Abstract
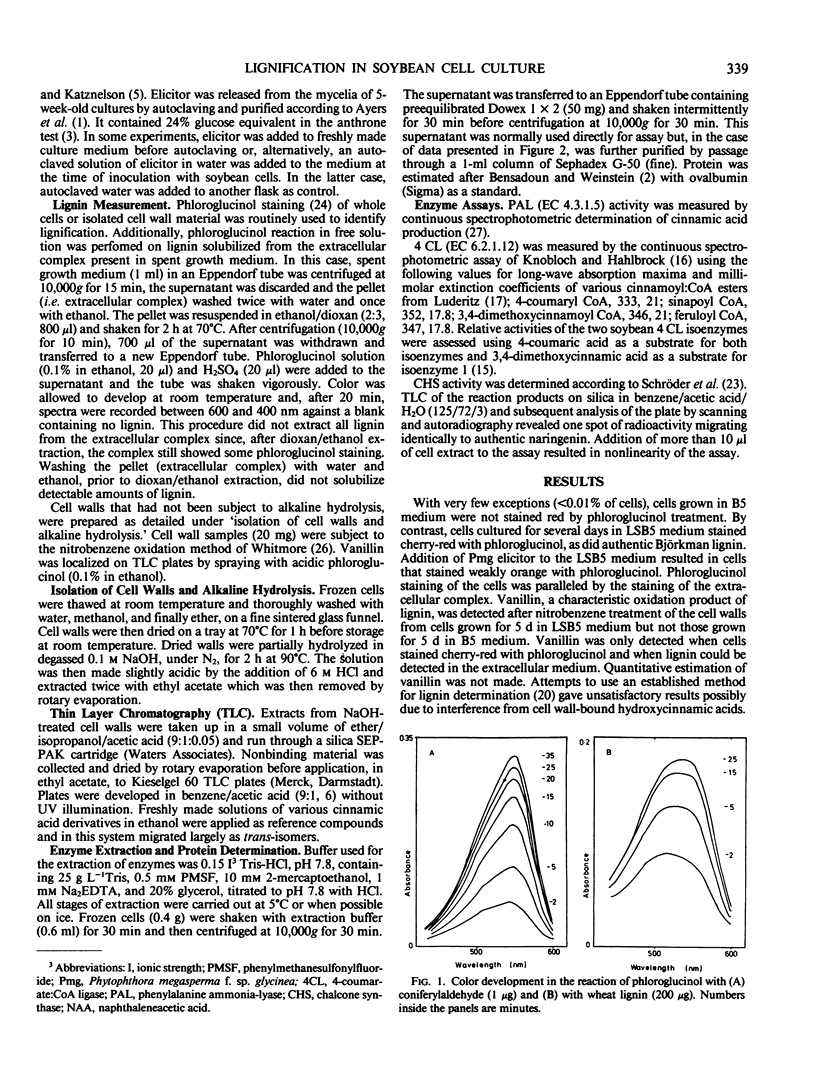
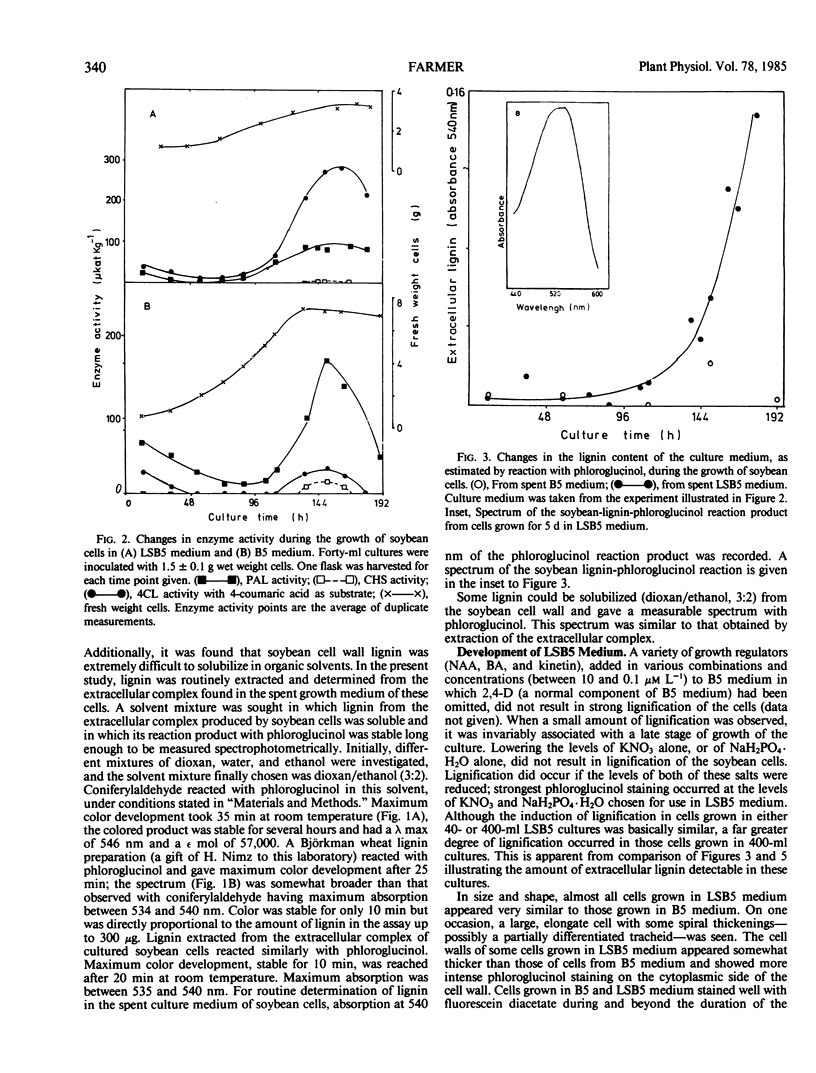
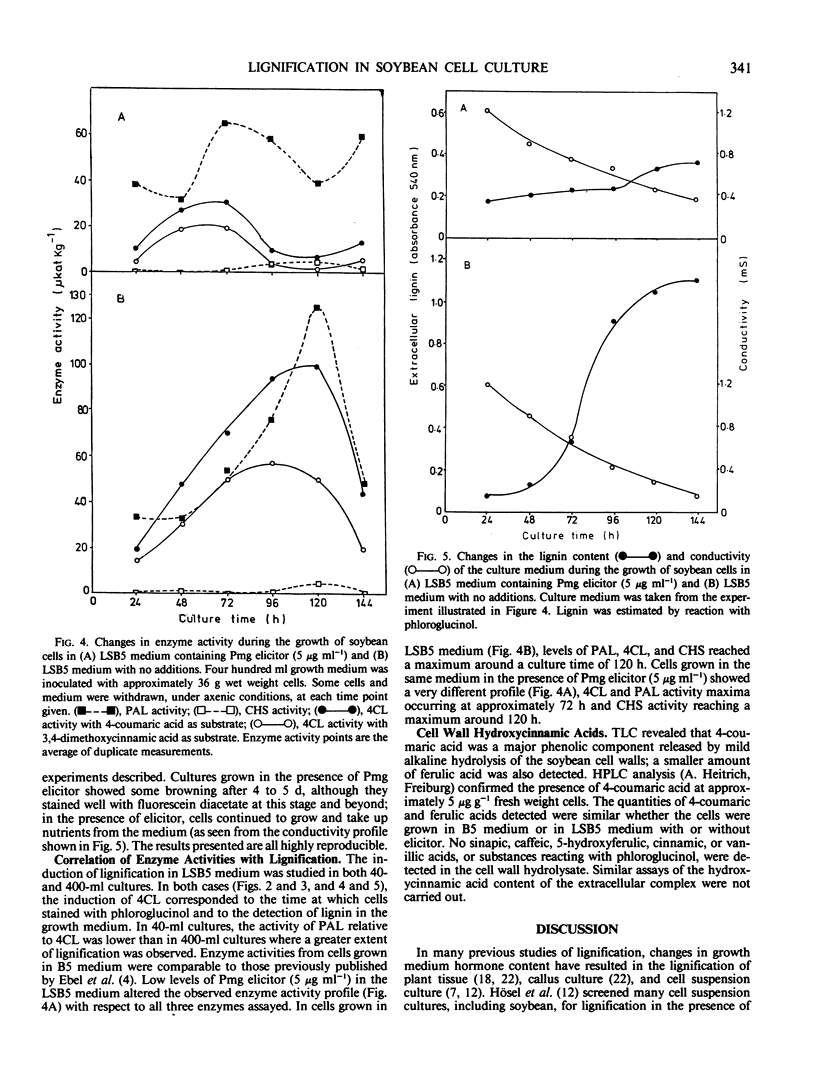
Soybean (Glycine max L.) cells cultured in B5 medium produce extremely low amounts of lignin. However, modification in the growth medium, by lowering the concentration of NO−3 and PO2−4, results in the lignification of these cells without affecting levels of cell wall-esterified 4-coumaric and ferulic acid. The production of an extracellular, macromolecular complex by the cultured soybean cells (Moore TS Jr 1973 Plant Physiol 51: 529-536) allows a rapid, nondestructive solubilization of the lignin which can be estimated by reaction with phloroglucinol in free solution. This system has been used to study the effects of fungal elicitor on the synthesis of lignin in soybean cells. The inclusion of very low levels of an elicitor fraction from the cell walls of Phytophthora megasperma in the medium in which lignification of the soybean cells occurs suppressed both the accumulation of extracellular lignin and phloroglucinol staining of the cell walls without affecting the levels of bound hydroxycinnamic acids. The activity profiles of phenylalanine ammonia-lyase (EC 4.3.1.5) and isoenzymes of 4-coumarate:CoA ligase (EC 6.2.1.12) were compared in lignifying and elicitor-treated cell cultures as was the activity of chalcone synthase, an enzyme of flavonoid biosynthesis. The measured activities of these enzymes in cell cultures treated with elicitor were considerably lower than in untreated cells.
Full text
PDF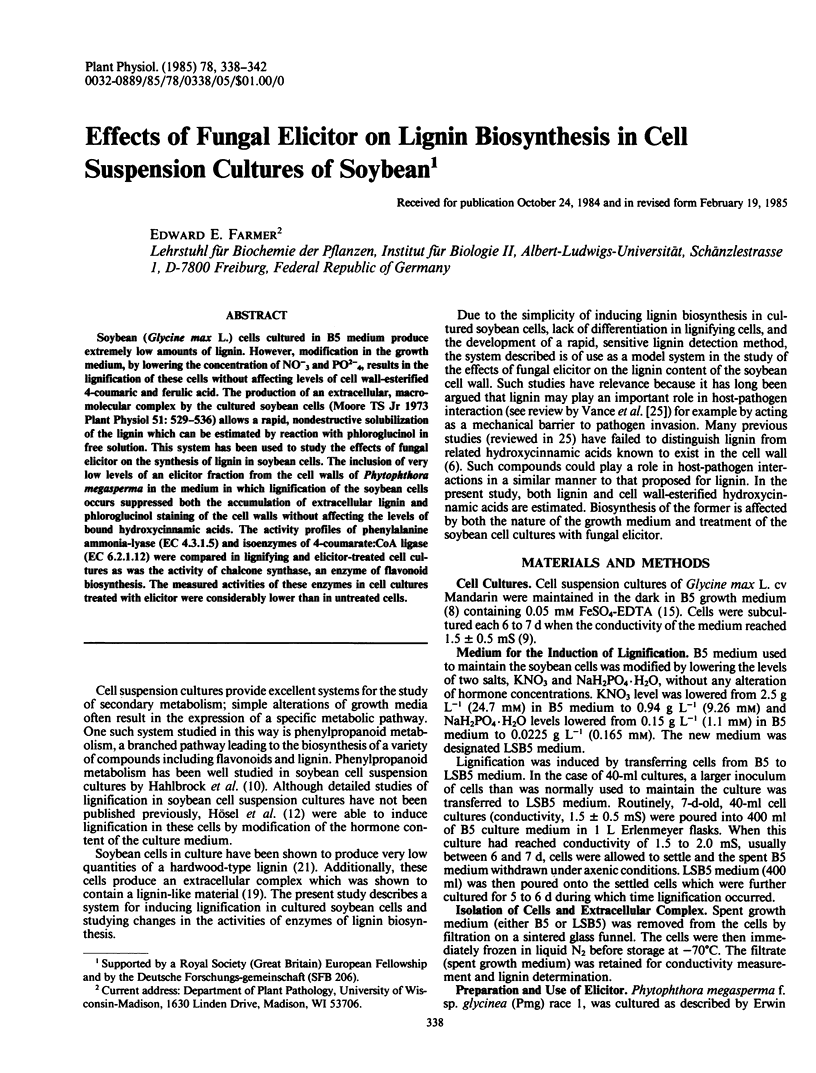

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- ERWIN D. C., KATZNELSON H. Studies on the nutrition of Phytophthora cryptogea. Can J Microbiol. 1961 Feb;7:15–25. doi: 10.1139/m61-003. [DOI] [PubMed] [Google Scholar]
- Ebel J., Schaller-Hekeler B., Knobloch K. H., Wellman E., Grisebach H., Hahlbrock K. Coordinated changes in enzyme activities of phenylpropanoid metabolism during the growth of soybean cell suspension cultures. Biochim Biophys Acta. 1974 Oct 8;362(3):417–424. doi: 10.1016/0304-4165(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Komamine A. Establishment of an Experimental System for the Study of Tracheary Element Differentiation from Single Cells Isolated from the Mesophyll of Zinnia elegans. Plant Physiol. 1980 Jan;65(1):57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Knobloch K. H., Hahlbrock K. 4-Coumarate:CoA ligase from cell suspension cultures of Petroselinum hortense Hoffm. Partial purification, substrate specificity, and further properties. Arch Biochem Biophys. 1977 Nov;184(1):237–248. doi: 10.1016/0003-9861(77)90347-2. [DOI] [PubMed] [Google Scholar]
- Knobloch K. H., Hahlbrock K. Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur J Biochem. 1975 Mar 17;52(2):311–320. doi: 10.1111/j.1432-1033.1975.tb03999.x. [DOI] [PubMed] [Google Scholar]
- Moore T. S. An extracellular macromolecular complex from the surface of soybean suspension cultures. Plant Physiol. 1973 Mar;51(3):529–536. doi: 10.1104/pp.51.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M. A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. J Sci Food Agric. 1972 Apr;23(4):455–463. doi: 10.1002/jsfa.2740230405. [DOI] [PubMed] [Google Scholar]
- Whitmore F. W. Lignin formation in wheat coleoptile cell walls: a possible limitation of cell growth. Plant Physiol. 1971 Nov;48(5):596–602. doi: 10.1104/pp.48.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]


