson SE. Automated topographic screening for keratoconus in refractive surgery candidates. CLAO Journal 1996;22(3):164-7. [PubMed] [Google Scholar]
Kamiya 2019 {published data only}
- Kamiya K, Ayatsuka Y, Kato Y, Fujimura F, Takahashi M, Shoji N, et al. Keratoconus detection using deep learning of colour-coded maps with anterior segment optical coherence tomography: a diagnostic accuracy study. BMJ Open 2019;9(9):e031313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamiya 2021 {published data only}
- Kamiya K, Ayatsuka Y, Kato Y, Shoji N, Mori Y, Miyata K. Diagnosability of keratoconus using deep learning with Placido disk-based corneal topography. Frontiers in Medicine 2021;8:724902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kojima 2020 {published data only}
- Kojima T, Nishida T, Nakamura T, Tamaoki A, Hasegawa A, Takagi Y, et al. Keratoconus screening using values derived from auto-keratometer measurements: a multicenter study. American Journal of Ophthalmology 2020;215:127-34. [DOI] [PubMed] [Google Scholar]
Kojima 2021 {published data only}
- Kojima T, Isogai N, Nishida T, Nakamura T, Ichikawa K. Screening of keratoconus using Autokeratometer and Keratometer Keratoconus Index. Diagnostics 2021;11(11):2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovacs 2016 {published data only}
- Kovacs I, Mihaltz K, Kranitz K, Juhasz E, Takacs A, Dienes L, et al. Accuracy of machine learning classifiers using bilateral data from a Scheimpflug camera for identifying eyes with preclinical signs of keratoconus. Journal of Cataract and Refractive Surgery 2016;42(2):275-83. [DOI] [PubMed] [Google Scholar]
Kuo 2020 {published data only}
- Kuo BI, Chang WY, Liao T S, Liu FY, Liu HY, Chu HS, et al. Keratoconus screening based on deep learning approach of corneal topography. Translational Vision Science and Technology 2020;9(2):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavric 2021 {published data only}
- Lavric A, Anchidin L, Popa V, Al-Timemy AH, Alyasseri Z, Takahashi H, et al. Keratoconus severity detection from elevation, topography and pachymetry raw data using a machine learning approach. IEEE Access 2021;9:84344-55. [Google Scholar]
Lopes 2018 {published data only}
- Lopes BT, Ramos IC, Salomao MQ, Guerra FP, Schallhorn SC, Schallhorn JM, et al. Enhanced tomographic assessment to detect corneal ectasia based on artificial intelligence. American Journal of Ophthalmology 2018;195:223-32. [DOI] [PubMed] [Google Scholar]
Lucena 2021 {published data only}
- Lucena AR, Araujo MO, Carneiro RF, Cavalcante TD, Ribeiro AB, Anselmo FJ. Development of an application for providing corneal topography reports based on artificial intelligence. Arquivos Brasileiros de Oftalmologia 2021;85(4):351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeda 1994 {published data only}
- Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated keratoconus screening with corneal topography analysis. Investigative Ophthalmology and Visual Science 1994;35(6):2749-57. [PubMed] [Google Scholar]
Maeda 1995a {published data only}
- Maeda N, Klyce SD, Smolek MK. Comparison of methods for detecting keratoconus using videokeratography. Archives of Ophthalmology 1995;113(7):870-4. [DOI] [PubMed] [Google Scholar]
Maeda 1995b {published data only}
- Maeda N, Klyce SD, Smolek MK. Neural network classification of corneal topography. Preliminary demonstration. Investigative Ophthalmology and Visual Science 1995;36(7):1327-35. [PubMed] [Google Scholar]
Mahmoud 2013 {published data only}
- Mahmoud AM, Nunez MX, Blanco C, Koch DD, Wang L, Weikert MP, et al. Expanding the cone location and magnitude index to include corneal thickness and posterior surface information for the detection of keratoconus. American Journal of Ophthalmology 2013;156(6):1102-11. [DOI] [PubMed] [Google Scholar]
Mahmoud 2021 {published data only}
- Mahmoud HA, Mengash HA. Automated keratoconus detection by 3D corneal images reconstruction. Sensors 2021;21(7):2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadpour 2022 {published data only}
- Mohammadpour M, Heidari Z, Hashemi H, Yaseri M, Fotouhi A. Comparison of artificial intelligence-based machine learning classifiers for early detection of keratoconus. European Journal of Ophthalmology 2022;32(3):1352-60. [DOI] [PubMed] [Google Scholar]
Pavlatos 2020 {published data only}
- Pavlatos E, Chen S, Yang Y, Wang Q, Huang D, Li Y. A coincident thinning index for keratoconus identification using OCT pachymetry and epithelial thickness maps. Journal of Refractive Surgery 2020;36(11):757-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabinowitz 1999 {published data only}
- Rabinowitz Y S, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. Journal of Cataract and Refractive Surgery 1999;25(10):1327-35. [DOI] [PubMed] [Google Scholar]
Ruiz 2016 {published data only}
- Ruiz Hidalgo I, Rodriguez P, Rozema JJ, Ni Dhubhghaill S, Zakaria N, Tassignon MJ, et al. Evaluation of a machine-learning classifier for keratoconus detection based on scheimpflug tomography. Cornea 2016;35(6):827-32. [DOI] [PubMed] [Google Scholar]
Ruiz 2017 {published data only}
- Ruiz Hidalgo I, Rozema JJ, Saad A, Gatinel D, Rodriguez P, Zakaria N, et al. Validation of an objective keratoconus detection system implemented in a scheimpflug tomographer and comparison with other methods. Cornea 2017;36(6):689-95. [DOI] [PubMed] [Google Scholar]
Saad 2014 {published data only}
- Saad A, Guilbert E, Gatinel D. Corneal enantiomorphism in normal and keratoconic eyes. Journal of Refractive Surgery 2014;30(8):542-7. [DOI] [PubMed] [Google Scholar]
Saad 2016 {published data only}
- Saad A, Gatinel D. Combining placido and corneal wavefront data for the detection of forme fruste keratoconus. Journal of Refractive Surgery 2016;32(8):510-6. [DOI] [PubMed] [Google Scholar]
Saika 2013 {published data only}
- Saika M, Maeda N, Hirohara Y, Mihashi T, Fujikado T, Nishida K. Four discriminant models for detecting keratoconus pattern using Zernike coefficients of corneal aberrations. Japanese Journal of Ophthalmology 2013;57(6):503-9. [DOI] [PubMed] [Google Scholar]
Shetty 2015 {published data only}
- Shetty R, Matalia H, Srivatsa P, Ghosh A, Dupps W J Jr, Sinha Roy A. A novel zernike application to differentiate between three-dimensional corneal thickness of normal corneas and corneas with keratoconus. American Journal of Ophthalmology 2015;160(3):453-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2020 {published data only}
- Shi C, Wang M, Zhu T, Zhang Y, Ye Y, Jiang J, et al. Machine learning helps improve diagnostic ability of subclinical keratoconus using Scheimpflug and OCT imaging modalities. Eye and Vision 2020;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sideroudi 2017 {published data only}
- Sideroudi H, Labiris G, Georgantzoglou K, Ntonti P, Siganos C, Kozobolis V. Fourier analysis algorithm for the posterior corneal keratometric data: clinical usefulness in keratoconus. Ophthalmic and Physiological Optics 2017;37(4):460-6. [DOI] [PubMed] [Google Scholar]
Smadja 2013 {published data only}
- Smadja D, Touboul D, Cohen A, Doveh E, Santhiago MR, Mello GR, et al. Detection of subclinical keratoconus using an automated decision tree classification. American Journal of Ophthalmology 2013;156(2):237-46. [DOI] [PubMed] [Google Scholar]
Smolek 1997 {published data only}
- Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Investigative Ophthalmology and Visual Science 1997;38(11):2290-9. [PubMed] [Google Scholar]
Souza 2010 {published data only}
- Souza MB, Medeiros FW, Souza DB, Garcia R, Alves MR. Evaluation of machine learning classifiers in keratoconus detection from orbscan II examinations. Clinics 2010;65(12):1223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramaniam 2022 {published data only}
- Subramaniam P, Ramesh GP. Keratoconus classification with convolutional neural networks using segmentation and index quantification of eye topography images by particle swarm optimisation. BioMed Research International 2022;2022:Article ID 8119685. [DOI: 10.1155/2022/8119685] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Twa 2005 {published data only}
- Twa MD, Parthasarathy S, Roberts C, Mahmoud AM, Raasch TW, Bullimore MA. Automated decision tree classification of corneal shape. Optometry and Vision Science 2005;82(12):1038-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2020 {published data only}
- Xie Y, Zhao L, Yang X, Wu X, Yang Y, Huang X, et al. Screening candidates for refractive surgery with corneal tomographic-based deep learning. JAMA Ophthalmology 2020;138(5):519-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu Z, Li W, Jiang J, Zhuang X, Chen W, Peng M, et al. Characteristic of entire corneal topography and tomography for the detection of sub-clinical keratoconus with Zernike polynomials using Pentacam. Scientific Reports 2017;7(1):16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2022a {published data only}
- Xu Y, Ren YR, Zhaung XY, Zhang XF. Predictive index based on minimum corneal thickness and symmetry index back of Sirius for early diagnosis of keratoconus. International Eye Science 2022;22(9):1426-35. [Google Scholar]
Yang 2021 {published data only}
- Yang Y, Pavlatos E, Chamberlain W, Huang D, Li Y. Keratoconus detection using OCT corneal and epithelial thickness map parameters and patterns. Journal of Cataract and Refractive Surgery 2021;47(6):759-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousefi 2018 {published data only}
- Yousefi S, Yousefi E, Takahashi H, Hayashi T, Tampo H, Inoda S, et al. Keratoconus severity identification using unsupervised machine learning. PLoS One 2018;13(11):e0205998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeboulon 2020a {published data only}
Zeboulon 2020b {published data only}
- Zeboulon P, Debellemaniere G, Gatinel D. Unsupervised learning for large-scale corneal topography clustering. Science Reports 2020;10(1):16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aatila 2021 {published data only}
- Aatila M, Lachgar M, Hamid H, Kartit A. Keratoconus severity classification using features selection and machine learning algorithms. Computational and Mathematical Methods in Medicine 2021;2021:9979560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Timemy 2022 {published data only}
- Al-Timemy A, Al-Zubaidi L, Ghaeb N, Takahashi H, Lavric A, Mosa Z, et al. A device-agnostic deep learning model for detecting keratoconus based on anterior elevation corneal maps. In: Investigative Ophthalmology and Visual Science. 7 edition. Vol. 63. 2022:2101.
Buehren 2018 {published data only}
- Buehren J, Kleinhans S, Herrmann E, Kohnen T. Comparison of metrics obtained with discriminant analysis and decision trees for the detection of subclinical keratoconus. Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 5724.
Cao 2021b {published data only}
- Cao K, Verspoor K, Chan E, Daniell M, Sahebjada S, Baird PN. Novel, high-performance machine learning model for detection of subclinical keratoconus. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 2157.
Castro‐Luna 2021 {published data only}
- Castro-Luna G Jimenez-Rodriguez D, Castano-Fernandez AB, Perez-Rueda A. Diagnosis of subclinical keratoconus based on machine learning techniques. Journal of Clinical Medicine 2021;10(18):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR2000037484 {published data only}
- ChiCTR2000037484. Study on artificial intelligence-assisted diagnosis of keratoconus diseases. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000037484 (first received 28 August 2020).
ChiCTR2000039070 {published data only}
- ChiCTR2000039070. Research on early warning diagnosis and prognosis model of keratoconus based on Artificial Intelligence. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000039070 (first received 15 October 2020).
DosSantos 2019 {published data only}
- Dos Santos VA, Schmetterer L, Stegmann H, Pfister M, Messner A, Schmidinger G, et al. CorneaNet: fast segmentation of cornea OCT scans of healthy and keratoconic eyes using deep learning. Biomedical Optics Express 2019;10(2):622-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsawy 2022 {published data only}
- Elsawy A, Abdel-Mottaleb M. PIPE-Net: A pyramidal-input-parallel-encoding network for the segmentation of corneal layer interfaces in OCT images. Computers in Biology and Medicine 2022;147:105595. [DOI] [PubMed] [Google Scholar]
Feng 2021 {published data only}
- Feng R, Xu Z, Zheng X, Hu H, Jin X, Chen DZ, et al. KerNet: a novel deep learning approach for keratoconus and sub-clinical keratoconus detection based on raw data of the Pentacam HR system. IEEE Journal of Biomedical and Health Informatics 2021;25(10):3898-910. [DOI] [PubMed] [Google Scholar]
Hazarbessanov 2022 {published data only}
- Hazarbassanov RM, Alyasseri ZA, Al-Timemy A, Lavric A, Abasid AK, Takahashi H, et al. Detecting keratoconus on two different populations using an unsupervised hybrid artificial intelligence model. In: Investigative Ophthalmology and Visual Science. 7 edition. Vol. 63. 2022:2088.
Hernandez 2020 {published data only}
- Hernandez LA, Sanchez-Huerta V, Ramirez-Fernandez M, Hernandez-Quintela E. Combinatorial approach to determine top performing keratometric features and machine learning algorithms for keratoconus detection. Investigative Ophthalmology and Visual Science 2020;61(7):ARVO E-abstract 4750.
Hidalgo 2014 {published data only}
- Hidalgo IR, Perez PR, Rozema JJ, Tassignon MJB. Comparison of machine learning methods to automatically classify keratoconus. Investigative Ophthalmology and Visual Science 2014;55(13):ARVO E-abstract 4206.
Hjordtal 1995 {published data only}
- Hjordtal JO, Erdmann L, Bek T. Fourier analysis of video-keratographic data. A tool for separation of spherical, regular astigmatic and irregular astigmatic corneal power components. Ophthalmic and Physiological Optics 1995;15(3):171-85. [PubMed] [Google Scholar]
Issarti 2018 {published data only}
- Issarti I, Consejo A, Rozema J. Elevation-based detection of keratoconus. Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 5810.
JPRN‐UMIN000034587 {published data only}
- JPRN-UMIN000034587. Diagnostic evaluation of keratoconus using anterior segment optical coherence tomography and machine learning. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000034587 (first received 1 November 2018).
JPRN‐UMIN000040128 {published data only}
- JPRN-UMIN000040128. Diagnostic evaluation of keratoconus using corneal topography and machine learning. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000040128 (first received 10 April 2020).
JPRN‐UMIN000040308 {published data only}
- JPRN-UMIN000040308. Prediction of keratoconus progression using anterior segment optical coherence tomography and deep learning. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000040308 (first received 1 October 2020).
JPRN‐UMIN000040321 {published data only}
- JPRN-UMIN000040321. Development of diagnostic artificial intelligence in the ophthalmology. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000040321 (first received 7 May 2020).
JPRN‐UMIN000043831 {published data only}
- JPRN-UMIN000043831. Screening for keratoconus using Smartphone and artificial intelligence. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000043831 (first received 3 April 2021).
Kleinhans 2019 {published data only}
- Kleinhans S, Herrmann E, Kohnen T, Buhren J. Comparison of discriminant analysis and decision trees for the detection of subclinical keratoconus. Klinische Monatsblatter fur Augenheilkunde 2019;236(6):798-805. [DOI] [PubMed] [Google Scholar]
Klyce 2005 {published data only}
- Klyce SD, Karon MD, Smolek MK. Screening patients with the corneal navigator. Journal of Refractive Surgery 2005;21(5):S617-22. [DOI] [PubMed] [Google Scholar]
Kundu 2021 {published data only}
- Kundu G, Shetty R, Khamar P, Mullick R, Gupta S, Nuijts R, et al. Universal architecture of corneal segmental tomography biomarkers for artificial intelligence-driven diagnosis of early keratoconus. British Journal of Ophthalmology 2021;16:319309. [DOI] [PubMed] [Google Scholar]
Lavric 2019 {published data only}
- Lavric A, Valentin P. Keratodetect: keratoconus detection algorithm using convolutional neural networks. Computational Intelligence and Neuroscience 2019;2019:8162567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li X, Yang H, Rabinowitz YS. Keratoconus: classification scheme based on videokeratography and clinical signs. Journal of Cataract and Refractive Surgery 2009;35(9):1597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2021 {published data only}
- Li DF, Dong YL, Xie, S, Guo Z, Li SX, Guo Y, et al. Deep learning based lesion detection from anterior segment optical coherence tomography images and its application in the diagnosis of keratoconus. Chinese Journal of Ophthalmology June 2021;57(6):447-53. [DOI] [PubMed] [Google Scholar]
Liu 2021 {published data only}
- Liu H, Anwar M, Koaik M, Taylor S, Karanjia R, Mintsioulis G, et al. Deep learning for detection of keratoconus and prediction of crosslinking efficacy. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 2044.
Malyugin 2021 {published data only}
- Malyugin B, Sakhnov S, Izmailova S, Boiko E, Pozdeyeva N, Axenova L, et al. Keratoconus diagnostic and treatment algorithms based on machine-learning methods. Diagnostics 2021;11(10):1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matalia 2020 {published data only}
- Matalia H, Matalia J, Pisharody A, Patel Y, Chinnappaiah N, Salomao M, et al. Unique corneal tomography features of allergic eye disease identified by OCT imaging and artificial intelligence. Journal of Biophotonics 2020;13(10):e202000156. [DOI] [PubMed] [Google Scholar]
Nasrin 2018 {published data only}
- Nasrin F, Iyer RV, Mathews SM. Simultaneous estimation of corneal topography, pachymetry, and curvature. IEEE Transactions on Medical Imaging 2018;37(11):2463-73. [DOI] [PubMed] [Google Scholar]
NCT01746823 {unpublished data only}
- NCT01746823. Identification and validation of functional biomarkers for keratoconus. clinicaltrials.gov/ct2/show/NCT01746823 (first received 11 December 2012).
NCT04313387 {published data only}
- NCT04313387. Efficiency of an algorithm derived from corneal tomography parameters to distinguish highly susceptible corneas to ectasia from healthy. clinicaltrials.gov/ct2/show/NCT04313387 (first received 18 March 2020).
NCT04763785 {published data only}
- NCT04763785. Development of a keratoconus detection algorithm by deep learning analysis and Its validation on eyestar images. clinicaltrials.gov/ct2/show/NCT04763785 (first received 21 February 2021).
Omidi 2022 {published data only}
- Omidi P, Cayless A, Langenbucher A. Evaluation of optimal Zernike radial degree for representing corneal surfaces. PLOS One 2022;17(5):e0269119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavlatos 2022 {published data only}
- Pavlatos E, Huang D, Li Y. Combining OCT corneal topography and thickness maps to diagnose keratoconus using a convolutional neural network. In: Investigative Ophthalmology and Visual Science. 7 edition. Vol. 63. 2022:2109.
Ramos‐Lopez 2011 {published data only}
- Ramos-Lopez D, Martinez-Finkelshtein A, Castro-Luna GM, Pinero D, Alio JL. Placido-based indices of corneal irregularity. Optometry and Vision Science 2011;88(10):1220-31. [DOI] [PubMed] [Google Scholar]
Rozema 2017 {published data only}
- Rozema JJ, Rodriguez P, Navarro R, Koppen C. Bigaussian wavefront model for normal and keratoconic eyes. Optometry and Vision Science 2017;94(6):680-87. [DOI] [PubMed] [Google Scholar]
Saad 2010 {published data only}
- Saad A, Gatinel D. Topographic and tomographic properties of forme fruste keratoconus corneas. Investigative Ophthalmology and Visual Science 2010;51(11):5546-55. [DOI] [PubMed] [Google Scholar]
Saad 2012 {published data only}
- Saad A, Gatinel D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Investigative Ophthalmology and Visual Science 2012;53(6):2978-92. [DOI] [PubMed] [Google Scholar]
Schatteburg 2022 {published data only}
- Schatteburg J, Langenbucher A. Protocol for the diagnosis of keratoconus using convolutional neural networks. PLOS One 2022;17(2):e0264219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souza 2008 {published data only}
- Souza MB, Medeiros FW, Souza DB, Alves MR. Detection of keratoconus based on a neural network with Orbscan. Arquivos Brasileiros de Oftalmologia 2008;71(6):65-8. [DOI] [PubMed] [Google Scholar]
Steinberg 2015a {published data only}
- Steinberg J, Aubke-Schultz S, Frings A, Hulle J, Druchkiv V, Richard G, et al. Correlation of the KISA% index and Scheimpflug tomography in 'normal', 'subclinical', 'keratoconus-suspect' and 'clinically manifest' keratoconus eyes. Acta Opthalmologica 2015;93(3):e199-207. [DOI] [PubMed] [Google Scholar]
Steinberg 2015b {published data only}
- Steinberg J, Casagrande MK, Frings A, Katz T, Druchkiv V, Richard G, et al. Screening for subclinical keratoconus using swept-source Fourier domain anterior segment optical coherence tomography. Cornea 2015;34(11):1413-9. [DOI] [PubMed] [Google Scholar]
Takahashi 2021 {published data only}
- Takahashi H, Al-Timemy AH, Mosa ZM, Alyasseri Z, Lavric A, Filho JA, et al. Detecting keratoconus severity from corneal data of different populations with machine learning. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 2145.
Tan 2019 {published data only}
- Tan A, Yu M, Chen X, Hu L. Application of deep learning in early diagnosis assistant system of keratoconus. Zhongguo Yiliao Qixie Zazhi 2019;43(2):83-5. [DOI] [PubMed] [Google Scholar]
Tas 2021 {published data only}
- Tas AY, Hasanreisoglu M, Balim H, Gonen M, Sahin A. Automated diagnosis of keratoconus from corneal topography. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 2021.
Toprak 2021 {published data only}
- Toprak I, Cavas F, Velazquez JS, Alio del Barrio JL, Alio JL. Three-dimensional morphogeometric and volumetric characterization of cornea in pediatric patients with early keratoconus. American Journal of Ophthalmology 2021;222:102-11. [DOI] [PubMed] [Google Scholar]
Velazquez‐Blazquez 2020 {published data only}
- Velazquez-Blazquez JS, Bolarin JM, Cavas-Martinez F, Alio JL. Emklas: A new automatic scoring system for early and mild keratoconus detection. Translational Vision Science and Technology 2020;9(2):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieira de Carvalho 2008 {published data only}
- Vieira de Carvalho LA, Barbosa MS. Neural networks and statistical analysis for classification of corneal videokeratography maps based on Zernike coefficients: a quantitative comparison. Arquivos Brasileiros de Oftalmologia 2008;71(3):337-41. [DOI] [PubMed] [Google Scholar]
Wang 2022 {published data only}
- Wang L, Shen M, Shi C, Zhou Y, Chen Y, Pu J, et al. EE-Net: An edge-enhanced deep learning network for jointly identifying corneal micro-layers from optical coherence tomography. Biomedical Signal Processing and Control 2022;71(Pt B):103213. [Google Scholar]
Xu 2022b {published data only}
Yucekul 2022 {published data only}
- Yücekul B, Dick HB, Taneri S. Systematic Detection of Keratoconus in Optical Coherence Tomography: Corneal and Epithelial Thickness Maps. Journal of Cataract & Refractive Surgery 2022;10:1097. [DOI] [PubMed] [Google Scholar]
Zghal 1997 {published data only}
- Zghal I, Saragoussi JJ, Cotinat J, Renard G, Pouliquen Y. Automated keratoconus detection in fellow eyes of unilateral clinically keratoconus. Journal Francais d'Ophtalmologie 1997;20(4):284-91. [PubMed] [Google Scholar]
Zou 2019 {published data only}
- Zou HH, Xu JH, Zhang L, Ji SF, Wang Y. Assistant diagnose for subclinical keratoconus by artificial intelligence. Chinese Journal of Ophthalmology 2019;55(12):911-5. [DOI] [PubMed] [Google Scholar]
Additional references
Abràmoff 2016
- Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investigative Ophthalmology and Visual Science 2016;57(13):5200-6. [DOI] [PubMed] [Google Scholar]
Brown 2018
- Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RV, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmology 2018;136(7):803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunner 2018
- Brunner M, Czanner G, Vinciguerra R, Romano V, Ahmad S, Batterbury M, et al. Improving precision for detecting change in the shape of the cornea in patients with keratoconus. Scientific Reports 2018;8(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2022
- Cao K, Verspoor K, Sahebjada S, Baird PN. Accuracy of machine learning assisted detection of keratoconus: a systematic review and meta-analysis. Journal of Clinical Medicine 2022;11(3):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne: Veritas Health Innovation, accessed 30 November 2022. Available at www.covidence.org.
Fan 2018
- Fan R, Chan TC, Prakash G, Jhanji V. Applications of corneal topography and tomography: a review. Clinical and Experimental Ophthalmology 2018;42(2):133-46. [DOI] [PubMed] [Google Scholar]
Ferdi 2019
- Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus natural progression: a systematic review and meta-analysis of 11 529 eyes. Ophthalmology 2019;126(7):935-45. [DOI] [PubMed] [Google Scholar]
Flynn 2016
- Flynn TH, Sharma DP, Bunce C, Wilkins MR. Differential precision of corneal Pentacam HR measurements in early and advanced keratoconus. British Journal of Ophthalmology 2016;100(9):1183-7. [DOI] [PubMed] [Google Scholar]
Gargeya 2017
- Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology 2017;124(7):962-9. [DOI] [PubMed] [Google Scholar]
Giri 2017
- Giri P, Azar DT. Risk profiles of ectasia after keratorefractive surgery. Current Opinion in Ophthalmology 2017;28(4):337-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomes 2015
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio RJ, Guell JL, et al. Global consensus on keratoconus and ectatic diseases. Cornea 2015;34(4):359-69. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 27 February 2023. Available from gradepro.org.
Grassmann 2018
- Grassmann F, Mengelkamp J, Brandl C, Harsch S, Zimmermann ME, Linkohr B, et al. A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology 2018;125(9):1410-20. [DOI] [PubMed] [Google Scholar]
Gulshan 2016
- Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016;316(22):2402-10. [DOI] [PubMed] [Google Scholar]
Harbord 2007
- A unification of models for meta-analysis of diagnostic accuracy studies. Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. Biostatistics 2007;8:239-51. [DOI] [PubMed] [Google Scholar]
Hashemi 2020
- Hashemi H, Heydarian S, Hooshmand E, Saatchi M, Yekta A, Aghamirsalim M, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea 2020;39(2):263-70. [DOI] [PubMed] [Google Scholar]
Hayes 2012
- Hayes S, Khan S, Boote C, Kamma-Lorger CS, Dooley E, Lewis J, et al. Depth profile study of abnormal collagen orientation in keratoconus corneas. Archives of Ophthalmology 2012;130(2):251-2. [DOI] [PubMed] [Google Scholar]
Hogarty 2019
- Hogarty DT, Mackey DA, Hewitt AW. Current state and future prospects of artificial intelligence in ophthalmology: a review. Clinical and Experimental Ophthalmology 2019;47(1):128-39. [DOI] [PubMed] [Google Scholar]
Kanellopoulos 2013a
- Kanellopoulos AJ, Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clinical Ophtholmology 2013;7:1539-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanellopoulos 2013b
- Kanellopoulos AJ, Moustou V, Asimellis G. Evaluation of visual acuity, pachymetry and anterior-surface irregularity in keratoconus and crosslinking intervention follow-up in 737 cases. International Journal of Keratoconus and Ectatic Corneal Diseases 2013;2(3):95. [Google Scholar]
Kelly 2011
- Kelly T, Williams KA, Coster DJ. Corneal transplantation for keratoconus: a registry study. Archives of Ophthalmology 2011;129(6):691-7. [DOI] [PubMed] [Google Scholar]
LeCun 2015
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521(7553):436-44. [DOI] [PubMed] [Google Scholar]
Leeflang 2022
- Leeflang MM, Steingart KR, Scholten RJ, Davenport C. Chapter 12: Drawing conclusions. In: Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2.0 (updated July 2023). Cochrane, 2023. Available from training.cochrane.org/handbook-diagnostic-test-accuracy/current.
Lin 2019
- Lin SR, Ladas JG, Bahadur GG, Al-Hashimi S, Pineda R. A review of machine learning techniques for keratoconus detection and refractive surgery screening. Seminars in Ophthalmology 2019;34(4):317-26. [DOI] [PubMed] [Google Scholar]
Lopes 2012
- Lopes BT, Ramos IC, Faria-Correia F, Luz A, Freitas Valbon B, Belin MW, et al. Correlation of topometric and tomographic indices with visual acuity in patients with keratoconus. International Journal of Keratoconus and Ectatic Corneal Diseases 2012;1(3):167-72. [Google Scholar]
Lopes 2019
- Lopes BT, Eliasy A, Ambrosio R. Artificial Intelligence in corneal diagnosis: where are we? Current Ophthalmology Reports 2019;7(3):204-11. [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from training.cochrane.org/handbook-diagnostic-test-accuracy/archive/v1.
Martínez‐Abad 2017
- Martínez-Abad A, Pinero DP. New perspectives on the detection and progression of keratoconus. Journal of Cataract and Refractive Surgery 2017;43(9):1213-27. [DOI] [PubMed] [Google Scholar]
Meek 2005
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Investigative Ophthalmology and Visual Science 2005;46(6):1948-56. [DOI] [PubMed] [Google Scholar]
Rabinowitz 2021
- Rabinowitz YS, Galvis V, Tello A, Rueda D, García JD. Genetics vs chronic corneal mechanical trauma in the etiology of keratoconus. Experimental Eye Research 2021;202:108328. [DOI] [PubMed] [Google Scholar]
Reitsma 2009
- Reitsma JB, Rutjes AW, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ. Chapter 9: Assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2009. Available from training.cochrane.org/handbook-diagnostic-test-accuracy/archive/v1.
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre: Cochrane Collaboration, accessed 20 March 2023.
Röck 2018
- Röck T, Bartz-Schmidt KU, Röck D. Trends in corneal transplantation at the University Eye Hospital in Tübingen, Germany over the last 12 years: 2004–2015. PLOS One 2018;13(6):e0198793. [DOI] [PMC free article] [PubMed] [Google Scholar]
SAS software [Computer program]
- SAS software. Version 9.2. North Carolina, USA: SAS Institute Inc, accessed 15 October 2022. www.sas.com.
Sedghipour 2012
- Sedghipour MR, Sadigh AL, Motlagh BF. Revisiting corneal topography for the diagnosis of keratoconus: use of Rabinowitz’s KISA% index. Clinical Ophthalmology 2012;6:181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shibata 2018
- Shibata N, Tanito M, Mitsuhashi K, Fujino Y, Matsuura M, Murata H, et al. Development of a deep residual learning algorithm to screen for glaucoma from fundus photography. Scientific Reports 2018;8(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata software [Computer program]
- StataCorp. 2021. Version Release 17. College Station, TX: StataCorp LLC, accessed 10 October 2022. www.stata.com.
Subhash 2013
- Subhash HM, Wang RK. Optical coherence tomography: technical aspects. In: Liang R, editors(s). Biomedical Optical Imaging Technologies. Biological and Medical Physics, Biomedical Engineering. Berlin, Heidelberg: Springer, 2013:163-212. [DOI: 10.1007/978-3-642-28391-8_5] [DOI] [Google Scholar]
Sykakis 2015
- Sykakis E, Karim R, Evans JR, Bunce C, Amissah‐Arthur KN, Patwary S, et al. Corneal collagen cross‐linking for treating keratoconus. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD010621. [DOI: 10.1002/14651858.CD010621.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2022
- Takwoingi Y, Dendukuri N, Schiller I, Rücker G, Jones HE, Partlett C, et al. Chapter 10: Undertaking meta-analysis. In: Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2.0 (updated July 2023). Cochrane, 2023. Available from training.cochrane.org/handbook-diagnostic-test-accuracy/current.
Ting 2017
- Ting DS, Cheung CY, Lim G, Tan GS, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017;318(22):2211-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ting 2019
- Ting DS, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, Wong TY. Artificial intelligence and deep learning in ophthalmology. British Journal of Ophthalmology 2019;103(2):167-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ting 2020
- Ting DS, Foo VH, Yang LW, Sia ST, Ang M, Lin H, et al. Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. British Journal of Ophthalmology 2020;105(2):158-68. [DOI] [PubMed] [Google Scholar]
Wojtkowski 2010
- Wojtkowski M. High-speed optical coherence tomography: basics and applications. Applied Optics 2010;49(16):D30-61. [DOI] [PubMed] [Google Scholar]
Yang 2021
- Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al, QUADAS-C Group. QUADAS-C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Annals of Internal Medicine 2021;174(11):1592-9. [DOI] [PubMed] [Google Scholar]
Zadnik 1996
- Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea 1996;15(2):139-46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Vandevenne 2021
- Vandevenne MM, Favuzza E, Veta M, Lucenteforte E, Berendschot T, Mencucci R, et al. Artificial intelligence for detecting keratoconus. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD014911. [DOI: 10.1002/14651858.CD014911] [DOI] [PMC free article] [PubMed] [Google Scholar]


