Abstract
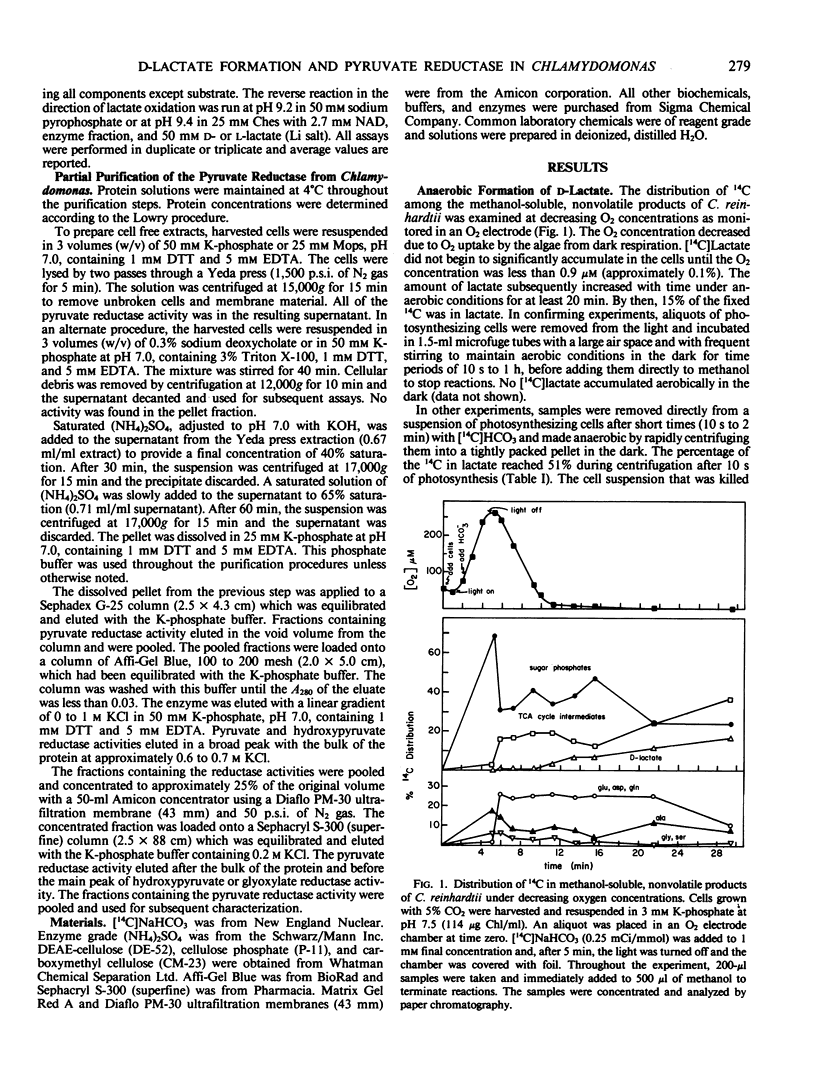
d-Lactate accumulation in Chlamydomonas reinhardtii was dependent on anaerobic conditions. As much as 50% of the 14C after 2 minutes of photosynthetic 14CO2 fixation moved into d-lactate from sugar phosphates if the cells became anaerobic for short time periods. No lactate accumulated in the dark until the O2 concentration decreased to less than 0.1%. Lactate was determined to be of the d-configuration using stereospecific lactate dehydrogenases. d-Lactate produced anaerobically by algae grown on 5% CO2 was only slowly metabolized aerobically in the light or dark, and in the dark, only a trace of the lactate was excreted.
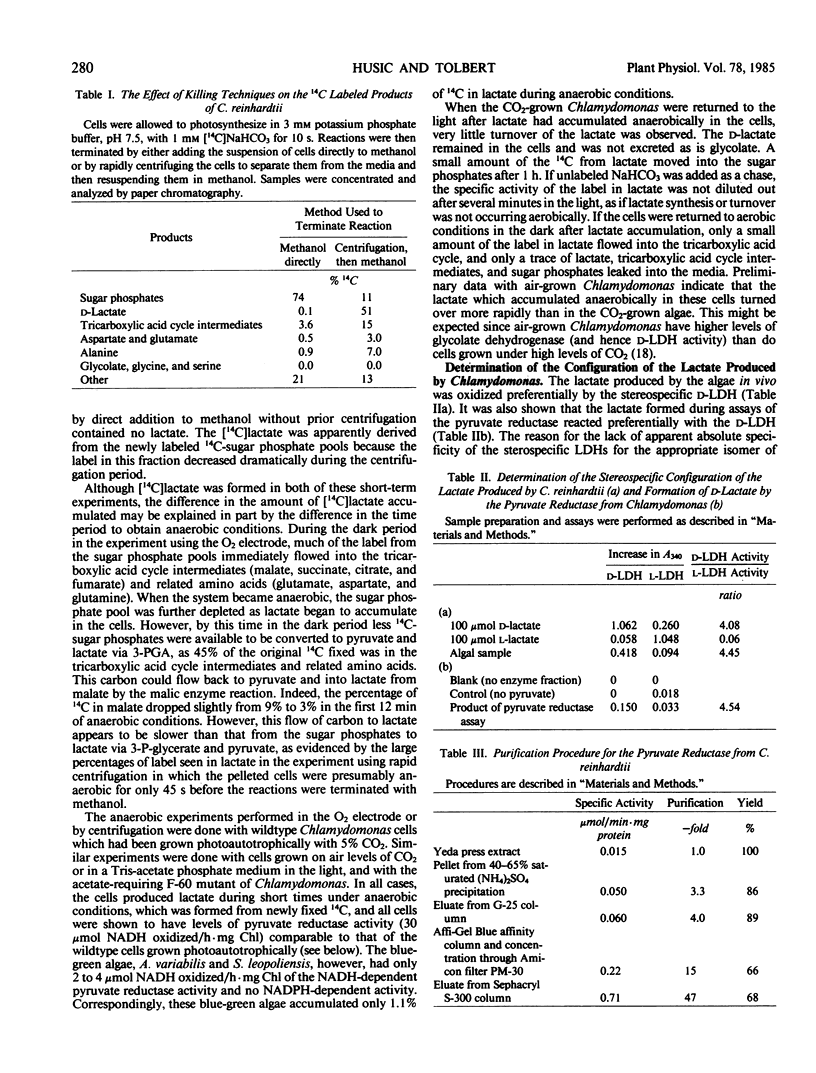
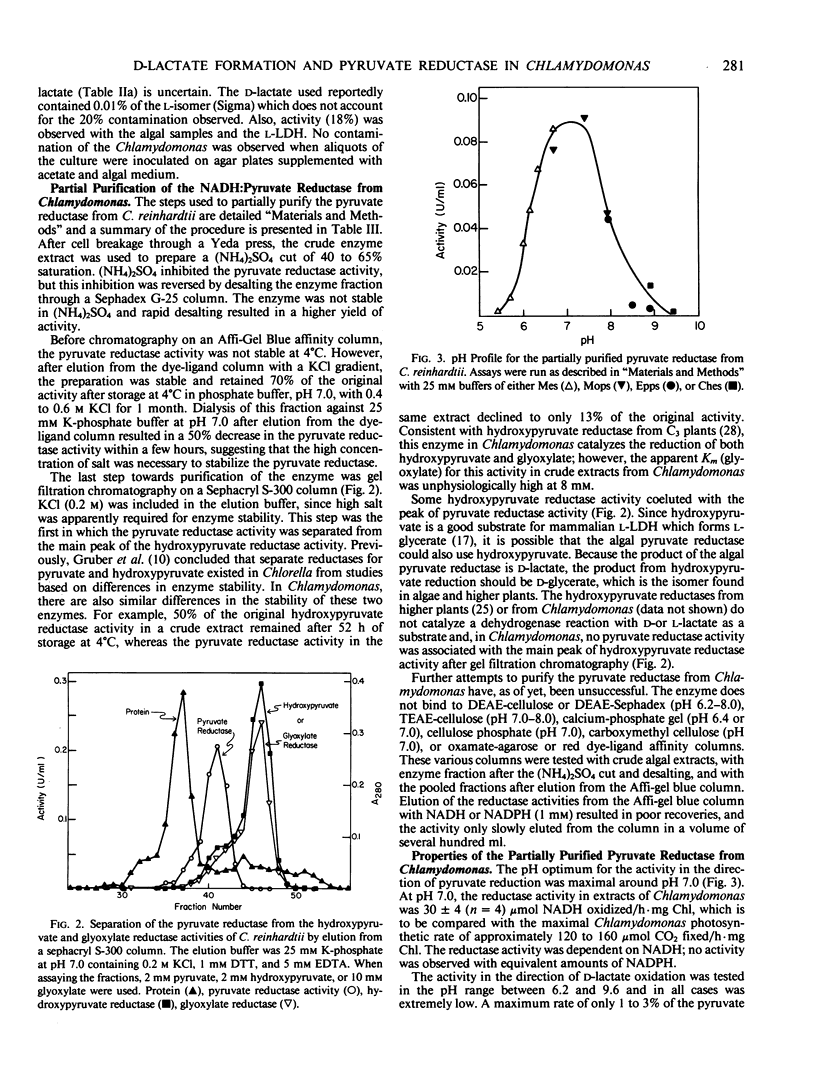
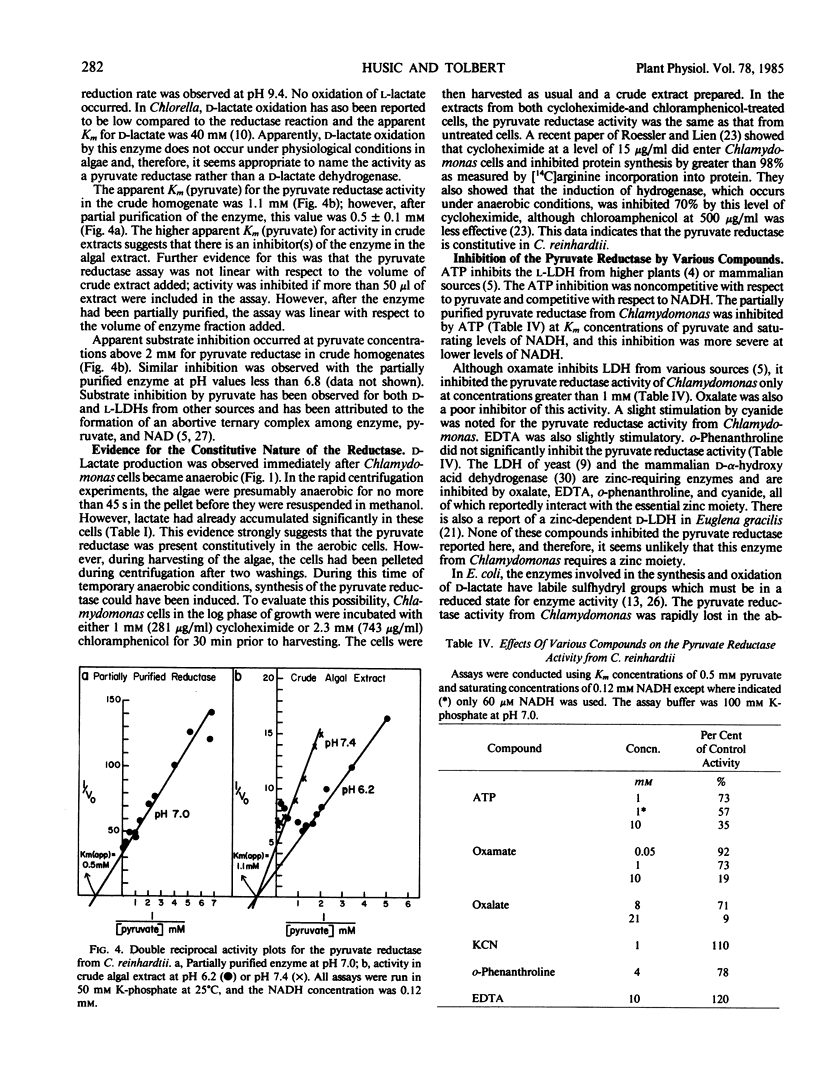
A pyruvate reductase (d-lactate: diphosphopyridine nucleotide oxidoreductase, EC 1.1.1.28) was partially purified 47-fold from Chlamydomonas. Because this enzyme catalyzes an essentially irreversible reaction in the direction of pyruvate reduction, it is considered to be a pyruvate reductase. The reductase activity in extracts of Chlamydomonas was 30 micromoles per hour per milligram chlorophyll. For the partially purified enzyme, the apparent Km (pyruvate) was 0.5 millimolar, and the pH optimum was 7.0. Studies with cycloheximide and chloramphenicol indicated that the enzyme was constitutive in aerobic cells. Potassium phosphate stimulated the reductase, and high salt and dithiothreitol were required for stability. The enzyme demonstrated substrate inhibition and was inhibited by ATP. Pyruvate reductase was separated from a hydroxypyruvate reductase by gel filtration chromatography, indicating the presence of separate reductases for these two substrates in Chlamydomonas.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beezley B. B., Gruber P. J., Frederick S. E. Cytochemical localization of glycolate dehydrogenase in mitochondria of chlamydomonas. Plant Physiol. 1976 Sep;58(3):315–319. doi: 10.1104/pp.58.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N., Brown R. H., Merrett M. J. Oxidative phosphorylation during glycollate metabolism in mitochondria from phototrophic Euglena gracilis. Biochem J. 1975 Sep;150(3):373–377. doi: 10.1042/bj1500373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D., Davies S. Purification and properties of L(+)-lactate dehydrogenase from potato tubers. Biochem J. 1972 Oct;129(4):831–839. doi: 10.1042/bj1290831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGOLIN C., SINGER T. P. The lactic dehydrogenase of yeast. III. D(-)Lactic cytochrome c reductase, a zinc-flavoprotein from aerobic yeast. Biochim Biophys Acta. 1963 Feb 12;67:201–218. doi: 10.1016/0006-3002(63)91818-3. [DOI] [PubMed] [Google Scholar]
- Gfeller R. P., Gibbs M. Fermentative Metabolism of Chlamydomonas reinhardtii: I. Analysis of Fermentative Products from Starch in Dark and Light. Plant Physiol. 1984 May;75(1):212–218. doi: 10.1104/pp.75.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber P. J., Frederick S. E., Tolbert N. E. Enzymes related to lactate metabolism in green algae and lower land plants. Plant Physiol. 1974 Feb;53(2):167–170. doi: 10.1104/pp.53.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt G., Tanner W., Kandler O. Effect of Light on the Rate of Glycolysis in Scenedesmus obliquus. Plant Physiol. 1971 Jun;47(6):841–843. doi: 10.1104/pp.47.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE C. A. A zinc-dependent lactate dehydrogenase in Euglena gracilis. Biochem J. 1962 Jan;82:61–66. doi: 10.1042/bj0820061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler P. G., Lien S. Activation and de novo synthesis of hydrogenase in chlamydomonas. Plant Physiol. 1984 Dec;76(4):1086–1089. doi: 10.1104/pp.76.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAFFORD H. A., MAGALDI A., VENNESLAND B. The enzymatic reduction of hydroxypyruvic acid to D-glyceric acid in higher plants. J Biol Chem. 1954 Apr;207(2):621–629. [PubMed] [Google Scholar]
- TUBBS P. K. Effects of metal-complexing agents on mitochondrial D-alpha-hydroxy acid dehydrogenase. Biochem Biophys Res Commun. 1960 Nov;3:513–517. doi: 10.1016/0006-291x(60)90166-2. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate-controlled change in conformation. J Biol Chem. 1968 May 25;243(10):2587–2596. [PubMed] [Google Scholar]
- Tolbert N. E., Harrison M., Selph N. Aminooxyacetate stimulation of glycolate formation and excretion by chlamydomonas. Plant Physiol. 1983 Aug;72(4):1075–1083. doi: 10.1104/pp.72.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]


