Abstract
The expression of genes encoding enzymes involved in xylan degradation and two endoglucanases involved in cellulose degradation was studied at the mRNA level in the filamentous fungus Aspergillus niger. A strain with a loss-of-function mutation in the xlnR gene encoding the transcriptional activator XlnR and a strain with multiple copies of this gene were investigated in order to define which genes are controlled by XlnR. The data presented in this paper show that the transcriptional activator XlnR regulates the transcription of the xlnB, xlnC, and xlnD genes encoding the main xylanolytic enzymes (endoxylanases B and C and β-xylosidase, respectively). Also, the transcription of the genes encoding the accessory enzymes involved in xylan degradation, including α-glucuronidase A, acetylxylan esterase A, arabinoxylan arabinofuranohydrolase A, and feruloyl esterase A, was found to be controlled by XlnR. In addition, XlnR also activates transcription of two endoglucanase-encoding genes, eglA and eglB, indicating that transcriptional regulation by XlnR goes beyond the genes encoding xylanolytic enzymes and includes regulation of two endoglucanase-encoding genes.
The two most abundant structural polysaccharides in nature are cellulose and the hemicellulose xylan, which are closely associated in plant cell walls (4). Filamentous fungi, particularly Aspergillus and Trichoderma species, are well-known and efficient producers of both cellulolytic and hemicellulolytic enzymes. The cellulase degradation system of these organisms consists of three classes of enzymes (2): endoglucanases (EC 3.2.1.4), cellobiohydrolases (EC 3.2.1.91), and β-glucosidases (EC 3.2.1.21). Members of all of these classes are necessary to degrade cellulose, a homopolymer of β-1,4-linked d-glucose. Xylan, however, is a heterogeneous polymer with a backbone consisting of β-1,4-linked d-xylose residues, which can be substituted at the C-2 and C-3 positions with various residues, such as acetic acid, α-l-arabinofuranose, (4-o-methyl)glucuronic acid, ferulic acid, and p-coumaric acid (5). Due to this heterogeneous composition, a more complex set of enzymes is required for xylan degradation. The following enzymes have been found to be necessary during the cooperative process of xylan breakdown: endoxylanase (EC 3.2.1.8), β-xylosidase (EC 3.2.1.37), acetylxylan esterase (EC 3.1.1.72), α-l-arabinofuranosidase (EC 3.2.1.55), arabinoxylan arabinofuranohydrolase, β-glucuronidase (EC 3.2.1.139), feruloyl esterase, and p-coumaroyl esterase (3).
The expression of cellulose- and xylan-degrading enzymes by Aspergillus and Trichoderma species has been studied extensively at the cellular level (1, 20, 21, 25). It has been shown that xylanase- and cellulase-encoding genes are regulated at the transcriptional level (10, 23, 31, 43). In the presence of d-glucose the genes are not expressed, and it has been shown that the carbon catabolite repressor protein CreA is involved in transcriptional repression of xylanase-encoding (10) and arabinase-encoding (38) genes in Aspergillus species. It has been demonstrated that in Trichoderma reesei the CreA counterpart Cre1 causes repression of transcription of cellulase-encoding (22, 23) and xylanase-encoding (30, 31) genes. However, far less is known about the mechanism by which cellulase- and xylanase-encoding genes are induced. The inducing abilities of various saccharides have been tested, and some saccharides induce the synthesis of both xylanases and cellulases (10, 21, 31, 37, 48). Nevertheless, on the basis of biochemical data (1, 20, 21) and mRNA expression analysis data (23, 31), a separate induction mechanism has been proposed for these systems in both Aspergillus and Trichoderma.
Recently, a selection system was developed to isolate Aspergillus niger strains having mutations in a transcription factor involved in induction of expression of xylanolytic genes. Complementation of such a mutation by transformation with a plasmid library led to the isolation of the A. niger xlnR gene, which encodes a transcriptional activator of the A. niger xylanolytic system (44). This xlnR gene encodes a zinc binuclear cluster protein, which is a member of the GAL4 family of transcription factors. Isolation of both the xlnR gene and A. niger xlnR loss-of-function mutants provided an opportunity to study the spectrum of genes that are controlled by XlnR at the transcriptional level.
MATERIALS AND METHODS
Aspergillus strains, transformation, and culture conditions.
All of the A. niger strains used were derived from wild-type strain N400 (= CBS 120.49). The strains used were A. niger N402 (cspA1), a short-conidiophore derivative, NW205::130 [argB13 cspA1 nicA1 pyrA6 UAS(xlnA)-pyrA], NXA1-4 [argB13 cspA1 nicA1 pyrA6 UAS(xlnA)-pyrA xlnR1] (strains NW205::130 and NXA1-4 are described more extensively in reference 44), and N902 (argB15 cspA1 fwnA1 metB10 pyrA5).
Strain N902::230-25.12 (argB15 cspA1 fwnA1 metB10), which contains approximately 20 additional copies of xlnR, as determined by a phosphorimager analysis of Southern blots, was obtained by cotransformation of A. niger N902. The cotransforming plasmids were pIM230 (44) and pGW635 (19), which contain the functional xlnR gene (EMBL accession no. AJ001909) and the pyrA gene (EMBL accession no. X96734), respectively. Transformation was carried out as described previously (27).
All media were based on Aspergillus minimal medium (36). The media contained the carbon sources indicated below, and the starting pH of each medium was 6. Spores were inoculated at a concentration of 106 spores ml−1. In transfer experiments the first culture containing d-fructose was supplemented with 0.2% (wt/vol) Casamino Acids and 0.1% (wt/vol) yeast extract. After overnight growth, mycelia were recovered by filtration and washed with saline. These mycelia were transferred to media containing d-xylose or xylan as a carbon source and 0.05% (wt/vol) Casamino Acids. The xylan used was birchwood xylan (Roth-7500).
Expression cloning of A. niger glucanases in Escherichia coli.
A xylan-induced cDNA library of A. niger (43) was screened for expression of endoglucanases by using a modified procedure (6, 46, 47). The plates contained 20 ml of 2× TY, 0.2% carboxymethyl cellulose (CMC) (Sigma), 1.5% agar, and 100 μg of ampicillin per ml. E. coli cells were plated in an overlay consisting of 5 ml of the same medium containing about 300 colonies per plate, and the plates were incubated for 48 h at 37°C. Next, 5 ml of 0.1% Congo red (Aldrich) was poured onto each plate. After it was stained for 1 to 2 h, each plate was destained with 5 ml of 5 M NaCl for 0.5 to 1 h. About 12,000 colonies from the A. niger cDNA library were plated. Screening on CMC resulted in 89 colonies that had halos after staining with Congo red. None of these colonies produced a halo when it was screened with Remazol brilliant blue-modified xylan. All colonies contained a full-length cDNA copy, which appeared to originate from two different genes. Both of the enzymes encoded were active on CMC and on β-glucan (unpublished data). The corresponding genes, eglA and eglB, were cloned by using these cDNA fragments.
Northern blot analysis.
Total RNA was isolated from powdered mycelia by using TRIzol reagent (Life Technologies) according to the supplier’s instructions. For Northern blot analysis 10 μg of total RNA was glyoxylated and separated on a 1.6% (wt/vol) agarose gel (39). After capillary blotting onto Hybond-N filters (Amersham), the amounts of RNA were checked by staining the rRNA on the Hybond filters with a 0.2% (wt/vol) methylene blue solution. The filters were hybridized at 42°C in a solution containing 50% (vol/vol) formamide, 10% (wt/vol) dextran sulfate, 0.9 M NaCl, 90 mM trisodium citrate, 0.2% (wt/vol) Ficoll, 0.2% (wt/vol) polyvinylpyrrolidone, 0.2% (wt/vol) bovine serum albumin, 0.1% (wt/vol) sodium dodecyl sulfate, and 100 μg of single-stranded herring sperm DNA per ml. Washing was done under homologous hybridization conditions with a solution containing 30 mM NaCl, 3 mM trisodium citrate, and 0.1% (wt/vol) sodium dodecyl sulfate at 68°C. The 32P-labelled DNA probes used were either cDNA or genomic fragments, as shown in Table 1.
TABLE 1.
Probes used in Northern blot analysis
| Gene | EMBL accession no. | Enzyme encoded | Fragment used | Reference |
|---|---|---|---|---|
| abfB | X74777 | α-l-Arabinofuranosidase B | 1.7-kb EcoRI-XhoI | 15 |
| aguA | Y15405 | α-Glucuronidase A | 0.8-kb EcoRV-KpnIa | 12 |
| axeA | A22880 | Acetylxylan esterase A | 1.5-kb HindIIIa,b | 9 |
| axhA | Z78011 | Arabinoxylan hydrolase A | 1.2-kb EcoRI-XhoI | 17 |
| bglA | β-Glucosidase A | 1.0-kb NcoI-SstI | This study | |
| eglA | AJ224451 | Endoglucanase A | 0.9-kb XhoI | This study |
| eglB | AJ224452 | Endoglucanase B | 1.1-kb EcoRI-XhoI | This study |
| faeA | Y09330 | Feruloyl esterase A | 0.5-kb EcoRV-XhoIa | 11 |
| xlnB | D38071 | Endoxylanase B | 0.9-kb EcoRI-XhoI | 24 |
| xlnC | Endoxylanase C | 1.2-kb EcoRI-XhoI | 18 | |
| xlnD | Z84377 | β-Xylosidase D | 2.8-kb PstI-NsiIa | 43 |
| 18S rRNA | X78538 | 18S rRNA subunit | 0.7-kb EcoRI | 33 |
Genomic fragment instead of cDNA.
Fragment from the Aspergillus tubingensis axeA gene.
A 1-kb β-glucosidase cDNA fragment of A. niger, as determined by sequence analysis, was isolated from a xylan-induced cDNA library (43) by using PCR with degenerate oligonucleotides based on the Aspergillus kawachii (EMBL accession no. AB003470) and Aspergillus aculeatus (EMBL accession no. P48825) sequences for β-glucosidase and cloned into pGEM-T (Promega).
Nucleotide sequence accession numbers.
The eglA and eglB sequences have been deposited in the GenBank-EMBL sequence database under accession no. AJ224451 and AJ224452, respectively.
RESULTS AND DISCUSSION
Induction of the xylanolytic system.
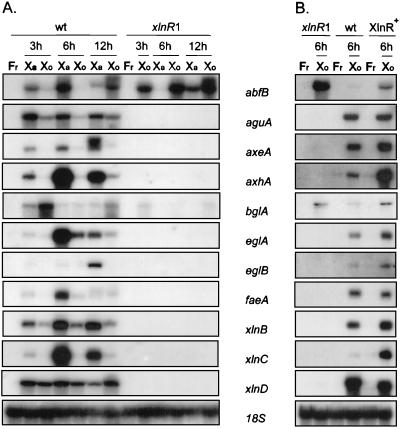
An A. niger mutant having a loss-of-function mutation in the xylanolytic transcriptional activator gene xlnR lacks transcription of the endoxylanase B- and β-xylosidase-encoding genes xlnB and xlnD (44). To investigate the spectrum of genes which are under control of the transcriptional activator xlnR, expression in an A. niger wild-type strain and expression in the strain with the xlnR loss-of-function mutation were analyzed by Northern blot analysis. To do this, we used fragments of genes cloned from A. niger encoding enzymes which are potentially involved in the breakdown of xylan (Table 1). A. niger NW205::130 (wild type) and NXA1-4 (a xlnR mutant) were precultured and subsequently transferred to media containing 1% birchwood xylan and to media containing 1% d-xylose (both birchwood xylan and d-xylose are known to be carbon sources that induce the xylanolytic system in A. niger) (10). Northern blot analysis of RNA obtained from the wild-type strain showed that xylanolytic, arabinanolytic, and cellulolytic genes were induced when the organism was grown on xylan, which is the natural substrate, and on d-xylose (Fig. 1A). High levels of expression were obtained in most cases after 6 h of growth on the polymeric carbon source xylan, although the patterns of expression for individual genes differed. Whereas some genes, including xlnD and aguA, had a high transcription level during the early phase of induction, other genes, including axeA and eglB, were highly transcribed at a later stage. The level of induction on d-xylose was usually lower than the level of induction on xylan, but some genes, including xlnD and xlnB, had a relatively high level of expression on d-xylose. As expected for extracellular enzyme systems under the control of carbon catabolite repression (10, 38), none of the genes was expressed on d-fructose.
FIG. 1.
Northern blot analysis of expression of A. niger genes encoding cellulose- and xylan-degrading enzymes. (A) Time course of induction in A. niger NW205::130 (wt) and NXA1-4 (xlnR1) (a loss-of-function mutant). Both strains were cultured for 18 h in medium containing 3% d-fructose (Fr), and mycelia were subsequently transferred to medium containing 1% xylan (Xa) or 1% d-xylose (Xo) and incubated for the times indicated. Each lane contained 10 μg of total RNA, which was checked by hybridization with the 18S rRNA probe. Blots were hybridized with gene-specific probes as indicated. (B) Comparison of expression of genes encoding cellulose- and xylan-degrading enzymes in A. niger N902 (wt), NXA1-4 (xlnR1), and N902-pIM230-25.12 (XlnR+) (N902 with multiple copies of xlnR) upon transfer to medium containing 1% d-xylose for 6 h after growth for 18 h in medium containing 1% d-fructose. A Northern blot analysis was performed exactly as described above. The signal intensities of the different blots cannot be compared to each other due to the unknown specific activities of the probes used and the different exposure times used for the various blots.
Effect of the xlnR loss-of-function mutation on expression.
An analysis of transcription in the xlnR loss-of-function mutant NXA1-4 revealed expression of only abfB and bglA upon growth on d-xylose and xylan (Fig. 1A). Mutant NXA1-4 lacks the ability to induce transcription of genes encoding xylanolytic enzymes which are involved in the degradation of the polyxylose backbone of xylan. Also, transcription of genes encoding accessory enzymes is absent in this mutant. These findings explain the previously described impaired growth of NXA mutants on xylan (44). Strains with an xlnR loss-of-function mutation are not able to express the xylanolytic enzymes, which results in impaired release of saccharides (and therefore carbon source) from the polymeric xylan. Although arabinofuranosidase B is expressed in these mutants, apparently the l-arabinose released from arabinoxylan by this enzyme is not sufficient to allow normal growth of the fungus. The inability of the xlnR loss-of-function mutants to degrade xylan also affects the release of inducer from the polymeric substrate. Induction by d-xylose, however, is independent of the presence of the xylanolytic enzyme system. Therefore, gene expression was reexamined in a second experiment by using mutant NXA1-4, wild-type strain N902, and xlnR multicopy strain N902::230-25.12; d-xylose was used as the inducing carbon source in this experiment.
Effect of multiple copies of xlnR.
In the wild-type strain all of the genes tested were induced on d-xylose (Fig. 1B), whereas in strain NXA1-4 only abfB and bglA transcription was observed. In xlnR multicopy strain N902::230-25.12 all of the genes were also expressed. Some genes (for example, aguA and faeA) had equal transcript levels in both the wild-type and the xlnR multicopy strain, whereas other genes (for example, abfB, axhA, bglA, xlnB, and xlnC) had increased transcription levels in the xlnR multicopy strain compared to that in the wild-type strain. From this finding we concluded that the transcriptional activator XlnR regulates the transcription of the xlnB, xlnC, and xlnD genes encoding the main xylanolytic enzymes (endoxylanases B and C and β-xylosidase, respectively). In addition, the aguA, axeA, axhA, and faeA genes encoding accessory enzymes (α-glucuronidase A, acetylxylan esterase A, arabinoxylan arabinofuranohydrolase A, and feruloyl esterase A) are controlled by the transcriptional regulator XlnR. The transcriptional activator XlnR also activates transcription of the eglA and eglB genes, which encode endoglucanases A and B. This indicates that regulation by the transcriptional activator XlnR goes beyond regulation of the genes encoding xylanolytic enzymes and also includes regulation of at least two endoglucanase-encoding genes.
All of the genes that were found to be controlled by the transcriptional activator XlnR exhibited differences in their levels of expression in response to increased xlnR gene copies. The differences in the responses to the xlnR gene copy number might originate from differences in the XlnR binding sites in the various xylanolytic promoters. The sequence 5′-GGCTAAA-3′ has been suggested previously to be a consensus binding site for the XlnR protein; this suggestion was based on the results of a comparison of a limited number of mainly endoxylanase promoters of different Aspergillus species (44). The results presented here show that expression of at least nine genes in A. niger is controlled by XlnR. The axeA, axhA, eglA, eglB, and xlnC genes, for which an increased xlnR copy number has a positive effect on the level of transcription, all have a nucleotide other than adenine at the last position. Transcription of xlnB, which has an adenine at the last position, however, is also positively influenced. A comparison of the sequences of these nine promoters (Table 2) suggests, therefore, that the last nucleotide in the proposed consensus sequence is less important in the binding site and that 5′-GGCTAA-3′ is a more appropriate consensus sequence, but the seventh nucleotide could play a role in XlnR binding.
TABLE 2.
Putative XlnR binding sites in the upstream region of XlnR-controlled genes
| Gene | XlnR binding site | Position(s)a |
|---|---|---|
| aguA | GGCTAAa | −276 |
| axeA | GGCTAAt | −261 (R) |
| axhA | GGCTAAt | −340 |
| GGCTAAg | −850 (R) | |
| eglA | GGCTAAg | −710 |
| eglB | GGCTAAg | −128 |
| faeA | GGCTAAa | −265, −225 |
| xlnB | GGCTAAa | −124, −216 |
| xlnC | GGCTAAt | −290 |
| GGCTAAg | −500 | |
| xlnD | GGCTAAa | −133, −147 |
Position relative to the ATG translation start codon. (R) indicates the opposite orientation of the putative XlnR binding site.
All of the A. niger genes for which XlnR transcriptional control has been demonstrated contain one or more copies of this consensus sequence in the promoter region. The different genes vary in the number of putative XlnR binding sites present, as four genes have two putative sites. Also, the orientation varies, as some genes have the opposite orientation or both orientations are present. The differences found in the effect of the xlnR copy number and the level of transcription of the individual genes cannot be explained by the differences in the presumed XlnR binding sites, since the mode of binding of XlnR is not known (44).
The context in which the sites are located in the promoter region may also play an important role. The putative XlnR binding site is not a direct or inverted repeat, while most zinc binuclear cluster proteins have a dimeric nature and bind to symmetric sites. However, some proteins (for example, the AlcR protein of Aspergillus nidulans) are thought to act as monomers. Two molecules of AlcR can simultaneously bind to symmetric sites, whereas only one molecule occupies a direct repeat (28).
It has been proposed that repression by CreA of the xylanolytic genes (10, 44) is analogous to the double lock mechanism described for the ethanol regulon in A. nidulans (13, 26). In this model CreA represses both the positive and autoregulated trans-acting gene alcR and structural genes, such as alcA and aldA (14, 26, 28, 32). Some CreA binding sites in the alcA and alcR upstream region are close to or overlap the AlcR targets (13, 26). Therefore, it has been suggested that competition between the AlcR and CreA proteins for the same region is a mechanism in the regulation of the ethanol regulon genes. This is also the case in the regulation of expression of amdS by the trans-acting factors AmdR, FacB, AmdA, AmdX, AreA, and CreA (8, 29, 34, 35). The overlap of AmdX binding sites with CreA and AmdA binding sites suggests that there is competition for binding sites by multiple factors (34). The repressor protein CreA has been shown to also have a function in xylanolytic gene expression in A. niger (10, 17). However, putative CreA sites in the XlnR-controlled genes in A. niger are generally at distances of more than 40 bp from the putative XlnR binding sites; an exception is the 1-bp distance in the xlnD upstream region (43). Thus, XlnR-CreA competition for all xylanolytic promoters is unlikely. Besides the trans-acting factors XlnR and CreA, other trans-acting factors may have a function in modulating the transcription of the various XlnR-controlled genes.
Transcription of the β-glucosidase-encoding gene bglA is under separate control, and therefore not all genes encoding cellulolytic enzymes are controlled by XlnR. Of the genes involved in xylan degradation, the abfB gene is the only gene whose transcription is not controlled by XlnR. The encoded enzyme, however, is involved in hydrolysis of l-arabinofuranosyl residues not only from arabinoxylan but also from arabinan (41) and pectin (40). abfB gene expression is under coordinate control with expression of the arabinofuranosidase A-encoding abfA gene and the endoarabinase-encoding abnA gene (16). Although it is clear from the results presented here that the abfB and bglA genes are not controlled by XlnR, the level of transcription is increased in both the NXA1-4 mutant and the xlnR multicopy transformant. The promoter sequence of the A. niger bglA gene is not available, and the abfB gene does not contain the XlnR binding site. The increase in the levels of expression of abfB and bglA on d-xylose may be an indirect effect of the xlnR loss-of-function mutation and gene dosage. For example, there could be an effect on pentose catabolism (45), thereby influencing the l-arabitol concentration, which is the inducer of the abfB gene (42).
The use of a loss-of-function mutation in the transcriptional activator XlnR is a powerful tool for understanding the fungal strategy for degrading the variety of xylan structures which occur in nature. The fact that expression of the xylanolytic enzymes and expression of some cellulolytic enzymes are coordinately regulated at the molecular level provides new insight into the regulation of expression of both enzyme systems. The findings presented here strengthen the hypothesis that there is an evolutionary relationship between some of the xylanolytic and cellulolytic enzyme systems. Xylanases and cellulases have been shown to be related at various levels. The three-dimensional structures of, for example, family 11 endoxylanases and family 12 endoglucanases are similar (7). Also, there are similarities in the primary structures of, for example, β-xylosidase XlnD and β-glucosidase BglA, both of which are members of the family 3 glycosyl hydrolases (43). Here we provide evidence that there is coordination in the regulation of xylanases and some cellulases.
ACKNOWLEDGMENT
We appreciate financial support from grant BIO2-CT93-0174 from the BIOTECH Programme of the European Commission to J.V.
REFERENCES
- 1.Bailey M J, Buchert J, Viikari L. Effect of pH on production of xylanase by Trichoderma reesei on xylan- and cellulose-based media. Appl Microbiol Biotechnol. 1993;40:224–229. [Google Scholar]
- 2.Béguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- 3.Biely P. Microbial xylanolytic systems. Trends Biotechnol. 1985;3:286–290. [Google Scholar]
- 4.Carpita N C, Gibeaut D M. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 5.Coughlan M, Hazlewood G P. β-1,4-d-Xylan-degrading enzyme systems: biochemistry, molecular biology and applications. Biotechnol Appl Biochem. 1993;17:259–289. [PubMed] [Google Scholar]
- 6.Dalbøge H. Expression cloning of fungal enzyme genes; a novel approach for efficient isolation of enzyme genes of industrial relevance. FEMS Microbiol Rev. 1997;21:29–42. doi: 10.1111/j.1574-6976.1997.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 7.Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3:853–859. doi: 10.1016/S0969-2126(01)00220-9. [DOI] [PubMed] [Google Scholar]
- 8.Davis M A, Kelly J M, Hynes M J. Fungal catabolic gene regulation: molecular genetic analysis of the amdS gene of Aspergillus nidulans. Genetica. 1993;90:133–145. doi: 10.1007/BF01435035. [DOI] [PubMed] [Google Scholar]
- 9.de Graaff, L. H., J. Visser, H. C. van den Broeck, F. Strozyk, F. J. M. Kormelink, and J. C. Boonman. March 1992. Cloning, expression and use of acetyl xylan esterases from fungal origin. European patent application 0507369-A/7.
- 10.de Graaff L H, van den Broek H C, van Ooijen A J J, Visser J. Regulation of the xylanase-encoding xlnA gene of Aspergillus tubingensis. Mol Microbiol. 1994;12:479–490. doi: 10.1111/j.1365-2958.1994.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 11.de Vries R P, Michelsen B, Poulsen C H, Kroon P A, van den Heuvel R H H, Faulds C B, Williamson G, van den Hombergh J P T W, Visser J. The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Appl Environ Microbiol. 1997;63:4638–4644. doi: 10.1128/aem.63.12.4638-4644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries R P, Poulsen C H, Madrid S, Visser J. aguA, the gene encoding an extracellular α-glucuronidase from Aspergillus tubingensis, is specifically induced on xylose and not on glucuronic acid. J Bacteriol. 1998;180:243–249. doi: 10.1128/jb.180.2.243-249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felenbok B. The ethanol utilization regulon of Aspergillus nidulans: the alcA-lacR system as a tool for the expression of recombinant proteins. J Biotechnol. 1991;17:11–18. doi: 10.1016/0168-1656(91)90023-o. [DOI] [PubMed] [Google Scholar]
- 14.Fillinger S, Felenbok B. A newly identified gene cluster in Aspergillus nidulans comprises five novel genes localized in the alc region that are controlled both by the specific transactivator AlcR and the general carbon-catabolite repressor CreA. Mol Microbiol. 1996;20:475–488. doi: 10.1046/j.1365-2958.1996.5301061.x. [DOI] [PubMed] [Google Scholar]
- 15.Flipphi M J A, van Heuvel M, van der Veen P, Visser J, de Graaff L H. Cloning and characterization of the abfB gene coding for the major α-l-arabinofuranosidase (AbfB) of Aspergillus niger. Curr Genet. 1993;24:525–532. doi: 10.1007/BF00351717. [DOI] [PubMed] [Google Scholar]
- 16.Flipphi M J A, Visser J, van der Veen P, de Graaff L H. Arabinase gene expression in Aspergillus niger: indications for coordinated regulation. Microbiology. 1994;140:2673–2682. doi: 10.1099/00221287-140-10-2673. [DOI] [PubMed] [Google Scholar]
- 17.Gielkens M M C, Visser J, de Graaff L H. Arabinoxylan degradation by fungi: characterisation of the arabinoxylan-arabinofuranohydrolase encoding genes from Aspergillus niger and Aspergillus tubingensis. Curr Genet. 1997;31:22–29. doi: 10.1007/s002940050172. [DOI] [PubMed] [Google Scholar]
- 18.Gielkens, M. M. C., J. Visser, and L. H. de Graaff. 1998. Unpublished data.
- 19.Goosen T, Bloemheuvel G, Gysler C, de Bie D A, van den Broek H W J, Swart K. Transformation of Aspergillus niger using the homologous orotidine-5′-phosphate-decarboxylase gene. Curr Genet. 1987;11:499–503. doi: 10.1007/BF00384612. [DOI] [PubMed] [Google Scholar]
- 20.Hrmová M, Biely P, Vrsanská M. Cellulose- and xylan-degrading enzymes of Aspergillus terreus and Aspergillus niger. Enzyme Microb Technol. 1989;11:610–616. [Google Scholar]
- 21.Hrmová M, Petráková E, Biely P. Induction of cellulose- and xylan-degrading enzyme systems in Aspergillus terreus by homo- and heterodisaccharides composed of glucose and xylose. J Gen Microbiol. 1991;137:541–547. doi: 10.1099/00221287-137-3-541. [DOI] [PubMed] [Google Scholar]
- 22.Ilmén M, Thrane C, Penttilä M. The glucose repressor gene cre1 of Trichoderma: isolation and expression of a full-length and a truncated mutant form. Mol Gen Genet. 1996;251:451–460. doi: 10.1007/BF02172374. [DOI] [PubMed] [Google Scholar]
- 23.Ilmén M, Saloheimo A, Onnela M, Penttilä M. Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl Environ Microbiol. 1997;63:1298–1306. doi: 10.1128/aem.63.4.1298-1306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita K, Takano M, Koseki T, Ito K, Iwano K. Cloning of the xynNB gene encoding xylanase B from Aspergillus niger and its expression in Aspergillus kawachii. J Ferment Bioeng. 1995;79:422–428. [Google Scholar]
- 25.Kubicek C P, Messner R, Gruber F, Mach R L, Kubicek-Pranz E M. The Trichoderma cellulase regulatory puzzle: from the interior life of a secretory fungus. Enzyme Microb Technol. 1993;15:90–99. doi: 10.1016/0141-0229(93)90030-6. [DOI] [PubMed] [Google Scholar]
- 26.Kulmburg P, Mathieu M, Dowzer C, Kelly J, Felenbok B. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol Microbiol. 1993;7:847–857. doi: 10.1111/j.1365-2958.1993.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 27.Kusters-van Someren M A, Harmsen J A M, Kester H C M, Visser J. Structure of the Aspergillus niger pelA gene and its expression in Aspergillus niger and Aspergillus nidulans. Curr Genet. 1991;20:293–299. doi: 10.1007/BF00318518. [DOI] [PubMed] [Google Scholar]
- 28.Lenouvel F, Nikolaev I, Felenbok B. In vivo recognition of specific DNA targets by AlcR, a zinc binuclear cluster activator different from the other proteins of this class. J Biol Chem. 1997;272:15521–15526. doi: 10.1074/jbc.272.24.15521. [DOI] [PubMed] [Google Scholar]
- 29.Lints R, Davis M A, Hynes M J. The positively acting amdA gene of Aspergillus nidulans encodes a protein with two C2H2 zinc-finger motifs. Mol Microbiol. 1995;15:965–975. doi: 10.1111/j.1365-2958.1995.tb02365.x. [DOI] [PubMed] [Google Scholar]
- 30.Mach R L, Strauss J, Zeilinger S, Schindler M, Kubicek C P. Carbon catabolite repression of xylanase I (xyn1) gene expression in Trichoderma reesei. Mol Microbiol. 1996;21:1273–1281. doi: 10.1046/j.1365-2958.1996.00094.x. [DOI] [PubMed] [Google Scholar]
- 31.Margolles-Clark E, Ilmén M, Penttilä M. Expression patterns of ten hemicellulase genes of the filamentous fungus Trichoderma reesei on various carbon sources. J Biotechnol. 1997;57:167–179. [Google Scholar]
- 32.Mathieu M, Felenbok B. The Aspergillus nidulans CREA protein mediates glucose repression of the ethanol regulon at various levels through competition with the ALCR-specific transactivator. EMBO J. 1994;13:4022–4027. doi: 10.1002/j.1460-2075.1994.tb06718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melchers W J G, Verweij P E, van den Hurk P, van Belkum A, de Pauw B E, Hoogkamp-Korstanje J A A, Meis J F G M. General primer-mediated PCR for detection of Aspergillus species. J Clin Microbiol. 1994;32:1710–1717. doi: 10.1128/jcm.32.7.1710-1717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy R L, Andrianopoulos A, Davis M A, Hynes M J. Identification of amdX, a new Cys-2-His-2 (C2H2) zinc-finger gene involved in the regulation of the amdS gene of Aspergillus nidulans. Mol Microbiol. 1997;23:591–602. doi: 10.1046/j.1365-2958.1997.d01-1872.x. [DOI] [PubMed] [Google Scholar]
- 35.Parsons L M, Davis M A, Hynes M J. Identification of functional regions of the positively acting regulatory gene amdR from Aspergillus nidulans. Mol Microbiol. 1992;6:2999–3007. doi: 10.1111/j.1365-2958.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 36.Pontecorvo G, Roper J A, Hemmons J L, MacDonald K D, Bufton A W J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- 37.Royer J C, Nakas J P. Interrelationship of xylanase induction and cellulase induction of Trichoderma longibranciatum. Appl Environ Microbiol. 1990;56:2535–2539. doi: 10.1128/aem.56.8.2535-2539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruijter G J G, Vanhanen S A, Gielkens M M C, van de Vondervoort P J I, Visser J. Isolation of Aspergillus niger creA mutants and effects of the mutations on expression of arabinases and l-arabinose catabolic enzymes. Microbiology. 1997;143:2991–2998. doi: 10.1099/00221287-143-9-2991. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Schols H A, Posthumus M A, Voragen A G J. Structural features of hairy regions of pectins isolated from apple juice produced by the liquefaction process. Carbohydr Res. 1990;206:117–126. [Google Scholar]
- 41.van der Veen P, Flipphi M J A, Voragen A G J, Visser J. Induction, purification and characterisation of arabinases produced by Aspergillus niger. Arch Microbiol. 1991;157:23–28. doi: 10.1007/BF00245330. [DOI] [PubMed] [Google Scholar]
- 42.van der Veen P, Flipphi M J A, Voragen A G J, Visser J. Induction of extracellular arabinases on monomeric substrates in Aspergillus niger. Arch Microbiol. 1993;159:66–71. doi: 10.1007/BF00244266. [DOI] [PubMed] [Google Scholar]
- 43.van Peij N N M E, Brinkmann J, Vrsanská M, Visser J, de Graaff L H. β-Xylosidase activity, encoded by xlnD, is essential for complete hydrolysis of xylan by Aspergillus niger but not for induction of the xylanolytic enzyme spectrum. Eur J Biochem. 1997;245:164–173. doi: 10.1111/j.1432-1033.1997.00164.x. [DOI] [PubMed] [Google Scholar]
- 44.van Peij N N M E, Visser J, de Graaff L H. Isolation and analysis of xlnR, encoding a transcriptional activator co-ordinating xylanolytic expression in Aspergillus niger. Mol Microbiol. 1998;27:131–142. doi: 10.1046/j.1365-2958.1998.00666.x. [DOI] [PubMed] [Google Scholar]
- 45.Witteveen C F B, Busink R, van de Vondervoort P, Dijkema C, Swart K, Visser J. l-Arabinose and d-xylose catabolism in Aspergillus niger. J Gen Microbiol. 1989;135:2163–2171. [Google Scholar]
- 46.Wood P J, Erfle J D, Teather R M. Use of complex formation between Congo Red and polysaccharides in detection and assay of polysaccharide hydrolases. Methods Enzymol. 1988;160:59–74. [Google Scholar]
- 47.Wood T M, Mahalingeshwara Bhat K. Methods for measuring cellulase activities. Methods Enzymol. 1988;160:87–130. [Google Scholar]
- 48.Zeilinger S, Mach R L, Schindler M, Herzog P, Kubicek C P. Different inducibility of expression of the two xylanase genes xyn1 and xyn2 in Trichoderma reesei. J Biol Chem. 1996;271:25624–25629. doi: 10.1074/jbc.271.41.25624. [DOI] [PubMed] [Google Scholar]