Abstract
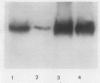
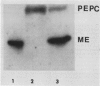
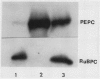
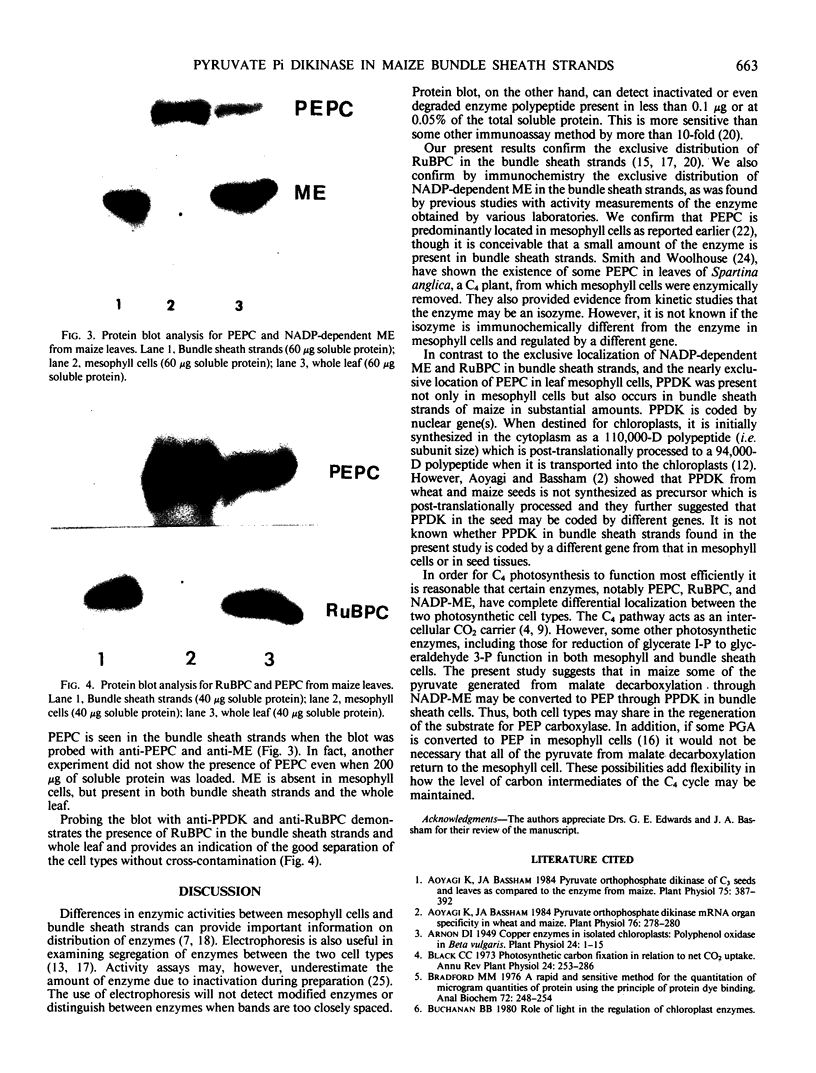
Mesophyll protoplasts and bundle sheath strands were isolated from maize leaves. Light microscopic observation showed the preparations were pure and without cross contamination. Protein blot analysis of mesophyll and bundle sheath cell soluble protein showed that the concentration of pyruvate orthophosphate dikinase (EC 2.7.9.1) is about one-tenth as much in the bundle sheath cells as in mesophyll cells, but about eight times greater than that found in wheat leaves, on the basis of soluble protein. Phosphoenolpyruvate carboxylase (EC 4.1.1.31) was barely detectable in the bundle sheath cells, while ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39) and NADP-dependent malic enzyme (EC 1.3.1.37) were exclusively present in the bundle sheath cells and were absent in the mesophyll cells. Whereas pyruvate, Pi dikinase was previously considered localized only in mesophyll cells of C4 plants, these results clearly demonstrate the presence of appreciable quantities of the enzyme in the bundle sheath cells of the C4 species maize.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase mRNA organ specificity in wheat and maize. Plant Physiol. 1984 Sep;76(1):278–280. doi: 10.1104/pp.76.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase of c(3) seeds and leaves as compared to the enzyme from maize. Plant Physiol. 1984 Jun;75(2):387–392. doi: 10.1104/pp.75.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M. L., Melis A. Localization of photosynthetic electron transport components in mesophyll and bundle sheath chloroplasts of Zea mays. Arch Biochem Biophys. 1983 Jul 1;224(1):19–28. doi: 10.1016/0003-9861(83)90186-8. [DOI] [PubMed] [Google Scholar]
- Hague D. R., Uhler M., Collins P. D. Cloning of cDNA for pyruvate, Pi dikinase from maize leaves. Nucleic Acids Res. 1983 Jul 25;11(14):4853–4865. doi: 10.1093/nar/11.14.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. A., Black C. C. Two-Dimensional Electrophoretic Mapping of Proteins of Bundle Sheath and Mesophyll Cells of the C(4) Grass Digitaria sanguinalis (L.) Scop. (Crabgrass). Plant Physiol. 1982 Nov;70(5):1359–1366. doi: 10.1104/pp.70.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Regulation of oxaloacetate, aspartate, and malate formation in mesophyll protoplast extracts of three types of c(4) plants. Plant Physiol. 1975 Aug;56(2):324–331. doi: 10.1104/pp.56.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Hall T. C., Edwards G. E. Differential Localization of Fraction I Protein between Chloroplast Types. Plant Physiol. 1976 May;57(5):730–733. doi: 10.1104/pp.57.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Harpster M. H., Mayfield S. P., Taylor W. C. Light-regulated gene expression during maize leaf development. J Cell Biol. 1984 Feb;98(2):558–564. doi: 10.1083/jcb.98.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]
- Usuda H., Ku M. S., Edwards G. E. Activation of NADP-Malate Dehydrogenase, Pyruvate,Pi Dikinase, and Fructose 1,6-Bisphosphatase in Relation to Photosynthetic Rate in Maize. Plant Physiol. 1984 Sep;76(1):238–243. doi: 10.1104/pp.76.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]