Abstract
A cluster of cases of pulmonary hemosiderosis among infants was reported in Cleveland, Ohio, during 1993 and 1994. These unusual cases appeared only in infants ranging in age from 1 to 8 months and were characterized by pulmonary hemorrhage, which caused the babies to cough up blood. A case-control study identified major home water damage (from plumbing leaks, roof leaks, or flooding) as a risk factor for development of pulmonary hemorrhage in these infants. Because of an interest in the possibility that trichothecene mycotoxins might be involved in this illness, a number of isolates of Stachybotrys chartarum were grown in the laboratory on rice, and extracts were prepared and analyzed both for cytotoxicity and for specific toxins. Two isolates of Memnoniella echinata, a fungus closely related to S. chartarum, were also included in these studies. S. chartarum isolates collected from the homes were shown to produce a number of highly toxic compounds, and the profiles of toxic compounds from M. echinata were similar; the most notable difference was the fact that the principal metabolites produced by M. echinata were griseofulvins.
Toxigenic and nontoxigenic strains of Stachybotrys chartarum (Ehrenb. ex Link) Hughes (= Stachybotrys atra Corda) have been isolated from cellulose-based agricultural materials, including hay and straw, and from contaminated moist building materials (30). S. chartarum is the etiologic agent of stachybotryotoxicosis, a severe disease of large domestic animals in eastern Europe. This disease is associated with contaminated straw, is characterized by leukopenia, hemorrhage, and arrhythmic heartbeat, and often leads to death (12). S. chartarum is known to produce several mycotoxins (Fig. 1 to 3), including the macrocyclic trichothecenes, which are potent cytotoxins (15), as well as a variety of immunosuppressant agents (2, 16, 24, 25, 34–37). Since the macrocyclic trichothecenes as a group are highly toxic and produce biological effects in experimental animals similar to those observed in stachybotryotoxicosis (15), they are considered the chemical agents responsible for the disease (8). These naturally occurring toxic sesquiterpene metabolites are potent inhibitors of protein synthesis in eukaryotic organisms, and the spores of S. chartarum have been shown to contain macrocyclic trichothecenes (39). Recently, there has been increased interest in this organism as a potential cause of adverse health responses in humans in agriculture, homes, and offices (1, 20–22) following the report of Croft et al. (5) that exposure to S. chartarum was the apparent cause of an unexplained outbreak of illness over a period of several years in a home located in suburban Chicago, Ill. In the mid-1990s, a cluster of cases of pulmonary hemosiderosis were reported in Cleveland, Ohio (10). These unusual cases occurred only in infants ranging in age from 1 to 8 months and were characterized by pulmonary hemorrhage, which caused the babies to cough up blood. A case-control study identified major home water damage (from plumbing leaks, roof leaks, or flooding) as a risk factor for development of pulmonary hemorrhage in these infants (29). Because of an interest in the possibility that trichothecene mycotoxins might be involved in this illness, a number of isolates of S. chartarum and Memnoniella echinata (a fungus closely related to S. chartarum) isolated from homes of infants in the case-control study were grown in the laboratory on rice, and extracts were prepared and analyzed both for cytotoxicity and for specific toxins.
FIG. 1.
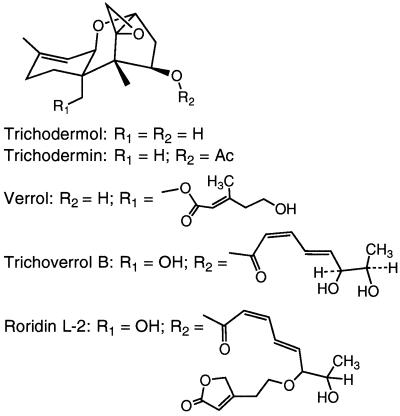
Chemical structures of trichoverroid trichothecences. Ac, acetyl.
FIG. 3.
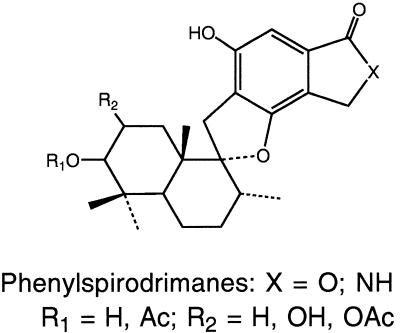
Structure of phenylspirodrimanes. Ac, acetyl; OAc, acetate.
MATERIALS AND METHODS
Sources of fungi and production of mycotoxins on rice.
Cultures of S. chartarum and M. echinata (Riv.) Galloway were isolated from air and surface samples from homes enrolled in a case-control study of pulmonary hemosiderosis in infants (10, 29). Sixteen representative isolates of S. chartarum and two isolates of M. echinata were grown on Uncle Ben’s Converted Rice (100 g per 500-ml Erlenmeyer flask) for 4 weeks at 24°C. Two isolates of S. chartarum (JS6301 and JS6307) were also grown for 2 weeks at 24°C and then for 2 weeks at 5°C. Subcultures of the following four isolates used in this study have been deposited in the American Type Culture Collection: M. echinata JS6308 (= ATCC 200581) and S. chartarum JS5802 (= ATCC 201210), JS5817 (= ATCC 201211), and JS5818 (= ATCC 201212).
Media and chemicals.
Media used for maintenance of fungus cultures were obtained from Difco Laboratories (Detroit, Mich.). Minimum essential medium (MEM), dimethyl sulfoxide, and MTT [1-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were obtained from Sigma Chemical Co. (St. Louis, Mo.), and the extraction solvents and solvents used for chromatography were obtained from Fisher Scientific Co. (Pittsburgh, Pa.) and were high-performance liquid chromatography (HPLC) grade.
Preparation of rice culture extracts.
All extraction procedures were done in a chemical fume hood. After incubation, the rice was air dried and was stored at 4°C. The rice was ground with a coffee grinder and covered with methanol (MeOH) overnight. The mixture was filtered, and the solid portion was reextracted twice with MeOH at 40 to 50°C with sonication (Bransonic 321; Branson Sonic Power Co., Danbury, Conn.) for 30 min each time. The MeOH extracts were combined, dried by rotary evaporation, and weighed. Then ca. 100 mg of the crude extract was taken up in ca. 1 ml of CHCl3 and applied to a short column containing silica (about 2.5 g; diameter, 40 to 60 μm) packed in hexane. Three 15-ml fractions were obtained by washing the column with 90% diethyl ether–hexane (fraction F-1), 6% MeOH–CH2Cl2 (fraction F-2), and MeOH (fraction F-3). Fractions F-1, F-2, and F-3 represented ca. 20, 10, and 70% of the weight, respectively.
Cytotoxicity testing of culture extracts.
Extracts were tested for cytotoxicity by using an inhibition-of-cell-proliferation assay with a continuous cell line of feline fetus lung cells (7). This procedure involved evaluation of mitochondrial activity after treatment with a tetrazolium dye. Extracts were dissolved in MEM containing 10% MeOH. Blank, solvent control wells and serial 1:2 dilutions of samples in MEM were prepared. Cells were added at a concentration of 4 × 103 cells/well. The plates were incubated for 5 days at 37°C in a humidified environment in the presence of 5% CO2. After incubation, 125 μg of MTT was added to each well, and the plates were incubated for an additional 4 h. The plates were centrifuged, the medium was removed, 100 μl of dimethyl sulfoxide was added, and the plates were shaken for 30 min and read with a plate reader (Dynatech Laboratories Inc., Chantilly, Va.) at 540 nm. The results were recorded as the lowest concentration that resulted in 50% inhibition.
HPLC analyses.
Fractions F-1 and F-2 were analyzed by reversed-phase (C18) HPLC with a Gilson model 303 gradient chromatograph. Two systems were used. (i) In the first system, the HPLC instrument was connected to a Knauer variable-wavelength detector (set at 260 nm) and a Shimadzu Chromatopac C-R3A, Supelco 5 μm C18 column (4.6 by 250 mm), and chromatography was performed with a 10-min 60 to 75% MeOH gradient in 5% aqueous acetic acid (AcOH) with a flow rate of 1.2 min (solvent system A). (ii) Alternatively, the HPLC instrument was connected to a Shimadzu model SPD-M10A diode array detector and a Beckman Ultrasphere 5 μm C18 column (4.6 by 250 mm), and chromatography was performed with a solvent system (flow rate, 0.8 ml/min) consisting of acetonitrile (AcCN) in water by using the following step gradient: 50% AcCN from zero time to 10 min, 75% AcCN from 10 to 15 min, and 85% AcCN from 15 to 22 min (solvent system B). Levels of trichothecenes were determined with standard curves prepared by injecting known amounts of standards and measuring the areas under the peaks. The following retention times were observed with solvent system B: trichoverrol B, 4.0 min; roridin L-2, 5.4 min; satratoxin G, 7.3 min; isosatratoxin G, 7.6 min; satratoxin H, 8.7 min; isosatratoxin F, 9.4 min; and roridin E, 16.3 min. The levels of the phenylspirodrimanes were estimated from peak areas obtained with solvent system A. These spirocyclic compounds typically had retention times in the 15- to 20-min range and often produced overlapping peaks. Under the same solvent conditions (solvent system A), the trichothecenes had retention times in the 4- to 9-min range.
Water extraction of large-scale culture.
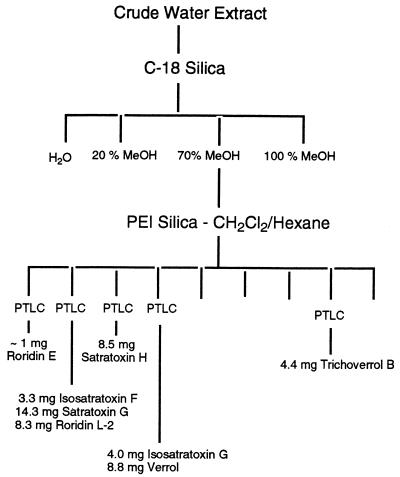
S. chartarum JS5818 was grown on rice at the ambient temperature for 30 days. The culture (1 kg) was coarsely ground and then slurried with water and treated with ultrasound for 30 min. The mixture was filtered by using Whatman no. 1 filter paper, and the rice material was reextracted twice, which resulted in 4.15 liters of a green opaque solution. The liquid was filtered once more and divided into three equal portions, and each portion was then passed through an RP Sep-Pak column (Waters C18, 10 g, 35 ml), in which the trapped compounds were visible as an orange band. The column was eluted with 50 ml of water and then with 100-ml portions of 20% MeOH–water, 70% MeOH–water, and 100% MeOH, and the solvent was removed by rotary evaporation (bath temperature, <35°C). HPLC analysis revealed that the majority of the macrocyclic trichothecenes eluted in the 70% MeOH–water fractions. The 70% MeOH–water fractions were combined and concentrated to dryness. This resulted in 1.5 g of brown gum, which was adsorbed onto polyethyleneimine (PEI) silica (particle size, 40 μm; 1.7 g; J. T. Baker, Phillipsburg, N.J.) and flash chromatographed with a PEI column (30 g; 25 by 180 mm; increasing CH2Cl2 in hexane). This resulted in nine fractions which were combined based on a thin-layer chromatography analysis (Fig. 4).
FIG. 4.
Flow chart depicting the procedure used for isolation of S. chartarum mycotoxins. PTLC, preparative thick-layer chromatography.
Fraction 1 was applied to a 1-mm Chromatotron plate (Harrison Research Inc., Palo Alto, Calif.) (radial chromatography), and a trace (<1 mg) of roridin E was eluted with 70% ethyl acetate (EtOAc)–hexane. Fraction 2 was purified by 1-mm radial chromatography (EtOAc/hexane/MeOH, 80:20:2), which gave isosatratoxin F (3.3 mg), satratoxin G (14.3 mg), and roridin L-2 (8.3 mg). In an identical manner, fraction 3 gave satratoxin H (8.5 mg). Fraction 4 was applied to a 2-mm chromatotron plate and eluted with 70% EtOAc–hexane, which gave isosatratoxin G (4.0 mg) and verrol (18) (8.8 mg). Fraction 8 was purified on a 1-mm plate (EtOAc/hexane/MeOH, 85:15:5), which gave trichoverrol B (4.4 mg). Further extraction of the rice culture with methanol and MeOH/CHCl3 (1:1) gave (after filtration and solvent removal) a brown gum (21.4 g). Column chromatography performed with a PEI silica (16) step as a key separatory step yielded, after radial chromatography cleanup, the following major macrocyclic trichothecene constituents: roridin E (23 mg), satratoxin H (16.0 mg), satratoxin G (16.9), and roridin L-2 (5.4 mg).
Isolation of isosatratoxin F.
MeOH extraction of a 250-g culture of S. chartarum JS5106 that had been grown at room temperature for 30 days gave 7.5 g of crude extract. This material was subjected to medium-pressure liquid chromatography with 150 g of silica gel (Whatman LPS-1). Elution with dichloromethane gave 520 mg in the first fraction, which was subjected to high-speed countercurrent chromatography with a model CCC-1000 instrument (Pharm-Tech Research Corp., Baltimore, Md.) (Vc, 355 ml; flow rate, 1.8 ml/min; solvent, MeOH/H2O/CCl4/hexane/CH2Cl2 [6:4:8:1:1]); this resulted in five fractions. Fraction 4 was recrystallized from CH2Cl2-hexane, which gave 20 mg of isosatratoxin F (mp, 153 to 155°C; [α]D25 = 46.4° [c = 0.4 acetone]; HRMS [CI] for C29H34O10, calculated 542.2152, found 542.2155; IR [CHCl3] 3478, 1747, 1713, and 1188 cm−1); 1H nuclear magnetic resonance (NMR) (CDCl3, Bruker AMX at 400 MHz) δ 0.80 (3H, s, H-14), 1.70 (3H, s, H-16), 1.80-2.00 (4H, m, H-7 and H-8), 2.20 (ddd, 1H, J = 4.7, 5.1, 15.5 Hz, H-3β), 2.50 (dd, 1H, J = 8.0, 15.5 Hz, H-3α), 2.80 and 3.12 (AB, 1H each, J = 4.0 Hz, H-13), 3.38 (1H, s, H-2′), 3.54 (1H, d, J = 5.0 Hz, H-11) 3.62 (1H, s, H-12′), 3.83 (1H, d, J = 5.1 Hz, H-2), 4.14 (2H, m, H-5′), 4.20 (2H, s, H-15), 5.39 (1H, d, J = 5.0 Hz, H-10), 5.55 (1H, dd, J = 1.6 and 16.4 Hz, H-7′), 5.84 (1H, dd, J = 4.7 and 8.0 Hz, H-4), 5.90 (1H, dd, J = 1.6 and 11.6 Hz, H-10′), 6.54 (1H, ddd, J = 1.6, 5.7, and 11.6 Hz, H-9′), and 6.72 (1H, ddd, J = 1.6, 5.7, and 16.4 Hz, H-8′); 13C NMR (CDCl3, Bruker AMX at 100 MHz) δ 79.1 (C-2), 34.4 (C-3), 74.1 (C-4), 49.4 (C-5), 43.0 (C-6), 20.0 (C-7), 27.4 (C-8), 140.5 (C-9), 118.5 (C-10), 67.7 (C-11), 65.3 (C-12), 47.9 (C-13), 7.9 (C-14), 65.0 (C-15), 23.3 (C-16), 166.0 (C-1′), 58.8 (C-2′), 63.8 (C-3′), 22.5 (C-4′), 61.1 (C-5′), 87.0 (C-6′), 129.8 (C-7′), 130.5 (C-8′), 143.2 (C-9′), 121.1 (C-10′), 166.9 (C-11′), 73.5 (C-12′), 208.6 (C-13′), and 27.4 (C-14′).
RESULTS AND DISCUSSION
In general, the levels of cytotoxicity observed (Table 1) correlated with the relative levels of macrocyclic and trichoverroid trichothecenes (Fig. 1 and 2); five of the six cultures that exhibited the highest levels of toxicity (50% inhibitory concentrations, 0.2 to 1.2 μg/g) contained the highest levels of trichothecenes, whereas cultures that exhibited low levels of cytotoxicity contained small amounts or no detectable amounts of trichothecenes. Of the isolates from case and control homes (Table 1), three belonging to each group were among the most toxic and three belonging to each group were essentially nontoxic. Thus, there was no perceived relationship between cytotoxicity and the origin of an isolate (a case home or a control home). Most of the rice culture extracts of S. chartarum were found to contain macrocyclic trichothecenes (e.g., satratoxins) and trichoverroid trichothecenes (e.g., roridin L-2 and trichoverrol B) (Table 1). In the HPLC analyses we used a diode array detector which detected the phenylspirodrimanes (Fig. 3) and only those trichothecenes that exhibit UV absorption (e.g., the satratoxins and trichoverroids). It should be noted that the analytical method employed (HPLC with UV detection) did not detect the normally UV-transparent simple trichothecenes, such as trichodermol and verrucarol and their acetates, compounds (Fig. 1) which are known to be produced by S. chartarum (17). The phenylspirodrimanes were present in all of the S. chartarum and M. echinata cultures and were generally present at higher levels than the trichothecenes, but there was no relationship between their levels and cytotoxicity. No attempt was made to determine the levels of the phenylspirodrimanes, but their levels could be estimated from the total peak areas observed in the chromatograms. The phenylspirodrimanes exhibit characteristic UV spectra (λmax, ca. 216, 257, and 300 nm) and are generally well-separated from the trichothecenes in terms of retention time.
TABLE 1.
Chemical and cytotoxicity analysis of Cleveland isolates of S. chartarum and M. echinata
| Strain | Toxicity (μg/ml) | Concn (μg/ml) of:
|
Concn of phenylspirodrimanesa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trichoverrol B | Roridin L-2 | Satratoxin G | Isosatratoxin G | Satratoxin H | Isosatratoxin F | Roridin E | All compounds | |||
| JS5106b | 1.2 | 20 | 25 | 10 | 25 | 80 | +++ | |||
| JS5119b | 1.2 | 20 | 15 | 35 | ++ | |||||
| JS5802b | 0.3 | 40 | 30 | 10 | 80 | ++ | ||||
| JS5108b | 40 | 0 | + | |||||||
| JS5807 | 160 | 20 | 20 | 40 | ++ | |||||
| JS5808 | 0.2 | 65 | 40 | 15 | 50 | 170 | + | |||
| JS5815 | 20 | 0 | + | |||||||
| JS5816 | >500 | 5 | 5 | + | ||||||
| JS5806b | 160 | 0 | + | |||||||
| JS5105b | >500 | 0 | +++ | |||||||
| JS5111b | 10 | 15 | 15 | ++++ | ||||||
| JS6307b | 320 | 0 | ++ | |||||||
| JS6307b,c | 40 | 0 | +++ | |||||||
| JS5817 | 0.2 | 40 | 55 | 65 | 160 | +++ | ||||
| JS5818 | 0.2 | 50 | 10 | 5 | 10 | 10 | 25 | 110 | ++ | |
| JS5819 | 10 | 8 | 8 | ++ | ||||||
| JS6301 | 80 | 5 | 5 | 10 | +++ | |||||
| JS6301c | 40 | 0 | +++ | |||||||
| JS6308d | 160 | 0 | + | |||||||
| JS6309d | 160 | 0 | + | |||||||
+, 10 to 20 ppm; ++, 40 to 90 ppm; +++, 100 to 200 ppm; ++++, 200 to 500 ppm.
Strains obtained from case homes. The other strains were obtained from control homes.
The culture was incubated at 10°C for the final 2 weeks.
M. echinata strains. The major products are ca. 5,000 ppm of dechlorogriseofulvin and ca. 5,000 ppm of epidechlorogriseofulvin, as well as trichodermol and trichodermin (total concentration, ca. 200 ppm) (19).
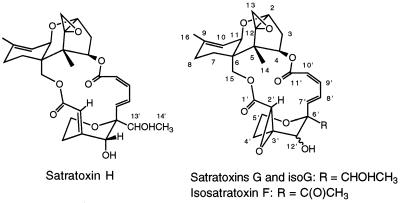
FIG. 2.
Structures of satratoxin G, satratoxin H, and isosatratoxins F and G.
Cytotoxicity assays were performed with crude extracts. The crude extracts were passed through a short silica gel column, which resulted in three fractions: fractions F-1 (90% ether–hexane), F-2 (6% MeOH–CH2Cl2), and F-3 (MeOH). Moderately polar fraction F-2, which contained the bulk of the macrocyclic and trichoverroid trichothecenes, as well as the phenylspirodrimanes, was subjected to HPLC analyses. Additional cytotoxicity investigations of the fractions demonstrated that the greatest cytotoxicity was found in fraction F-2, although fractions F-1 and F-3 were also appreciably cytotoxic (data not shown). Further studies are under way to determine the origin of the cytotoxicities in these fractions. The results suggest that we are far from characterizing all of the toxins produced by the fungi studied.
Following a series of chromatography procedures (see above), a 250-g rice culture of S. chartarum JS5106 gave 20 mg of a compound which appeared to be the previously described compound satratoxin F (9). This amount is significantly more of this compound than we expected based on our analysis of a small culture (Table 1), which indicates that there may be significant variation in toxin production by a given isolate. The NMR spectral data for this compound matched very closely the NMR spectral data for satratoxin F. Most of the carbon chemical shifts for this compound (isosatratoxin F) (Fig. 2) are within 0.2 ppm of the carbon chemical shifts for the corresponding carbons in satratoxin F (9); the only exceptions are the carbon 13′ shift (for satratoxin F, C-13′ is 217.0 ppm; for isosatratoxin F, C-13′ is 208.6 ppm) and the carbon 14′ shift (for satratoxin F, C-14′ is 29.7 ppm; for isosatratoxin F, C-14′ is 27.4 ppm). However, in the proton spectrum, most of the signals for the two compounds are virtually the same; the only exceptions are the H-15 signal (for satratoxin F, H-15 is 3.38 ppm; for isosatratoxin F, H-15 is 4.20 ppm) and the H-12′ signal (for satratoxin F, H-12′ is 4.24 ppm; for isosatratoxin F, H-12′ is 3.62 ppm). Although these data indicate that satratoxin F and isosatratoxin F are diastereomers, they do not allow us to say at which stereogenic center(s) (C-12′ or C-6′) they differ.
Previous investigators reported that some macrocyclic trichothecenes could be extracted from grain artificially contaminated with S. chartarum by using swine stomach and intestinal fluid (14, 33). Sorenson et al. (40) reported that aqueous wash preparations of conidia of both S. chartarum and M. echinata were nearly as toxic as MeOH extracts of the spores themselves. These findings suggest that the somewhat lipophilic trichothecenes can be extracted with water. To examine this further, a 1-kg rice culture of S. chartarum JS5818 (not dried) was extracted with water by using sonication. We obtained only about 5% of the normal weight of extract, but the extract was very rich in trichothecenes and more than 50 mg of pure trichothecenes was isolated (see above). Based on the total amount of trichothecenes isolated from this culture, this represented about 50% of the trichothecenes produced by S. chartarum. The H-13 epoxide hydrogens, which exhibit a characteristic set of AB resonances centered around 3.0 ppm, could be clearly seen in the 1H NMR spectrum of the crude aqueous extract; there was no discernible sign of the trichothecenes in the crude MeOH extract. The trichothecenes are exported to the surface of a fungal spore, where they become water soluble, perhaps because they are imbedded in the water-soluble surface polysaccharides. This could be highly relevant to pulmonary hemosiderosis in infants since the highly potent toxins would be readily released into the microenvironment of the developing lung cells in vivo. There were only traces of the lipophilic phenylspirodrimanes in the aqueous extract, which may have been a result of poorer transport of these compounds across the outer fungal cell membrane. There are, in fact, reasons to believe that toxigenic fungi in general export their toxins to the surface, where they can effectively inhibit competition from other microorganisms (6). JS5818 was the only S. chartarum isolate found to produce appreciable amounts of roridin E. Interestingly, this isolate also produced both satratoxin G and isosatratoxin G, which proved to be easily separable by chromatography with PEI silica, a chromatographic medium that we have found to be particularly useful for isolation of natural products (16).
S. chartarum and M. echinata are morphologically and physiologically closely related cellulolytic fungi; both of these organisms have worldwide distributions, are often found together, and are commonly found in soil (23). In both the genus Stachybotrys and the genus Memnoniella conidia are found on clusters of unbranched phialides borne on simple or branched conidiophores. The conidia are dark green to black. The two genera differ primarily in the arrangement of the conidia, which are aggregated in slimy heads in the genus Stachybotrys and in long persistent chains in the genus Memnoniella. Although these similarities have led some authors to suggest that the species of these genera should be combined in the same genus, most authors agree that the two genera are distinct (23). The conidia of S. chartarum are ellipsoidal and are 7 to 12 by 4 to 6 μm. Although these measurements seem to suggest that the conidia are too large to enter the respiratory tract, the aerodynamic diameter is ca. 5 μm (39, 40). This is consistent with studies which have shown that nonspherical particles, such as fibers, orient themselves in an air stream in the long dimension; i.e., the aereodynamic diameter corresponds to the narrow dimension. M. echinata conidia have a smaller aerodynamic diameter (40) and would be expected to have an even greater potential to penetrate deep into lungs than the conidia of S. chartarum.
The genus Stachybotrys has received considerable attention in the scientific literature, especially in recent years, as a possible health risk in indoor air, but little information is available on the genus Memnoniella. Our results indicate that M. echinata can have toxicity similar to that of some isolates of S. chartarum, although the isolates of M. echinata which we studied were less toxic than the most toxic isolates of S. chartarum studied. We found macrocyclic trichothecenes in cultures of S. chartarum but not in cultures of M. echinata, demonstrating that the latter species contains some of the toxins of S. chartarum but perhaps not all. On the basis of our results, it is not possible to say that M. echinata cannot produce macrocyclic trichothecenes since only two isolates were studied (40). M. echinata was found in only one sample from the Cleveland homes, and the two isolates, isolates JS6308 and JS6309 (Table 1), appeared to be indistinguishable. These isolates were moderately cytotoxic and produced the simple trichothecenes trichodermol and trichodermin, as well as substantial amounts of dechlorogriseofulvins (19). The latter metabolites are well-known antifungal compounds produced by several Penicillium species but have not been reported to be produced by members of other fungal genera (3), although there is a preliminary report that suggests that griseofulvins may be produced by Aspergillus species (13). A careful survey showed that only one of the S. chartarum isolates (JS5806) produced any detectable quantities of dechlorogriseofulvins. However, this isolate produced only about 5 ppm of dechlorogriseofulvins. Like other workers (25), we found that our isolates of M. echinata also produce phenylspirodrimanes, although at relatively low levels. It should be noted that the macrocyclic trichothecenes are typically considerably more cytotoxic than either the simple trichothecenes, the phenylspirodrimanes, or the griseofulvins. Thus, in terms of their chemical products, both S. chartarum and M. echinata produce phenylspirodrimanes, but these two organisms differ in that the former produces macrocyclic and trichoverroid trichothecenes and the latter produces griseofulvins. Both produce varying amounts of simple trichothecenes, such as trichodermol and trichodermin. The smaller aerodynamic diameter of the conidia of M. echinata and the fact that M. echinata produces many of the same toxins suggest that M. echinata should also be considered potentially dangerous in indoor air (40). In cases in which both species are present in the same samples, their combined toxic potential should be considered.
It is now appreciated that the principal nonpathogenic biological agents responsible for the health problems associated with damp buildings are fungi rather than bacteria or viruses (26, 27). Although fungi have been viewed traditionally as allergens (and in unusual circumstances, pathogens) in this context, data have accumulated which show that the adverse health effects resulting from inhalation of fungal spores are due to multiple factors. One factor associated with certain fungi is low-molecular-weight toxins (mycotoxins) produced by the fungi (28). Reports have indicated that airborne spores of toxigenic S. chartarum, Aspergillus versicolor, and several Penicillium species are potentially hazardous, especially when air-handling systems are heavily contaminated (11, 38). Nikulin and coworkers have provided powerful evidence supporting the notion that mycotoxins in the spores of S. chartarum are hazardous. In their work, mice treated intranasally with highly toxic spores of S. chartarum died very rapidly compared with mice which received the same dosage of relatively noncytotoxic spores. Both the in vitro cytotoxicities and the in vivo toxicities correlated closely with the levels of satratoxins in the spores (31, 32). Interestingly, studies of inhalation of T-2 toxin (a simple trichothecene with toxicity similar to that of the satratoxins) demonstrated that although this mode of toxin administration was about 1 order of magnitude more effective than intravenous administration, lung tissue of the treated animals remained essentially unchanged (4). This is in stark contrast to what was observed in the lung tissue of mice treated with spores of the highly cytotoxic organism S. chartarum; in the latter case the lung tissue had profound lesions (31, 32).
There is extensive literature concerning toxicoses (usually in animals) associated with S. chartarum that dates back to the 1930s (12). The reports came mainly from Eastern Europe, although there have been scattered reports of stachybotryotoxicosis in other parts of the world. The fact that no similar cases of stachybotryotoxicosis have been reported in North America may leave the impression that the toxigenic potential of North American isolates of S. chartarum differ substantially from the toxigenic potential of S. chartarum strains isolated in Europe. Our data obtained with the Cleveland isolates clearly show that North American isolates of S. chartarum have about the same spectrum of toxigenicity as the isolates described in European studies, although the specific toxins may vary with locality. Eastern European isolates of S. chartarum appear to produce commonly satratoxin H as the major macrocyclic trichothecene metabolite, whereas in the Cleveland isolates of S. chartarum satratoxin H was the major macrocyclic trichothecene metabolite in only 3 of the 16 isolates examined. Roridin L-2 was found to be somewhat more common in the Cleveland isolates. Although there was a clear association between the presence of S. chartarum and the incidence of pulmonary hemosiderosis in the Cleveland cases, much work has to be done to establish a cause-and-effect relationship.
ACKNOWLEDGMENT
B.B.J. acknowledges the partial support provided by the Center for Indoor Air Research.
REFERENCES
- 1.Anonymous. Guidelines on assessment and remediation of Stachybotrys atra in indoor environments. In: Johanning E, Yang C S, editors. Fungi and bacteria in indoor air environments. Latham, N.Y: Eastern New York Occupational Health Program; 1995. pp. 201–207. [Google Scholar]
- 2.Ayer W A, Miao S. Secondary metabolites of the aspen fungus Stachybotrys cylindrospora. Can J Chem. 1993;71:487–493. [Google Scholar]
- 3.Cole R J, Cox R H. Handbook of toxic fungal metabolites. New York, N.Y: Academic Press; 1981. [Google Scholar]
- 4.Creasia D A, Thurman J D, Wannemacher R W, Jr, Bunner D L. Acute inhalation toxicity of T-2 mycotoxin in the rat and guinea pig. Fundam Appl Toxicol. 1990;53:1370–1375. doi: 10.1016/0272-0590(90)90230-h. [DOI] [PubMed] [Google Scholar]
- 5.Croft W A, Jarvis B B, Yatawara C S. Airborne outbreak of trichothecene toxicosis. Atmos Environ. 1986;20:549–552. [Google Scholar]
- 6.Demain A I. Why do microorganisms produce antimicrobials? In: Hunter P A, Darby G K, Russell N J, editors. Fifty years of antimicrobials: past perspectives and future trends. New York, N.Y: Cambridge University Press; 1995. pp. 205–239. [Google Scholar]
- 7.Dombrink-Kurtzman M A, Bennet G A, Richard J L. An optimized MTT bioassay for determination of cytotoxicity of fumonisin in turkey lymphocytes. J Assoc Off Anal Chem Int. 1994;72:512–516. [PubMed] [Google Scholar]
- 8.Eppley R M, Bailey W J. 12,13-Epoxy-9-trichothecenes as the probable mycotoxins responsible for stachybotryotoxicosis. Science. 1973;181:758–760. doi: 10.1126/science.181.4101.758. [DOI] [PubMed] [Google Scholar]
- 9.Eppley R M, Mazzola E P, Stack M E, Dreifus P A. Structures of satratoxin F and satratoxin G, metabolites of Stachybotrys atra: application of proton and carbon-13 nuclear magnetic resonance spectroscopy. J Org Chem. 1980;45:2522–2523. doi: 10.1021/jo00422a014. [DOI] [PubMed] [Google Scholar]
- 10.Etzel R A, Montana E, Sorenson W G, Kullman G, Miller J D, Jarvis B, Dearborn D G. Pulmonary hemosiderosis associated with exposure to Stachybotrys atra. Epidemiology. 1996;7:S38. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- 11.Flannigan B, Miller J D. Health implications of fungi in indoor environments—an overview. In: Samson R, Flannigan B, Flannigan M, Graveson S, editors. Health implication of fungi in indoor environments. Amsterdam, The Netherlands: Elsevier; 1994. pp. 3–28. [Google Scholar]
- 12.Forgacs J. Stachybotrytoxicosis. In: Kadis S, Ciegler A, Ajl S J, editors. Microbial toxins. VIII. New York, N.Y: Academic Press, Inc.; 1972. pp. 95–128. [Google Scholar]
- 13.Frisvad J. The connection between the penicillia and aspergilli and mycotoxins with special emphasis on misidentified isolates. J Arch Environ Contam Toxicol. 1989;18:452–467. doi: 10.1007/BF01062373. [DOI] [PubMed] [Google Scholar]
- 14.Harrach B, Nummi M, Niku-Paavola M L, Mirocha C J, Palyusik M. Identification of “water-soluble” toxins produced by a Stachybotrys atra strain from Finland. Appl Environ Microbiol. 1982;44:494–495. doi: 10.1128/aem.44.2.494-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvis B B. Macrocyclic trichothecenes. In: Sharma R P, Salunkhe D K, editors. Mycotoxins and phytoalexins in human and animal health. Boca Raton, Fla: CRC Press; 1991. pp. 361–421. [Google Scholar]
- 16.Jarvis B B. Macrocyclic trichothecenes from Brazilian Baccharis species: from microanalysis to large scale isolation. Phytochem Anal. 1992;3:241–249. [Google Scholar]
- 17.Jarvis B B, Salemme J, Morais A. Stachybotrys toxins. 1. Nat Toxins. 1995;3:10–16. doi: 10.1002/nt.2620030104. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis B B, Vrudhula V M, Midiwo J O, Mazzola E P. New trichothecenes from Myrothecium verrucaria: verrol and 12,13-deoxytrichodermadiene. J Org Chem. 1983;48:2576–2580. [Google Scholar]
- 19.Jarvis B B, Zhou Y, Jiang J, Wang S, Sorenson W G, Hintikka E, Nikulin M, Parikka P, Etzel R A, Dearborn D G. Toxigenic molds in water-damaged buildings: dechlorogriseofulvins from Memnoniella echinata. J Nat Prod. 1996;59:553–554. doi: 10.1021/np960395t. [DOI] [PubMed] [Google Scholar]
- 20.Johanning E. Health problems related to fungal exposure—the example of toxigenic Stachybotrys charatum (atra) In: Johnanning E, Yang C S, editors. Fungi and bacteria in indoor air environments. Latham, N.Y: Eastern New York Occupational Health Program; 1995. pp. 169–182. [Google Scholar]
- 21.Johanning E, Biagini R, Hull D, Morey P, Jarvis B B, Landsbergis P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int Arch Occup Environ Health. 1996;68:207–218. doi: 10.1007/BF00381430. [DOI] [PubMed] [Google Scholar]
- 22.Johanning E, Morey P R, Jarvis B B. Clinical-epidemiological investigation of health effects caused by Stachybotrys atra building contamination. Indoor Air ’93. 1993;1:225–230. [Google Scholar]
- 23.Jong S C, Davis E E. Contribution to the knowledge of Stachybotrys and Memnoniella in culture. Mycotaxon. 1976;3:409–485. [Google Scholar]
- 24.Kaneto R, Dobashi K, Kojima I, Sakai K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, Akagawa H, Mizuno S. Mer-NF5003B, E and F, novel sesquiterpenoids as avian myeloblastosis virus protease inhibitors produced by Stachybotrys sp. J Antibiot. 1994;47:727–730. doi: 10.7164/antibiotics.47.727. [DOI] [PubMed] [Google Scholar]
- 25.Lam Y, Wichmann K T, Meinz M S, Guariglia L, Giacobbie R A, Mochales S, Kong L, Honeycutt S S, Zink D, Bills G F, Huang L, Burg R W, Monaghan R L, Jackson R, Reid G, Maguire J J, McKnight A T, Ragan C I. A novel inositol mono-phosphatase inhibitor from Memnoniella echinata: producing organism, fermentation, isolation, physiochemical and in vitro biological properties. J Antibiot. 1992;45:1397–1403. doi: 10.7164/antibiotics.45.1397. [DOI] [PubMed] [Google Scholar]
- 26.Miller J D. Fungi as contaminants in indoor air. Atmos Environ. 1992;26:2163–2172. [Google Scholar]
- 27.Miller J D. Approaches to assessment of the microbial flora of buildings. ASHRAE publication IAQ 92. Environments for Healthy People. Atlanta, Ga: American Society of Heating, Refrigeration and Air-Conditioning Engineers; 1993. Fungi and the building engineer; pp. 147–159. [Google Scholar]
- 28.Miller J D. Quantification of health effects of combined exposures: a new beginning. In: Morawska L, editor. Indoor air quality—an integrated approach. Amsterdam, The Netherlands: Elsevier; 1995. pp. 159–168. [Google Scholar]
- 29.Montana E, Etzel R A, Allan T, Horgan T E, Dearborn D G. Environmental risk factors associated with pediatric idiopathic pulmonary hemorrhage/hemosiderosis in a Cleveland community. Pediatrics. 1997;99:117–124. doi: 10.1542/peds.99.1.e5. [DOI] [PubMed] [Google Scholar]
- 30.Nikulin M, Pasenen A-L, Berg S, Hintikka E-L. Stachybotrys atra growth and toxin production in some building materials and fodder under different relative humidities. Appl Environ Microbiol. 1994;60:3421–3424. doi: 10.1128/aem.60.9.3421-3424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikulin M, Reijula K E, Jarvis B B, Hintikka E-L. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int J Exp Pathol. 1996;77:213–218. doi: 10.1046/j.1365-2613.1996.9250323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikulin M, Reijula K E, Jarvis B B, Veijalainen P, Hintikka E-L. Effects of internasal exposure to spores of Stachybotrys atra in mice. Fundam Appl Toxicol. 1997;35:182–188. [PubMed] [Google Scholar]
- 33.Nummi M, Niku-Paavola M L. Water soluble toxins of Stachybotrys alternans. Ann Nutr Aliment. 1977;31:761–770. [PubMed] [Google Scholar]
- 34.Ogawa K, Nakamura M, Hayashi M, Yaginuma S, Yamamoto S, Furihata F, Shinya K, Seto H. Stachybocins, novel endothelin antagonists produced by Stachybotrys sp. M6222.2. Structure determination of stachybocin-A, stachybocin-B and stachybocin-C. J Antibiot. 1995;48:1396–1400. doi: 10.7164/antibiotics.48.1396. [DOI] [PubMed] [Google Scholar]
- 35.Roggo B E, Hug P, Moss S, Stämpeli A, Kriempler H-P, Peter H H. Novel spirodihydrobenzofuranlactams as antagonists of endothelin and as inhibitors of HIV-1 protease produced by Stachybotrys sp. II. Structure determination. J Antibiot. 1996;49:374–379. doi: 10.7164/antibiotics.49.374. [DOI] [PubMed] [Google Scholar]
- 36.Roggo B E, Petersen F, Silla M, Roesel J L, Moerker T, Peter H H. Novel spirodihydrobenzofuranlactams as antagonists of endothelin and as inhibitors of HIV-1 protease produced by Stachybotrys sp. I. Fermentation, isolation and biological activity. J Antibiot. 1996;49:13–19. doi: 10.7164/antibiotics.49.13. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto K, Tsujii E, Miyauchi M, Nakanishi T, Yamashita M, Shigematsu N, Tada T, Izumi S, Okuhara M. FR901459, a novel immunosuppressant isolated from Stachybotrys chartarum no. 19392. J Antibiot. 1993;46:1788–1798. doi: 10.7164/antibiotics.46.1788. [DOI] [PubMed] [Google Scholar]
- 38.Smith J E, Anderson J G, Lewis C W, Murad Y M. Cytotoxic fungal spores in the indoor air atmosphere of the damp domestic environment. FEMS Microbiol Lett. 1992;100:337–344. doi: 10.1111/j.1574-6968.1992.tb14061.x. [DOI] [PubMed] [Google Scholar]
- 39.Sorenson W G, Frazer D G, Jarvis B B, Simpson J, Robinson V A. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl Environ Microbiol. 1987;53:1370–1375. doi: 10.1128/aem.53.6.1370-1375.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorenson W G, Jarvis B B, Zhou Y, Jiang J, Wang S, Hintikka E-L, Nikulin M. Toxine im Zusammenhang mit Stachybotrys and Memnoniella in Häusern mit Wasserschaden. In: Gareis M, Scheuer R, editors. 18. Mykotoxin Workshop. Kulmbach, Germany: Institut für Mikobiologie und Toxikologie der Bundesanstalt für Fleischforschung; 1996. pp. 207–214. [Google Scholar]