Abstract
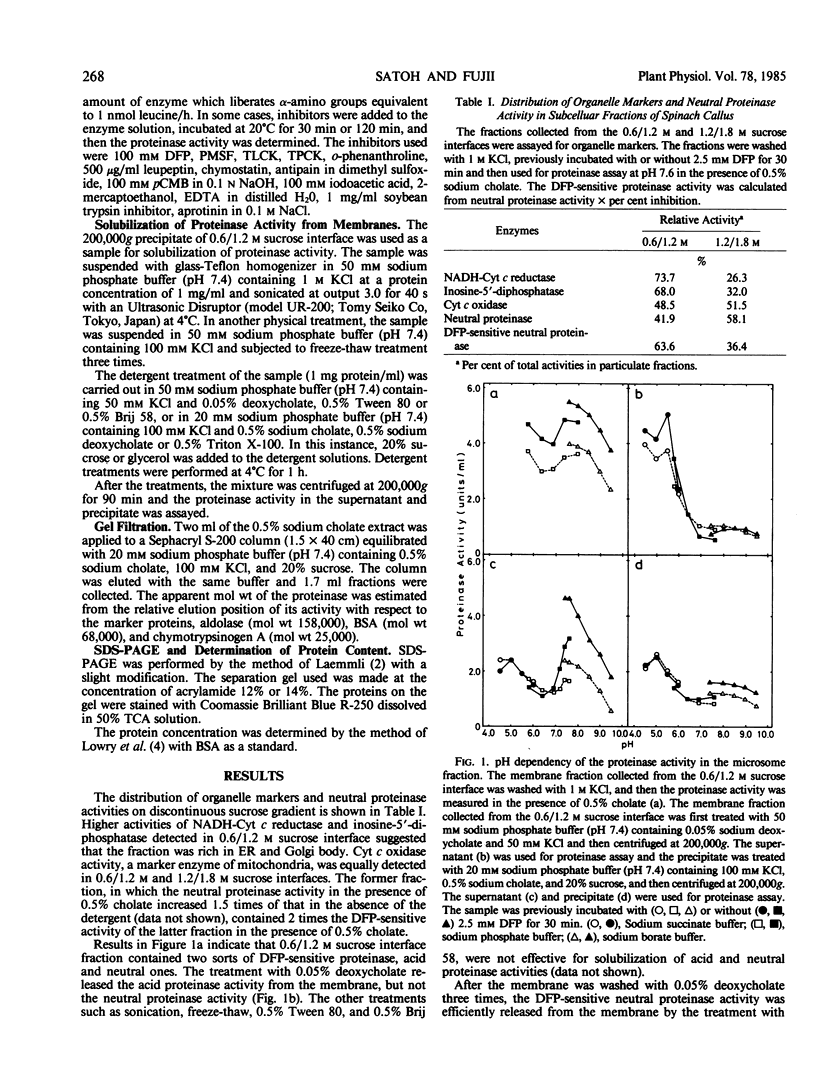
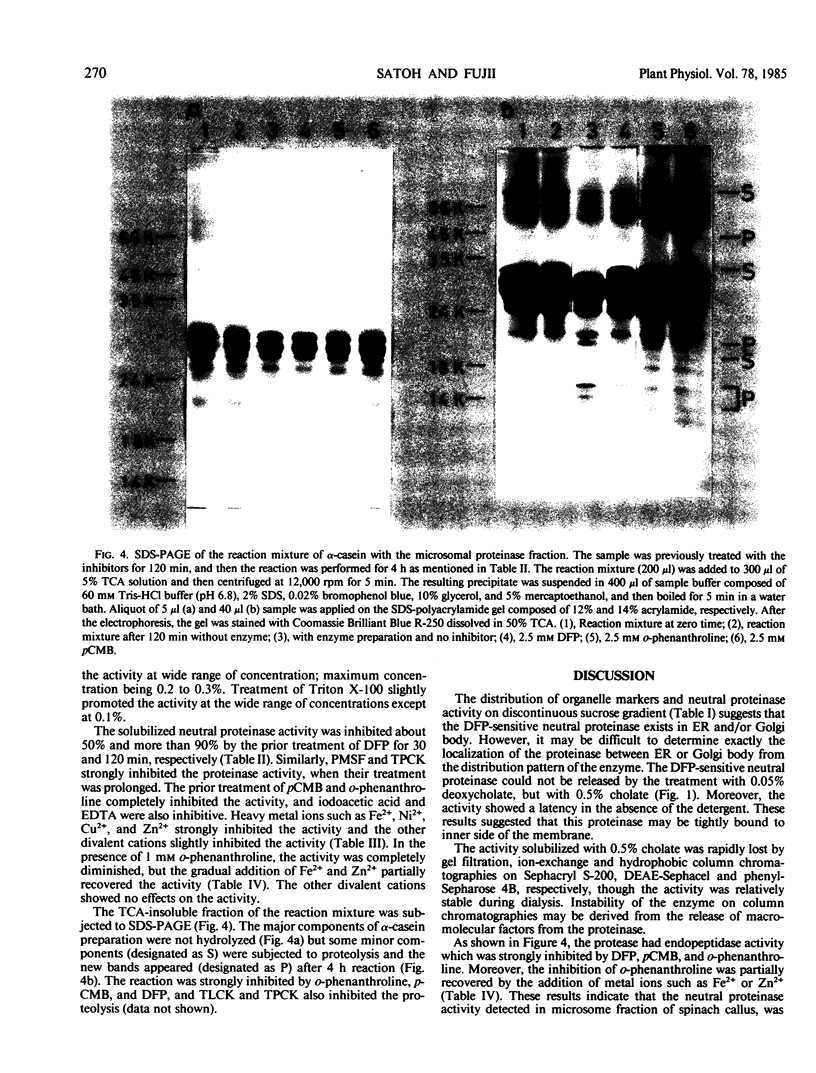
A di-isopropyl phosphorofluoridate-sensitive endopeptidase activity against some minor components of heat-denatured α-casein was detected in the endoplasmic reticulum and Golgi body-rich fraction of spinach callus. The activity was not solubilized with 0.05% sodium deoxycholate, but with 0.5% sodium cholate. The activity was strongly inhibited by deoxycholate (0.2-0.5%), di-isopropyl phosphorofluoridate, p-chloro-mercuric benzoate, o-phenanthroline, NiCl2, CuCl2, and ZnSO4, and moderately by phenylmethylsulfonyl fluoride, l-1-tosylamide-2-phenylethyl chloromethyl ketone, iodoacetic acid, ethylenediaminetetraacetate, and FeSO4, and slightly by chymostatin. The inhibitory effect of o-phenanthroline was partially recovered with the addition of FeSO4 and ZnSO4.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lively M. O., Walsh K. A. Hen oviduct signal peptidase is an integral membrane protein. J Biol Chem. 1983 Aug 10;258(15):9488–9495. [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971 Dec;48(6):671–674. doi: 10.1104/pp.48.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath H. Evolution of proteolytic enzymes. Science. 1984 Apr 27;224(4647):350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Takahashi K. A novel neutral protease(s) from monkey liver. J Biochem. 1976 Dec;80(6):1443–1446. doi: 10.1093/oxfordjournals.jbchem.a131419. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Zimmerman M., Boime I., Ashe B., Mumford R. A., Alberts A. W. Characterization of an endopeptidase involved in pre-protein processing. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4225–4229. doi: 10.1073/pnas.76.9.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]