Abstract
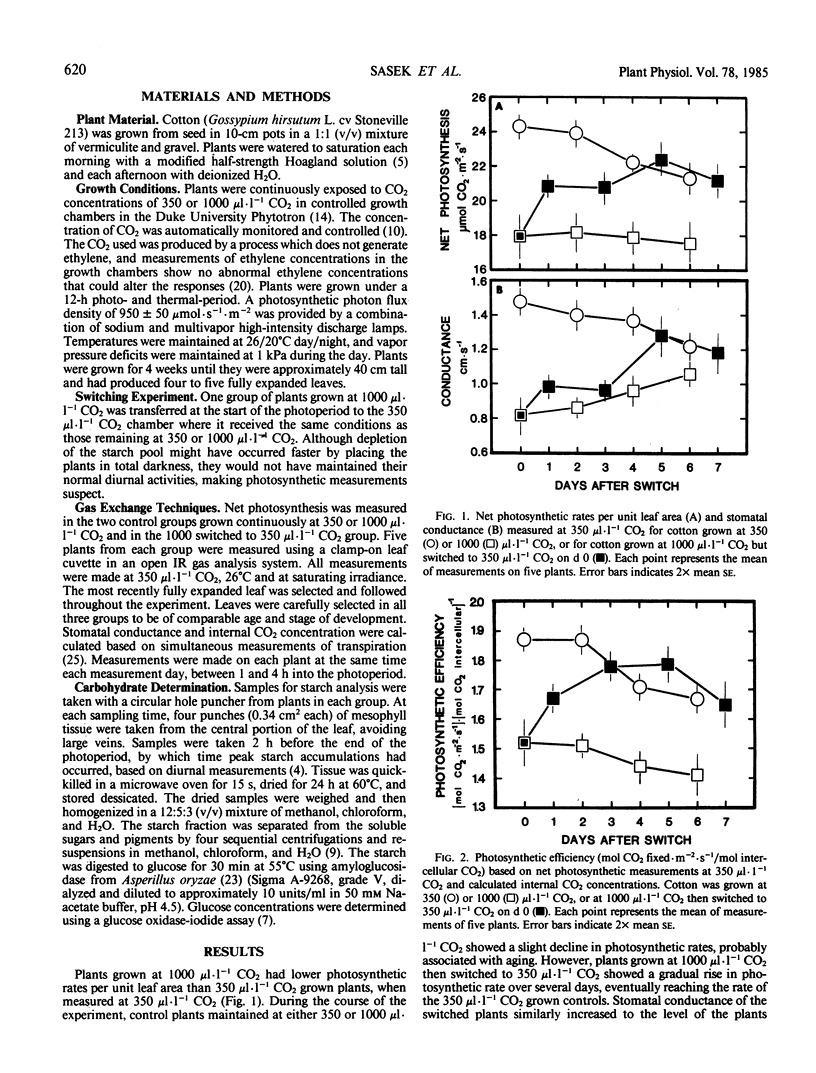
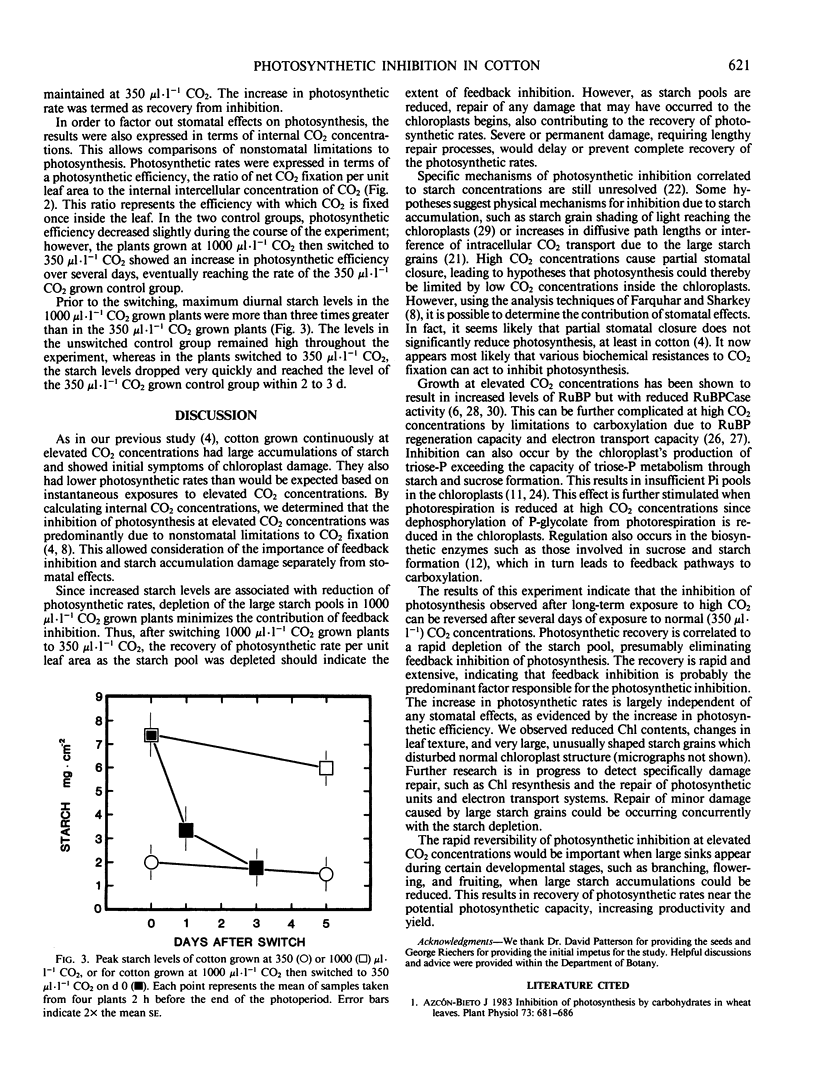
Cotton (Gossypium hirsutum L. cv Stoneville 213) was grown at 350 and 1000 microliters per liter CO2. The plants grown at elevated CO2 concentrations contained large starch pools and showed initial symptoms of visible physical damage. Photosynthetic rates were lower than expected based on instantaneous exposure to high CO2.
A group of plants grown at 1000 microliters per liter CO2 was switched to 350 microliters per liter CO2. Starch pools and photosynthetic rates were monitored in the switched plants and in the two unswitched control groups. Photosynthetic rates per unit leaf area recovered to the level of the 350 microliters per liter CO2 grown control group within four to five days. To assess only nonstomatal limitations to photosynthesis, a measure of photosynthetic efficiencies was calculated (moles CO2 fixed per square meter per second per mole intercellular CO2). Photosynthetic efficiency also recovered to the levels of the 350 microliters per liter CO2 grown controls within three to four days.
Recovery was correlated to a rapid depletion of the starch pool, indicating that the inhibition of photosynthesis is primarily a result of feedback inhibition. However, complete recovery may involve the repair of damage to the chloroplasts caused by excessive starch accumulation. The rapid and complete reversal of photosynthetic inhibition suggests that the appearance of large, strong sinks at certain developmental stages could result in reduction of the large starch accumulations and that photosynthetic rates could recover to near the theoretical capacity during periods of high photosynthate demand.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clough J. M., Peet M. M., Kramer P. J. Effects of High Atmospheric CO(2) and Sink Size on Rates of Photosynthesis of a Soybean Cultivar. Plant Physiol. 1981 May;67(5):1007–1010. doi: 10.1104/pp.67.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs M. P. The identification of congenital deafness. Trans Am Acad Ophthalmol Otolaryngol. 1970 Nov-Dec;74(6):1208–1214. [PubMed] [Google Scholar]
- Morison J. I., Gifford R. M. Ethylene Contamination of CO(2) Cylinders: Effects on Plant Growth in CO(2) Enrichment Studies. Plant Physiol. 1984 May;75(1):275–277. doi: 10.1104/pp.75.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger E. D., Koller H. R. Influence of Leaf Starch Concentration on CO(2) Assimilation in Soybean. Plant Physiol. 1976 Apr;57(4):560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharr D. M., Huber S. C., Sox H. N. Leaf Carbohydrate Status and Enzymes of Translocate Synthesis in Fruiting and Vegetative Plants of Cucumis sativus L. Plant Physiol. 1985 Jan;77(1):104–108. doi: 10.1104/pp.77.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Imai K., Farquhar G. D., Cowan I. R. A Direct Confirmation of the Standard Method of Estimating Intercellular Partial Pressure of CO(2). Plant Physiol. 1982 Mar;69(3):657–659. doi: 10.1104/pp.69.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu C. V., Allen L. H., Bowes G. Effects of Light and Elevated Atmospheric CO(2) on the Ribulose Bisphosphate Carboxylase Activity and Ribulose Bisphosphate Level of Soybean Leaves. Plant Physiol. 1983 Nov;73(3):729–734. doi: 10.1104/pp.73.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]


