Abstract
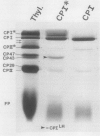
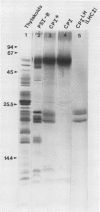
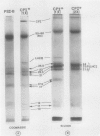
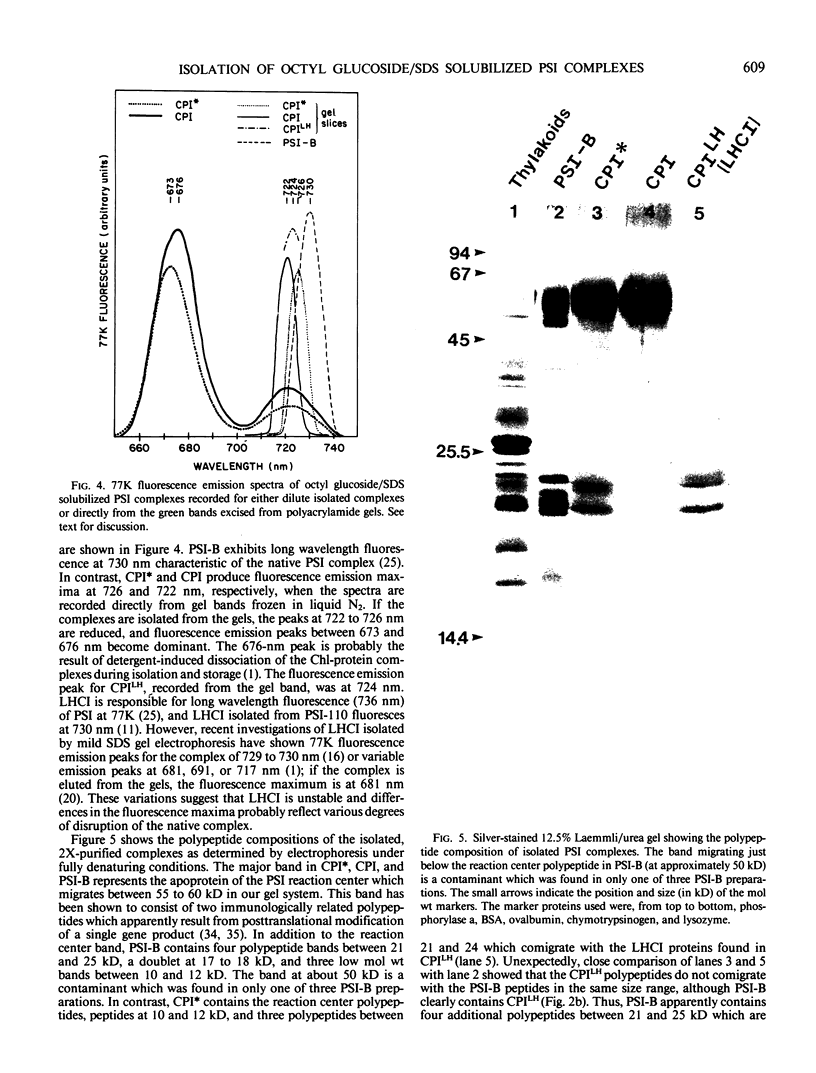
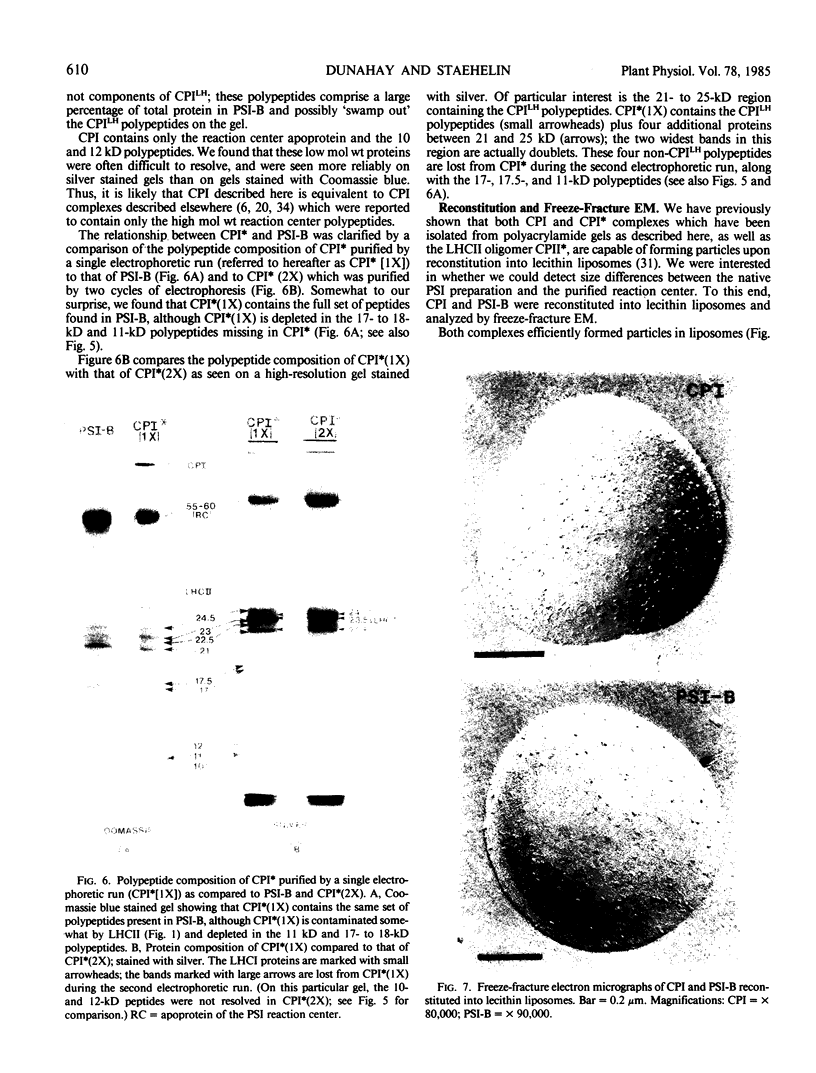
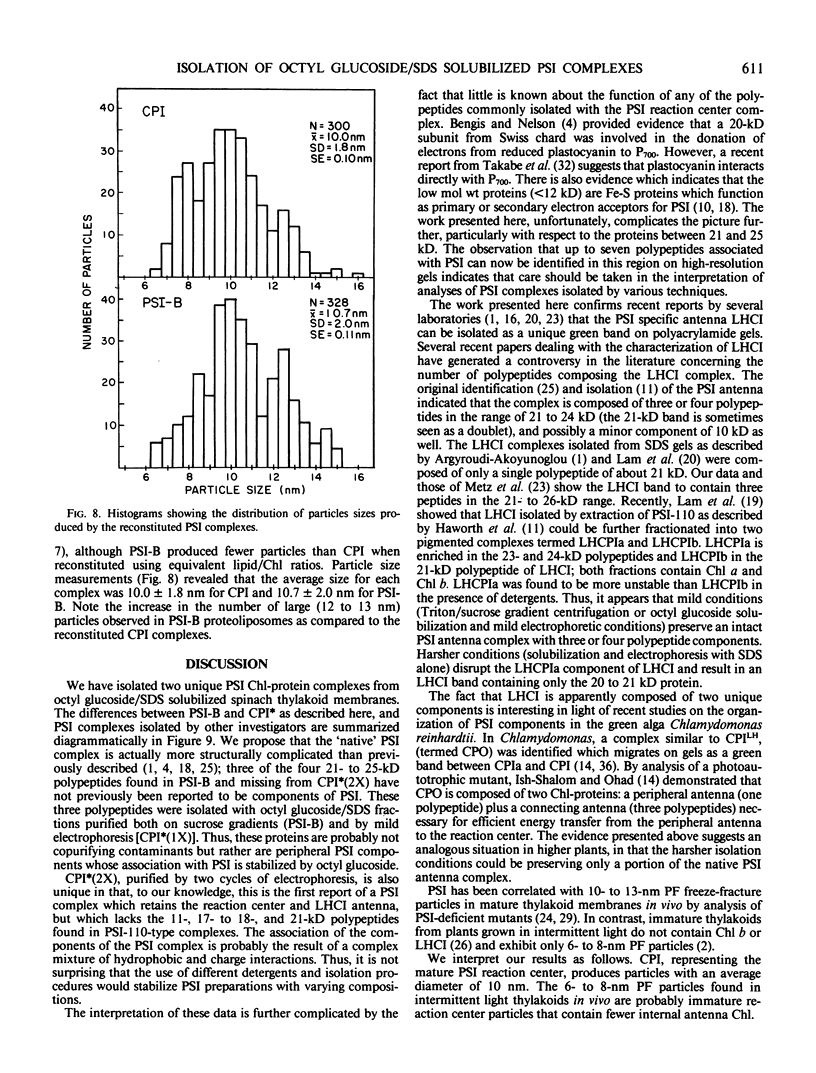
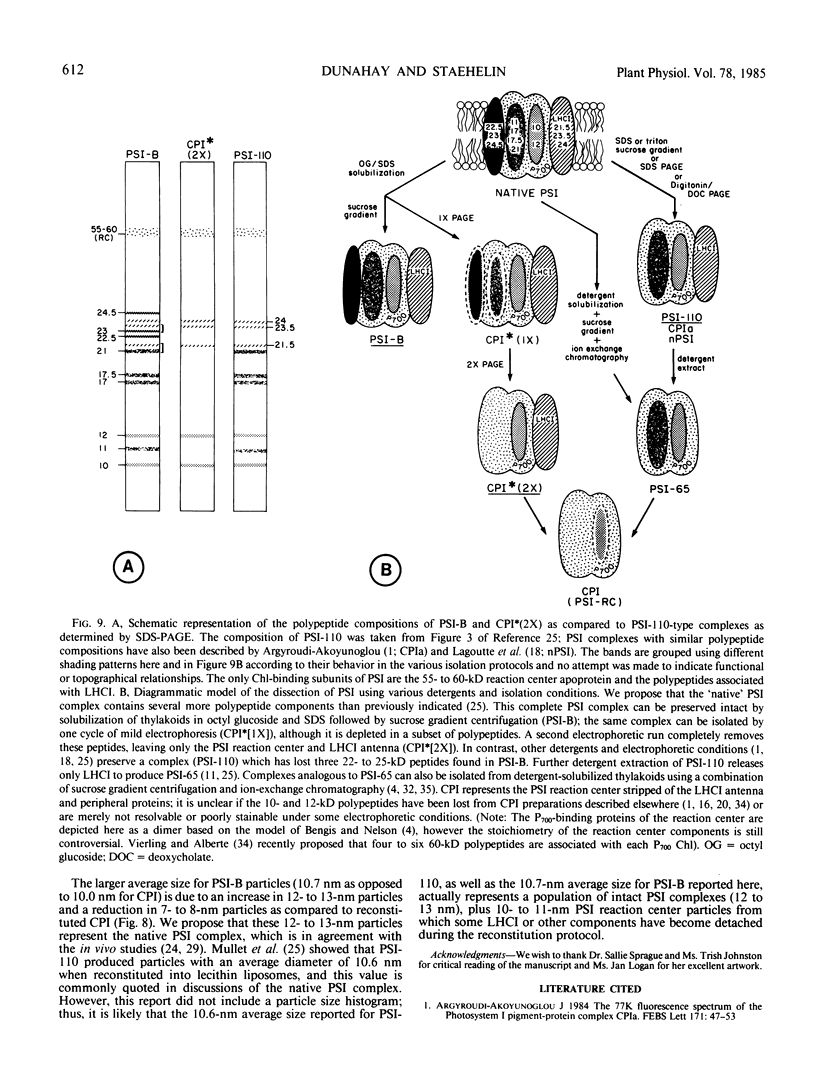
We have used the nonionic detergent octyl-β-d-glucopyranoside in combination with sodium dodecyl sulfate to isolate two novel Photosystem I (PSI) complexes from spinach (Spinacea oleracea L.) thylakoid membranes. These complexes have been characterized as to their spectral properties, content of PSI reaction center chlorophyll P700, and protein composition. PSI-B, purified from solubilized membranes by sucrose density gradient centrifugation, is a putative native PSI complex. PSI-B contains four polypeptides between 21 and 25 kilodaltons in addition to the components of the PSI antenna complex (LHCI); three of these polypeptides have not previously been associated with PSI. A second complex, CPI*, is purified from octyl glucoside/sodium dodecyl sulfate solubilized thylakoids by two cycles of preparative gel electrophoresis under mildly denaturing conditions. Electrophoresis under these conditions releases a discrete set of polypeptides from PSI producing a complex composed only of the PSI reaction center and the LHCI antenna.
In addition, the PSI reaction center complex CPI isolated from preparative gels and PSI-B were reconstituted into lecithin liposomes for structural analysis using freeze-fracture electron microscopy. The results suggest that the native PSI complex produces 12- to 13-nanometer particles, while the PSI reaction center, depleted of LHCI and peripheral proteins, produces particles with an average diameter of 10 nanometers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Staehelin L. A., Arntzen C. J. Spatial relationship of photosystem I, photosystem II, and the light-harvesting complex in chloroplast membranes. J Cell Biol. 1977 May;73(2):400–418. doi: 10.1083/jcb.73.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W. Sensitive fluorescence method for the determination of chlorophyll a-chlorophyll b ratios. Biochim Biophys Acta. 1971 Nov 2;253(1):222–231. doi: 10.1016/0005-2728(71)90248-9. [DOI] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Fractionation of Thylakoid Membranes with the Nonionic Detergent Octyl-beta-d-glucopyranoside: RESOLUTION OF CHLOROPHYLL-PROTEIN COMPLEX II INTO TWO CHLOROPHYLL-PROTEIN COMPLEXES. Plant Physiol. 1980 Sep;66(3):428–432. doi: 10.1104/pp.66.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. The effects of cations and trypsin on extraction of chlorophyll-protein complexes by octyl glucoside. Arch Biochem Biophys. 1982 Apr 1;214(2):563–572. doi: 10.1016/0003-9861(82)90061-3. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Ikegami I., Kato S. Enrichment of photosystem I reaction center chlorophyll from spinach chloroplasts. Biochim Biophys Acta. 1975 Mar 20;376(3):588–592. doi: 10.1016/0005-2728(75)90181-4. [DOI] [PubMed] [Google Scholar]
- Kirchanski S. J., Park R. B. Comparative Studies of the Thylakoid Proteins of Mesophyll and Bundle Sheath Plastids of Zea mays. Plant Physiol. 1976 Sep;58(3):345–349. doi: 10.1104/pp.58.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam E., Oritz W., Mayfield S., Malkin R. Isolation and Characterization of a Light-Harvesting Chlorophyll a/b Protein Complex Associated with Photosystem I. Plant Physiol. 1984 Mar;74(3):650–655. doi: 10.1104/pp.74.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel A., Staehelin L. A. Adhesion between liposomes mediated by the chlorophyll a/b light-harvesting complex isolated from chloroplast membranes. J Cell Biol. 1980 Jan;84(1):40–56. doi: 10.1083/jcb.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Metz J. G., Krueger R. W., Miles D. Chlorophyll-Protein Complexes of a Photosystem II Mutant of Maize : Evidence that Chlorophyll-Protein a-2 and a Chlorophyll-Protein Complex Derived from a Photosystem I Antennae System Comigrate on Polyacrylamide Gels. Plant Physiol. 1984 May;75(1):238–241. doi: 10.1104/pp.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. R. A chloroplast membrane lacking photosystem I. Changes in unstacked membrane regions. Biochim Biophys Acta. 1980 Aug 5;592(1):143–152. doi: 10.1016/0005-2728(80)90121-8. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. A developmental study of photosystem I peripheral chlorophyll proteins. Plant Physiol. 1980 May;65(5):823–827. doi: 10.1104/pp.65.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Simpson D. J. Freeze-fracture studies on barley plastid membranes. VI. Location of the P700-chlorophyll a-protein 1. Eur J Cell Biol. 1983 Sep;31(2):305–314. doi: 10.1007/BF02906493. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol. 1976 Oct;71(1):136–158. doi: 10.1083/jcb.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T., Ishikawa H., Niwa S., Itoh S. Electron transfer between plastocyanin and P700 in highly-purified photosystem I reaction center complex. Effects of pH, cations, and subunit peptide composition. J Biochem. 1983 Dec;94(6):1901–1911. doi: 10.1093/oxfordjournals.jbchem.a134544. [DOI] [PubMed] [Google Scholar]
- Vierling E., Alberte R. S. P(700) Chlorophyll a-Protein : Purification, Characterization, and Antibody Preparation. Plant Physiol. 1983 Jul;72(3):625–633. doi: 10.1104/pp.72.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]