Abstract
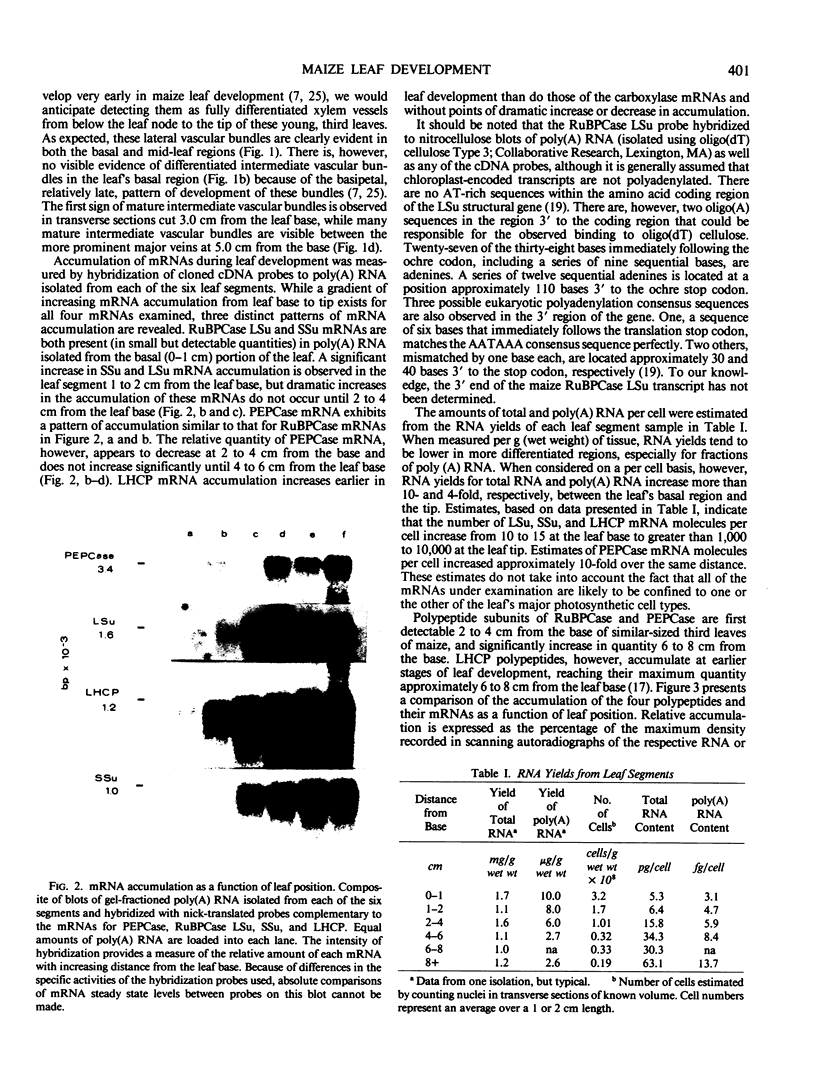
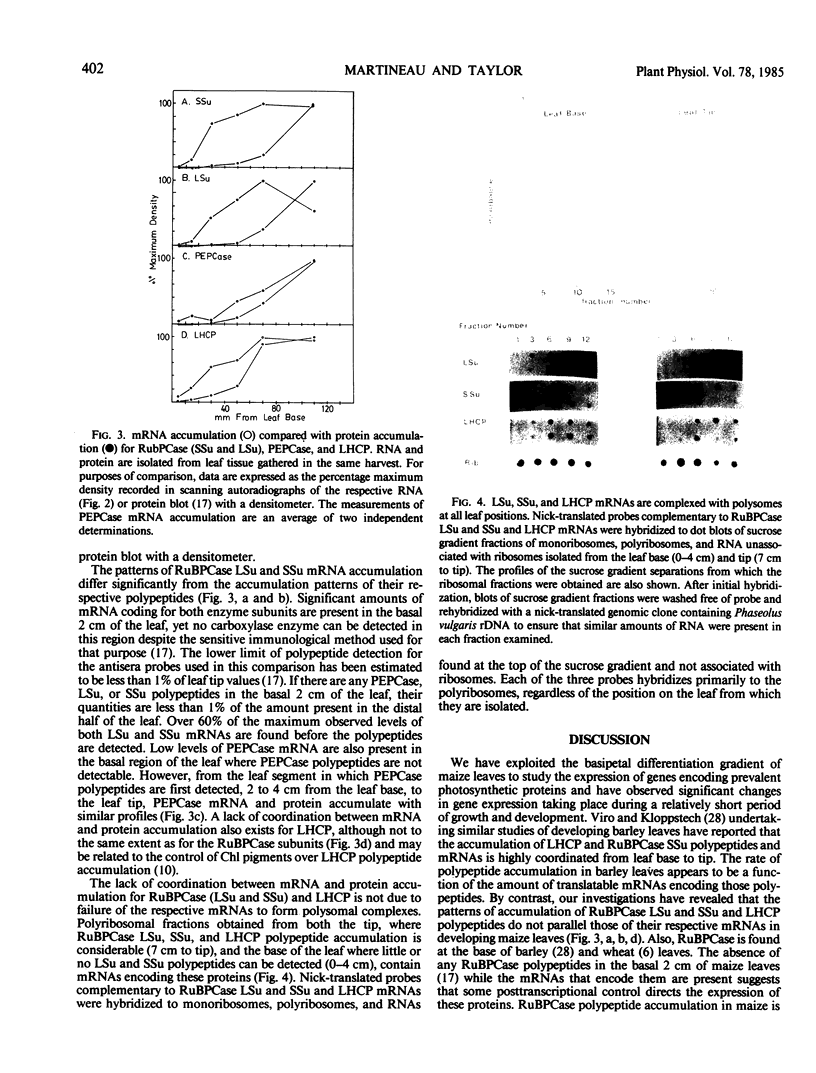
We have exploited the positional gradient of cellular differentiation in Zea mays leaves to study the accumulation of mRNAs encoding subunits of the two CO2-fixing enzymes and the major chlorophyll-binding protein. These three proteins are differentially compartmentalized in the two photosynthetically active cell types of the leaf. Previous studies have shown that accumulation of the two carboxylases commences 2 to 4 cm from the base of the leaf (Mayfield SP, WC Taylor Planta 161: 481-486) at a position where bundle sheath and mesophyll cells show morphological evidence of maturation. The light-harvesting chlorophyll a/b protein accumulates progressively from the leaf base, as does its mRNA, in spite of its localization in mesophyll cells after cellular differentiation occurs. While small quantities of phosphoenolpyruvate carboxylase mRNA are detectable in the basal region of the leaf, significant mRNA accumulation is coincident with that of the polypeptide at 4 to 6 cm from the leaf base, the region where bundle sheath and mesophyll cells exhibit fully differentiated morphologies. mRNAs encoding the small and large subunits of ribulose 1,5-bisphosphate carboxylase accumulate to significant levels before bundle sheath cells are fully differentiated and before their polypeptides are detectable. Cytological examination indicates that this is the position at which the maturation of intermediate vascular bundles is first evident. Cytosolically localized small subunit mRNA and chloroplast-localized large subunit mRNA are complexed with polyribosomes at all positions of the leaf.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Leech R. M. Development of Photosystem I and Photosystem II Activities in Leaves of Light-grown Maize (Zea mays). Plant Physiol. 1977 Oct;60(4):640–644. doi: 10.1104/pp.60.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Leech R. M. Genome Expression during Normal Leaf Development : I. CELLULAR AND CHLOROPLAST NUMBERS AND DNA, RNA, AND PROTEIN LEVELS IN TISSUES OF DIFFERENT AGES WITHIN A SEVEN-DAY-OLD WHEAT LEAF. Plant Physiol. 1982 Apr;69(4):904–910. doi: 10.1104/pp.69.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Galau G. A., Britten R. J., Davidson E. H. Nonrepetitive DNA sequence representation in sea urchin embryo messenger RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3516–3520. doi: 10.1073/pnas.70.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. Regulation of enzymes in C4 photosynthesis. Curr Top Cell Regul. 1978;14:1–27. [PubMed] [Google Scholar]
- Huber S. C., Hall T. C., Edwards G. E. Differential Localization of Fraction I Protein between Chloroplast Types. Plant Physiol. 1976 May;57(5):730–733. doi: 10.1104/pp.57.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Gibbs M. Carbon dioxide fixation and related properties in sections of the developing green maize leaf. Plant Physiol. 1980 May;65(5):802–809. doi: 10.1104/pp.65.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]