Abstract
Background and objectives:
Safe and effective management of cancer-related pain is a worldwide challenge. In the search for treatment options, natural products used in Chinese herbal medicines (CHMs) have received attention in clinical studies for their effects on cancer-related pain. The objective of this systematic review is to evaluate the clinical evidence for topically applied CHMs as adjunctive treatments for cancer pain management.
Methods:
Nine biomedical databases and 4 clinical trial registries were searched for randomized-controlled trials (RCTs) that reported measures of pain and/or quality of life. Risk of bias was assessed using the Cochrane tool. Meta-analysis employed mean difference (MD) with 95% confidence intervals (random effects).
Results:
Twenty (20) RCTs (1636 participants) met the inclusion criteria. Meta-analyses were grouped based on the comparisons and outcome measures. For pain intensity, there was a greater reduction in the topical CHM group versus placebo (MD −0.72 [−1.04, −0.40]), no difference when compared to tramadol (MD −0.15 [−0.38, 0.08]), and a greater reduction when topical CHMs were combined with conventional analgesic medications (MD −0.67 [−0.93, −0.40]). Analgesic onset time was reduced in the CHM group compared to tramadol (MD −26.02 [−27.57, −24.47] minutes), and for CHMs combined with conventional medications (MD −19.17 [−21.83, −16.52] minutes). When CHMs were combined with analgesic medications, improvements were found for duration of analgesia (MD 1.65 [0.78, 2.51] hours), analgesic maintenance dose (MD −31.72 [−50.43, −13.01] milligrams/day), and quality of life.
Conclusion:
Addition of topical CHMs to conventional analgesic medications was associated with improved outcomes for pain intensity, some other pain-related outcomes, and measures of quality of life. Limitations included methodological issues in some studies and considerable heterogeneity in some pooled results.
Keywords: cancer pain, traditional medicine, natural products, herbal medicine, meta-analysis, topical analgesic, Chinese medicine
Introduction
Cancer-related pain remains prevalent and undertreated throughout the world.1,2 The prevalence has been estimated at over 70% in advanced disease, 3 with 38% experiencing moderate to severe pain, 4 and there remain inadequacies in cancer pain management. 5 The World Health Organization (WHO) strongly advocates pain relief for moderate and severe cancer pain for which the analgesic ladder remains a mainstay of therapy.6,7 Besides oral opioids and other analgesics, topical preparations appear effective, 8 can be targeted at the pain area, and may avoid the adverse effects of systemic medications.9,10 Adjunctive and non-pharmacologic interventions, including acupuncture and other complementary therapies, can also be considered.11 -17 Nevertheless, it has been suggested that new pharmaceuticals are needed. 18 One area of research has been the effect of natural products on pain.19 -22
A review of topical analgesics found they were well tolerated and had lesser side effects and undesirable drug–drug interactions than some oral interventions, but were underused in clinical practice.23,24 Their greater use has been proposed, particularly for localized neuropathic pain associated with malignancy. 9 A Cochrane review on topical capsaicin, a natural product from plants in the Capsicum genus, found evidence of effectiveness for chronic neuropathic pain. 8 In a recent review, evidence was found for multiple topical analgesics, although this included case series and case reports. 25
The topical application of natural products has been used for pain relief in traditional medicine. 26 In China and other countries in eastern Asia, topical interventions are typically multi-ingredient formulations that are composed of natural products of diverse origin, which have been prepared and processed for medical use. These are listed in major pharmacopeia. 27
Systematic reviews of traditional medicines for various types of cancer pain suggested that combining traditional medicines with conventional therapies may improve pain outcomes.28,29 A systematic review of externally applied Chinese herbal medicines (CHMs) combined with the 3-step analgesic ladder reported improved pain relief and reduced adverse reactions to conventional analgesics. 30 A book chapter that included 17 randomized controlled trials (RCTs) of topical CHMs for cancer pain found improvements in pain and other outcomes. 31 A meta-analysis of 23 RCTs reported that topical application of CHMs at painful locations combined with 3-step analgesia improved cancer pain and quality of life and reduced the rate of some adverse reactions. 32 For bone cancer pain, a meta-analysis of the results of 6 RCTs reported that combining topically applied CHMs with conventional management resulted in improved pain relief. 33 In a recent clinical study of a traditional herbal topical analgesic conducted in the United States, participants reported effectiveness and convenience of use. 34
The aims of this systematic review and meta-analysis were to determine the effects of topically applied CHMs on cancer-related pain, inform clinicians of the best available evidence, assess the safety and tolerability of these interventions based on reported adverse events and dropouts, and identify directions for future research.
Methods
This review followed methods outlined in the PRISMA guidelines,35,36 the PRISMA Extension for Chinese Herbal Medicines 2020, 37 and Cochrane Collaboration.38,39 Its protocol was registered with PROSPERO (CRD42021264676).
Information Sources and Search Strategy
Comprehensive literature searches were conducted to identify studies that used topically applied natural products as traditional medicines in eastern Asia to treat cancer pain. Searches used methods outlined in the Cochrane Handbook of Systematic Reviews. 38 English-language databases included PubMed, Excerpta Medica Database (Embase), Cumulative Index of Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine Database (AMED), and Cochrane Central Register of Controlled Trials (CENTRAL), including the Cochrane Library. Chinese-language databases included China Biomedical Literature (CBM), China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), and Wanfang.
Clinical trial registries, including the Australian New Zealand Clinical Trial Registry (ANZCTR), Chinese Clinical Trial Registry (ChiCTR), EU Clinical Trials Register (EU-CTR), ClinicalTrials.gov, and International Clinical Trials Registry Platform (ICTRP), were searched to identify ongoing or completed trials. Where required, trial investigators were contacted to obtain data. Databases and registries were searched from their respective inceptions to May 2022, with an updated search in November 2022.
In addition to databases and clinical trial registries, reference lists of systematic reviews and included RCTs were searched for additional publications (see Table S1 for search terms).
Selection Criteria
Inclusion criteria for study participants included, inpatient or outpatient adult participants (aged 18 years and over) diagnosed with a malignant tumor based on pathology tests, or diagnosed with liver cancer based on the American Association for the Study of Liver Disease (AASLD) 40 or the European Association for the Study of the Liver (EASL) guidelines, 41 and with chronic pain syndromes caused by cancer or cancer-related treatment, such as surgery, chemotherapy, radiotherapy, and/or other conventional treatments. Exclusion criteria were studies that included participants with pain unrelated to cancer or cancer-related treatments, postoperative pain occurring within a short time (≤2 months), or phlebitis.
Inclusion criteria for test interventions included topically applied CHM in the form of a hot compress, cataplasm (poultice), and/or other external application. The topically applied CHM could be combined with a conventional therapy for cancer-related pain as an integrative approach. Co-interventions were allowed provided the same co-intervention was used in at least 2 arms of the study. Exclusion criteria included, novel synthetic compounds or isolated chemical compounds (such as capsaicin), homeopathic preparations, nutritional supplements, plant-based products not used in traditional medicine, topical CHMs combined with oral CHMs, CHM combined with acupuncture, or other non-topical therapy. Studies in which the details of the topical intervention were unclear were excluded.
Inclusion criteria for control interventions included putatively inactive controls, such as no treatment, supportive care, or placebo; conventional therapies recommended in guidelines, such as analgesics, radiation, neurolytic block, and intrathecal pump; or other conventional treatments aimed at managing cancer pain. Exclusion criteria included use of pethidine or other discontinued medications; and use of CHM or other complementary medicine in the control group.
Inclusion criteria for outcomes included, measures of pain, analgesia, performance status, and quality of life (QOL). There was no limit on the treatment duration. Exclusion criteria were studies that did not report numerical outcome data.
Data Screening and Extraction
Search results were downloaded to spreadsheets, which were merged and duplicates were removed. Abstracts were screened by 2 reviewers (YHL & BHM). Full texts of potential inclusions were obtained for further assessment. For studies that satisfied all inclusion and exclusion criteria, the study characteristics, funding sources, and outcome data were extracted to predefined spreadsheets by YHL and checked by BHM. Any issues or disagreements were resolved by discussion and consultation with a third person (ALZ and/or XFG). Species’ names were based on the Chinese pharmacopeia. 27
Risk of Bias Assessment
Risk of bias using the Cochrane Collaboration’s tool 38 was assessed independently by 2 researchers (YHL & BHM), and any disagreement was resolved by discussion and consultation with a third person (XFG). Potential reporting bias was assessed using funnel plots and Egger’s test when 10 or more studies were available.
Data Analysis
Meta-analyses were conducted where feasible using Stata 12.0 and Review Manager 5.3. Dichotomous data were assessed as risk ratio (RR), and continuous data were assessed as mean difference (MD) or standardized mean difference (SMD), all with 95% confidence intervals (CI). Available case analysis with a random effects model was used in all analyses. This provides a conservative estimate of difference between groups and is applicable to data in which heterogeneity is likely. Heterogeneity was assessed using the I2 statistic. Where possible and appropriate, planned subgroup analyses included type of control medication, pain severity at baseline (numerical rating scale [NRS] ≥4 or visual analog scale [VAS] ≥4), treatment duration, and/or the specific topically applied CHM formulation. Sensitivity analyses were undertaken to explore potential sources of heterogeneity.
Results
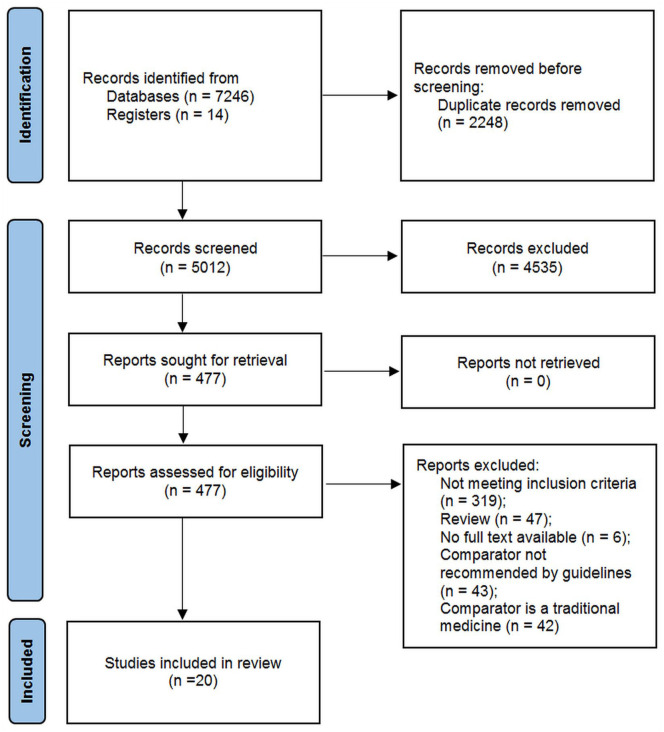
The searches identified 20 RCTs that met all the selection criteria (Figure 1). All were conducted in China and enrolled 1636 participants. Participants ranged from 18 to 85 years of age. Study durations ranged from 7 to 28 days (Table 1). Following dropouts, 1601 participants completed the studies. One study compared topical CHM with placebo, 42 one compared a topical CHM with a conventional analgesic, 43 three studies compared topical CHMs plus conventional analgesics with placebo plus conventional analgesics,44 -46 one study compared a topical CHM plus a conventional analgesic plus pamidronate disodium with the same analgesic plus pamidronate disodium, 47 and 14 studies compared topical CHMs plus conventional analgesics with conventional analgesics alone.
Figure 1.
PRISMA flow diagram of study selection.
Table 1.
Characteristics of Included Studies.
| Author, year | Study population | Pain intensity | Sample size (T/C); dropouts (T/C) | Age range; male/female | CHM group intervention; duration (in days) | Control intervention | Outcome (MD [95% CI] P value) |
|---|---|---|---|---|---|---|---|
| Che 2018 | Miscellaneous cancers | NRS ≥ 4 | 50/50; 0/0 | 18-74; 59/41 | Unnamed cataplasm (poultice) +intrathecal injections of morphine; 28 | Intrathecal injections of morphine | VAS (−1.27 [−1.55, −0.99] P < .05); QOL: QLQ-C30 (3.91 [2.40, 5.42] P < .05); average frequency of breakthrough pain (−1.21 [−1.49, −0.93] P < .05); analgesic dose (−3.37 [−3.77, −2.97] P < .05); AE |
| Ding 2015 | Miscellaneous cancers | NRS ≥ 4 | 34/31; 0/0 | 25-78; 35/30 | Xiao Ji Zhi Tong Tie + morphine sulfate sustained-release tablets; 7 | Morphine sulfate sustained-release tablets | NRS (−0.70 [−1.57, .17] P > .05); KPS (3.14 [−3.97, 10.25] P > .05); QOL: CS (−0.52 [−3.61, 2.57] P > .05); analgesic dose (−17.52 [−25.60, −9.44] P < .05); AE |
| Feng 2012 | Liver cancer | 1 ≤ NRS ≤ 3 | 73/67; 0/0 | 18-85; 121/19 | Shuang Bai San; 7 | Placebo | NRS (−0.72 [−1.04, −0.40] P < .05); AE |
| Huang 2013 | Miscellaneous cancers | NRS ≥ 4 | 26/25; 0/0 | 36-74; 24/27 | Xiao Zheng Zhi Tong Gao + oxycodone HCl extended-release tablets; 7 | Oxycodone HCl extended-release tablets | NRS (−0.25 [−1.28, .78] P > .05); KPS (7.97 [.52, 15.42] P < .05); QOL: QLQ-C30 (15.05 [5.38, 24.72] P < .05); analgesic onset time (−19.00 [−24.01, −13.99] P < .05); analgesic dose (−22.68 [−43.36, −1.99] P < .05); analgesia duration (1.70 [.36, 3.04] P < .05); AE |
| Li 2016 | Miscellaneous cancers | NRS ≥ 2 | 40/40; 0/0 | 33-70; 44/36 | Jian Zhong Xiao Ba Bu Ji + three-step analgesics; 28 | Three-step analgesics | NRS (−0.62 [−1.07, −0.17] P < .05); KPS (4.16 [.23, 8.09] P < .05); AE |
| Li 2006 | Miscellaneous cancers | VAS ≥ 4 | 25/25; 0/0 | >18; 30/20 | Zhen Tong number 1 + three-step analgesics; 7 | Three-step analgesics | VAS (−0.33 [−0.94, .28] P > .05); KPS (8.40 [1.55, 15.24] P < .05); QOL: CS (1.68 [−0.17, 3.53] P > .05); AE |
| Liu 2015 | Bone metastatic cancers | All degrees | 30/30; 0/0 | 18-69; 37/23 | Die Da Gao + oxycodone HCl extended-release tablets + pamidronate disodium; 28 | Oxycodone HCl extended-release tablets + pamidronate disodium | NRS (−0.30 [−0.64, .04] P > .05); KPS (RR 1.27 [1.005, 1.61] P < .05); 1 AE |
| Liu 2017 | Liver cancer | NRS ≤ 3 | 17/19; 0/0 | 28-70; 24/12 | Shuang Bai San + celecoxib capsules; 7 | Celecoxib capsules | NRS (−1.52 [−1.77, −1.27] P < .05); AE |
| Luo 2020 | Miscellaneous cancers | VRS ≥ grade II | 43/43; 0/0 | 39-67; 45/41 | Su Luo Zhi Tong Gao + three-step analgesics; 20 | Three-step analgesics | NRS (−0.46 [−0.57, −0.34] P < .05); AE |
| Song 2017 | Advanced lung cancer | NRS ≥ 7 | 30/30; 0/0 | 35-71; 36/24 | Xiao Pi Zhen Tong Gao + oxycodone HCl extended-release tablets; 7 | Placebo + oxycodone HCl extended-release tablets | NRS (−0.87 [−1.42, −0.32] P < .05); KPS (.73 [−2.00, 3.46] P > .05); average analgesic dose (−60.00 [−73.77, −46.23] P < .05); AE |
| Tang 2013 | Advanced cancer | NRS ≥ 4 | 40/40; 1/2 | 30-75; 33/44 | Bing Chong Zhi Tong Gao + morphine sulfate sustained-release tablets; 7 | Placebo + morphine sulfate sustained-release tablets | NRS (−0.43 [−1.00, .14] P > .05); KPS (.09 [−5.94, 6.12] P > .05); QOL: NCCN (−5.24 [−10.33, −0.15] P < .05); average frequency of breakthrough pain (−1.41 [−220, −0.62] P < .05); analgesic maintenance dose (−6.24 [−20.53, 8.05] P =.392); AE |
| Tian 2015 | Bone metastatic cancers | NRS ≥ 4 | 33/33; 2/3 | 31-79; 33/33 | Gu Tong Tie + oxycodone HCl extended-release tablets; 10 | Oxycodone HCl extended-release tablets | NRS (−1.01 [−2.08, .06] P > .05); KPS (5.35 [−0.42, 11.12] P > .05); QOL: NCCN (−5.84 [−13.92, 2.24], P = .157); average frequency of breakthrough pain (−0.26 [−0.73, .21], P = .281); analgesic dose (91.57 [−226.40, 409.54] P > .05); AE |
| Wang 2016 | Ovarian cancer | All degrees | 30/30; 0/0 | 32-71; NS | Ai Tong Xiao Wai Yong Tie + three-step analgesics; 14 | Three-step analgesics | NRS (−0.68 [−0.90, −0.46] P < .05); KPS (10.22 [6.82, 13.62] P < .05) |
| Wang 2014 | Miscellaneous cancers | NRS ≥ 4 | 40/40; 0/0 | 38-70; 50/30 | Wu Xiang Tong Xiao Gao + three-step analgesics; 7 | Placebo + three-step analgesics | NRS (−0.10 [−0.71, .51] P > .05); analgesic onset time (−24.72 [−67.04, 17.62] P = .253); total analgesic dose (−138.00 [−234.22, −41.78] P < .05); analgesia duration (3.66 [1.13, 6.18] P < .05) |
| Xu 2013 | Miscellaneous cancers | All degrees | 45/38; 5/0 | 30-70; 43/35 | Wu Sheng Ding + three-step analgesics; 7 | Three-step analgesics | NRS (.05 [−0.66, .76] P > 0. 05); QOL-CS (−0.22 [−1.92, 1.48] P > .05); AE |
| Zhang 2004 | Miscellaneous cancers | NRS < 7 | 200/60; 0/0 | 23-73; 141/119 | Hua Jian Ba Du Mo; 7 | Tramadol (oral) | NRS (−0.15 [−0.38, .08] P = .211); analgesic onset time (−26.02 [−27.57, −24.47] P < .05); analgesia duration (.32 [−0.26, .90] P = .283); AE |
| Zhao 2018 | Miscellaneous cancers | NRS ≥ 4 | 40/40; 1/1 | 47-70; 41/37 | Xiao Zhong Zhi Tong San + morphine hydrochloride sustained-release tablets; 14 | Morphine hydrochloride sustained-release tablets | NRS (−0.36 [−0.67, −0.05] P < .05); KPS (−0.41 [−4.08, 3.26] P > .05); average frequency of breakthrough pain (−1.46 [−1.79, −1.13] P < .05); analgesic dose (−33.84 [−58.61, −9.07] P < .05) |
| Zhao 2016 | Malignant neuropathic pain | NRS ≥ 4 | 18/18; 1/1 | 42-80; 19/15 | Zhi Tong San + fentanyl transdermal system; 7 | Fentanyl transdermal system | NRS (−0.05 [−0.91, .81] P > .05); QOL: QLQ-C30 (6.86 [1.24, 12.48] P < .05); analgesic dose (−72.94 [−107.30, −38.58] P < .05) |
| Zhong 2009 | Miscellaneous cancers | NRS ≥ 4 | 31/32; 0/2 | >18; 19/15 | Qi Zheng Xiao Tong Tie + morphine sulfate sustained-release tablets; 7 | Morphine sulfate sustained-release tablets | NRS (−1.15 [−1.30, −1.00] P < .05); KPS (.05 [−1.37, 1.46] P > .05); QOL: CS (2.74 [2.24, 3.25] P < .05); analgesic onset time (−19.21 [−22.34, −16.07] P < .05); analgesia duration (1.32 [1.14, 1.50] P < .05) |
| Zhou 2020 | Miscellaneous cancers | All degrees | 50/50; 9/7 | 18-74; 46/38 | Long Teng Tong Luo alcohol tincture + three-step analgesics; 8 | Three-step analgesics | NRS (−0.26 [−0.61, .09] P > .05); average frequency of breakthrough pain (−0.94 [−5.36, 3.48], P = .677); analgesic dose (−13.81 [−30.72, 3.10] P > .05); AE |
Incidence of 10 points or more improvement in KPS.
Abbreviations: AE, adverse event; C, control group; CHM, Chinese herbal medicine; CI, confidence interval; CS, Chinese scale; MD, mean difference; NCCN, National Comprehensive Cancer Network; NRS, numeric rating scale; QLQ-C30, 30-item Quality of Life of Questionnaire for Cancer Patients; QOL, quality of life; KPS, Karnofsky Performance Status; oxycodone HCl, oxycodone hydrochloride; RR, risk ratio; T, test group; VAS, visual analog scale.
Outcome data were available for pain intensity (20 studies), analgesic onset time (4 studies), frequency of breakthrough pain (5 studies), analgesia duration (4 studies), analgesic dose (10 studies), quality of life (9 studies), and Karnofsky Performance Status (KPS; 10 studies).
Two studies used the same multi-ingredient formulation, Shuang Bai San,42,48 while the others each tested a different multi-ingredient formulation. The ingredients most frequently used in the formulations were: Borneolum (bing pian; 11 studies), Asarum species (xi xin; 10 studies), Corydalis yanhusuo W.T.Wang (yan hu suo; 9 studies), Rheum species (da huang; 8 studies), Commiphora myrrha Engl. (mo yao; 8 studies), Boswellia carteri Birdw. (ru xiang; 8 studies), Aconitum carmichaelii (chuan wu; 7 studies), and Buthus martensii Karsch. (quan xie; 7 studies) (see Tables S2 and S3 for the ingredients used in each study).
Risk of Bias
Risk of bias for sequence generation was judged “low risk” in 12 RCTs because an appropriate method of randomization was described. The others were judged “unclear risk.” Only one study described a suitable method of allocation concealment and was judged “low risk”; the remaining studies did not mention allocation concealment and were judged “unclear risk.” Four studies that used a placebo for the topical CHM in the control groups were judged “low risk” for blinding of participants. The remaining studies were judged “high risk.” All studies were judged “high risk” for blinding of personnel due to lack of clear descriptions and “unclear risk” for blinding of outcome assessors, since it is common practice for attending physicians and nurses not connected with the RCT to collect routine data on pain. Each study had few or no dropouts, so all were judged as “low risk” for incomplete outcome data. For selective outcome reporting only one protocol could be located and all outcomes were reported, so it was judged “low risk” 42 ; all other studies were judged “unclear risk” (Table S4).
Results of Meta-Analysis
Pain intensity
Twenty studies reported on pain intensity: 2 used a 10-point VAS49,50 and the others used the NRS. There was a greater reduction in pain intensity between the CHM fomentation Shuang bai san and placebo fomentation (MD −0.72 [−1.04, −0.40], n = 140) after 7 days of treatment. 42 There was no difference in pain intensity between the group that used the topical CHM paste, Hua jian ba du mo, and the group that used the conventional analgesic tramadol (Figure 2(a)) (MD −0.15 [−0.38, 0.08], n = 260) after 7 days of treatment. 43
Figure 2.
Forest Plots. (a) Cancer Pain Intensity. (b) Analgesic Onset Time (minutes). (c) Analgesia Duration (hours). (d) Analgesic dose. (e) Quality of Life. (f) Karnofsky Performance Status.
When topical CHMs were combined with conventional analgesic medications, there was a greater reduction in pain intensity in the integrative medicine group in the pooled result of 3 placebo-controlled RCTs (MD −0.48 [−0.92, −0.05], I2 = 41.4%, n = 217), with moderate heterogeneity.44 -46 There was also a significantly greater reduction in pain intensity in the pooled result of 14 open-label studies (MD −0.67 [−0.93, −0.40], I2 = 89.8%, n = 924), but heterogeneity was considerable.48 -61
A sensitivity analysis included 5 studies53 -56,58 that compared topical CHMs plus conventional analgesics with conventional analgesics alone and which had treatment durations of more than 7 days, except for the study that used intrathecal injections of morphine. 50 The results of this sensitivity analysis showed a significant difference between groups, without important heterogeneity (MD −0.52 [−0.64, −0.39], I2 = 18.2%, n = 365).
There was no significant difference in pain intensity in the single RCT of a topical CHM plus oxycodone HCl extended-release tablets plus pamidronate disodium versus the same pharmacotherapies (MD −0.30 [−0.64, 0.04], n = 60). 47
The pooled result of all 18 RCTs of topical CHMs plus conventional pharmacotherapies versus the same pharmacotherapies showed significantly greater reduction in pain in the integrative medicine groups (MD −0.61 [−0.84, −0.38], I2 = 87.8%, n = 1201), but heterogeneity was considerable. The funnel plot of all 18 RCTs showed no apparent asymmetry and the Egger’s test was not significant (Figure S1a). A similar result was found for the 14 open label RCTs (Figure S1b). This suggested that the risk of publication bias was not high.
In a subgroup analysis that included the 9 studies that enrolled participants with moderate to severe pain (NRS ≥ 4 or VAS ≥ 4). The pooled result showed a significant difference between groups, with slightly higher heterogeneity (MD −0.67 [−1.01, −0.33], I2 = 89.5%, n = 586). A sensitivity analysis was conducted with 7 RCTs47,53 -56,58,61 that compared topical CHMs plus conventional pharmacotherapies (i.e., analgesics and other treatments) with conventional pharmacotherapies alone and had treatment durations of more than 7 days, excluding the study that used intrathecal injections of morphine. 50 This sensitivity analysis showed a significant difference between groups without important heterogeneity (MD −0.47 [−0.59, −0.35], I2 = 21.7%, n = 509).
One CHM, Shuang bai san, was used in 2 RCTs of pain due to liver cancer. When the results were pooled, there was significantly less pain in the Shuang bai san group (MD −1.13 [−1.91, −0.34], I2 = 93.3%, n = 176), with considerable heterogeneity.
Analgesic onset time
Four studies reported on analgesic onset time. For the comparison of topical CHM versus oral tramadol (Figure 2(b)), there was significantly greater reduction in analgesic onset time in the topical CHM group (MD −26.02 [−27.57, −24.47] minutes, n = 260). 43 When topical CHMs were used in combination with conventional analgesic medications according to the 3-step ladder, there was no significant reduction in analgesic onset time in one placebo-controlled RCT (MD −24.72 [−67.04, 17.62] minutes, n = 80). 45 However, there was significantly greater reduction in the topical CHM group in the pool of 2 open-label RCTs that combined CHMs with morphine sulfate sustained-release tablets (MD −19.15 [−21.81, −16.49] minutes, I2 = 0%, n = 111), with no heterogeneity.52,60 When results from all 3 integrative medicine studies were pooled, there was significantly greater reduction in analgesic onset time in the groups that received the CHMs for 7 days (MD −19.17 [−21.83, −16.52] minutes, I2 = 0%, n = 191), with no heterogeneity.
Analgesia duration
Analgesia duration was not significantly different when comparing a topical CHM and tramadol for 7 days (MD 0.32 [−0.26, 0.90] hours, n = 260). 43 One placebo-controlled RCT of a topical CHM plus 3-step analgesics (Figure 2(c)) showed a significantly greater increase in analgesia duration in the combination therapy group (MD 3.66 [1.13, 6.18] hours, n = 80). 45 In addition, the pooled effect for 2 open-label RCTs of topical CHMs plus morphine sulfate sustained-release tablets for 7 days showed significantly greater increases (MD 1.32 [1.15, 1.50] hours, I2 = 0%, n = 112), with no heterogeneity.52,60 When all 3 integrative medicine studies were pooled, there was a greater increase in analgesia duration in the groups that received CHMs for 7 days (MD 1.65 [0.78, 2.51] hours, I2 = 44.1%, n = 192), with moderate heterogeneity.
Analgesic dose
Three placebo-controlled RCTs of topical CHMs combined with conventional analgesics reported results for analgesic dose as morphine equivalents (Figure 2(d)). One study reported a significant reduction in total analgesic dose (MD −138.00 [−234.22, −41.78] mg, n = 80) after 7 days. 45 Another found a significant reduction in average analgesic dose per day after 7 days (MD −60.00 [−73.77, −46.23] mg per day, n = 60), 46 and the third study found no significant difference between groups in the analgesic maintenance dose (MD −6.24 [−20.53, 8.05] mg per day, n = 77) after 7 days. 44
In the 7 open-label RCTs, one study found no difference between groups in the total dose (MD 91.57 [−226.40, 409.54] mg, n = 61) 55 after 10 days. The pooled result of 2 studies that reported average dose per day showed no significant difference (MD −5.03 [−12.5, 2.45] mg per day, I2 = 31.7%, n = 184) after 8 to 28 days, without important heterogeneity.50,61 The pooled result for the 4 studies that reported analgesic maintenance dose found a significantly greater reduction after 7 to 14 days (MD −31.72 [−50.43, −13.01] mg per day, I2 = 71.4%, n = 228) in the combined therapy groups, with substantial heterogeneity.51,52,58,59
When the results for the placebo controlled and open label studies were pooled, in the 2 studies that reported total dose, there was no significant difference (MD −75.29 [−275.78, 125.20] mg, I2 = 45.5%, n = 141), with moderate heterogeneity. For the 3 studies that reported average dose per day, the pooled result showed no significant difference (MD −25.44 [−61.17, 10.28] mg per day, I2 = 97.0%, n = 244), with considerable heterogeneity. For analgesic maintenance dose, the pooled result of all 5 studies showed a significantly greater reduction in the groups that received CHMs for 7 to 14 days (MD −24.95 [−39.77, −10.14] mg per day, I2 = 71.7%, n = 305), with substantial heterogeneity.
Frequency of breakthrough pain
Five studies reported data on frequency of breakthrough pain, but the units were different. One placebo-controlled RCT of a topical CHM plus morphine sulfate sustained-release tablets showed greater reduction in average frequency per person (MD −1.41 [−2.20, −0.62], n = 77) in the combined therapy group after 7 days. 44 Two open-label RCTs showed a significantly greater reduction in average frequency per person per week (MD −1.21 [−1.49, −0.93], n = 100) after 28 days 50 and in average frequency per person from days 4 to 14 (MD −1.46 [−1.79, −1.13], n = 61) 58 in the groups that received the topical CHMs. However, another 2 open-label RCTs found no significant differences in average frequency per person per day after 10 days (MD −0.26 [−0.73, 0.21], n = 61) 55 or total frequency per person (MD −0.94 [−5.36, 3.48], n = 21). 61
Quality of life
Nine studies reported data on QOL (Figure 2(e)). For topical CHMs combined with conventional analgesic medications, one placebo-controlled study reported significant improvement on the National Comprehensive Cancer Network (NCCN) impact of pain measurement after 7 days (MD −5.24 [−10.33, −0.15], n = 77). 44 However, the single open-label study that used the NCCN impact of pain measurement found no significant difference between groups after 10 days (MD −5.84 [−13.92, 2.24], n = 61). 55 The pooled result for these 2 studies showed a significantly greater improvement in the groups that received the CHMs (MD −5.41 [−9.72, −1.10], I2 = 0%, n = 138), with no heterogeneity.
For the 30-item Quality of Life of Questionnaire for cancer patients (QLQ-C30), there were improvements in the pooled results of 3 open-label RCTs (MD 6.85 [1.80, 11.89], I2 = 65.5%, n = 185) in favor of the topical CHM groups at 7 to 28 days, but heterogeneity was substantial.50,52,59
The pooled result of 4 RCTs that used the Chinese QOL scale found no significant difference between groups after 7 days (MD 1.18 [−0.57, 2.93], I2 = 79.8%, n = 254), but the heterogeneity was considerable.49,51,57,60 In the subgroup analysis that included the three 7-day studies with NRS ≥ 4 or VAS ≥ 4 at baseline, there was less heterogeneity and a significantly greater improvement in the CHM groups (MD 1.86 [0.32, 3.40], I2 = 61.3%, n = 176).49,51,60
Karnofsky performance status
When the topical CHMs were combined with conventional analgesic medications (Figure 2(f)), there was no difference between groups in KPS after 7 days in the pooled result of 2 placebo-controlled RCTs (MD 0.62 [−1.86, 3.11], I2 = 0%, n = 137), with no heterogeneity.44,46 However, there was a significantly greater improvement in the pooled results of 8 open-label RCTs (MD 4.53 [1.13, 7.93], I 2= 82.4%, n = 506) after 7 to 28 days, although heterogeneity was considerable.49,51 -53,55,56,58,60
When all 10 studies were pooled, there was significant improvement in KPS after 7 to 28 days (MD 3.59 [0.96, 6.23], I2 = 78%, n = 643), but heterogeneity was considerable. The funnel plot showed no apparent asymmetry, and the Egger’s test was not significant, suggesting publication bias was not likely (Figure S1c).
In a subgroup analysis of the 6 studies that enrolled participants with moderate to severe pain (NRS ≥ 4 or VAS ≥ 4), the pooled result showed no difference between groups after 7–14 days with reduced heterogeneity (MD 2.91 [−0.07, 5.89], I2 = 60.3%, n = 366).49,51,52,55,58,60
Safety of Topical Chinese Herbal Medicines
Eight RCTs reported no adverse events (AEs) associated with topical CHMs during the study period. Skin reactions were reported in 7 RCTs, and in one study skin allergies led to 5 dropouts. 57 Except for one study that mentioned skin allergy due to the adhesive plaster, 44 AEs in the other 6 studies were likely due to the CHMs (Table S5). Overall, there were 35 dropouts: 19 in CHM groups and 16 in control groups.
Discussion
Summary of Meta-Analysis Results
Pain intensity was an outcome in all 20 studies of topical CHMs for cancer pain management. In the single comparison of CHM with a placebo, there was a greater reduction in pain intensity in the CHM group after 7 days of treatment. When CHM was compared with the analgesic tramadol, there was no significant difference between groups after 7 days (Figure 2(a)). The remaining 18 studies combined CHMs with conventional medications, and 3 of these studies used a placebo for the CHM in the control group to facilitate blinding. The pooled result for the 3 blinded studies (n = 217) showed a greater reduction in pain intensity after 7 days in the groups that received CHM, with moderate heterogeneity. This result appears to be the most meaningful, but, at an average of −0.48 points on the NRS, it was a small change. The pooled result for 14 open-label studies (n = 924) showed a greater reduction (MD −0.67 points), as did the pooled result of all 18 studies (MD −0.61 points) after 7 to 28 days, but heterogeneity was considerable in each of these pools. For specific topical formulations, the best available evidence was for Shuang bai san in pain due to liver cancer, but this was based on only 2 RCTs.
Analgesic onset time is another clinically important outcome, but it was only reported in 4 studies. When compared with oral tramadol, the topical CHM group showed an average decrease of 26.02 minutes (Figure 2(b)). In the comparisons between CHMs combined with conventional analgesics as integrative medicine and conventional analgesics alone, there was no significant difference in the single placebo-controlled study, but the pooled result of 2 open-label studies showed significantly reduced onset time in the CHM groups. These results are difficult to interpret, since the placebo-controlled study allowed a variety of options for analgesia based on the 3-step ladder. While the average onset time was 24.72 minutes faster in the CHM group, the standard deviation was wide, leading to no significant difference. In contrast, both open-label studies used morphine sulfate sustained-release tablets; the average onset time was 19.15 minutes faster, the standard deviations were narrower, and there was no heterogeneity in the pooled result. When we pooled the results of all 3 studies (n = 191), the result was significant in favor of adding topical CHMs, with an average of 19.17 minutes faster onset time, with no heterogeneity. Therefore, these results were not as contradictory as they appear and suggest that adding topical CHMs may result in faster cancer pain relief.
The same four seven-day studies that reported analgesic onset time also reported duration of analgesia. This was not different between groups in the comparison with oral tramadol, but the other 3 studies reported longer durations in the groups that received additional topical CHMs (Figure 2(c)). Notably, there was a significant difference in the placebo-controlled study and the pooled result of three seven-day studies (n = 192) that showed an average increase of 1.65 hours in duration of analgesia, with moderate heterogeneity.
Studies used different approaches to calculate analgesic dose. The largest meta-analysis pool was for 5 studies that reported analgesic maintenance dose (n = 305). This suggested an average reduction of about 25 mg (morphine equivalent) per day after 7 to 14 days (Figure 2(d)), but the heterogeneity was substantial. Data on frequency of breakthrough pain were reported in 5 studies, but no results were poolable. The only placebo-controlled study (n = 77) found a greater reduction in average frequency per person in the group that received the CHM in addition to morphine sulfate sustained-release tablets for 7 days.
Quality of life was reported in 9 studies, but 3 different measures were used (Figure 2(e)). One study that employed a placebo found a significant improvement on NCCN impact of pain after 7 days. The pooled result of 2 RCTs (n = 138) found a similar result after 7 to 10 days, without heterogeneity. For QLQ-C30, the pooled results of 3 open-label studies (n = 185) showed an average improvement of 6.85 points for combining topical CHMs with conventional analgesics. This difference appears clinically important, 62 but the study durations ranged from 7 to 28 days, and the heterogeneity was substantial. The meta-analysis of the Chinese QOL scale showed no significant difference between groups for all 4 RCTs (n = 254), with considerable heterogeneity. When only the 3 studies of participants with moderate to severe pain at baseline were included (n = 176), there was a significantly greater improvement in QOL after 7 days in the groups that received CHMs, with reduced heterogeneity. For KPS (Figure 2(f)), the pooled result of 2 placebo-controlled RCTs (n = 137) found no significant difference between groups after 7 days. This was not surprising, given the short duration of these studies. In the pooled result for all 10 RCTs, there was a greater improvement in the groups that received topical CHMs after 7 to 28 days, but the heterogeneity was considerable.
Interpretation of Meta-Analysis Results
In the meta-analysis pools, heterogeneity marred some estimates of the treatment effect. This was likely due to variation in the CHMs and conventional therapies used, along with variation in the duration of studies and in the type of cancer. Notably, 12 studies were of 7 days duration, while the remainder ranged from 8 to 28 days, and 14 studies included multiple cancer types. Where possible, subgroup analyses were used to address these variations; there were some reductions in heterogeneity, but this was at the expense of sample size.
In most studies, the CHM was used in addition to conventional pain management. In some studies, the conventional medication was fixed, but in others the 3-step approach was used, so a range of oral and/or injected medications may have been administered. Considering that active pain management was used, and was likely to have been effective, there may have been little scope for the additional CHMs to further reduce pain intensity, hence the small change in the NRS. For analgesic onset time, the addition of the CHMs appeared to reduce this by an average of 19.17 minutes when compared to oral medication, probably due to the relatively faster effect of the topical route. For duration of analgesia, the average increase of 1.65 hours in the pooled result of 3 studies suggested that the effects of the topical CHMs were not transient and may have complemented the effects of the conventional analgesics. The mixed results for frequency of breakthrough pain are difficult to interpret, except to say that the CHMs were focused on pain relief, were applied according to the trial protocols, and would not have affected the course of the disease. Also, it is probable that breakthrough pain events were managed according to the individual’s condition using additional opioids or other interventions as needed. The studies did not mention the application of additional CHMs to manage breakthrough pain events.
Concerns have been expressed about possible interactions between herbal medicines and conventional medications. 63 In this review, no serious AEs were reported, but the safety data were inadequate in some studies. The total dropout rates were similar between groups even though some topical CHMs were associated with skin reactions. This points to a need for test patches to assess individual reactions. Assessments of drug–herb interactions may not have been feasible in some studies due to their short duration, but this aspect needs to be considered in future studies.
Biological Activities of the Main Ingredients
There has been considerable research into the analgesic effects of compounds, notably alkaloids, contained in CHMs. 64 The included studies often tested multi-ingredient formulations, each of which contained multiple compounds, so it was not possible to determine which, if any, contributed to the clinical effect. In addition, the studies of multi-ingredient topical formulations generally did not provide the rationale for including each ingredient, so we cannot be certain that they were intended to exert anti-nociceptive effects. However, we can infer some reasons based on traditional use and published studies. Due to the large number of ingredients in the formulations, we have limited this discussion to the 3 most common (Table S3) and to papers published in English.
The most frequently included ingredient, borneol (bing pian), can be used in topical formulations as a skin penetration enhancer. Borneol is non-toxic and non-irritating with a long history of use in CHM. Animal studies have shown it enhances permeation and drug delivery across the brain capillary endothelium, stratum corneum, corneal epithelium, and mucosal membranes. 65 In a comparison with the standard skin penetration enhancer laurocapram (Azone ®), borneol showed low skin irritation potential and promoted penetration of multiple pharmaceuticals. 66 In addition, borneol has anti-nociceptive effects. A clinical study of 122 postoperative patients found that when compared to placebo, a single topical application of 25% (+)-borneol for approximately 30 to 60 minutes showed a significantly greater reduction in postoperative pain on a VAS, without any adverse effects. 67 In mouse models of inflammation and pain, borneol reduced pain but did not produce a sedative effect. 68 In mice with neuropathic pain, the systemic or local administration of (+)-borneol reduced pain responses without inhibition of motor function.69,70
The second most common ingredient was xi xin. The official sources are Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim) Kitag., Asarum sieboldii Miq. var. seoulense Nakai, and Asarum sieboldii Miq. 27 No human studies of this ingredient alone were located, but animal studies have demonstrated anti-nociceptive actions for its extracts.71,72 Methyl eugenol is a major component of the essential oil of Asarum species, and asarinin is a major lignin.73,74 In mice, methyl eugenol inhibited pain-related behaviors. 75 In a series of experiments in rats, an alkamide-enriched fraction of an aqueous extract of Asarum heterotropoides var. mandshuricum, its essential oil, and the compound asarinin all showed reductions in pain-related behaviors. 76
The third most common herb ingredient in the topical CHMs, yan hu suo, is sourced from the rhizomes of Corydalis yanhusuo W.T. Wang.27,77 Of the alkaloids from yan hu suo, levo-tetrahydropalmatine (l-THP) has received research attention in humans and animals for its effects on pain and drug addiction.78,79 Studies in mice have shown dose-dependent reductions in pain responses.80 -83 No human studies of the following 3 compounds derived Corydalis yanhusuo were located. The alkaloid dehydrocorybulbine (DHCB), showed dose-dependent antinociceptive activity in mice without showing sedative effects or tolerance. 84 In rodent models of cancer pain, levo-corydalmine (l-CDL) showed antinociceptive effects,85 -87 and dehydrocorydaline (DHC) showed reductions in pain-related behaviors in mice.88,89
This brief review of the 3 most frequently included herbs showed anti-nociceptive effects in multiple experimental studies. In addition, there has been considerable research into the other herbs used frequently in the topical CHMs. 90 This experimental evidence suggests the effects reported in the clinical studies may have plausible physiological bases.
Strengths and Limitations of This Review
A strength of this review was the comprehensive nature of the searches, which included multiple databases and clinical trial registries. Therefore, the data set was as complete as was feasible. Further strengths were the inclusion criteria, which required a pathological diagnosis of cancer, the presence of pain at baseline, and assessments using established outcome measures. These aspects ensured the studies all measured pain associated with cancer, and the measurement methods were meaningful. A strength of our approach to meta-analysis was the subgrouping (where possible) of placebo-controlled studies and open-label studies. In addition, we provided a subgroup (where possible) for the studies that required at least moderate pain severity at baseline, since such studies were more likely to provide clinically meaningful assessments. A major limitation was the lack of blinding in all but the 4 participant-blind studies. All studies were judged high risk for blinding of personnel, which we acknowledge could be difficult when a topical preparation was used, but it appears that assessments were not blinded even though this would be feasible. Furthermore, only one study provided a protocol. These deficiencies reduced our confidence in the meta-analysis results. Future studies of topical CHMs should employ blinding where feasible and adhere to established standards for clinical trial reporting. 91
Conclusion
Overall, the evidence suggested that the addition of some topical CHM preparations to conventional therapies may assist in the alleviation of cancer pain in the short term, without major safety concerns. Considering that people with cancer pain should be involved in decisions regarding the application of multimodal approaches to the management of their pain, 5 healthcare professionals could consider patient requests to use topical CHM preparations as part of their pain management plan when the CHM has been clinically evaluated.
The results of the 20 included RCTs suggest that the topical application of certain traditional CHMs used in east Asia improved scores on pain intensity, onset, and duration in people with cancer-related pain in comparison with placebo and when used as additions to conventional analgesic medications. The CHMs included ingredients that have received research attention for their effects on pain. Future studies could consider including these ingredients in test formulations. Further rigorously designed studies are needed to investigate the possible roles of these topical CHMs as additions to cancer pain management.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354231210870 for Topical Traditional Chinese Medicines for Cancer Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials by Yihong Liu, Brian H. May, Anna J. Hyde, Yihan He, Xinfeng Guo, Anthony Lin Zhang, Chuanjian Lu, Charlie Changli Xue and Haibo Zhang in Integrative Cancer Therapies
Acknowledgments
We wish to thank Dr Iris WY Zhou and Dr Meaghan Coyle for their assistance with searches, and Louise Pobjoy and Dr Meaghan Coyle for their comments and assistance with editing.
Footnotes
Author Contributions: Searches, data extraction and data analyses were conducted by Yihong Liu and Brian H. May. The manuscript was drafted by Yihong Liu, Brian H. May and Anna J Hyde with input from all other authors during the revision process. Yihan He assisted in the searches and data evaluation. Anthony Lin Zhang and Xinfeng Guo provided expert contributions in methodology and data interpretation. Charlie C. Xue, Chuanjian Lu and Haibo Zhang provided substantial contributions to the conception, design and supervision of the project and critical revision of the manuscript. Haibo Zhang provided expert commentary on clinical aspects and interpretation of results during the production of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support was provided by the China-Australia International Research Centre for Chinese Medicine (CAIRCCM)—a joint initiative of RMIT University, Australia and Guangdong Provincial Academy of Chinese Medical Sciences, China; the National Natural Science Foundation of China (82004447); Guangdong Basic and Applied Basic Research Foundation (2021A1515011597); the Research Fund for Zhaoyang Talents of Guangdong Provincial Hospital of Chinese Medicine (ZY2022KY07); and the Research Fund of Qingmiao Talents of Guangdong Provincial Hospital of Chinese Medicine (SZ2022Q03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Statement: No ethical approval was required. This study involved no participation of human or nonhuman subjects.
ORCID iD: Haibo Zhang  https://orcid.org/0000-0001-8104-4280
https://orcid.org/0000-0001-8104-4280
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Yoon SY, Oh J. Neuropathic cancer pain: prevalence, pathophysiology, and management. Korean J Intern Med. 2018;33(6):1058-1069. doi: 10.3904/kjim.2018.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamieh N, Akel R, Anouti B, et al. Cancer-related pain: prevalence, severity and management in a tertiary care center in the Middle East. Asian Pac J Cancer Prev. 2018;19(3):769-775. doi: 10.22034/APJCP.2018.19.3.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fallon M, Giusti R, Aielli F, et al. ; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl 4):iv166-iv191. doi: 10.1093/annonc/mdy152 [DOI] [PubMed] [Google Scholar]
- 4. van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag. 2016;51(6):1070-1090.e9. doi: 10.1016/j.jpainsymman.2015.12.340 [DOI] [PubMed] [Google Scholar]
- 5. Bennett MI, Eisenberg E, Ahmedzai SH, et al. Standards for the management of cancer-related pain across Europe-A position paper from the EFIC Task Force on Cancer Pain. Eur J Pain. 2019;23(4):660-668. doi: 10.1002/ejp.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. World Health Organization; 2018. [PubMed] [Google Scholar]
- 7. Thapa D, Rastogi V, Ahuja V. Cancer pain management-current status. J Anaesthesiol Clin Pharmacol. 2011;27(2):162-168. doi: 10.4103/0970-9185.81820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Derry S, Wiffen PJ, Kalso EA, et al. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;5:CD008609. doi: 10.1002/14651858.CD008609.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paisley P, Serpell M. The role of topiceuticals in cancer pain. Curr Opin Support Palliat Care. 2017;11(2):93-98. doi: 10.1097/SPC.0000000000000271 [DOI] [PubMed] [Google Scholar]
- 10. Winegarden JA, Carr DB, Bradshaw YS. Topical ketamine with other adjuvants: underutilized for refractory cancer pain? A case series and suggested revision of the World Health Organization stepladder for cancer pain. J Palliat Med. 2020;23(9):1167-1171. doi: 10.1089/jpm.2019.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas EM, Weiss SM. Nonpharmacological interventions with chronic cancer pain in adults. Cancer Control. 2000;7(2):157-164. doi: 10.1177/107327480000700206 [DOI] [PubMed] [Google Scholar]
- 12. Duncan M, Moschopoulou E, Herrington E, et al. ; SURECAN Investigators. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open. 2017;7(11):e015860. doi: 10.1136/bmjopen-2017-015860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Portenoy RK. Treatment of cancer pain. Lancet. 2011;377(9784):2236-2247. doi: 10.1016/S0140-6736(11)60236-5 [DOI] [PubMed] [Google Scholar]
- 14. Deng G. Integrative medicine therapies for pain management in cancer patients. Cancer. 2019;25(5):343-348. doi: 10.1097/PPO.0000000000000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pugh TM, Squarize F, Kiser AL. A comprehensive strategy to pain management for cancer patients in an inpatient rehabilitation facility. Front Pain Res. 2021;2:688511. doi: 10.3389/fpain.2021.688511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He Y, Guo X, May BH, et al. Clinical evidence for association of acupuncture and acupressure with improved cancer pain: a systematic review and meta-analysis. JAMA Oncol. 2020;6(2):271-278. doi: 10.1001/jamaoncol.2019.5233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He Y, Zhang H, Li Y, et al. Acupuncture combined with opioids for cancer pain: a pilot pragmatic randomized controlled trial. Acupunct Med. 2022;40(2):133-141. doi: 10.1177/09645284211056016 [DOI] [PubMed] [Google Scholar]
- 18. Chwistek M. Recent advances in understanding and managing cancer pain. F1000Res. 2017;6:945. doi: 10.12688/f1000research.10817.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gouveia DN, Guimarães AG, Santos WBDR, Quintans-Júnior LJ. Natural products as a perspective for cancer pain management: a systematic review. Phytomedicine. 2019;58:152766. doi: 10.1016/j.phymed.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 20. Quintans JS, Antoniolli AR, Almeida JR, Santana-Filho VJ, Quintans-Júnior LJ. Natural products evaluated in neuropathic pain models - a systematic review. Basic Clin Pharmacol Toxicol. 2014;114(6):442-450. doi: 10.1111/bcpt.12178 [DOI] [PubMed] [Google Scholar]
- 21. Scuteri D, Hamamura K, Sakurada T, et al. Efficacy of essential oils in pain: a systematic review and meta-analysis of preclinical evidence. Front Pharmacol. 2021;12:640128. doi: 10.3389/fphar.2021.640128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1:80. doi: 10.4103/2152-7806.73804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part one: skin physiology and delivery systems. Pain Ther. 2015;4(1):17-32. doi: 10.1007/s40122-015-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part two: treatments and applications. Pain Ther. 2015;4(1):33-50. doi: 10.1007/s40122-015-0032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tayeb BO, Winegarden JA, Alashari RA, et al. Scoping review of off-label topical analgesia in palliative, hospice and cancer care: towards flexibility in evidence-based medicine. J Pain Res. 2021;14:3003-3009. doi: 10.2147/JPR.S263845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adams JD, Wang X. Control of pain with topical plant medicines. Asian Pac J Trop Biomed. 2015;5(4):268-273. [Google Scholar]
- 27. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China [Zhong Hua Ren Min Gong He Guo Yao Dian]. China Medical Science Press; 2015. [Google Scholar]
- 28. Lee J-W, Lee WB, Kim W, et al. Traditional herbal medicine for cancer pain: a systematic review and meta-analysis. Complement Ther Med. 2015;23(2):265-274. [DOI] [PubMed] [Google Scholar]
- 29. Jo HG, Seo J, Choi S, Lee D. East Asian herbal medicine to reduce primary pain and adverse events in Cancer Patients : a systematic review and meta-analysis with association rule mining to identify core herb combination. Front Pharmacol. 2021;12:800571. doi: 10.3389/fphar.2021.800571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Z, Tan W, Lian B, et al. Systematic review of external application of traditional Chinese medicine combined with three-step analgesia in the treatment of cancer pain. Liaoning J Tradit Chin Med. 2016;43(9):1816-1821. [Google Scholar]
- 31. May BH, Liu YH. Clinical evidence for Chinese herbal medicine. In: Xue CC, Lu CJ. (eds) Evidence-Based Clinical Chinese Medicine - Volume 18: Cancer Pain. World Scientific Publishing Co; 2020;89-151. [Google Scholar]
- 32. Wang J, Lu T, Wang M, et al. Cancer pain treated by external application of Chinese medicine to painful points combined with three-step pain relief method: a meta-analysis. Chin Med Emergentology. 2020;29(4):608-612. [Google Scholar]
- 33. Yan X, Yan Z, Liu W, et al. External application of traditional Chinese medicine in the treatment of bone cancer pain: a meta-analysis. Support Care Cancer. 2016;24(1):11-17. doi: 10.1007/s00520-015-2737-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liou KT, Chen C, Emard N, et al. Herbal topical analgesic for pain management: perspectives from cancer patients. Pain Med. 2021;22(6):1435-1440. doi: 10.1093/pm/pnab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang X, Tan R, Lam WC, et al. PRISMA (Preferred reporting items for systematic reviews and meta-analyses) extension for Chinese herbal medicines 2020 (PRISMA-CHM 2020). Am J Chin Med. 2020;48(6):1279-1313. doi: 10.1142/S0192415X20500639 [DOI] [PubMed] [Google Scholar]
- 38. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 39. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of Liver Diseases. Hepatology. 2018;68(2):723-750. [DOI] [PubMed] [Google Scholar]
- 41. European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. [DOI] [PubMed] [Google Scholar]
- 42. Feng L. The Effect of Shuang Bai San on Relieving Mild Pain in Patients with Primary Hepatic Carcinoma. [in Chinese] Guangzhou University of Chinese Medicine; 2012. [Google Scholar]
- 43. Zhang Y. Intervention Using Hua-jian-ba-du Membrane for Cancer Pain and Its Analgesic Mechanisms. [in Chinese] Tianjin University of Traditional Chinese Medicine; 2004. [Google Scholar]
- 44. Tang Q. Clinical Study on the Adjuvant Treatment of Local Cancer Pain with Bing Chong Zhi Tong Gao. [in Chinese] Beijing University of Chinese Medicine; 2013. [Google Scholar]
- 45. Wang Y, Wang X, Li R, Hui J. Clinical trial of Wuxiang Tongxiao paste for cancer pain. J Tradit Chin Med. 2014;33(3):188-189. [Google Scholar]
- 46. Song L, Jiang Y, Wang R. Clinical observation of Xiaopizhentong Gao combined with oxycodone for lung cancer patients with severe pain. J Tradit Chin Med. 2017;58(21):1846-1849. [Google Scholar]
- 47. Liu P. Clinical trial of Die da Gao combined with pamidronate disodium for bone metastasis pain. Pr Clin J Integr Tradit Chin West -med. 2015;15(1):31-32. [Google Scholar]
- 48. Liu Z. Evaluation of the clinical effects of external application of Shuangbai powder in the treatment of mild liver cancer pain. Chin J Mod Drug Appl. 2017;11(3):177-179. [Google Scholar]
- 49. Li G. Treatment of Moderate-Severe Cancer Pain with External Traditional Chinese Medicine (Zhengtong I) plus Analgesic Guidelines Published by WHO Based on the 3-Step Ladder. [in Chinese] Guangzhou University of Chinese Medicine; 2006. [Google Scholar]
- 50. Che J, Xu Y, Cao W, et al. Effects of intrathecal injection of opioids combined with traditional Chinese medicine acupoint application on moderate and severe cancer pain, sleep and quality of life in patients with advanced malignant tumors. Mod J Integr Tradit Chin West -med. 2018;27(18):1949-1953. [Google Scholar]
- 51. Ding Z. Clinical Research on Xiaojizhitong Plaster with MS Contin in the Treatment of Moderate to Severe Cancer Pain [in Chinese]. Hubei University of Chinese Medicine; 2015. [Google Scholar]
- 52. Huang Y. Clinical Research on Xiaozheng Zhitong Paste with Oxycontin in Moderate and Severe Cancer Pain [in Chinese]. Nanjing University of Chinese Medicine; 2013. [Google Scholar]
- 53. Li C, Luo X, Wang Q, et al. Clinical research on Jianzhongxiao cataplasm in the treatment of cancer pain. J Hubei Univ Chin Med. 2016;18(3):87-89. [Google Scholar]
- 54. Luo W, Luo Z. Clinical observation of Shuluo Zhitong Gao in the treatment of cancer pain. Chin J Tradit -med Sci Technol. 2020;27(5):791-792. [Google Scholar]
- 55. Tian J. Clinical Observation of Gutong Tie for Cancerous Somatic Pain Patients with Stagnation of Yin-Cold [in Chinese] Beijing University of Chinese Medicine; 2015. [Google Scholar]
- 56. Wang Y, Li Y, Wei Z. Clinical observation of Aitongxiao cataplasm for ovarian cancer pain. China’s Naturop. 2016;24(8):29-30. [Google Scholar]
- 57. Xu L. Clinical Research into the Treatment of Cancer Pain with External Traditional Chinese Medicine (Wushen Tincture) [in Chinese] Guangzhou University of Chinese Medicine; 2013. [Google Scholar]
- 58. Zhao M. Clinical Study of Xiao Zhong Zhitong San Combined with Morphine Hydrochloride Sustained-Release Tablets in Treating Moderate and Severe Cancer Pain [in Chinese]. Southwest Medical University; 2018. [Google Scholar]
- 59. Zhao W. Clinical Trial of Zhitong San for Cancer Pain [in Chinese]. Beijing University of Chinese Medicine; 2016. [Google Scholar]
- 60. Zhong X. Clinical Trial of Qizheng Xiaotong Tie Combined with MS Contin for Cancer Pain [in Chinese]. Beijing University of Chinese Medicine; 2009. [Google Scholar]
- 61. Zhou D, Dong C, Xu L, et al. Clinical observation on external application of Longteng Tongluo Tincture combined with three-step analgesic ladder in treating cancer pain. Shanghai J TCM. 2020;54(11):38-42. [Google Scholar]
- 62. Giesinger JM, Kuijpers W, Young T, et al. Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes. 2016;14:87. doi: 10.1186/s12955-016-0489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jahromi B, Pirvulescu I, Candido KD, Knezevic NN. Herbal medicine for pain management: efficacy and drug interactions. Pharmaceutics. 2021;13(2):251. doi: 10.3390/pharmaceutics13020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jiang W, Tang M, Yang L, et al. Analgesic alkaloids derived from traditional Chinese medicine in pain management. Front Pharmacol. 2022;13:851508. doi: 10.3389/fphar.2022.851508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kulkarni M, Sawant N, Kolapkar A, Huprikar A, Desai N. Borneol: a promising monoterpenoid in enhancing drug delivery across various physiological barriers. AAPS PharmSciTech. 2021;22(4):145. doi: 10.1208/s12249-021-01999-8 [DOI] [PubMed] [Google Scholar]
- 66. Yi QF, Yan J, Tang S-Y, Huang H, Kang LY. Effect of borneol on the transdermal permeation of drugs with differing lipophilicity and molecular organization of stratum corneum lipids. Drug Dev Ind Pharm. 2016;42(7):1086-1093. doi: 10.3109/03639045.2015.1107095 [DOI] [PubMed] [Google Scholar]
- 67. Wang S, Zhang D, Hu J, et al. A clinical and mechanistic study of topical borneol-induced analgesia. EMBO Mol Med. 2017;9(6):802-815. doi: 10.15252/emmm.201607300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Almeida JR, Souza GR, Silva JC, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. ScientificWorldJournal. 2013;2013:808460. doi: 10.1155/2013/808460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang J, Shen YY, Li J, et al. (+)-Borneol alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Eur J Pharmacol. 2015;757:53-58. doi: 10.1016/j.ejphar.2015.03.056 [DOI] [PubMed] [Google Scholar]
- 70. Zhou HH, Zhang L, Zhou QG, Fang Y, Ge WH. (+)-Borneol attenuates oxaliplatin-induced neuropathic hyperalgesia in mice. Neuroreport. 2016;27(3):160-165. doi: 10.1097/WNR.0000000000000516 [DOI] [PubMed] [Google Scholar]
- 71. Kim S-J, Gao Zhang C, Taek Lim J. Mechanism of anti-nociceptive effects of Asarum sieboldii miq. Radix: potential role of bradykinin, histamine and opioid receptor-mediated pathways. J Ethnopharmacol. 2003;88(1):5-9. doi: 10.1016/s0378-8741(03)00181-8 [DOI] [PubMed] [Google Scholar]
- 72. Suzuki Y, Yuzurihara M, Hibino T, Yano S, Kase Y. Aqueous extract of asiasari radix inhibits formalin-induced hyperalgesia via NMDA receptors. J Ethnopharmacol. 2009;123(1):128-133. doi: 10.1016/j.jep.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 73. Li C, Xu F, Cao C, et al. Comparative analysis of two species of Asari Radix et Rhizoma by electronic nose, headspace GC-MS and chemometrics. J Pharm Biomed Anal. 2013;85:231-238. doi: 10.1016/j.jpba.2013.07.034 [DOI] [PubMed] [Google Scholar]
- 74. Liu H, Wang C. The genus Asarum: a review on phytochemistry, ethnopharmacology, toxicology and pharmacokinetics. J Ethnopharmacol. 2022;282:114642. doi: 10.1016/j.jep.2021.114642 [DOI] [PubMed] [Google Scholar]
- 75. Yano S, Suzuki Y, Yuzurihara M, et al. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur J Pharmacol. 2006;553(1-3):99-103. doi: 10.1016/j.ejphar.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 76. Liu H, Li S, Huan X, et al. The antinociceptive and anti-inflammatory potential and pharmacokinetic study of significant alkamides ingredients from Asarum linn. J Ethnopharmacol. 2022;297:115569. doi: 10.1016/j.jep.2022.115569 [DOI] [PubMed] [Google Scholar]
- 77. Bensky D, Clavey S, Stöger E. (eds.). Chinese Herbal Medicine: Materia Medica, 3rd ed. Eastland Press; 2004. [Google Scholar]
- 78. Chu H, Jin G, Friedman E, Zhen X. Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol. 2008;28(4):491-499. doi: 10.1007/s10571-007-9179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang JB, Mantsch JR. L-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Med Chem. 2012;4(2):177-186. doi: 10.4155/fmc.11.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guo Z, Man Y, Wang X, et al. Levo-tetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci Rep. 2014;4:3905. doi: 10.1038/srep03905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhou H-H, Wu DL, Gao L-Y, Fang Y, Ge WH. L-tetrahydropalmatine alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Neuroreport. 2016;27(7):476-480. doi: 10.1097/WNR.0000000000000560 [DOI] [PubMed] [Google Scholar]
- 82. Kang D-W, Moon J-Y, Choi J-G, et al. Antinociceptive profile of Levo-tetrahydropalmatine in acute and chronic pain mice models: role of spinal sigma-1 receptor. Sci Rep. 2016;6:37850. doi: 10.1038/srep37850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang MY, Liu YP, Zhang LY, et al. Levo-tetrahydropalmatine attenuates bone cancer pain by inhibiting microglial cells activation. Mediators Inflamm. 2015;2015:752512. doi: 10.1155/2015/752512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Y, Wang C, Wang L, et al. A novel analgesic isolated from a traditional Chinese medicine. Curr Biol. 2014;24(2):117-123. doi: 10.1016/j.cub.2013.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dai W-L, Yan B, Jiang N, et al. Simultaneous inhibition of NMDA and mGlu1/5 receptors by levo-corydalmine in rat spinal cord attenuates bone cancer pain. Int J Cancer. 2017;141(4):805-815. doi: 10.1002/ijc.30780 [DOI] [PubMed] [Google Scholar]
- 86. Hu Y, Kodithuwakku N, Zhou L, et al. Levo-corydalmine alleviates neuropathic cancer pain induced by tumor compression via the CCL2/CCR2 pathway. Molecules. 2017;22(6):937. doi: 10.3390/molecules22060937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou L, Hu Y, Li C, et al. Levo-corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway. Neuropharmacol. 2018;135:34-47. doi: 10.1016/j.neuropharm.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 88. Yin Z-Y, Li L, Chu S-S, et al. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci Rep. 2016;6:27129. doi: 10.1038/srep27129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huo W, Zhang Y, Liu Y, et al. Dehydrocorydaline attenuates bone cancer pain by shifting microglial M1/M2 polarization toward the M2 phenotype. Mol Pain. 2018;14:1744806918781733. doi: 10.1177/1744806918781733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. May BH, Liu YH. Pharmacological actions of the common herbs. In: Xue CC, Lu CJ. (eds) Evidence-Based Clinical Chinese Medicine - Volume 18: Cancer Pain. World Scientific Publishing Co; 2020;89-151. [Google Scholar]
- 91. Bian Z, Liu B, Moher D, et al. Consolidated standards of reporting trials (CONSORT) for traditional Chinese medicine: current situation and future development. Front Med. 2011;5(2):171-177. doi: 10.1007/s11684-011-0132-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354231210870 for Topical Traditional Chinese Medicines for Cancer Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials by Yihong Liu, Brian H. May, Anna J. Hyde, Yihan He, Xinfeng Guo, Anthony Lin Zhang, Chuanjian Lu, Charlie Changli Xue and Haibo Zhang in Integrative Cancer Therapies