Abstract
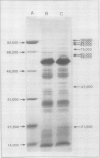
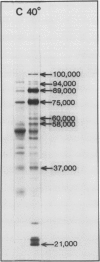
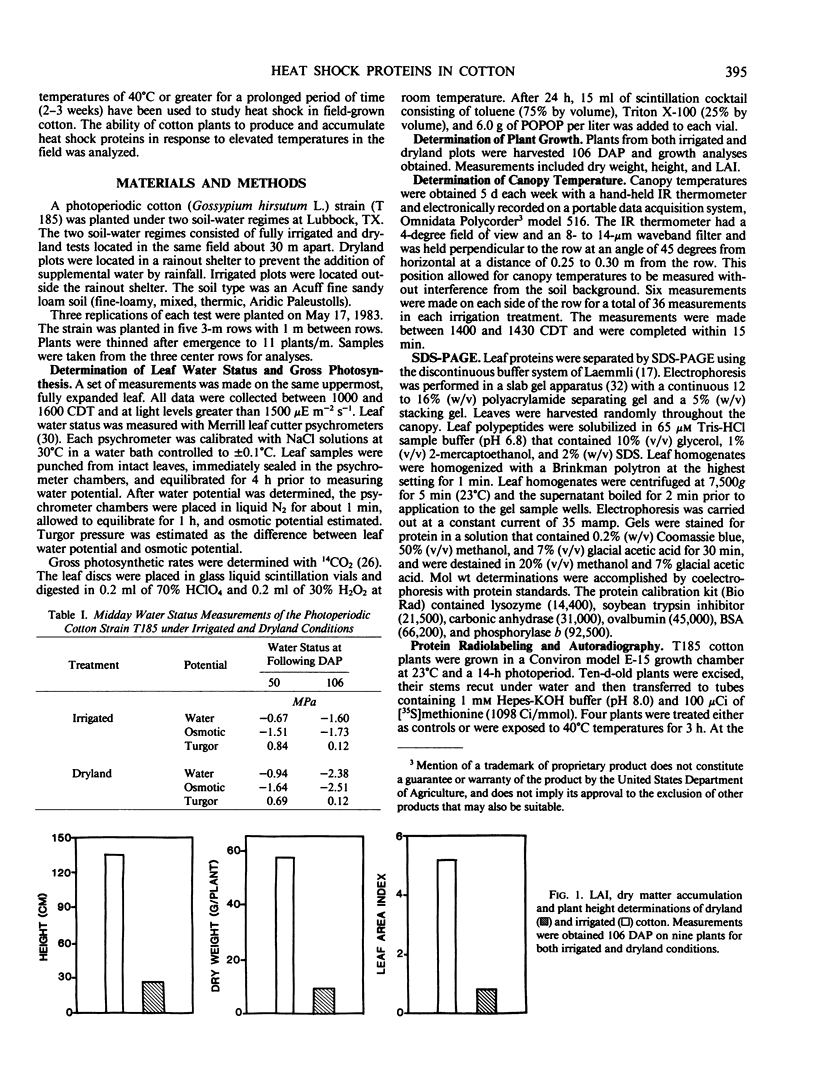
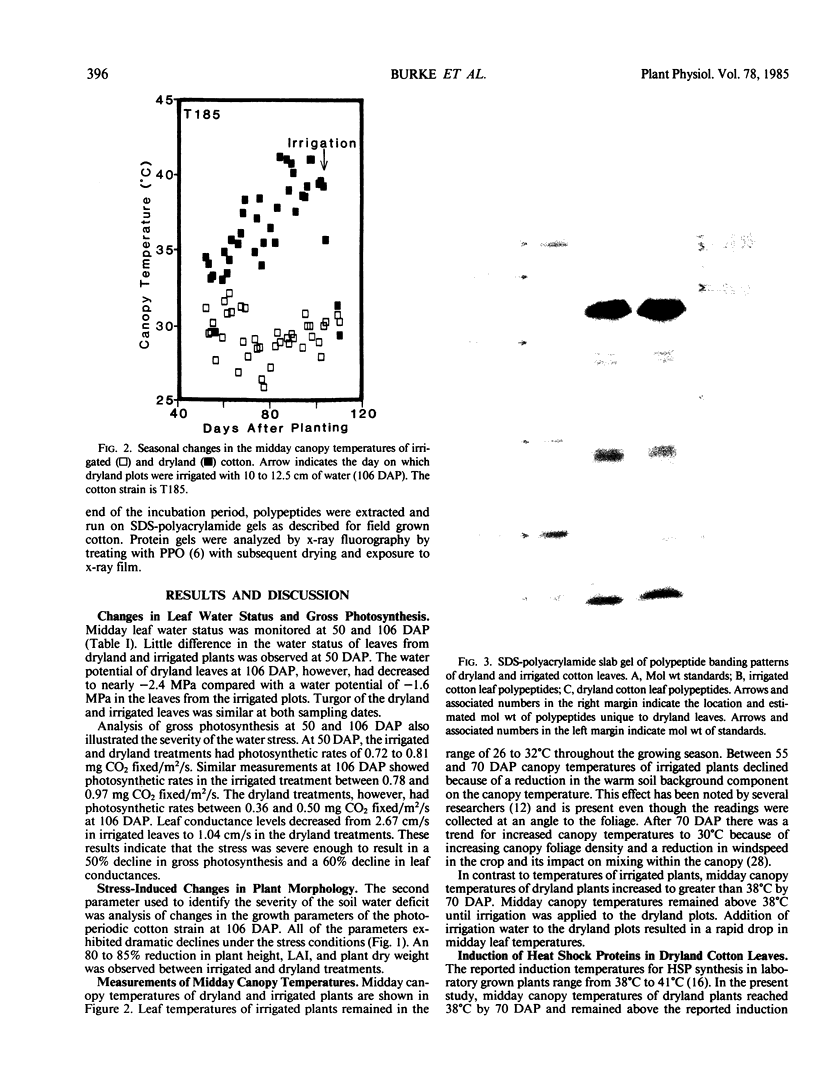
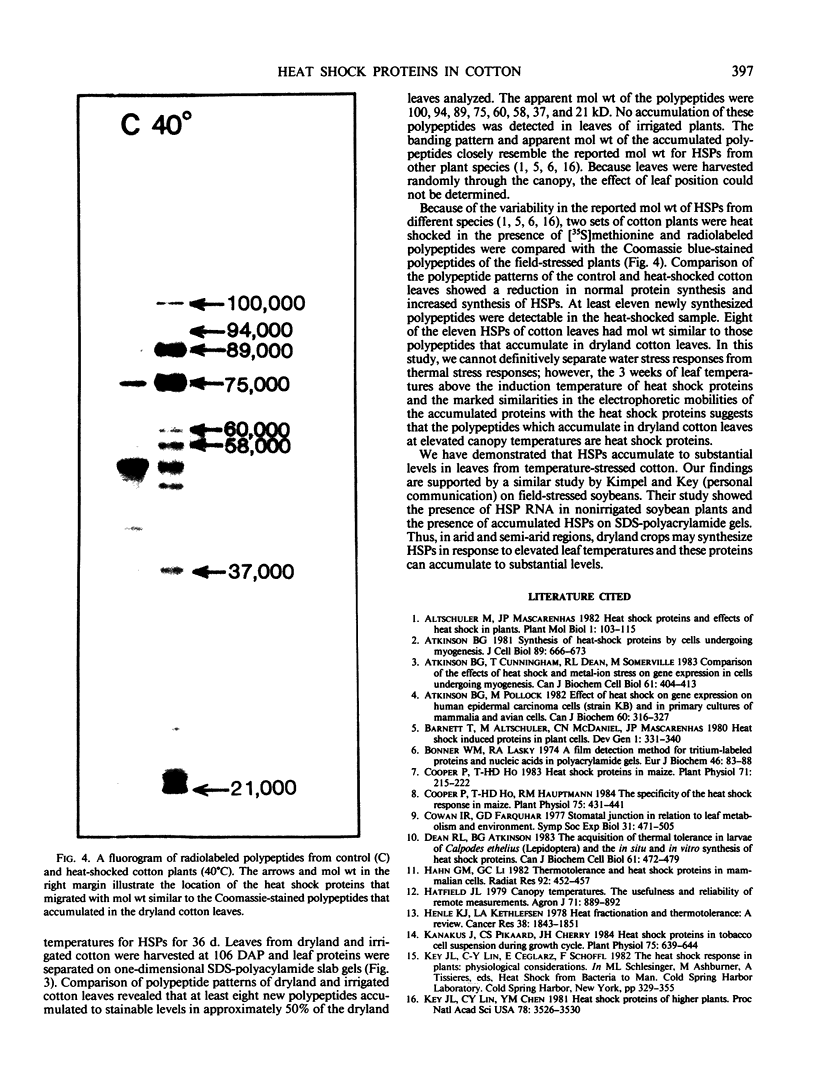
Cotton (Gossypium hirsutum L.) plants grown under field water deficits exhibited an 80 to 85% reduction in leaf area index, plant height, and dry matter accumulation compared with irrigated controls. Midday photosynthetic rates of dryland plants decreased 2-fold, and canopy temperatures increased to 40°C at 80 days after planting compared with canopy temperatures of 30°C for irrigated plants. Leaves from dryland plants which had exhibited canopy temperatures of 40°C for several weeks accumulated stainable levels of polypeptides with apparent molecular weights of 100, 94, 89, 75, 60, 58, 37, and 21 kilodaltons. These polypeptides did not accumulate in leaves from irrigated plants.
Addition of [35S]methionine to leaves of growth chamber-grown cotton plants and subsequent incubation at 40°C for 3 hours radiolabeled polypeptides with molecular weights similar to those that accumulate in dryland cotton leaves. These data suggest that the proteins which accumulate in water-stressed cotton leaves at elevated temperatures (40°C) are heat shock proteins and that these proteins can accumulate to substantial levels in field-stressed plants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson B. G., Cunningham T., Dean R. L., Somerville M. Comparison of the effects of heat shock and metal-ion stress on gene expression in cells undergoing myogenesis. Can J Biochem Cell Biol. 1983 Jun;61(6):404–413. doi: 10.1139/o83-055. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G., Pollock M. Effect of heat shock on gene expression in human epidermoid carcinoma cells (strain KB) and in primary cultures of mammalian and avian cells. Can J Biochem. 1982 Mar;60(3):316–327. doi: 10.1139/o82-038. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G. Synthesis of heat-shock proteins by cells undergoing myogenesis. J Cell Biol. 1981 Jun;89(3):666–673. doi: 10.1083/jcb.89.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H., Hauptmann R. M. Tissue specificity of the heat-shock response in maize. Plant Physiol. 1984 Jun;75(2):431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan I. R., Farquhar G. D. Stomatal function in relation to leaf metabolism and environment. Symp Soc Exp Biol. 1977;31:471–505. [PubMed] [Google Scholar]
- Dean R. L., Atkinson B. G. The acquisition of thermal tolerance in larvae of calpodes ethlius (lepidoptera) and the in situ and in vitro synthesis of heat-shock proteins. Can J Biochem Cell Biol. 1983 Jun;61(6):472–479. doi: 10.1139/o83-063. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., Li G. C. Thermotolerance and heat shock proteins in mammalian cells. Radiat Res. 1982 Dec;92(3):452–457. [PubMed] [Google Scholar]
- Henle K. J., Dethlefsen L. A. Heat fractionation and thermotolerance: a review. Cancer Res. 1978 Jul;38(7):1843–1851. [PubMed] [Google Scholar]
- Kanabus J., Pikaard C. S., Cherry J. H. Heat Shock Proteins in Tobacco Cell Suspension during Growth Cycle. Plant Physiol. 1984 Jul;75(3):639–644. doi: 10.1104/pp.75.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Landry J., Chrétien P., Bernier D., Nicole L. M., Marceau N., Tanguay R. M. Thermotolerance and heat shock proteins induced by hyperthermia in rat liver cells. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):59–62. doi: 10.1016/0360-3016(82)90385-6. [DOI] [PubMed] [Google Scholar]
- Li G. C. Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983 May;115(2):116–122. doi: 10.1002/jcp.1041150203. [DOI] [PubMed] [Google Scholar]
- Li G. C., Petersen N. S., Mitchell H. K. Induced thermal tolerance and heat shock protein synthesis in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):63–67. doi: 10.1016/0360-3016(82)90386-8. [DOI] [PubMed] [Google Scholar]
- Li G. C., Shrieve D. C. Thermal tolerance and specific protein synthesis in Chinese hamster fibroblasts exposed to prolonged hypoxia. Exp Cell Res. 1982 Dec;142(2):464–468. doi: 10.1016/0014-4827(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982 Apr;90(2):412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol. 1983 Sep;3(9):1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccheri M. C., Di Bernardo M. G., Giudice G. Synthesis of heat-shock proteins in developing sea urchins. Dev Biol. 1981 Apr 15;83(1):173–177. doi: 10.1016/s0012-1606(81)80020-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]