Abstract
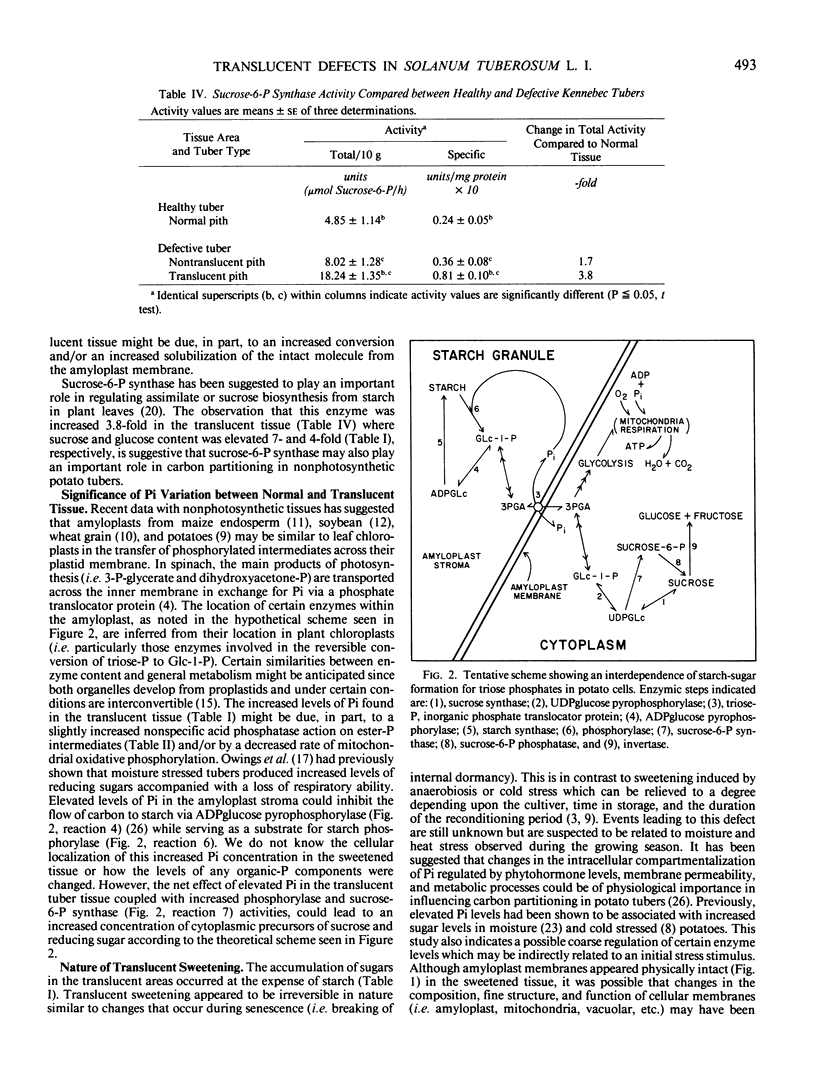
Kennebec (cv) potatoes randomly developed translucent areas in their centrally located pith-parenchymal cells during storage. These defective areas were characterized as having reduced starch concentration and increased levels of free sugars (i. e. sucrose and glucose) and inorganic phosphate. Electron micrographs of potato tubers stored at 10° ± 1°C for 8 months indicated that the amyloplast membrane was still intact and continuous around starch granules in both normal and prematurely sweetened tissue. The total activities of phosphorylase and sucrose-6-P synthase were elevated 5.4- and 3.8-fold, respectively, in the defective tissue compared to healthy nonsweetened tubers while there were no significant differences in the levels of sucrose synthase, UDPglucose pyrophosphorylase, invertase, or α-amylase. Total and specific activities of acid phosphatase were only slightly elevated in translucent tissue but their increase was significant (P < 0.05, t test) over that seen in healthy tubers. The premature sweetening in storage may have been indirectly triggered by moisture and heat stress experienced during development. Translucency eventually led to physical deterioration of the tissue.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Liu T. T., Shannon J. C. Measurement of Metabolites Associated with Nonaqueously Isolated Starch Granules from Immature Zea mays L. Endosperm. Plant Physiol. 1981 Mar;67(3):525–529. doi: 10.1104/pp.67.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Friedberg I., Ne'eman Z., Schramm M. Biogenesis and Degradation of Starch: I. The Fate of the Amyloplast Membranes during Maturation and Storage of Potato Tubers. Plant Physiol. 1971 Apr;47(4):465–477. doi: 10.1104/pp.47.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Huber S. C. Changes in Starch Formation and Activities of Sucrose Phosphate Synthase and Cytoplasmic Fructose-1,6-bisphosphatase in Response to Source-Sink Alterations. Plant Physiol. 1983 Jun;72(2):474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R., Preiss J. Pyrophosphorylases in Solanum tuberosum: III. PURIFICATION, PHYSICAL, AND CATALYTIC PROPERTIES OF ADPGLUCOSE PYROPHOSPHORYLASE IN POTATOES. Plant Physiol. 1982 Jun;69(6):1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R. Pyrophosphorylases in Solanum tuberosum: II. CATALYTIC PROPERTIES AND REGULATION OF ADP-GLUCOSE AND UDP-GLUCOSE PYROPHOSPHORYLASE ACTIVITIES IN POTATOES. Plant Physiol. 1981 Oct;68(4):924–929. doi: 10.1104/pp.68.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Williams M. G., Adrian E. K. The use of elemental iodine to enhance staining of thin sections to be viewed in the electron microscope. Stain Technol. 1977 Sep;52(5):269–272. doi: 10.3109/10520297709116792. [DOI] [PubMed] [Google Scholar]



