Abstract
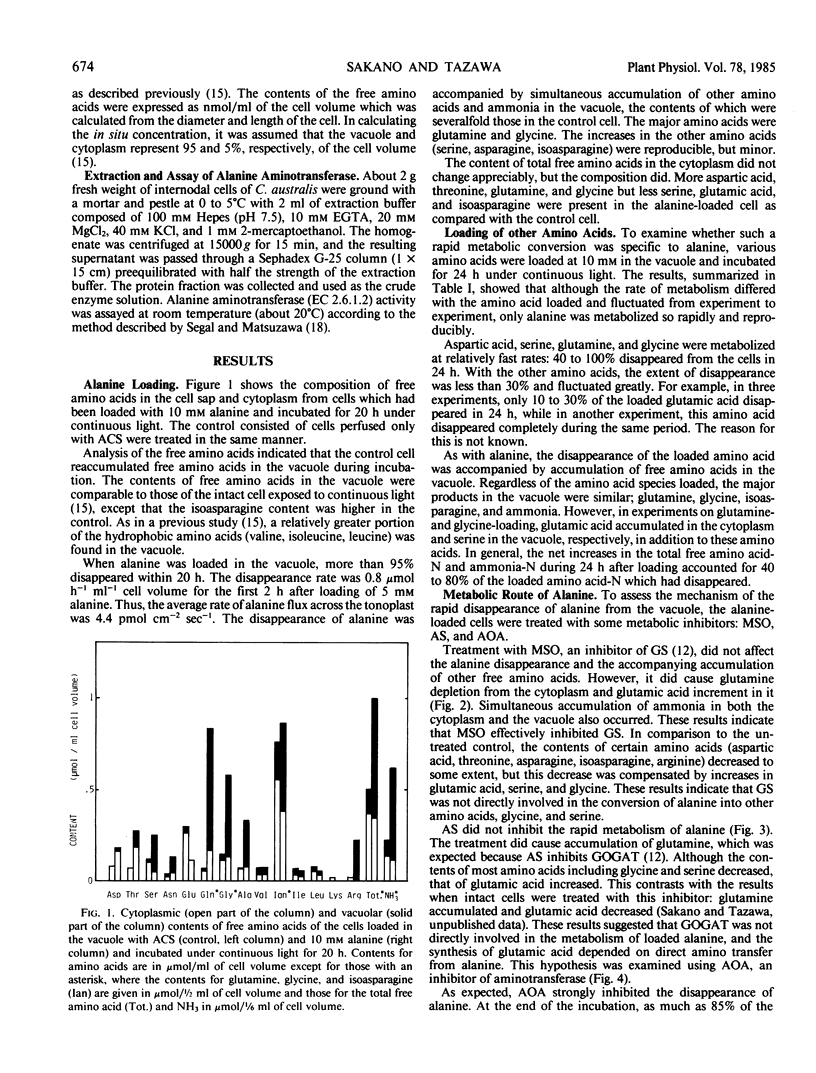
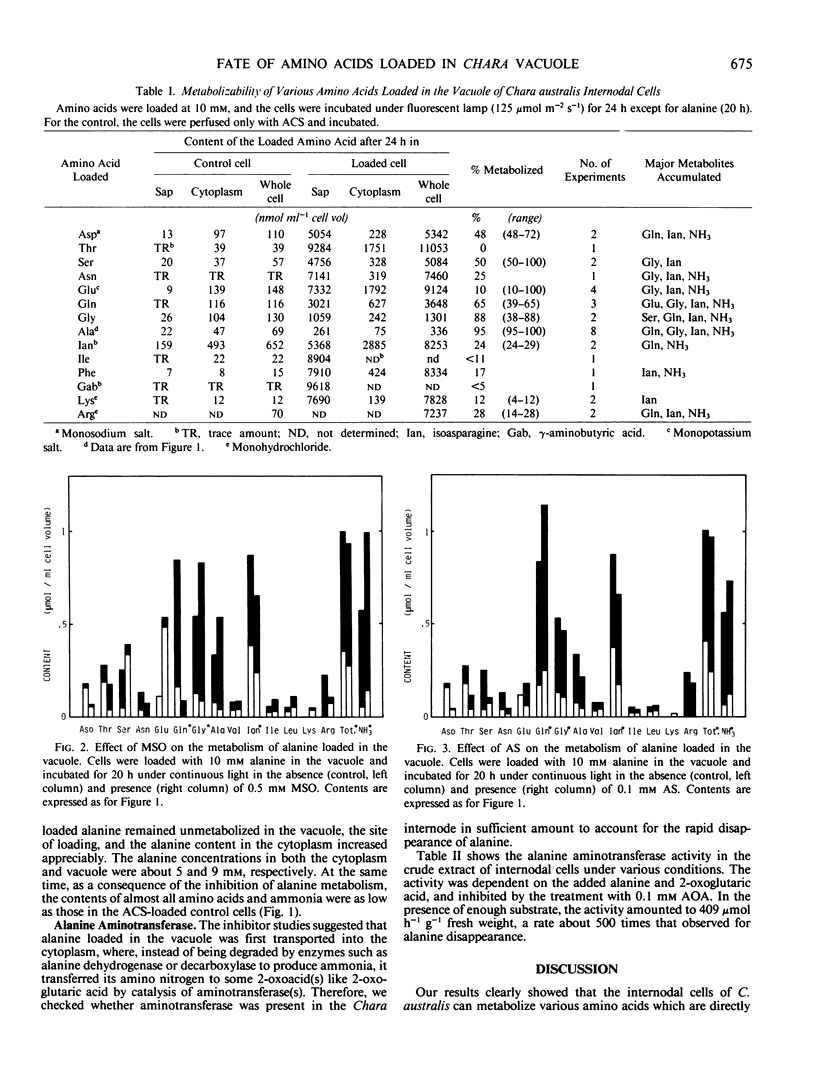
Vacuoles of internodal cells of Chara australis (or Chara corallina) were loaded with a 10 millimolar amount of various amino acids by a perfusion method and incubated under continuous light. After 20 to 24 hours, the cell sap was collected, and free amino acids in it and the rest of the cell (cytoplasm) were analyzed. The only amino acid metabolized completely was alanine. About 40 to 80% of the aspartic acid, glutamine, serine, and glycine were metabolized, whereas less than 30% of the threonine, asparagine, isoasparagine, isoleucine, phenylalanine, γ-aminobutyric acid, lysine, and arginine were metabolized. The figure for glutamic acid fluctuated between 10 and 100%. The main metabolites of alanine were glutamine, glycine and ammonia, which accumulated in the vacuole. Alanine utilization was not affected by l-methionine-d,l-sulfoximine or azaserine, but was strongly inhibited by aminooxyacetate. The cell extract contained enough alanine aminotransferase activity to account for the rate of alanine metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boudet A. M., Canut H., Alibert G. Isolation and Characterization of Vacuoles from Melilotus alba Mesophyll. Plant Physiol. 1981 Dec;68(6):1354–1358. doi: 10.1104/pp.68.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman J. M., Key J. L. Inactive and protein precursor pools of amino acids in the soybean hypocotyl. Plant Physiol. 1967 Jan;42(1):29–36. doi: 10.1104/pp.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Thom M., Komor E., Maretzki A. Vacuoles from Sugarcane Suspension Cultures : II. CHARACTERIZATION OF SUGAR UPTAKE. Plant Physiol. 1982 Jun;69(6):1320–1325. doi: 10.1104/pp.69.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Maretzki A., Komor E. Vacuoles from Sugarcane Suspension Cultures : I. ISOLATION AND PARTIAL CHARACTERIZATION. Plant Physiol. 1982 Jun;69(6):1315–1319. doi: 10.1104/pp.69.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]


