Abstract
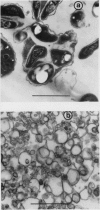
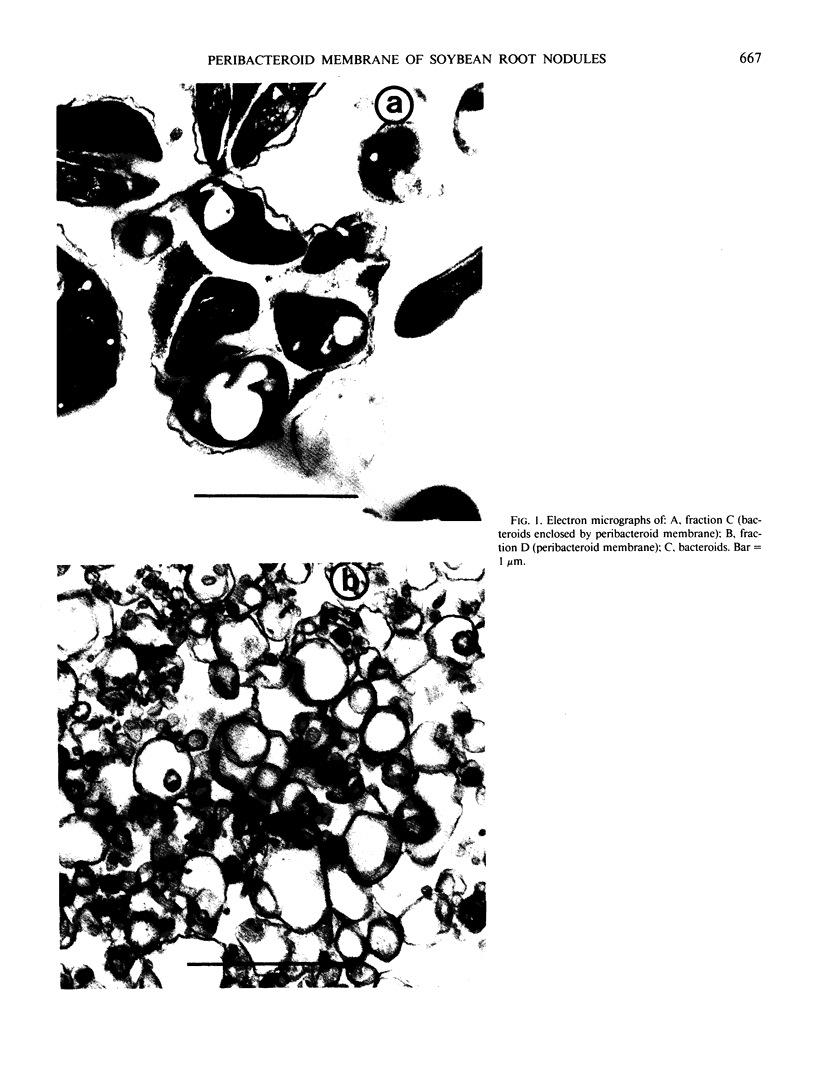
An improved method is described for the isolation of membrane envelope enclosing the bacteroids (peribacteroid membrane) from soybean (Glycine max L.) root nodules. The ATPase activity of the peribacteroid membrane from infected roots is compared with that of the plasma membrane from uninfected roots. The two ATPases are similar in terms of their vanadate sensitivities, pH optima, and mineral cation requirements, and show antigenic cross-reactivity. However, the ATPase of peribacteroid membrane is more sensitive to stimulation by NH4+. ATP-dependent proton translocation across the peribacteroid membrane was demonstrated in broken protoplasts of infected cells, by the use of fluorescence microscopy with acridine orange. It is suggested that acidification of the peribacteroid space by the peribacteroid membrane ATPase results in the conversion of NH3 to NH4+ in this space and thereby facilitates the removal of fixed-nitrogen from the bacteroid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Kennedy R. M. Adenosine triphosphatase from soybean callus and root cells. Plant Physiol. 1977 Feb;59(2):264–267. doi: 10.1104/pp.59.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. F., Morré D. J. Action and Inhibition of Endogenous Phospholipases during Isolation of Plant Membranes. Plant Physiol. 1978 Dec;62(6):933–937. doi: 10.1104/pp.62.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Kazazian V., Zogbi V., Bal A. K. Isolation and characterization of the membrane envelope enclosing the bacteroids in soybean root nodules. J Cell Biol. 1978 Sep;78(3):919–936. doi: 10.1083/jcb.78.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]