Abstract
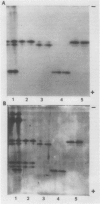
Polyphenol oxidase (PPO) was extensively purified to homogeneity from d'Anjou pear (Pyrus communis L.) by extraction in the presence of the phenolic binder AG 2-X8 andTriton X-100. Chlorophyll pigment was removed by chromatography resulting in a clear, colorless enzyme extract. Purification of pear PPO was achieved after chromatography on Phenyl Sepharose CL-4B, DEAE-cellulose, and hydroxylapatite columns. Only after the columns were run at room temperature rather than at 4°C were sharp peaks and good resolution obtained. Reproducibility of the entire scheme was excellent with chromatography on the hydrophobic resin as a key to successful purification. Three separate fractions of pear PPO were homogeneous when stained for protein with the silver stain after polyacrylamide slab gel electrophoresis and corresponded to relative mobilities of 0.41, 0.43, and 0.73. The effect of dimethylsulfoxide on enzyme activity was investigated and found to increase significantly the activity of purified pear PPO over the control.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H., Cammarata K. V. Spinach Thylakoid Polyphenol Oxidase : ISOLATION, ACTIVATION, AND PROPERTIES OF THE NATIVE CHLOROPLAST ENZYME. Plant Physiol. 1981 May;67(5):977–984. doi: 10.1104/pp.67.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Parish R. W. The intracellular location of phenol oxidases, peroxidase and phosphatases in the leaves of spinach beet (beta vulgaris L. subspecies vulgaris). Eur J Biochem. 1972 Dec 18;31(3):446–455. doi: 10.1111/j.1432-1033.1972.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yon R. J. Recent developments in protein chromatography involving hydrophobic interactions. Int J Biochem. 1978;9(6):373–379. doi: 10.1016/0020-711x(78)90048-4. [DOI] [PubMed] [Google Scholar]
- de Jesus Rivas N., Whitaker J. R. Purification and some properties of two polyphenol oxidases from bartlett pears. Plant Physiol. 1973 Nov;52(5):501–507. doi: 10.1104/pp.52.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]