Abstract
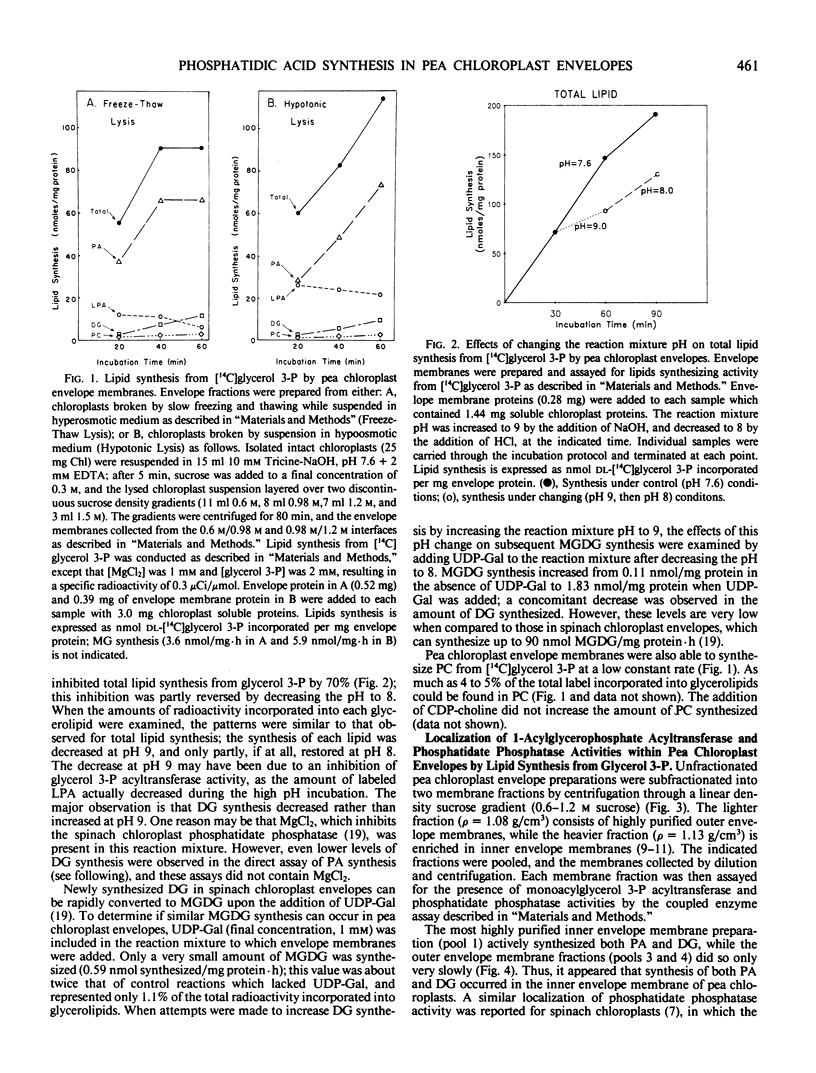
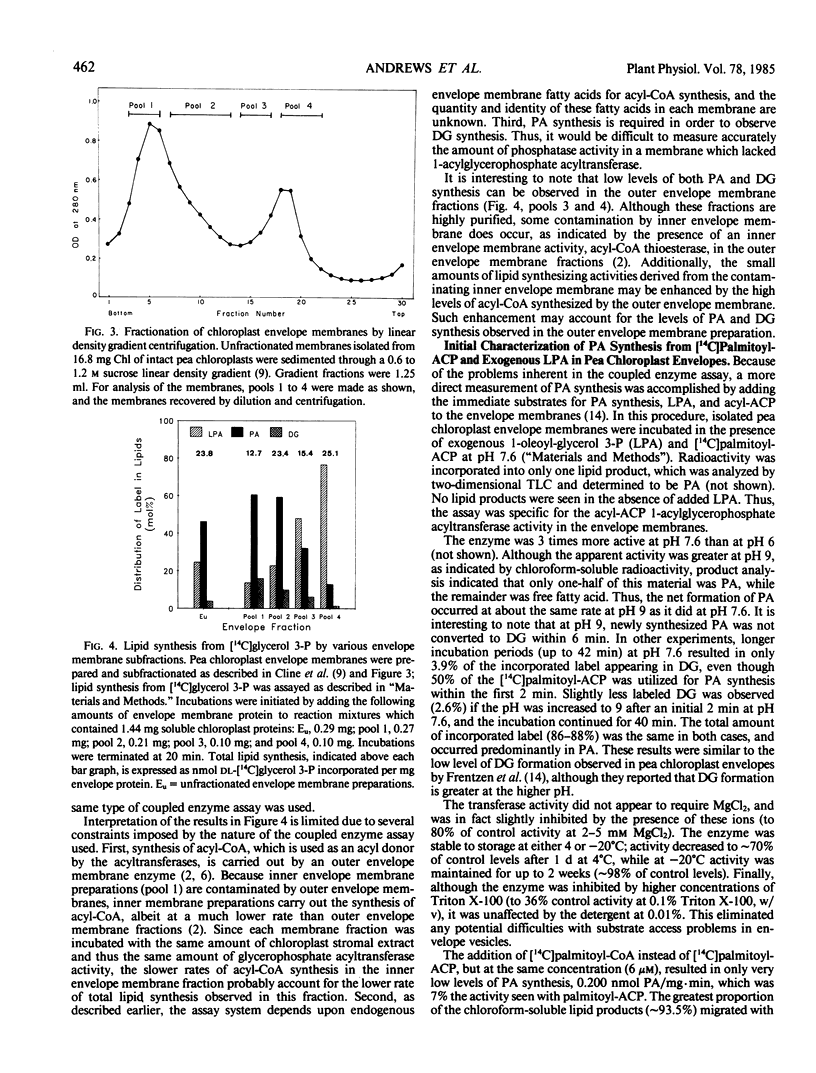
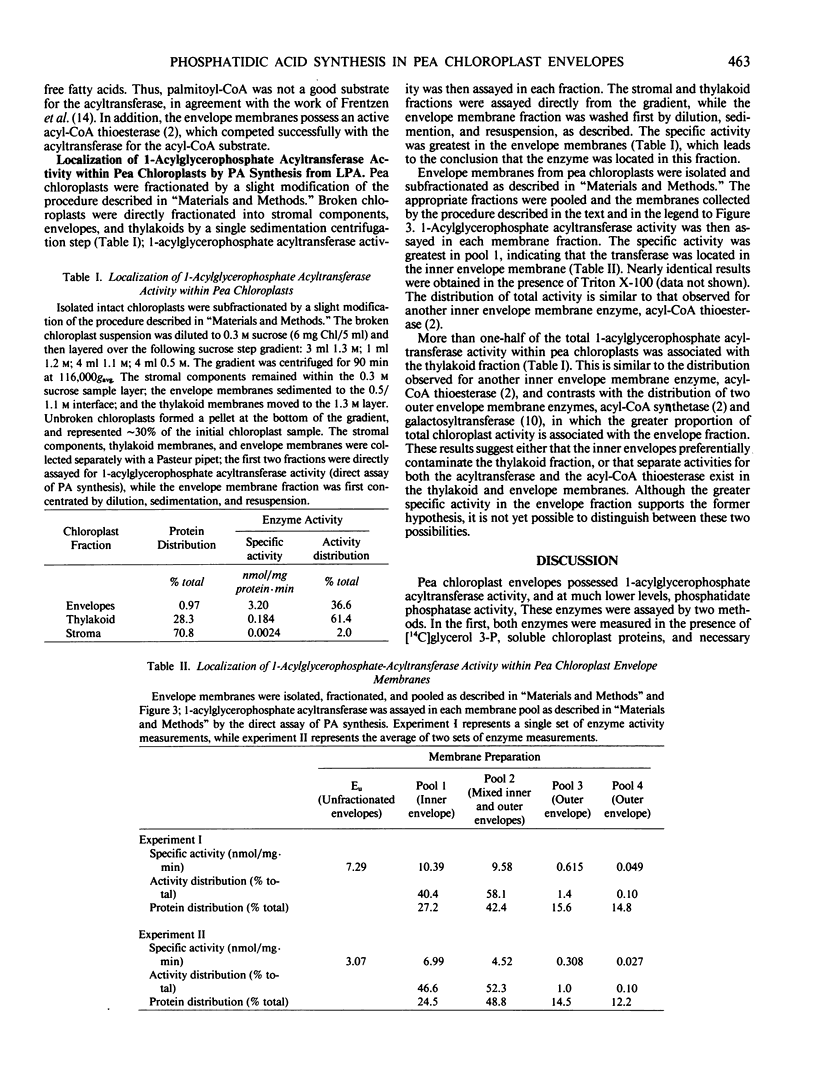
The second enzyme of phosphatidic acid synthesis from glycerol-3-phosphate, 1-acylglycerophospate acyltransferase, was localized to the inner envelope membrane of pea chloroplasts. The activity of this enzyme was measured by both a coupled enzyme assay and a direct enzyme assay. Using the coupled enzyme assay, phosphatidic acid phosphatase was also localized to the inner envelope membrane, although this enzyme has very low activity in pea chloroplasts. The addition of UDP-galactose to unfractionated pea chloroplast envelope preparations did not result in significant conversion of newly synthesized diacylglycerol to monogalactosyldiacylglycerol. Thus, the envelope synthesized phosphatidic acid may not be involved in galactolipid synthesis in pea chloroplasts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J., Keegstra K. Acyl-CoA Synthetase Is Located in the Outer Membrane and Acyl-CoA Thioesterase in the Inner Membrane of Pea Chloroplast Envelopes. Plant Physiol. 1983 Jul;72(3):735–740. doi: 10.1104/pp.72.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrams M., Heinz E. Positional Specificity and Fatty Acid Selectivity of Purified sn-Glycerol 3-Phosphate Acyltransferases from Chloroplasts. Plant Physiol. 1981 Sep;68(3):653–657. doi: 10.1104/pp.68.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Keegstra K. Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 1983 Feb;71(2):366–372. doi: 10.1104/pp.71.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G. Relationship between fatty-acyl composition of diacylgalactosylglycerol and turnover of chloroplast phosphatidate. Biochem J. 1983 Mar 15;210(3):949–952. doi: 10.1042/bj2100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Characterization of phosphatidate phosphohydrolase activity associated with chloroplast envelope membranes. FEBS Lett. 1979 Jun 1;102(1):147–150. doi: 10.1016/0014-5793(79)80947-3. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Improved purification of acyl carrier protein. Anal Biochem. 1980 Mar 1;102(2):362–364. doi: 10.1016/0003-2697(80)90168-2. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Solubilization, purification, and salt activation of acyl-acyl carrier protein synthetase from Escherichia coli. J Biol Chem. 1979 Aug 10;254(15):7116–7122. [PubMed] [Google Scholar]
- Rock C. O., Garwin J. L. Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J Biol Chem. 1979 Aug 10;254(15):7123–7128. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker H., Brandt H. P. Netzhautarterienverschluss und Wetter. Klin Monbl Augenheilkd. 1966;148(2):238–244. [PubMed] [Google Scholar]


