Abstract
Aspergillus oryzae was found to secrete two distinct β-glucosidases when it was grown in liquid culture on various substrates. The major form had a molecular mass of 130 kDa and was highly inhibited by glucose. The minor form, which was induced most effectively on quercetin (3,3′,4′,5,7-pentahydroxyflavone)-rich medium, represented no more than 18% of total β-glucosidase activity but exhibited a high tolerance to glucose inhibition. This highly glucose-tolerant β-glucosidase (designated HGT-BG) was purified to homogeneity by ammonium sulfate precipitation, gel filtration, and anion-exchange chromatography. HGT-BG is a monomeric protein with an apparent molecular mass of 43 kDa and a pI of 4.2 as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and isoelectric focusing polyacrylamide gel electrophoresis, respectively. Using p-nitrophenyl-β-d-glucoside as the substrate, we found that the enzyme was optimally active at 50°C and pH 5.0 and had a specific activity of 1,066 μmol min−1 mg of protein−1 and a Km of 0.55 mM under these conditions. The enzyme is particularly resistant to inhibition by glucose (Ki, 1.36 M) or glucono-δ-lactone (Ki, 12.5 mM), another powerful β-glucosidase inhibitor present in wine. A comparison of the enzyme activities on various glycosidic substrates indicated that HGT-BG is a broad-specificity type of fungal β-glucosidase. It exhibits exoglucanase activity and hydrolyzes (1→3)- and (1→6)-β-glucosidic linkages most effectively. This enzyme was able to release flavor compounds, such as geraniol, nerol, and linalol, from the corresponding monoterpenyl-β-d-glucosides in a grape must (pH 2.9, 90 g of glucose liter−1). Other flavor precursors (benzyl- and 2-phenylethyl-β-d-glucosides) and prunin (4′,5,7-trihydroxyflavanone-7-glucoside), which contribute to the bitterness of citrus juices, are also substrates of the enzyme. Thus, this novel β-glucosidase is of great potential interest in wine and fruit juice processing because it releases aromatic compounds from flavorless glucosidic precursors.
β-Glucoside glucohydrolases, commonly called β-glucosidases, catalyze the hydrolysis of alkyl- and aryl-β-glucosides, as well as diglucosides and oligosaccharides. These enzymes are widely used in various biotechnological processes, including the production of fuel ethanol from cellulosic agricultural residues (4, 27, 48) and the synthesis of useful β-glucosides (21, 38). In the flavor industry, β-glucosidases are also key enzymes in the enzymatic release of aromatic compounds from glucosidic precursors present in fruits and fermentating products (13, 39). Indeed, many natural flavor compounds, such as monoterpenols, C-13 norisoprenoids, and shikimate-derived compounds, accumulate in fruits as flavorless precursors linked to mono- or diglycosides and require enzymatic or acidic hydrolysis for the liberation of their fragrances (41, 45). Finally, β-glucosidases can also improve the organoleptic properties of citrus fruit juices, in which the bitterness is in part due to a glucosidic compound, naringin (4′,5,7-trihydroxyflavanone-7-rhamnoglucoside), whose hydrolysis requires, in succession, an α-rhamnosidase and a β-glucosidase (33).
It is now well-established that certain monoterpenols of grapes (e.g., linalol, geraniol, nerol, citronelol, α-terpineol, and linalol oxide), which are linked to diglycosides, such as 6-O-α-l-rhamnopyranosyl-, 6-O-α-l-arabinofuranosyl-, and 6-O-β-d-apiofuranosyl-β-d-glucosides, contribute significantly to the flavor of wine (15, 44). The enzymatic hydrolysis of these compounds requires a sequential reaction; first, an α-l-rhamnosidase, an α-l-arabinofuranosidase, or a β-d-apiofuranosidase cleaves the (1→6) osidic linkage, and then, the flavor compounds are liberated from the monoglucosides by the action of a β-glucosidase (18, 19). Unlike acidic hydrolysis, enzymatic hydrolysis is highly efficient and does not result in modifications of the aromatic character (16). However, grape and yeast glucosidases exhibit limited activity on monoterpenyl-glucosides during winemaking, and a large fraction of the aromatic precursors remains unprocessed (9, 16, 35). The addition of exogenous β-glucosidase during or following fermentation has been found to be the most effective way to improve the hydrolysis of the glycoconjugated aroma compounds in order to enhance wine flavor (2, 14, 39, 40). The ideal β-glucosidase should function and be stable at a low pH value (pH 2.5 to 3.8) and should be active at a high concentration of glucose (10 to 20%) and in the presence of 10 to 15% ethanol. However, most microbial β-glucosidases are very sensitive to glucose inhibition (4, 12, 47), as well as to inhibition by glucono-δ-lactone, another powerful β-glucosidase inhibitor produced by grape-attacking fungi which can be found in wine must at concentrations up to 2 g/liter (10).
The need for more suitable enzymes has led us and other workers to search for novel β-glucosidases with the desired properties. Recently, we showed that an extracellular glucose-tolerant and pH-stable β-glucosidase can be produced by Aspergillus strains (17). However, the enzyme of interest represented only a minor fraction of total β-glucosidase activity, and the major form was highly sensitive to glucose inhibition. Aspergillus oryzae appeared to be the best producer of the minor form when it was grown on quercetin (3,3′,4′,5,7-pentahydroxyflavone), a phenolic flavonoid found in plant cell walls. This paper presents further data on the production and characterization of this novel highly glucose-tolerant β-glucosidase (designated HGT-BG) purified from the extracellular culture filtrate of A. oryzae grown on quercetin.
MATERIALS AND METHODS
Organism and culture conditions.
A. oryzae CBS 12559, which was used in this study, was obtained from the Centraalbureau voor Schimmelcultures (Baarn, The Netherlands) and was maintained on potato dextrose agar (Difco). For enzyme production, A. oryzae was grown on minimal medium [0.2% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.1% NH4NO3, 0.1% (NH4)H2PO4, 0.05% MgSO4 · 7H2O, 0.05% yeast extract; pH 6.0] supplemented with an appropriate carbon source at a concentration of 0.5% (wt/vol). Quercetin, rutin, cellobiose, glucose, lactose, sucrose, maltose, arabinose, and xylose were purchased from Sigma. A 1-liter flask containing 500 ml of liquid medium was inoculated with 5 × 107 A. oryzae viable spores resuspended in 0.15% (vol/vol) Tween 80. The culture was incubated at 28°C for 14 days on an orbital shaker (120 rpm) and was harvested by filtration through Whatman GF/A glass microfiber filters. The filtrate was used as the crude β-glucosidase preparation.
Purification of HGT-BG.
All steps in the purification of HGT-BG were carried out at 4°C.
(i) Ammonium sulfate precipitation.
The protein of the culture filtrate was precipitated overnight with 85% (NH4)2SO4. The resulting precipitate was collected by centrifugation at 10,000 × g for 30 min and dissolved in the smallest possible volume of 100 mM acetate buffer (pH 6.0).
(ii) Gel filtration.
The concentrated enzyme solution was loaded onto an Ultrogel AcA 44 column (exclusion range, 10 to 130 kDa; BioSepra; 1.6 by 100 cm) equilibrated with 10 mM acetate buffer (pH 6.0). Elution was performed with the same buffer containing 50 mM NaCl and 8 mM EDTA at a flow rate of 20 ml h−1, and 2.5-ml fractions were collected. The β-glucosidase activity was eluted in two peaks (designated BGI and HGT-BG). The molecular weights of the native enzymes were determined by the method of Andrews (1) by using blue dextran and molecular weight markers from Sigma as standards. The fractions containing HGT-BG activity were pooled, concentrated, and desalted by ultrafiltration on Centriplus PM 10 membranes (Amicon).
(iii) Ion-exchange chromatography.
HGT-BG was further purified by high-pressure liquid chromatography on a TSK DEAE-5PW column (7.5 by 75 mm; Beckman) equilibrated with 10 mM citrate-phosphate buffer (pH 6.0) containing 1 mM EDTA. The column was washed with the same buffer and then again with the same buffer containing 0.13 M NaCl. A linear 0.13 to 0.25 M NaCl gradient was then applied at a flow rate of 2 ml min−1, and 1-ml fractions were collected. The protein content in the column effluent was monitored by determining the absorbance at 280 nm (A280). The active fractions were pooled, concentrated, and desalted as described above. This enzyme solution was the purified HGT-BG preparation used for subsequent studies.
Enzyme assay.
β-Glucosidase activity was routinely assayed by using a 1-ml reaction mixture containing 5 mM p-nitrophenyl-β-d-glucoside (pNPβG) (Sigma), 100 mM acetate buffer (pH 5.0), and an appropriate dilution of enzyme preparation. After 30 min of incubation at 50°C, the reaction was stopped by adding 2 ml of 1 M Na2CO3, and the p-nitrophenol release was monitored at A400. One unit of β-glucosidase activity corresponded to release of 1 μmol of p-nitrophenol min−1 under these conditions. Activities on other aryl substrates and in the presence of cations and reagents, all purchased from Sigma, were determined under the same conditions. The Km and Vmax values were calculated by the double-reciprocal plot method of Lineweaver and Burk (24) by using the SIGMA-PLOT software program. Glucose inhibition and glucono-δ-lactone (Sigma) inhibition were tested by adding the inhibitors at different concentrations to the standard reaction mixture and then performing the reaction experiment under the optimal conditions (30 min, 50°C, pH 5.0), using purified HGT-BG, the BGI active peak, or Klerzyme β-glucosidase (Gist-Brocades). The release of reducing sugars resulting from hydrolysis of natural substrates was determined by monitoring the A540 by the method of Miller (26), using glucose as the standard. Activity on insoluble β-glucans in synergism with β-glucanases was tested in 100 mM acetate buffer (pH 5.0) containing 2 mg of substrate ml−1 and 0.1. U of purified HGT-BG ml−1 alone or in combination with 0.1. U of cellulase ml−1 or 0.1 U of laminarinase ml−1 (Sigma). Activity on monoterpenyl glucosides was tested by adding 0.1 U of purified HGT-BG ml−1 or 0.1 U of Klerzyme β-glucosidase ml−1 to 100 mM acetate buffer (pH 5.0) containing 1.5 mM geranyl-β-glucoside and 100 g of glucose liter−1 or to a grape must (Ugni Blanc; pH 2.9, 90 g of glucose liter−1) supplemented with 0.15 mM geranyl-, neryl-, and linalyl-β-glucosides synthesized as described by Voirin et al. (42). After incubation at 20°C for 24 h in acetate buffer or for 1 week in grape must, the hydrolysis products were analyzed by performing high-pressure liquid chromatography on a reverse-phase column (C18, 5 μm; 220 by 4.6 mm; Brownlee) with water-acetonitrile (30:70, vol/vol) as the eluent at a flow rate of 1 ml min−1 (3). The release of glucose and monoterpenol was monitored at A200. Hydrolysis of naringin (4′,5,7-trihydroxyflavanone-7-rhamnoglucoside) (Sigma) was determined in 100 mM acetate buffer (pH 5.0) containing 2 mM substrate and 0.1 U of purified HGT-BG ml−1 alone or in combination with 0.1 U of α-rhamnosidase purified from naringinase ml−1 (Sigma) (19). Hydrolysis was checked by thin-layer chromatography analysis. Hydrolysis of benzyl- and 2-phenylethyl-β-d-glucosides was also studied by performing a thin-layer chromatography analysis after 24 h of incubation at 40°C in 100 mM acetate buffer (pH 5.0) containing 2 mM substrate and 0.1 U of purified HGT-BG ml−1 or 0.1 U of Klerzyme β-glucosidase ml−1.
Other analytical methods.
The protein content was determined at A595 by using the Bio-Rad protein assay based on the Bradford procedure (5) with bovine serum albumin as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 10% gels by the method of Laemmli (23). Low-molecular-weight standards (Bio-Rad) were used to determine the subunit molecular weight of the enzyme, and a Quick-Silver stain kit (Amersham) was used to locate the proteins. Analytical isoelectric focusing (IEF) was performed with Servalyt Precotes (Serva) containing ampholytes with a pH range of 3.0 to 6.0 or 10.0. Purified HGT-BG was stained with Coomassie brilliant blue R-250 (Serva) in order to determine the pI with pI standards (Protein Test Mix 9; Serva). Analytical IEF was also used to distinguish β-glucosidase activities revealed on the gel after 5 min of incubation at room temperature in 100 mM acetate buffer (pH 5.0) containing 1 mM 4-methylumbelliferyl-β-d-glucoside (Sigma). The hydrolyzed substrate was revealed by fluorescence under UV light (A365). Polyclonal antibodies against purified HGT-BG were raised in a rabbit. Western blotting was performed after SDS-PAGE on nitrocellulose filters by using a Protoblot Western blot AP kit (Promega) and an appropriate dilution (1:2,000) of anti-HGT-BG antiserum. Ponceau Red staining was used to locate high-molecular-weight standard proteins (Bio-Rad) and to estimate the specificity of anti-HGT-BG antibodies.
RESULTS
Production of HGT-BG.
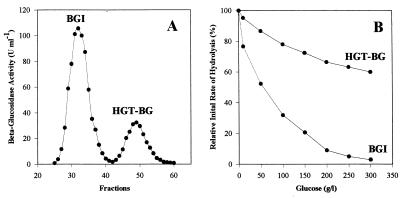
A. oryzae produced at least two distinct extracellular β-glucosidases, one which had a molecular mass of about 130 kDa and was highly inhibited by glucose (BGI) and one which had a molecular mass of about 40 kDa and was highly glucose tolerant (HGT-BG) (Fig. 1). The effects of a variety of carbon sources on HGT-BG production by A. oryzae were investigated (Table 1). The organism grew well on every substrate tested, although the cell mass yield was about five times lower on quercetin- or lactose-containing media than on glucose-containing media. Differences in the growth yield were, however, not related to differences in total or relative enzyme production. The greatest amount of β-glucosidase activity was recovered from the culture filtrate of strain CBS 12559 grown on cellobiose or rutin, a quercetin-3-rutinoside from plants. A high yield was also obtained on non-β-glucosidic substrates, such as glucose or maltose, but the β-glucosidase activity corresponded almost exclusively to the major form BGI. The best substrate for the production of HGT-BG form was found to be quercetin. Rutin, lactose, and sucrose were also good inducers of HGT-BG, but in no case did the HGT-BG activity exceed 18% of the total β-glucosidase activity.
FIG. 1.
Extracellular β-glucosidase production by A. oryzae CBS 12559 grown on 0.5% (wt/vol) quercetin. (A) β-Glucosidase activities separated by gel filtration on an Ultrogel AcA44 column. (B) Glucose inhibition of β-glucosidase activities.
TABLE 1.
Ratios of HGT-BG produced by A. oryzae CBS 12559 grown on various carbon sourcesa
| Carbon source | Total β-glucosidase activity (U) | Ratio of HGT-BG (%) |
|---|---|---|
| Quercetin | 1,142 | 17.8 |
| Rutin | 1,228 | 13.7 |
| Lactose | 396 | 12.7 |
| Sucrose | 1,047 | 10.4 |
| Cellobiose | 1,336 | 8.5 |
| Maltose | 836 | 4.6 |
| Arabinose | 796 | 3.4 |
| Xylose | 585 | 2.3 |
| Glucose | 1,146 | 1.5 |
Cultures were grown at 28°C for 14 days in 1-liter flasks containing 500 ml of minimal medium supplemented with 0.5% (wt/vol) carbon source. The precipitated proteins obtained from the different culture filtrates were resuspended in the same volume and loaded onto an Ultrogel AcA44 gel filtration column to separate the two distinct β-glucosidases.
Purification of HGT-BG.
HGT-BG was purified to homogeneity from a 10-liter culture filtrate of A. oryzae grown on quercetin. The purification results are summarized in Table 2. After separation of the two β-glucosidase peaks by gel filtration, HGT-BG was retained on an anion-exchange column and eluted at NaCl concentrations ranging from 0.198 to 0.212 M, with a maximum at 0.203 M NaCl. After SDS-PAGE analysis and silver staining, the protein was detected as a single band (Fig. 2A). The purified enzyme preparation had a specific activity of 1,066 U mg of total protein−1. Thus, HGT-BG was purified 177-fold to homogeneity. About 5 μg of pure enzyme were recovered per liter of culture; this yield corresponded to 4.5% of the total extracellular β-glucosidase activity and 0.026% of the total extracellular proteins measured in the culture filtrate.
TABLE 2.
Purification of HGT-BG from A. oryzae CBS 12559
| Purification step | Total protein (mg) | Total activity (U) | Sp act (U mg of protein−1) | % Recovery | Purification (fold) |
|---|---|---|---|---|---|
| Culture filtrate | 193.8 | 1,142 | 5.9 | 100 | 1 |
| Ammonium sulfate | 80.4 | 1,040 | 12.9 | 91 | 2.2 |
| Ultrogel AcA 44 | 2.4 | 140 | 58.3 | 12.5 | 9.9 |
| TSK DEAE-5PW | 0.05 | 53.3 | 1,066 | 4.5 | 176.9 |
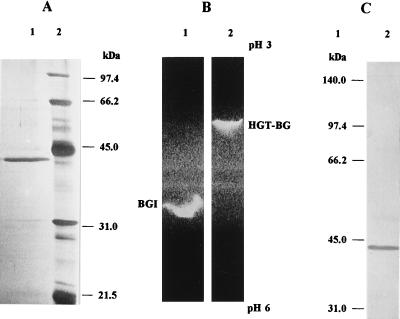
FIG. 2.
Molecular mass, pI, and immunological specificity analysis of purified HGT-BG from A. oryzae CBS 12559. (A) SDS-PAGE analysis. Lane 1, 3 μg of purified HGT-BG; lane 2, low-molecular-mass markers. Proteins were revealed by silver staining. (B) IEF-PAGE analysis. Lane 1, 10 μg of proteins from the BGI active peak; lane 2, 3 μg of purified HGT-BG. Activities were revealed by methylumbelliferone fluorescence under UV light. (C) Western blot analysis. Lane 1, high-molecular-mass markers; lane 2, 50 μg of total proteins from quercetin culture filtrate. Preparations were probed with anti-HGT-BG rabbit antiserum (1:2,000) as described in Materials and Methods.
Characterization of purified HGT-BG. (i) Molecular weight, pI, and immunological specificity.
The molecular mass of native HGT-BG was estimated to be around 40 kDa by gel filtration and was determined to be 43 kDa by SDS-PAGE analysis, indicating that the purified enzyme is a monomeric protein (Fig. 2A). The pI value of the purified enzyme was determined by analytical IEF to be 4.2 (data not shown). The two β-glucosidases could be easily distinguished after the enzymatic activity was visualized in the IEF gel (Fig. 2B); the pI value of BGI was estimated to be 4.9. SDS-PAGE followed by immunoblotting was performed in order to determine the immunological specificity of rabbit antiserum raised against the purified enzyme. The ability of anti-HGT-BG antibodies to cross-react with other proteins from the culture filtrate of A. oryzae grown on quercetin was examined. As shown in Fig. 2C, only one band was detected, and this band corresponded to the molecular mass of the purified enzyme. The antibodies did not cross-react with any band around 130 kDa that would have corresponded to BGI. Similar Western blotting experiments were performed with the protein extracts recovered from culture filtrates of the organism grown on different carbon sources as described in Table 1. The relative intensity of the single band which was detected in each case clearly confirmed the variations in HGT-BG-specific production that depended on the carbon source; quercetin was found to be the best substrate (data not shown).
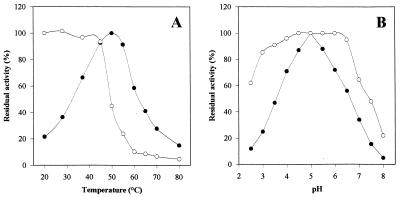
(ii) Temperature and pH.
The temperature dependence and pH dependence of purified HGT-BG are shown in Fig. 3. The temperature optimum for maximal HGT-BG activity was 50°C when preparations were incubated for 30 min in 100 mM acetate buffer (pH 5.0). Under optimal temperature conditions, the purified enzyme had a pH optimum of 5.0, although the activity was 50% of the maximal activity at pH 3.5 and 25% of the maximal activity at pH 3.0. The thermostability of the enzyme was investigated by measuring the residual activity after 4 h of incubation at temperatures ranging from 20 to 80°C. Under the conditions used (100 mM acetate buffer, pH 5.0), HGT-BG was highly stable at temperatures up to 45°C but was almost inactivated at temperatures above 60°C. It retained full activity after storage for 6 months at 4°C. The pH stability was also investigated by measuring the residual activity after 24 h of incubation at 20°C at pH values ranging from 2.5 to 8.0. The enzyme was fairly stable at pH 3.0 to 7.0, and 60% of the activity remained after incubation at pH 2.5. The stability of the enzyme in grape must (pH 2.9) was tested at 20°C. Full activity remained after 1 week of incubation under these biotechnological conditions.
FIG. 3.
Effects of temperature (A) and pH (B) on activity (•) and stability (○) of purified HGT-BG from A. oryzae CBS 12559. For the temperature stability determinations, residual activity was assayed after 4 h of incubation of the enzyme in 100 mM acetate buffer (pH 5) at different temperatures. For the pH stability determinations, residual activity was assayed after 24 h of incubation of the enzyme at 20°C in 100 mM citrate-phosphate buffer at different pH values. The enzyme was used at a concentration of 0.1 U ml−1 in both studies.
(iii) Substrate specificity and catalytic properties.
The relative rates of hydrolysis of various substrates by HGT-BG are presented in Table 3. The enzyme efficiently hydrolyzed natural oligosaccharides having (1→4)-β-glycosidic linkages, such as cellobiose, lactose, and xylobiose, but it hydrolyzed laminaribiose and gentiobiose, which have (1→3)-β- and (1→6)-β-glucosidic linkages, respectively, even more efficiently. The enzyme could also hydrolyze cellooligosaccharides (cellotriose to cellopentaose), but the efficacy decreased as the chain length increased. Laminarin, the only soluble polysaccharide with (1→3)-β-glucosidic linkage which could be tested, was poorly hydrolyzed, confirming the exo type of activity of the glucosidase. Insoluble β-glucans, such as cellulose, curdlan, pustulan, lichenan, laminaran, and yeast glucan, were also poor substrates for the purified enzyme alone. However, when used in combination with cellulase or laminarinase, HGT-BG increased the relative rates of reducing sugar release by 20 to 100% (data not shown). A comparison of the enzyme activities on various aryl-glycosides confirmed that HGT-BG has broad specificity for mono-β-d-glycosides, with a preference for glucose at the nonreducing end. However, the enzyme had no activity on salicin, which is usually a good substrate for β-glucosidase. Even more surprisingly, the enzyme could efficiently hydrolyze nigerose, maltose, and isomaltose, which have (1→3)-, (1→4)-, and (1→6)-α-glucosidic linkages, respectively, although pNPαG was very poorly cleaved.
TABLE 3.
Relative initial rates of hydrolysis of various substrates by purified HGT-BG from A. oryzae CBS 12559
| Substratea | Linkage of glycosyl group | Relative initial rate of hydrolysis (%)b |
|---|---|---|
| Saccharides (2 mg ml−1) | ||
| Laminaribiose (3-O-β-d-glucopyranosyl-d-glucose) | β(1,3)Glc | 100 |
| Gentiobiose (6-O-D-β-d-glucopyranosyl-β-d-glucose) | β(1,6)Glc | 100 |
| Cellobiose (4-O-β-d-glucopyranosyl-d-glucose) | β(1,4)Glc | 88 |
| Cellotriose ([β-d-Glc-1,4)]2-d-Glc) | β(1,4)Glc | 75 |
| Cellotetraose ([β-d-Glc-1,4)]3-d-Glc) | β(1,4)Glc | 62 |
| Cellopentaose ([β-d-Glc-1,4)]4-d-Glc) | β(1,4)Glc | 48 |
| Xylobiose (4-O-β-d-xylopyranosyl-d-xylose) | β(1,4)Xyl | 43 |
| Lactose (4-O-β-d-galactopyranosyl-d-glucose) | β(1,4)Gal | 73 |
| Sophorose (2-O-β-d-glucopyranosyl-d-glucose) | β(1,2)Glc | 26 |
| Nigerose (3-O-α-d-glucopyranosyl-d-glucose) | α(1,3)Glc | 72 |
| Isomaltose (6-O-α-d-glucopyranosyl-d-glucose) | α(1,6)Glc | 70 |
| Maltose (4-O-α-d-glucopyranosyl-d-glucose) | α(1,4)Glc | 68 |
| Laminarin [soluble linear β-(1,3)-d-glucan] | β(1,3)Glc | 11 |
| Aryl-glycosides (5 mM) | ||
| pNPβG | βGlc | 100 |
| p-Nitrophenyl-β-d-xyloside | βXyl | 50 |
| p-Nitrophenyl-β-d-galactoside | βGal | 8.2 |
No activity or poor activity was detected with sucrose, trehalose, isotrehalose, salicin (β-salicyl alcohol glucoside), methyl-β-glucoside, insoluble β-glucans, such as cellulose, curdlan, pustulan, lichenan, pullulan, barley β-glucan, and yeast glucan, and p-nitrophenyls, such as α-d-glucoside, β-d-cellobioside, β-d-gentiobioside, β-d-maltoside, α-l-maltoside, α-l-arabinofuranoside, and β-d-glucuronide.
Depending on the substrate, activity was determined under optimal conditions (50°C, pH 5) by measuring the release of reducing sugar (A540) or the release of p-nitrophenol (A400). The relative initial rates of hydrolysis of saccharides and aryl-glucosides are expressed as percentages of the initial rates of hydrolysis obtained with laminaribiose and pNPβG, respectively, and correspond to 0.1 U of HGT-BG activity ml−1.
The reaction kinetics of the purified enzyme were determined from Lineweaver-Burk plots under optimal conditions (30 min, pH 5.0, 50°C). The enzyme had apparent Km values of 0.55, 3.5, 5, 7, and 15.8 mM and Vmax values of 3,040, 380, 367, 353, and 283 μmol min−1 mg of protein−1 for hydrolysis of pNPβG, laminaribiose, gentiobiose, cellobiose, and geranyl-β-glucoside, respectively.
(iv) Inhibition by glucose, glucono-δ-lactone, and other sugars.
Glucose and glucono-δ-lactone acted as competitive inhibitors of pNPβG hydrolysis with Ki values of 1.36 M and 12.5 mM, respectively, which were obtained from the intersections of the lines on Dixon plots. The BGI active peak and Klerzyme β-glucosidase were completely inhibited by 1 g of glucono-δ-lactone liter−1, whereas purified HGT-BG still retained 20% of its initial activity in the presence of 10 g of this powerful β-glucosidase inhibitor liter−1. Sugar inhibition was not observed with 15% (wt/vol) fructose, sophorose, galactose, sucrose, lactose, arabinose, or xylose. Substrate inhibition was also not observed with 50 mM pNPβG, but the hydrolysis was 50% of the initial rate with 15% (wt/vol) laminariobiose, gentiobiose, cellobiose, or maltose.
(v) Potential inhibitors and activators.
The effects of various cations and reagents on HGT-BG activity were investigated (Table 4). Significant inactivation was observed with Ag+, Hg2+, Cu2+, Zn2+, and Fe3+, as well as with group-specific potential inhibitors, such as SDS, N-bromosuccinimide (NBS), diethylpyrocarbonate (DEPC), castanospermine, deoxynojirimycin, and methyldeoxynojirimycin. The presence of substrate (10 mM pNPβG) slightly decreased inhibition by NBS and DEPC. However, enzyme activity was either not affected or only slightly affected by other potential inhibitors, such as EDTA, p-chloromercuribenzoic acid (pCMB), dicyclohexyl carbodiimide, Woodward’s reagent K, dithiothreitol (DTT), dimethyl sulfoxide (DMSO), and N-acetylimidazole. The enzyme did not require Ca2+, Mg2+, or Co2+ for activity but was significantly stimulated by Mn2+. pNPβG hydrolysis was increased by 77% in the presence of 5 mM Mn2+. However, Ca2+ and Mn2+ had no effect on the stability of the enzyme.
TABLE 4.
Effects of cations and reagents on purified HGT-BG activity from A. oryzae CBS 12559
| Cation or reagenta | Relative initial rate of hydrolysis (%) |
|---|---|
| Control | 100 |
| AgCl | 3.1 |
| HgCl2 | 0 |
| CuCl2 | 3 |
| ZnCl2 | 17 |
| FeCl3 | 13 |
| MnCl2 | 177 |
| SDS | 2.5 |
| NBS | 0 (2)b |
| DEPC | 4.5 (9) |
| Castanospermine | 4.5 |
| Deoxynojirimycin | 5.5 |
| Methyldeoxynojirimycin | 14.5 |
| N-Acetylimidazole | 82 |
Activity of the purified enzyme was assayed in the presence of various cations at a concentration of 5 mM and reagents at a concentration of 10 mM. The activity observed in the absence of an added substance (control) was considered to be 100%. No effect on activity was observed with NaCl, KCl, CaCl2, MgCl2, LiCl2, CoCl2, EDTA, pCMB, dicyclohexyl carbodiimide, Woodward’s reagent K, DTT, and DMSO.
The values in parentheses are values from substrate protection experiments and are the relative residual activities after incubation of NBS or DEPC and 0.1 U of HGT-BG in the presence of 10 mM pNPβG.
At the optimal concentration found in wine, ethanol had a stimulating effect on HGT-BG activity. Under optimal conditions (30 min, pH 5.0, 50°C), pNPβG hydrolysis by purified HGT-BG increased 30% in the presence of 15% (vol/vol) ethanol but only 15% in the presence of 20% (vol/vol) ethanol.
(vi) Monoterpenyl-glucoside and naringin hydrolysis.
HGT-BG from A. oryzae and Klerzyme β-glucosidase from Aspergillus niger were assayed to determine whether they hydrolyzed monoterpenyl- (geranyl-, neryl-, linalyl-), benzyl-, and 2-phenylethyl-β-glucosides in rich glucose-containing media. Previously, Klerzyme β-glucosidase was found to efficiently catalyze the hydrolysis of monoterpenyl-glucosides during wine processing (20). However, after 24 h of incubation at 20°C in 100 mM acetate buffer (pH 5.0) supplemented with 100 g of glucose liter−1, 50% of the geranyl-β-glucoside was hydrolyzed in the presence of HGT-BG, compared to the 9% of geranyl-β-glucoside that was hydrolyzed in the presence of Klerzyme β-glucosidase. The activities of the two enzymes were also compared after 1 week of incubation at 20°C in grape must (pH 2.9, 90 g of glucose liter−1) supplemented with monoterpenyl-glucosides at a concentration 10 times higher than the concentration usually found. Under these conditions, 50% of the geranyl- and neryl-β-glucosides was hydrolyzed and 90% of the linalyl-β-glucoside was hydrolyzed in the presence of HGT-BG, whereas Klerzyme β-glucosidase exhibited no hydrolyzing activity (data not shown).
Finally, the activity of HGT-BG on naringin was tested. Naringin hydrolysis involves an α-rhamnosidase, which releases prunin, which is cleaved by a β-glucosidase; this process diminishes the bitterness of citrus juices (33). As expected, HGT-BG alone did not hydrolyze this substrate. However, naringin was completely hydrolyzed when HGT-BG was supplemented with an α-rhamnosidase (data not shown).
DISCUSSION
We identified two distinct active forms of β-glucosidase in the culture filtrate of A. oryzae CBS 12559 grown on various carbon sources. The two enzymes could be clearly distinguished on the basis of molecular weight, pI, immunological reactivity, and tolerance to glucose. The major form, BGI (molecular mass, 130 kDa; pI 4.9), could correspond to the β-glucosidase from A. oryzae described previously (25), although the latter enzyme was reported to have a different molecular mass (218 kDa) and a pI of 4.3. It could be that we underestimated the molecular mass of BGI due to a higher exclusion limit of the gel filtration procedure and that the pI value appeared to be different under the conditions which we used. However, fungi are known to secrete many forms of the same enzyme depending on the strain and environmental conditions. The two forms of A. oryzae β-glucosidases which were identified here, BGI and HGT-BG, were produced in different total and relative amounts depending on the carbon source upon which the strain was grown (Table 1). As expected, β-glucosidic substrates, such as cellobiose and rutin, resulted in the highest yields of total β-glucosidase activity, although a high level of activity was also observed on glucose-containing medium. It is known that β-glucosidases play an important role in the solubilization and reconstitution of biological membranes (21, 38). It was therefore not surprising to observe β-glucosidase production after 14 days of growth in very dense and miscellaneous cell cultures. Secretion of the minor form, HGT-BG, seemed, however, to be more specifically induced on complex phenolic flavonoidic compounds from plant cell walls, such as quercetin and rutin, suggesting that HGT-BG could play a specific role during plant attack and degradation by the fungus. On all of the substrates tested here, the fraction of HGT-BG in the cell-free culture filtrate was limited compared to the fraction of BGI, and even on quercetin it did not exceed 18% of the total β-glucosidase activity. After separation of the two β-glucosidase forms by gel filtration, HGT-BG was purified to homogeneity in a single step by anion-exchange chromatography. The overall purification procedure resulted in recovery of 4.5% of the enzyme activity and a 177-fold increase in specific activity (Table 2). The low level of enzyme recovery was due to the fact that the β-glucosidase activity measured in the culture filtrate was mostly the undesired BGI activity, which was discarded during purification of the HGT-BG activity. The specific activity of the purified enzyme preparation under optimal conditions (50°C, pH 5.0) was 1,066 U mg of protein−1 on pNPβG, whereas the specific activities reported for β-glucosidases from other fungi range from 2.7 to 979 U mg of protein−1 (28, 43). The purified enzyme from A. oryzae is therefore among the most efficient fungal β-glucosidases described so far. As a monomeric 43-kDa protein, HGT-BG is also among the smallest known β-glucosidases from aerobic fungi, whose molecular masses range from 39.8 to 480 kDa (8, 46). HGT-BG is, however, similar to other fungal β-glucosidases with respect to pI (pI 4.2) and optimal activity conditions (50°C, pH 5.0), as well as pH stability and thermal stability (7, 22, 28, 43, 46, 49).
β-Glucosidases may be divided into three groups on the basis of substrate specificity: (i) aryl-β-glucosidases, which have a strong affinity for aryl-β-glucosides; (ii) cellobiases, which hydrolyze only oligosaccharides; and (iii) broad-specificity β-glucosidases, which exhibit activity on many substrate types and are the most commonly observed β-glucosidases (34). The purified β-glucosidase from A. oryzae is a broad-specificity type, since it can hydrolyze a range of (1→3)-, (1→4)-, and (1→6)-β-diglycosides, as well as aryl- and alkyl-β-glycosides (Table 3). β-Glucosidases with very broad specificity have been isolated from many fungi (7, 12, 22, 30, 43, 46, 49). Surprisingly though, HGT-BG can also rather efficiently hydrolyze maltose and other diglucosides having (1→3)-, (1→4)-, or (1→6)-α linkages, although it exhibits almost no activity on pNPαG compared to the activity on pNPβG. Additional studies are required to characterize the unusual specificity of this β-glucosidase. To our knowledge, only one other fungal β-glucosidase, a β-glucosidase from Botrytis cinerea, has been reported to have the ability to hydrolyze both β- and α-glucosides (12). The fact that HGT-BG also exhibits substantial activity on β-glucans, such as laminarin, or cellooligosaccharides but exhibits decreased efficiency as the number of glucose units increases indicates that the enzyme possesses some exoglucanase activity. Furthermore, a synergetic effect was observed during β-glucan hydrolysis by HGT-BG in combination with cellulase or laminarinase, suggesting that HGT-BG might be implicated in β-glucan degradation in concert with efficient β-glucanases, as previously reported for Acremonium persicinum β-glucosidase (30). The results of previous studies suggest, however, that many broad-specificity enzymes that exhibit exo-β-(1,3)- and exo-β-(1,6)-glucanase activities with glucose as the only product of hydrolysis are β-glucosidases rather than exo-β-glucanases (7, 11, 29–31).
Various metal cations and potential inhibitors modified the activity of the purified enzyme. The enzyme was indeed greatly inhibited by Ag+, Cu2+, Hg2+, Zn2+, and Fe3+. This may indicate that thiol groups are involved in the active catalytic site. However, HGT-BG activity was not affected by pCMB or DTT, well-known thiol group inhibitors. Sulfhydryl groups may not be involved in the catalytic center of the enzyme but rather may be essential for maintenance of the three-dimensional structure of the active protein. Surprisingly, HGT-BG activity was not affected by dicyclohexyl carbodiimide and Woodward’s reagent K, suggesting that aspartyl and glutamyl residues are not involved at the active site of the enzyme. Slight inhibition by N-acetylimidazole also eliminated the possibility that tyrosine participates in catalysis, whereas the complete inactivation by NBS and DEPC observed indicates that tryptophan and histidine residues are important in the catalytic action of the enzyme (Table 4). However, pNPβG provided only a little protection against both inhibitors, suggesting that tryptophan and histidine residues are not involved in the binding site of the substrate. The three well-characterized β-glucosidase inhibitors (castanospermine, deoxynojirimycin, and methyldeoxynojirimycin) also inhibited HGT-BG activity, suggesting again that HGT-BG is a β-glucosidase rather than an exo-β-glucanase (32). The chelating agent EDTA did not inhibit HGT-BG activity, indicating that divalent cations are not required for enzyme activation. However, Mn2+ did significantly stimulate enzyme activity. Since Mn2+ is not involved in the stability of the enzyme, this specific cation could play a role in the enzyme function (e.g., by modulating its activity according to environmental conditions). It was also interesting to observe 30% stimulation of enzyme activity in the presence of 15% (vol/vol) ethanol. A similar effect was observed for A. niger β-glucosidase (49), whereas the activity of Fusarium oxysporum was increased 1.5-fold by ethanol (6). It has been proposed that alcohol activation of some β-glucosidases may be due to their glycosyltransferase activities (27). The enzyme could preferentially utilize alcohol rather than water as an acceptor for the glycosyl moiety during the catalysis of pNPβG, resulting in elevated reaction rates.
Its high resistance to glucose inhibition is surely what makes the newly purified β-glucosidase of great interest for biotechnological applications. Competitive inhibition by glucose is a common characteristic of fungal β-glucosidases that limits their use in enzymatic hydrolysis of plant products (12, 22, 36, 37, 43, 47, 49). Most microbial β-glucosidases have glucose inhibition constants (Ki) ranging from as low as 0.5 mM to no more than 100 mM (37, 47). For enzymes from Aspergillus species, Ki values have been reported to range from 3 to 14 mM (22, 49). Therefore, the Ki calculated here, 1.36 M, gives HGT-BG an outstanding position among fungal β-glucosidases. To our knowledge, in only one other study have workers described the purification and characterization (from Candida peltata) of a β-glucosidase having such a high tolerance to glucose (36). Recently, a glucose-tolerant β-glucosidase was also purified from A. niger, and this enzyme had a somewhat lower Ki (0.543 M) (49). Substrate inhibition is also a common property of fungal β-glucosidases (37, 47). A. oryzae HGT-BG was not inhibited by 50 mM pNPβG, and the hydrolysis was 50% of the initial rate in the presence of 15% laminaribiose, 15% gentiobiose, 15% cellobiose, or 15% maltose. This indicates that A. oryzae HGT-BG is tolerant to substrate inhibition, although to a lesser extent than C. peltata β-glucosidase, which was not inhibited by 40 mM pNPβG or 15% cellobiose (36). The properties of the glucose-tolerant β-glucosidases from A. oryzae, C. peltata, and A. niger are summarized in Table 5. As Table 5 shows, the three enzymes have many distinct features, especially in their catalytic properties. A. oryzae HGT-BG is the most efficient catalyst and the only enzyme with (1→3)-(1→6)-β specificity. HGT-BG is also more tolerant than most fungal β-glucosidases to inhibition by glucono-δ-lactone, the most powerful β-glucosidase inhibitor found in wine. Glucono-δ-lactone is produced at concentrations up to 2 g liter−1 from glucose by a glucose oxidase produced by grape-attacking fungi, such as B. cinerea (10). Like other fungal β-glucosidases, HGT-BG was competitively inhibited by glucono-δ-lactone (7, 30, 31). However, it still exhibited 20% of its initial rate of hydrolysis in the presence of 10 g of glucono-δ-lactone liter−1, whereas the other fungal β-glucosidases are totally inhibited by 1 g of this powerful inhibitor liter−1, Ki values ranging from 0.035 to 0.05 mM (7, 30, 31). The Ki value calculated here (12.5 mM), therefore, gives HGT-BG an outstanding position among fungal β-glucosidases. To our knowledge, only one other study has reported the characterization (from Sclerotium glucanicum) of an exo-β-1,6-glucanase having such a high tolerance to glucono-δ-lactone (Ki, 12.79 mM) (31).
TABLE 5.
Characteristics of glucose-tolerant β-glucosidases from A. oryzae CBS 12559, C. peltata NRRL Y-6888, and A. niger CCRC 31494
| Strain | Sp act (U mg of protein−1) | Mol wt (103) | Optimum temp (°C) | Optimum pH | pI | % Stimulation by ethanol (15%, vol/vol) | Cation stimulation |
|---|---|---|---|---|---|---|---|
| A. oryzae CBS 12559 | 1,066 | 43 | 50 | 5.0 | 4.2 | 30 | Mn2+ (5 mM) |
| C. peltata NRRL Y-6888 | 108 | 43 | 50 | 5.0 | NDa | None | None |
| A. niger CCRC 31494 | 198.5 | 49 (×2) | 55 | 5.0 | 3.2 | 30 | None |
| Thiol group inhibition | Substrate inhibition (%)
|
Substrate specificity |
Km (mM)
|
Ki for glucose (M) | Reference | ||
|---|---|---|---|---|---|---|---|
| pNPβG (40 mM) | Cellobiose (15%) | pNPβG | Cellobiose | ||||
| None | None | 50 | Laminariobiose, gentiobiose | 0.55 | 7.0 | 1.36 | This study |
| None | None | None | Cellooligosaccharides | 2.3 | 66 | 1.4 | 36 |
| None | ND | ND | Cellooligosaccharides | 21.7 | ND | 0.543 | 49 |
ND, not determined.
The present data clearly indicate the potential of the novel β-glucosidase HGT-BG to release flavor compounds in a natural medium rich in glucose. The higher efficiency of HGT-BG than of Klerzyme β-glucosidase for hydrolysis of monoterpenyl-glucosides in grape must is probably due to the broad specificity and higher glucose tolerance of HGT-BG rather than to differences in stability at acidic pH values, since Klerzyme β-glucosidase was described to be stable in grape must (20). Finally, HGT-BG, together with an α-rhamnosidase, was able to fully degrade the complex β-glucosidic naringin present in citrus juices.
In conclusion, the novel β-glucosidase purified from A. oryzae shows great potential for several biotechnological uses; it may be used to (i) increase the aromatic character of wines and fruit juices through the hydrolysis of flavor glucosidic precursors, (ii) decrease the bitterness of citrus juices through the hydrolysis of prunin, and (iii) improve the enzymatic conversion of cellulosic or noncellulosic materials to glucose through synergetic action with β-glucanases.
ACKNOWLEDGMENTS
This work was supported by the Development Department (DRIV) of the French National Institute for Agronomical Research (INRA).
We thank Anne Charpentier for her interest and support. We are particularly grateful to Nathalie Declerck for helpful discussions and careful reading of the manuscript. The help of Kathy Vinson in proofreading the manuscript is acknowledged.
REFERENCES
- 1.Andrews P. Estimation of the molecular weights of proteins by Sephadex gel filtration. Biochem J. 1965;91:595–606. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aryan A P, Wilson B, Strauss C R, Williams P J. The properties of glycosidases of Vitis vinifera and a comparison of their β-glucosidase activity with that of exogenous enzymes. An assessment of possible applications in enology. Am J Enol Vitic. 1987;38:182–188. [Google Scholar]
- 3.Bitteur S, Günata Z, Brillouet J M, Bayonove C, Cordonnier R. GC and HPLC of grape monoterpenyl glycosides. J Sci Food Agric. 1989;47:341–352. [Google Scholar]
- 4.Bothast R J, Saha B C. Ethanol production from agricultural biomass substrates. Adv Appl Microbiol. 1997;44:261–286. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Christakopoulos P, Goodenough P, Kekos D, Macris B J, Claeyssens M, Bhat M K. Purification and characterization of an extracellular β-glucosidase with transglycosylation and exo-glucosidase activities from Fusarium oxysporum. Eur J Biochem. 1994;224:379–385. doi: 10.1111/j.1432-1033.1994.00379.x. [DOI] [PubMed] [Google Scholar]
- 7.Copa-Patino J L, Broda P. A Phanerochaete chrysosporium β-d-glucosidase/β-d-xylosidase with specificity for (1→3)-β-d-glucan linkages. Carbohydr Res. 1994;253:265–275. doi: 10.1016/0008-6215(94)80071-5. [DOI] [PubMed] [Google Scholar]
- 8.Dekker R F H. Induction, localization and characterization of β-glucosidases produced by a species of Monilia. J Gen Microbiol. 1981;127:177–184. [Google Scholar]
- 9.Delcroix A, Günata Z, Sapis J C, Salmon J M, Bayonove C. Glycosidase activities of three enological yeast strains during wine making. Effect on the terpenol content of Muscat wine. Am J Enol Vitic. 1994;45:291–296. [Google Scholar]
- 10.Dubourdieu D, Desplanques C, Villetaz J C, Ribereau-Gayon P. Investigations of an industrial β-d-glucanase from Trichoderma harzianum. Carbohydr Res. 1985;144:277–287. [Google Scholar]
- 11.Fontaine T, Hartland R P, Diaquin M, Simenel C, Latgé J P. Differential patterns of activity displayed by two exo-β-1,3-glucanases associated with the Aspergillus fumigatus cell wall. J Bacteriol. 1997;179:3154–3163. doi: 10.1128/jb.179.10.3154-3163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gueguen Y, Chemardin P, Arnaud A, Galzy P. Purification and characterization of an intracellular β-glucosidase from Botrytis cinerea. Enzyme Microb Technol. 1995;78:900–906. [Google Scholar]
- 13.Guegen Y, Chemardin P, Janbon G, Arnaud A, Galzy P. A very efficient β-glucosidase catalyst for the hydrolysis of flavor precursors of wines and fruit juices. J Agric Food Chem. 1996;44:2336–2340. [Google Scholar]
- 14.Gueguen Y, Chemardin P, Pien S, Arnaud A, Galzy P. Enhancement of aromatic quality of Muscat wine by the use of immobilized β-glucosidase. J Biotechnol. 1997;55:151–156. [Google Scholar]
- 15.Günata Y Z, Bayonove C L, Baumes R L, Cordonnier R E. The aroma of grapes. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J Chromatogr. 1985;331:83–90. [Google Scholar]
- 16.Günata Z, Bayonove C, Tapiro C, Cordonnier R. Hydrolysis of grape monoterpenyl β-glucosides by various β-glucosidases. J Agric Food Chem. 1990;38:1232–1236. [Google Scholar]
- 17.Günata, Z., M. J. Vallier, R. Baumes, and C. Bayonove. January 1997. β-Glucosidase from filamentous fungi, and uses thereof. Institut National de la Recherche Agronomique patent PCT WO 97/02341.
- 18.Günata Z, Bitteur S, Brillouet J M, Bayonove C, Cordonnier R. Sequential enzymatic hydrolysis of potential aromatic glycosides from grape. Carbohydr Res. 1988;184:139–149. [Google Scholar]
- 19.Günata Z, Dugelay I, Sapis J C, Baumes R, Bayonove C. Role of enzymes in the use of the flavour potential from grape glycosides in winemaking. In: Scheirer P, Winterhalter P, editors. Progress in flavour precursor studies. Carol Stream, Ill: Allured Publishing Corporation; 1993. pp. 219–234. [Google Scholar]
- 20.Günata Z, Dugelay I, Vallier M J, Sapis J C, Bayonove C. Multiple forms of glycosidases in an enzyme preparation from Aspergillus niger: partial characterization of an apiosidase. Enzyme Microb Technol. 1997;21:305–312. [Google Scholar]
- 21.Günata Z, Vallier M J, Sapis J C, Baumes R, Bayonove C. Enzymatic synthesis of monoterpenyl β-d-glucosides by various β-glucosidases. Enzyme Microb Technol. 1994;16:1055–1058. [Google Scholar]
- 22.Kwon K-S, Kang H G, Hah Y C. Purification and characterization of two extracellular β-glucosidases from Aspergillus nidulans. FEMS Microbiol Lett. 1992;97:149–154. doi: 10.1016/0378-1097(92)90378-2. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. [Google Scholar]
- 25.Mega T, Matsushima Y. Comparative studies of three exo-β-glycosidases of Aspergillus oryzae. J Biochem. 1979;85:335–341. doi: 10.1093/oxfordjournals.jbchem.a132338. [DOI] [PubMed] [Google Scholar]
- 26.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;21:426–428. [Google Scholar]
- 27.Pemberton M S, Brown R D, Jr, Emert G H. The role of β-glucosidase in the bioconversion of cellulose to ethanol. Can J Chem Eng. 1980;58:723–729. [Google Scholar]
- 28.Peralta R M, Kadowaki M K, Terenzi H F, Jorge J A. A highly thermostable β-glucosidase activity from the thermophilic fungus Humicola grisea var. thermoidea: purification and biochemical characterization. FEMS Microbiol Lett. 1997;146:291–295. [Google Scholar]
- 29.Pitson S M, Seviour R J, McDougall B M. Noncellulolytic fungal β-glucanases: their physiology and regulation. Enzyme Microb Technol. 1993;15:178–192. doi: 10.1016/0141-0229(93)90136-p. [DOI] [PubMed] [Google Scholar]
- 30.Pitson S M, Seviour R J, McDougall B M. Purification and characterization of an extracellular β-glucosidase from the filamentous fungus Acremonium persicinum and its probable role in β-glucan degradation. Enzyme Microb Technol. 1997;21:182–190. doi: 10.1016/s0141-0229(96)00263-3. [DOI] [PubMed] [Google Scholar]
- 31.Rapp P. 1,3-β-Glucanase, 1,6-β-glucanase and β-glucosidase activities of Sclerotium glucanicum: synthesis and properties. J Gen Microbiol. 1989;135:2847–2858. [Google Scholar]
- 32.Ridruejo J C, Muñoz M D, Andaluz E, Larriba G. Inhibition of yeast exoglucanases by glucosidase inhibitors. Biochim Biophys Acta. 1989;993:179–185. doi: 10.1016/0304-4165(89)90161-x. [DOI] [PubMed] [Google Scholar]
- 33.Roitner M, Schalkhammer T, Pittner F. Characterization of naringinase from Aspergillus niger. Monatsch Chem. 1984;115:1255–1267. [Google Scholar]
- 34.Rojas A, Arola L, Romeu A. β-Glucosidase families revealed by computer analysis of protein sequences. Biochem Mol Biol Int. 1995;35:1223–1231. [PubMed] [Google Scholar]
- 35.Rosi I, Vinella M, Domizio P. Characterization of β-glucosidase activity in yeasts of oenological origin. J Appl Bacteriol. 1994;77:519–527. doi: 10.1111/j.1365-2672.1994.tb04396.x. [DOI] [PubMed] [Google Scholar]
- 36.Saha B C, Bothast R J. Production, purification, and characterization of a highly glucose-tolerant novel β-glucosidase from Candida peltata. Appl Environ Microb. 1996;62:3165–3170. doi: 10.1128/aem.62.9.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha B C, Feer S N, Bothast R J. Thermostable β-glucosidases. In: Saddler J N, Penner N H, editors. Enzymatic degradation of insoluble carbohydrates. Washington, D.C: American Chemical Society; 1995. pp. 197–207. [Google Scholar]
- 38.Shinoyama H, Takei K, Ando A, Fujii T, Sasaki M, Doi Y, Yasui T. Enzymatic synthesis of useful alkyl-β-glucosides. Agric Biol Chem. 1991;55:1679–1681. [Google Scholar]
- 39.Shoseyov O, Bravdo B A, Ikan R, Chet I. Immobilized endo-β-glucosidase enriches flavor of wine and passion fruit juice. J Agric Food Chem. 1990;27:1973–1976. [Google Scholar]
- 40.Vasserot Y, Arnaud A, Galzy P. Evidence for muscat marc monoterpenol glucosides hydrolysis by free or immobilized yeast β-glucosidase. Bioresource Technol. 1993;43:269–271. [Google Scholar]
- 41.Vasserot Y, Arnaud A, Galzy P. Monoterpenyl glycosides in plants and their biotechnological transformation. Acta Biotechnol. 1995;15:77–95. [Google Scholar]
- 42.Voirin S, Baumes R, Bayonove C, M’Bairaroua O, Tapiero C. Synthesis and NMR spectral properties of grape monoterpenyl glycosides. Carbohydr Res. 1990;207:39–56. [Google Scholar]
- 43.Watanabe T, Sato T, Yoshioka S, Kushijima T, Kuwahara M. Purification and properties of Aspergillus niger β-glucosidase. J Biochem. 1992;209:651–659. doi: 10.1111/j.1432-1033.1992.tb17332.x. [DOI] [PubMed] [Google Scholar]
- 44.Williams P J, Sefton M A, Wilson B. Nonvolatile conjugates of secondary metabolites as precursors of varietal grape flavor components. In: Teranishi R, Buttery R, Shahidi R, editors. Flavor chemistry: trends and developments. Washington, D.C: American Chemical Society; 1989. pp. 35–48. [Google Scholar]
- 45.Winterhalter P, Skouroumounis G K. Glycoconjugated aroma compounds: occurrence, role and biotechnological transformation. Adv Biochem Eng Biotechnol. 1997;55:73–105. doi: 10.1007/BFb0102063. [DOI] [PubMed] [Google Scholar]
- 46.Wood T M, McCrae S I. Purification and some properties of the extracellular β-glucosidase of the cellulolytic fungus Trichoderma koningii. J Gen Microbiol. 1982;128:2973–2982. [Google Scholar]
- 47.Woodward J, Wiseman A. Fungal and other β-glucosidases—their properties and applications. Enzyme Microb Technol. 1982;4:73–79. [Google Scholar]
- 48.Xin Z, Yinbo Q, Peiji G. Acceleration of ethanol production from paper mill waste fiber by supplementation with β-glucosidase. Enzyme Microb Technol. 1993;15:62–65. [Google Scholar]
- 49.Yan T-R, Lin C-L. Purification and characterization of a glucose-tolerant β-glucosidase from Aspergillus niger CCRC 31494. Biosci Biotechnol Biochem. 1997;61:965–970. doi: 10.1271/bbb.61.965. [DOI] [PubMed] [Google Scholar]