Abstract
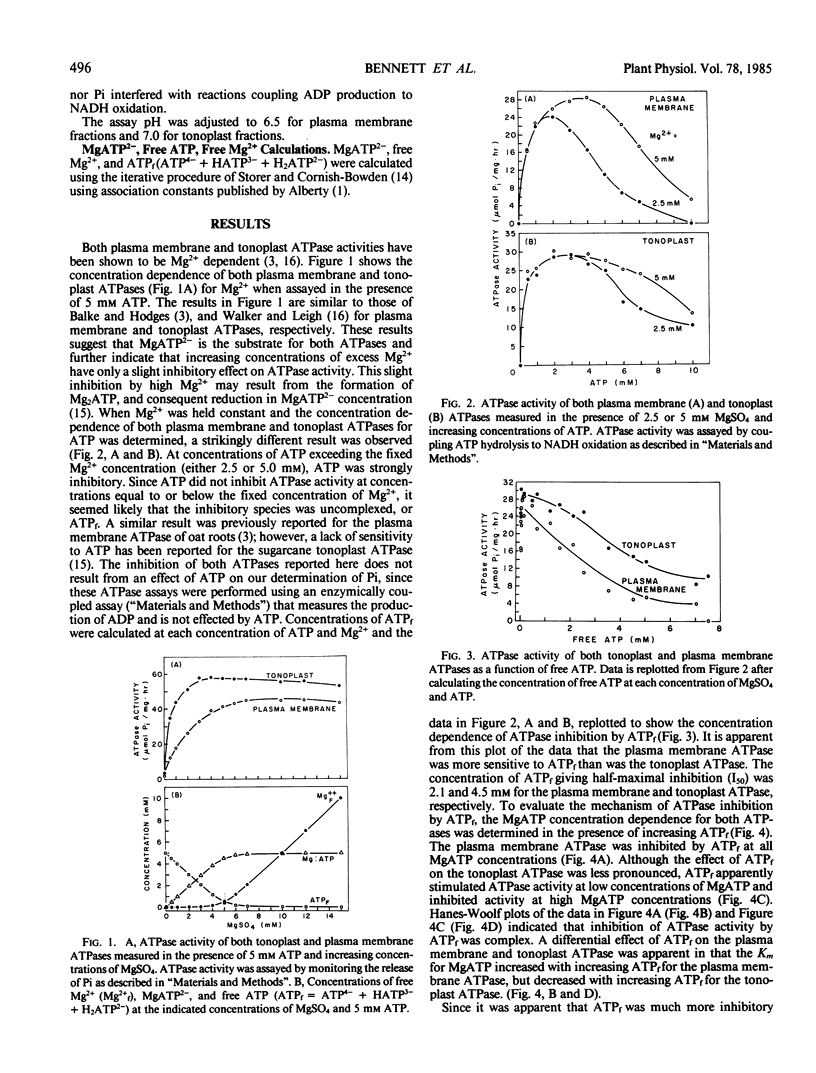
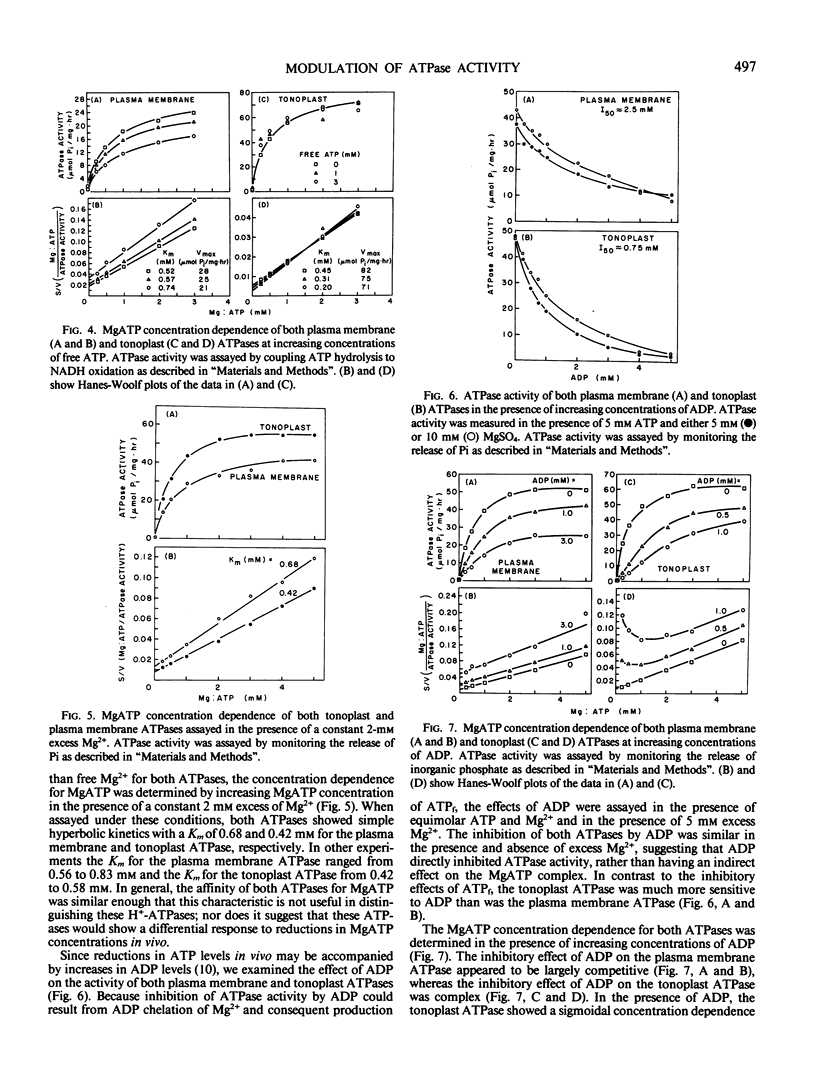
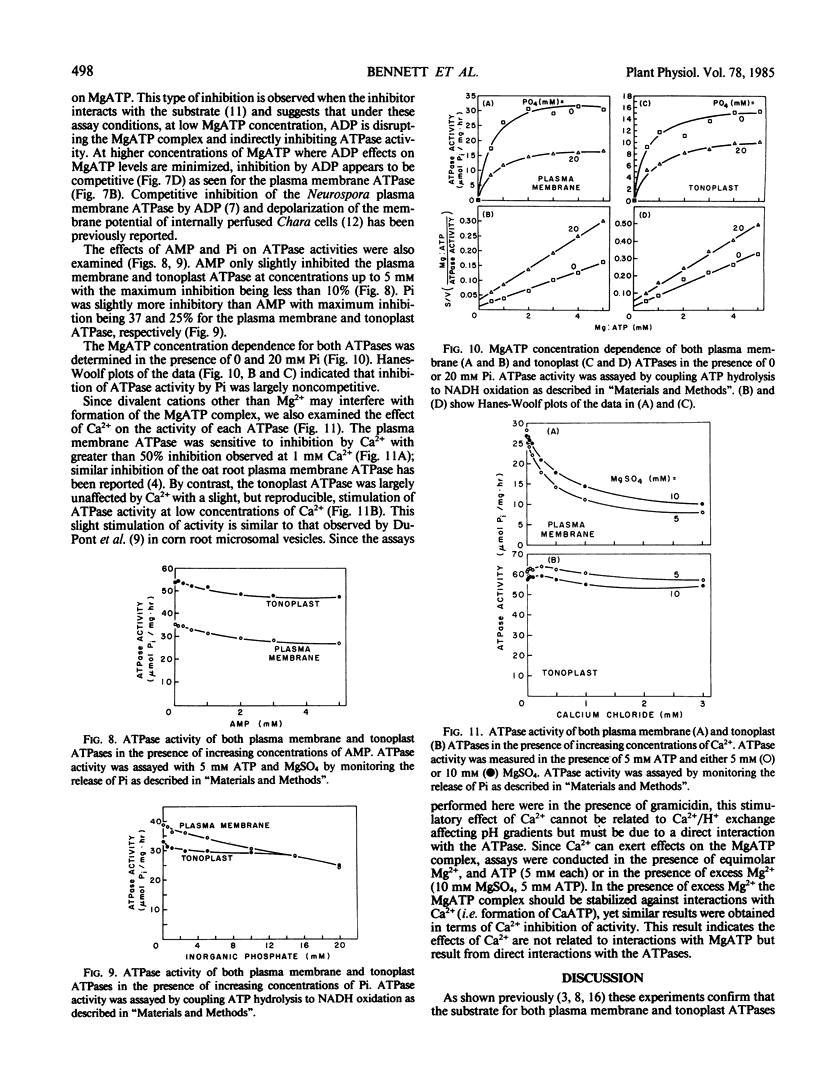
Two distinct membrane fractions containing H+-ATPase activity were prepared from red beet. One fraction contained a H+-ATPase activity that was inhibited by NO3− while the other contained a H+-ATPase inhibited by vanadate. We have previously proposed that these H+-ATPases are associated with tonoplast (NO3−-sensitive) and plasma membrane (vanadate-sensitive), respectively. Both ATPase were examined to determine to what extent their activity was influenced by variations in the concentration of ATPase substrates and products. The substrate for both ATPase was MgATP2−, and Mg2+ concentrations in excess of ATP had only a slight inhibitory effect on either ATPase. Both ATPases were inhibited by free ATP (i.e. ATP concentrations in excess of Mg2+) and ADP but not by AMP. The plasma membrane ATPase was more sensitive than the tonoplast ATPase to free ATP and the tonoplast ATPase was more sensitive than the plasma membrane ATPase to ADP.
Inhibition of both ATPases by free ATP was complex. Inhibition of the plasma membrane ATPase by ADP was competitive whereas the tonoplast ATPase demonstrated a sigmoidal dependence on MgATP2− in the presence of ADP. Inorganic phosphate moderately inhibited both ATPases in a noncompetitive manner.
Calcium inhibited the plasma membrane but not the tonoplast ATPase, apparently by a direct interaction with the ATPase rather than by disrupting the MgATP2− complex.
The sensitivity of both ATPases to ADP suggests that under conditions of restricted energy supply H+-ATPase activity may be reduced by increases in ADP levels rather than by decreases in ATP levels per se. The sensitivity of both ATPases to ADP and free ATP suggests that modulation of cytoplasmic Mg2+ could modulate ATPase activity at both the tonoplast and plasma membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Balke N. E., Hodges T. K. Plasma membrane adenosine triphosphatase of oat roots: activation and inhibition by mg and ATP. Plant Physiol. 1975 Jan;55(1):83–86. doi: 10.1104/pp.55.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: II. H/ATP Stoichiometry of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):545–548. doi: 10.1104/pp.74.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R. J., Slayman C. W. Inhibition of the plasma membrane [H+]-ATPase of Neurospora crassa by N-ethylmaleimide. Protection by nucleotides. J Biol Chem. 1982 Oct 25;257(20):12051–12055. [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]


