Abstract
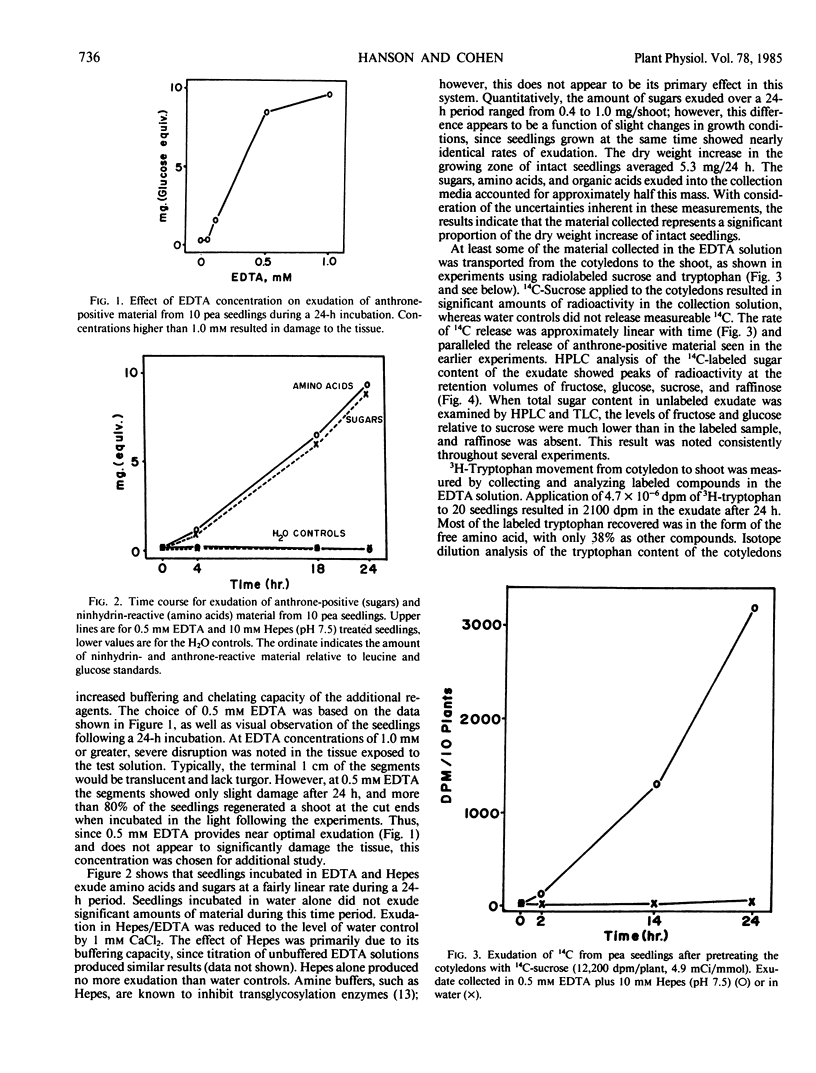
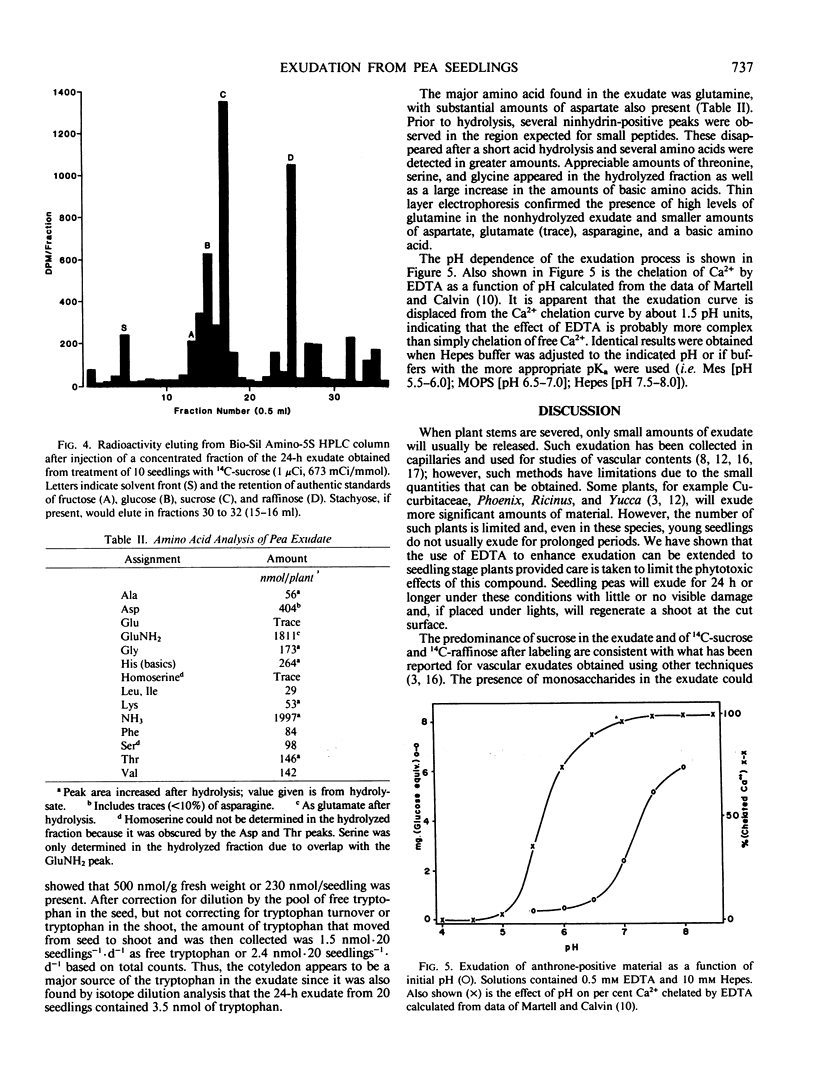
Ethylenediaminetetraacetic acid (EDTA), at concentrations higher than 1.0 millimolar, is phytotoxic to etiolated seedlings of Pisum sativum. Substantial vascular exudation from pea epicotyls could be obtained without tissue damage at 0.5 millimolar EDTA if the solution was buffered at pH 7.5 with sodium N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid. Treated seedlings exuded 950 micrograms (leucine equivalents) of ninhydrin-positive material per day and 870 micrograms (glucose equivalents) of anthrone-positive material per day. Amino acid analysis showed the exudate to have glutamine as the major amido nitrogen containing compound and sucrose was shown to be the major sugar. Radiolabeled tryptophan and sucrose applied to cotyledons were transferred through the epicotyl and into the collection medium. The pH profile for exudation shows half maximal exudation at pH 7.2, indicating the promotion of exudation by EDTA is probably not due simply to Ca2+ chelation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Hall P. L., Bandurski R. S. Movement of Indole-3-acetic Acid and Tryptophan-derived Indole-3-acetic Acid from the Endosperm to the Shoot of Zea mays L. Plant Physiol. 1978 Mar;61(3):425–429. doi: 10.1104/pp.61.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. E., Kolb E., Larner J. Purification and properties of rabbit muscle amylo-1,6-glucosidase-oligo-1,4-1,4-transferase. Biochemistry. 1969 Apr;8(4):1419–1428. doi: 10.1021/bi00832a017. [DOI] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Hanson A. D. Amino Acids Translocated from Turgid and Water-stressed Barley Leaves: I. Phloem Exudation Studies. Plant Physiol. 1979 Sep;64(3):460–466. doi: 10.1104/pp.64.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Transport, metabolism, and redistribution of xylem-borne amino acids in developing pea shoots. Plant Physiol. 1982 May;69(5):1226–1232. doi: 10.1104/pp.69.5.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Use of Phloem exudate technique in the study of amino Acid transport in pea plants. Plant Physiol. 1981 Sep;68(3):750–754. doi: 10.1104/pp.68.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


