Abstract
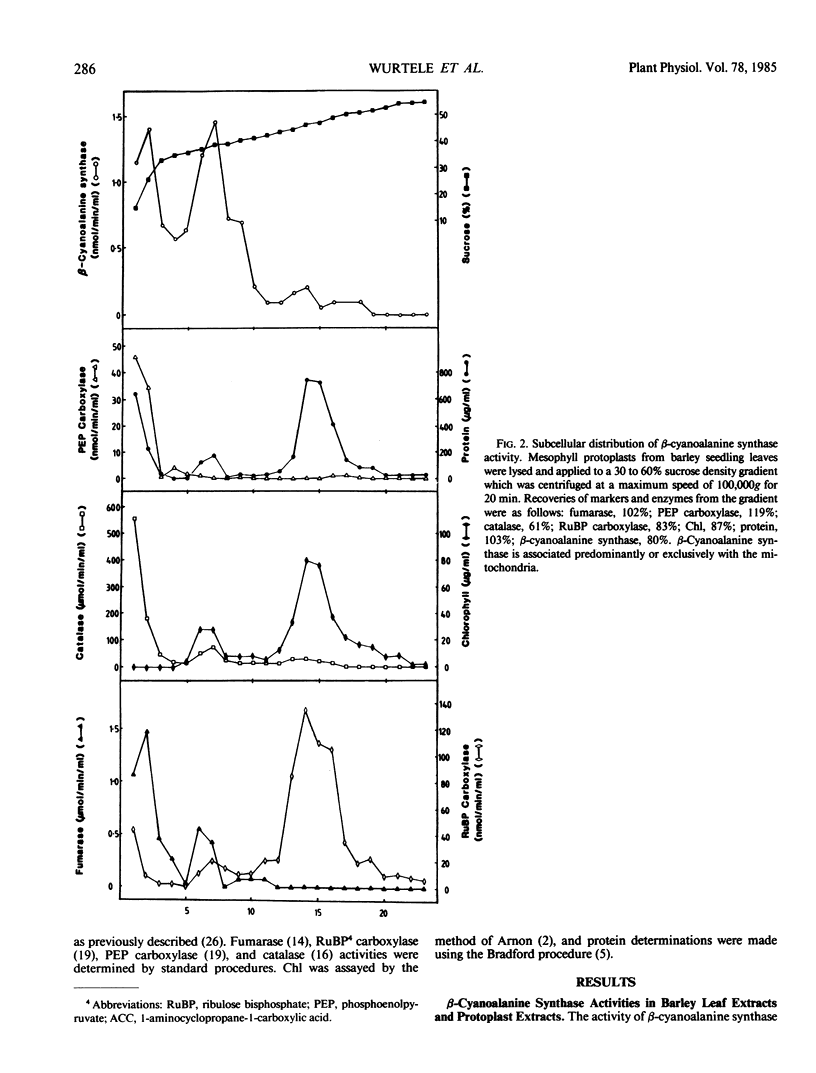
The subcellular and developmental distribution of β-cyanoalanine synthase (EC 4.4.1.9), which catalyzes the reaction between cysteine and HCN to form β-cyanoalanine and H2S, were investigated in barley (Hordeum vulgare) leaves. Total leaf activity was 1.1 micromoles per minute per gram fresh weight. Sucrose density gradients of lysed mesophyll protoplasts of barley revealed the exclusive or predominant localization of β-cyanoalanine synthase in the mitochondria. The enzyme was absent from both vacuole and chloroplast fractions.
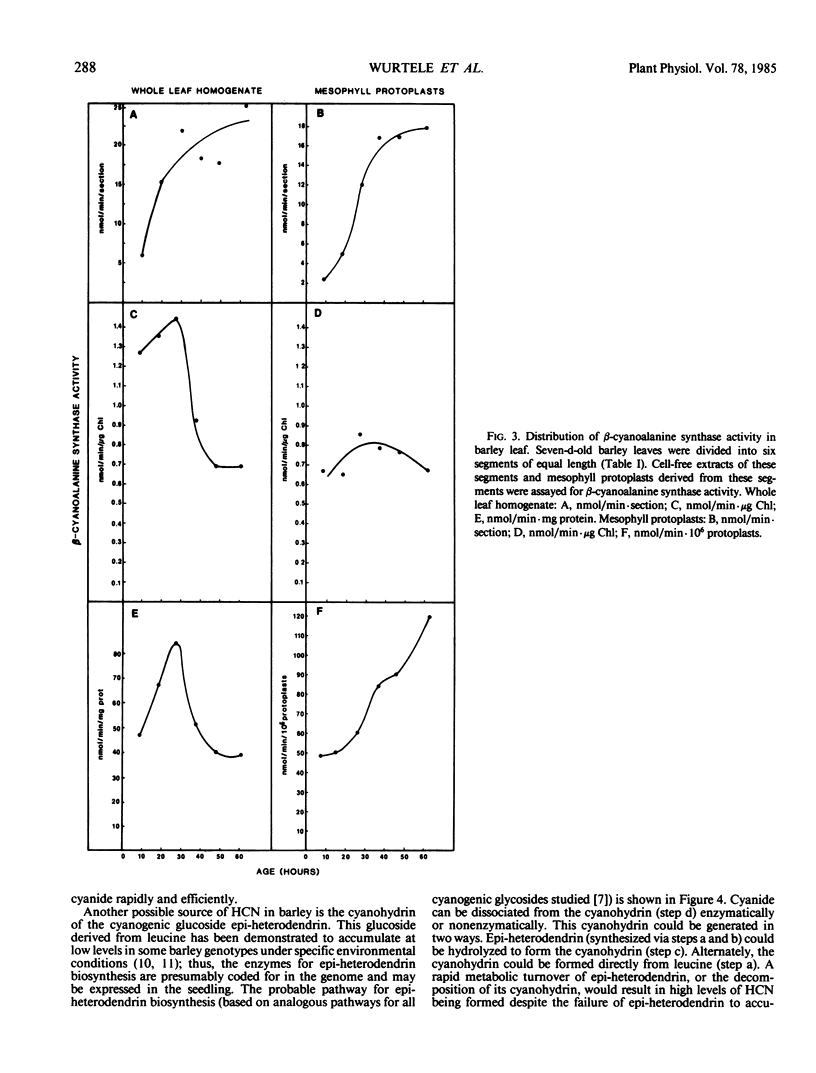
β-Cyanoalanine synthase activity was distributed over the entire length of the barley leaf. Activity was dependent on the developmental stage, with a 3.5-fold higher activity in the oldest (apical) compared to the youngest (basal) parts of the leaf. The corresponding difference in activity for mesophyll protoplasts isolated from these parts was 7.5-fold. In younger leaf seagments, the nonchlorophyllous tissues accounted for up to 70% of the total β-cyanoalanine synthase activity. These results are discussed with reference to the formation of HCN as a substrate in barley leaves.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyan T. N., Braunstein A. E., Goryachenkova E. V. Beta-cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1617–1621. doi: 10.1073/pnas.72.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal S. G., Hendrickson H. R., Abrol Y. P., Conn E. E. Cyanide metabolism in higher plants. 3. The biosynthesis of beta-cyanolanine. J Biol Chem. 1968 Oct 25;243(20):5302–5307. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castric P. A., Farnden K. J., Conn E. E. Cyanide metabolism in higher plants. V. The formation of asparagine from -cyanoalanine. Arch Biochem Biophys. 1972 Sep;152(1):62–69. doi: 10.1016/0003-9861(72)90193-2. [DOI] [PubMed] [Google Scholar]
- Conn E. E. Biosynthesis of cyanogenic glycosides. Naturwissenschaften. 1979 Jan;66(1):28–34. doi: 10.1007/BF00369352. [DOI] [PubMed] [Google Scholar]
- Cooney D. A., Jayaram N., Swengros S. G., Alter S. C., Levine M. The metabolism of L-asparagine in Asparagus officinalis. Int J Biochem. 1980;11(1):69–83. doi: 10.1016/0020-711x(80)90281-5. [DOI] [PubMed] [Google Scholar]
- Floss H. G., Hadwiger L., Conn E. E. Enzymatic formation of beta-cyanoalanine from cyanide. Nature. 1965 Dec 18;208(5016):1207–1208. doi: 10.1038/2081207a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. R., Conn E. E. Cyanide metabolism in higher plants. IV. Purification and properties of the beta-cyanolanine synthase of blue lupine. J Biol Chem. 1969 May 25;244(10):2632–2640. [PubMed] [Google Scholar]
- Miller J. M., Conn E. E. Metabolism of hydrogen cyanide by higher plants. Plant Physiol. 1980 Jun;65(6):1199–1202. doi: 10.1104/pp.65.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Subcellular distribution of acetyl-coenzyme A carboxylase in mesophyll cells of barley and sorghum leaves. Arch Biochem Biophys. 1984 Dec;235(2):555–561. doi: 10.1016/0003-9861(84)90229-7. [DOI] [PubMed] [Google Scholar]
- Peiser G. D., Wang T. T., Hoffman N. E., Yang S. F., Liu H. W., Walsh C. T. Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc Natl Acad Sci U S A. 1984 May;81(10):3059–3063. doi: 10.1073/pnas.81.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J., Conn E. E. Tissue Distribution of beta-Cyanoalanine Synthase in Leaves. Plant Physiol. 1984 Aug;75(4):979–982. doi: 10.1104/pp.75.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]



