Abstract
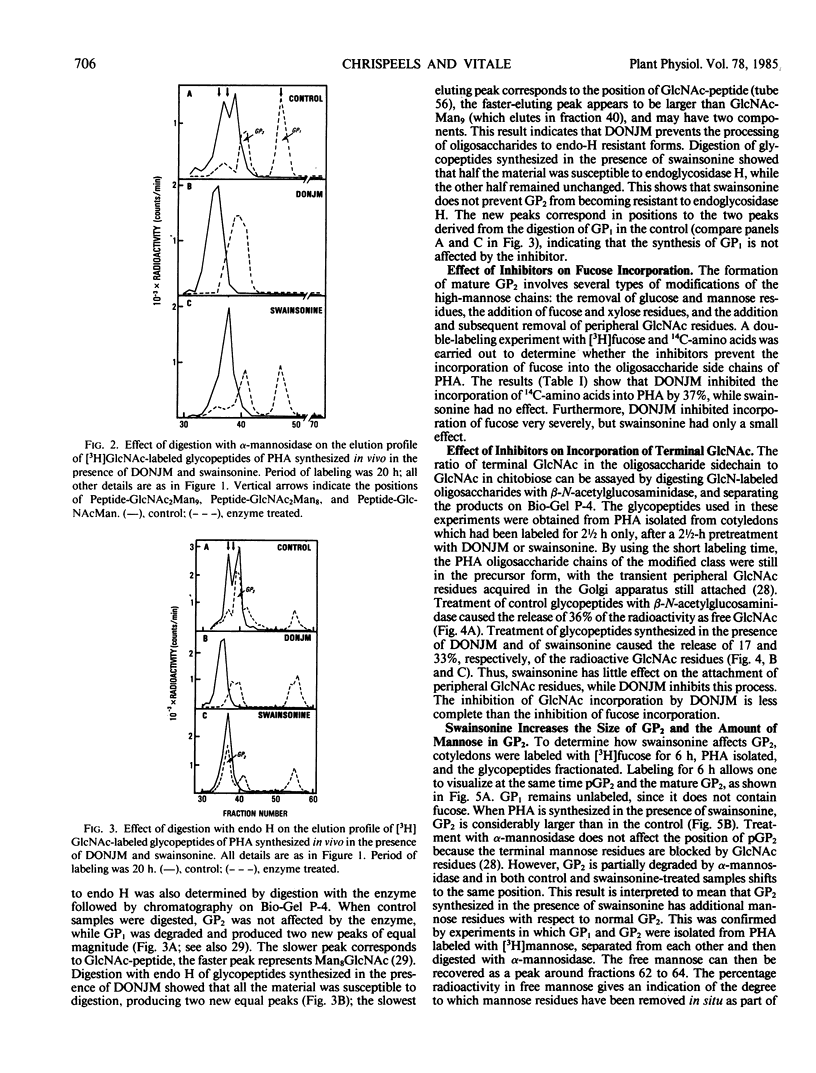
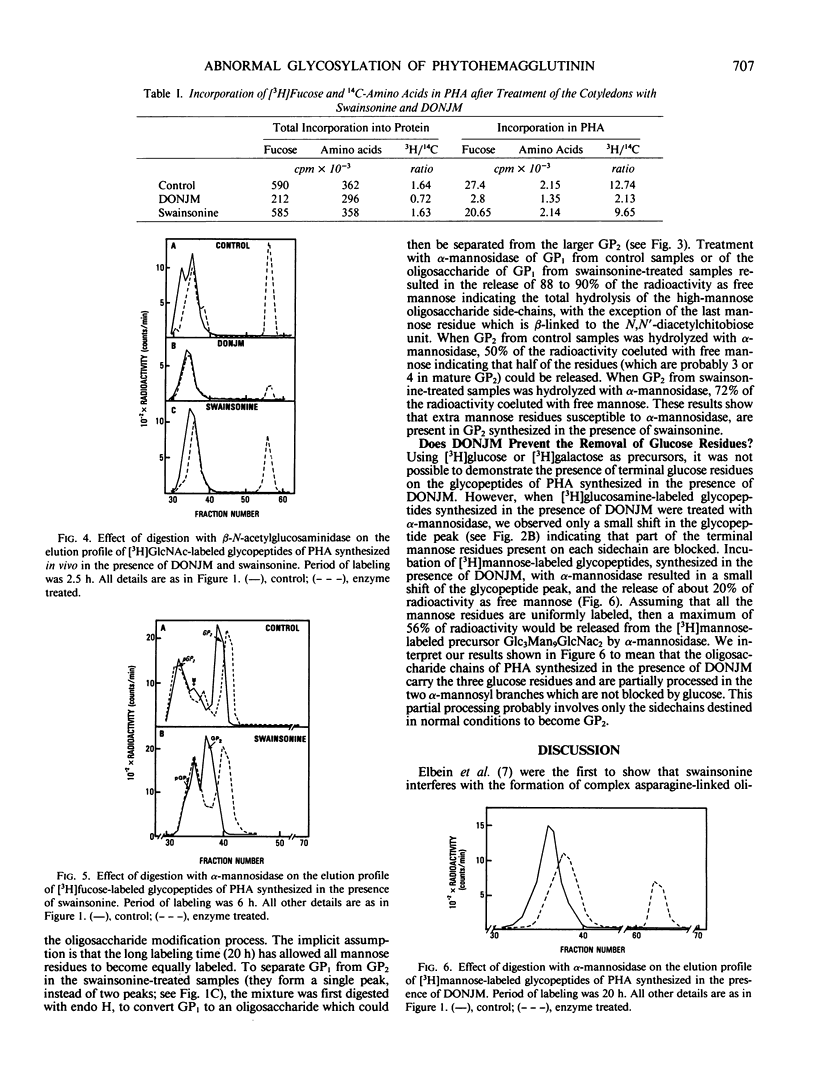
Phytohemagglutinin, the glycoprotein lectin of the common bean, Phaseolus vulgaris, has both high-mannose (Man8-9GlcNAc2) and modified oligosaccharide side chains. The modified side chains have glucosamine, mannose, fucose, and xylose in the molar ratios 2:3.8:0.6:0.5, and are resistant to hydrolysis by endoglycosidase H. Synthesis and processing of side chains in the presence of 1-deoxynojirimycin, an inhibitor of α-glucosidase, results in the formation of chains which are all alike. They are sensitive to endoglycosidase H, do not contain fucose, and are largely resistant to α-mannosidase. This indicates that they are probably high-mannose chains blocked by terminal glucose residues. Synthesis and processing of side chains in the presence of swainsonine, an inhibitor of α-mannosidase II, results in the formation of normal high-mannose chains, and of modified chains which contain fucose residues, are resistant to endoglycosidase H, and can be distinguished from normal modified chains only by the presence of extra mannose residues.
Processing of the phytohemagglutinin modified chains of PHA under normal conditions involves the attachment of peripheral N-acetylglucosamine residues in the Golgi complex and their subsequent removal in the protein bodies. The attachment of the N-acetylglucosamine residues is largely inhibited by deoxynojirimycin but still occurs in the presence of swainsonine. The results presented in this work show that processing of the asparagine-linked oligosaccharides is under the control of several glycosidases and glycosyltransferases and involves the formation of intermediate products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. D., Tsai D., Schachter H. Control of glycoprotein synthesis. The in vitro synthesis by hen oviduct membrane preparations of hybrid asparagine-linked oligosaccharides containing 5 mannose residues. J Biol Chem. 1984 Jun 10;259(11):6984–6990. [PubMed] [Google Scholar]
- Arumugham R. G., Tanzer M. L. Abnormal glycosylation of human cellular fibronectin in the presence of swainsonine. J Biol Chem. 1983 Oct 10;258(19):11883–11889. [PubMed] [Google Scholar]
- Davies H. M., Delmer D. P. Two Kinds of Protein Glycosylation in a Cell-Free Preparation from Developing Cotyledons of Phaseolus vulgaris. Plant Physiol. 1981 Aug;68(2):284–291. doi: 10.1104/pp.68.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Dorling P. R., Vosbeck K., Horisberger M. Swainsonine prevents the processing of the oligosaccharide chains of influenza virus hemagglutinin. J Biol Chem. 1982 Feb 25;257(4):1573–1576. [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsted R. L., Leavitt R. D., Bachur N. R. Purification of the phytohemagglutinin family of proteins from red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim Biophys Acta. 1975 Sep 9;405(1):72–81. doi: 10.1016/0005-2795(75)90316-5. [DOI] [PubMed] [Google Scholar]
- Gross V., Tran-Thi T. A., Vosbeck K., Heinrich P. C. Effect of swainsonine on the processing of the asparagine-linked carbohydrate chains of alpha 1-antitrypsin in rat hepatocytes. Evidence for the formation of hybrid oligosaccharides. J Biol Chem. 1983 Mar 25;258(6):4032–4036. [PubMed] [Google Scholar]
- Hettkamp H., Bause E., Legler G. Inhibition by nojirimycin and 1-deoxynojirimycin of microsomal glucosidases from calf liver acting on the glycoprotein oligosaccharides Glc1-3Man9GlcNAc2. Biosci Rep. 1982 Nov;2(11):899–906. doi: 10.1007/BF01114896. [DOI] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Processing of N-linked oligosaccharides in soybean cultured cells. Arch Biochem Biophys. 1983 Feb 1;220(2):415–425. doi: 10.1016/0003-9861(83)90431-9. [DOI] [PubMed] [Google Scholar]
- Hori H., James D. W., Jr, Elbein A. D. Isolation and characterization of the Glc3Man9GlcNAc2 from lipid-linked oligosaccharides of plants. Arch Biochem Biophys. 1982 Apr 15;215(1):12–21. doi: 10.1016/0003-9861(82)90273-9. [DOI] [PubMed] [Google Scholar]
- Hori H., Pan Y. T., Molyneux R. J., Elbein A. D. Inhibition of processing of plant N-linked oligosaccharides by castanospermine. Arch Biochem Biophys. 1984 Feb 1;228(2):525–533. doi: 10.1016/0003-9861(84)90019-5. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Takahashi N., Oguri S., Tejima S. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979 Nov 10;254(21):10715–10719. [PubMed] [Google Scholar]
- Kang M. S., Elbein A. D. Mechanism of Inhibition of Jack Bean alpha-Mannosidase by Swainsonine. Plant Physiol. 1983 Mar;71(3):551–554. doi: 10.1104/pp.71.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1984 May;98(5):1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. A., Datema R., Schwarz R. T. N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology. 1983 Oct 15;130(1):238–242. doi: 10.1016/0042-6822(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Swainsonine causes the production of hybrid glycoproteins by human skin fibroblasts and rat liver Golgi preparations. J Biol Chem. 1983 Jun 25;258(12):7578–7585. [PubMed] [Google Scholar]
- Vitale A., Ceriotti A., Bollini R., Chrispeels M. J. Biosynthesis and processing of phytohemagglutinin in developing bean cotyledons. Eur J Biochem. 1984 May 15;141(1):97–104. doi: 10.1111/j.1432-1033.1984.tb08162.x. [DOI] [PubMed] [Google Scholar]
- Vitale A., Chrispeels M. J. Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: attachment in the Golgi apparatus and removal in protein bodies. J Cell Biol. 1984 Jul;99(1 Pt 1):133–140. doi: 10.1083/jcb.99.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


