Abstract
Nano-range bioactive colloidal carrier systems are envisaged to overcome the challenges associated with treatments of numerous diseases. Lipid nanoparticles (LNPs), one of the extensively investigated drug delivery systems, not only improve pharmacokinetic parameters, transportation, and chemical stability of encapsulated compounds but also provide efficient targeting and reduce the risk of toxicity. Over the last decades, nature-derived polyphenols, vitamins, antioxidants, dietary supplements, and herbs have received more attention due to their remarkable biological and pharmacological health and medical benefits. However, their poor aqueous solubility, compromised stability, insufficient absorption, and accelerated elimination impede research in the nutraceutical sector. Owing to the possibilities offered by various LNPs, their ability to accommodate both hydrophilic and hydrophobic molecules and the availability of various preparation methods suitable for sensitive molecules, loading natural fragile molecules into LNPs offers a promising solution. The primary objective of this work is to explore the synergy between nature and nanotechnology, encompassing a wide range of research aimed at encapsulating natural therapeutic molecules within LNPs.
Keywords: phytochemicals, dietary supplements, functional food, SLN, NLC, liposome, nanoemulsion, herbs and spices
1. Introduction
Nutraceuticals, natural substances that could be found in food or as part of food, are considered to be effective in terms of increasing the nutritional value of diets. They provide specific nutrients to malnourished populations, avoiding adverse effects of conventional therapy, and contribute to the prevention of certain ailments. The term ‘Nutraceutical’, conceived from Pharmaceutics and Nutrition, was introduced by Stephen Defelice in 1989 and has transformed the concept of traditional nutrition [1]. Nutraceuticals cover a great variety of substances, including phytochemicals, herbs, spices, functional foods, and dietary supplements [2]. Nutraceuticals have been classified in more than one way based on their chemical nature, source, properties, and/or disease interventions. A single nutraceutical may fall into more than one category; for example, vitamins are classified as functional foods, but some of them may also be considered dietary supplements on account of their antioxidant properties. These pharmaceutically dispensed bioactives do not solely fall in the food or medicine categories, but an area in between these two domains that is receiving a good share of attention from health practitioners [3].
The nutraceutical industry is flourishing at an incredible rate, offering a wide range of therapeutics against cancer, diabetes, hypertension, hypercholesterolemia, anxiety, depression, insomnia, analgesia, fever, cold, and cough [4]. Nonetheless, the number of processed natural ingredients is limited due to their poor solubility, low stability, and high degradation. Additionally, they often exhibit low bioavailability due to incomplete gastric absorption, excessive first-pass metabolism, and rapid clearance; thus, they face the challenges of erratic pharmacokinetic (PK) profiles [5], which are the main hurdles to deliver and achieve the desired therapeutic outcomes of bioactive natural agents. In order to exploit the maximum therapeutic potential of these natural ingredients, scientists are exploring novel encapsulation methods [6].
Recently, nanotechnology has been successfully employed in the pharmaceutical industry for improving drug solubility, bioavailability, and stability during processing and storage [7]. Nanopreparations based on lipids (LNPs), which are naturally abundant and part of the human diet, have been found to be superior to polymeric nanoparticles in preserving and enhancing the biological activity of the encapsulated payloads [8]. Low production cost, ease of scale-up, better stability profile, and ease of modifications make LNPs a more preferable option among colloidal carriers [9]. LNPs are feasible and effective for administration via different routes, including oral, parenteral, and topical [10].
Solid lipid nanoparticles (SLNs), a primary type of LNPs, are made up of solid lipids with a crystalline nature, which encapsulate the active substance within a solid lipid matrix, allowing for controlled drug release [11]. SLNs can easily penetrate through biological membranes and facilitate the penetration of loaded drugs, hence improving their concentration in target tissues [12]. Nanostructured lipid carriers (NLCs), introduced as the next generation LNPs, are composed of solid and liquid lipids. They aim to overcome the shortcomings of SLNs, for instance, increased drug loading capacity and improved stability by preventing drug leakage from the nanoparticles during storage [13].
Liposomes, nanosized lipid bilayer vesicular systems composed of natural phospholipids and cholesterol, can incorporate higher loads of both hydrophilic and hydrophobic drugs, protect the loaded substances, and efficiently cross biological membranes [14,15]. Moreover, liposomes can be easily modified for targeted delivery with controlled release and have minimal intrinsic toxicity due to the natural phospholipids. Structurally, liposomes may have one or multiple bilayer membranes. On the basis of number of bilayers, liposomes are classified as multilamellar vesicles (MLV) and unilamellar vesicles (ULV). ULV are further divided into three types: giant unilamellar vesicles (GUV), large unilamellar vesicles (LUV), and small unilamellar vesicles (SUV). ULV is comprised of a single bilayer sphere, while MLV is a structure of concentric phospholipid spheres with multiple layers enclosing the aqueous medium. Vesicular size and number of bilayers together effect the drug encapsulation efficiency [16].
Nanoemulsions (NE) or microemulsions (ME) are well-established lipid based colloidal nanoformulations stabilized with a mixture of surfactants to maintain the stability of immiscible phases (oil and water) in a colloidal state. These are mainly of three types: oil in water (o/w), water in oil (w/o), and multiphase [17]. NEs possess several advantages, including enhanced solubility, increased bioavailability, and bioaccessibility of poorly soluble drugs and nutraceuticals, as well as straightforward easy manufacturing procedure and steps. NE provide protection to natural and synthetic active compounds from adverse storage conditions, degradation, light, pH, and elevated temperature [18].
To mitigate the adverse effects of therapeutics and reduce the length of treatment procedures, nanomedicines containing bioactive materials are being launched in the global pharmaceutical market. Products that incorporate natural compounds driven by nanotechnology present better pharmacoeconomic options in both therapeutic and diagnostic fields [19]. The global nutraceuticals market size was USD 540 billion in 2022 and is estimated to reach USD 1025 billion by 2030. While the nanomedicine market size was USD 377 billion in 2021, it is projected to exceed USD 964 billion by the end of 2030 [20,21,22].
Our review profoundly covers recent developments in the use of LNPs for encapsulating natural active ingredients for remedial, food fortification, and diagnostic purposes.
We are discussing SLN, NLC, liposomes, and NE as examples of lipid nanocarriers to load natural compounds in the main text. The most widely and extensively used compounds have been detailed in this review, while the less commonly used or newly established natural drugs have been summarized into tables. Special attention was also paid to formulation methods, characterization, and advantages in delivering nutraceuticals via LNPs.
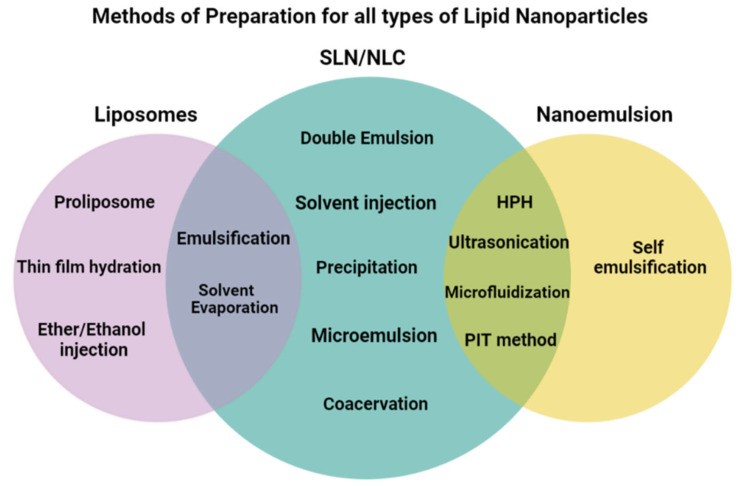
Lipid nanoparticles are prepared by different methods, including high-pressure homogenization, ultrasonication, emulsification, evaporation, hydration, and both high and low-energy methods. Every method has its own advantages and disadvantages that assist researchers in selecting the most appropriate option accordingly. Different methods of preparation for NLC, SLN, liposomes, and NE have been enlisted in Figure 1, while their advantages and disadvantages of each method are presented in Table 1. Moreover, a brief description of the most commonly used preparation methods has also been included in this article.
Figure 1.
Methods of preparation for LNPs.
Table 1.
Advantages and disadvantages of preparation methods of LNPs.
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hot High-Pressure Homogenization | Large scale production, absence of organic solvent, incorporation of lipophilic and insoluble drugs | Temperature dependent degradation, intricacy of crystallization, unsuitable for heat-sensitive or hydrophilic drugs |
| Cold High-Pressure Homogenization | Suitable method for thermolabile compounds | Large particle size and broader size distribution |
| Ultrasonication | Common laboratory equipment | Wider size distribution, large amount of surfactant required, physical instability |
| Phase Inversion Temperature | Temperature can be reduced by surfactant incorporation, narrow size distribution, high stability | Thermolabile ingredients degradation |
| Precipitation | Rapid and reproducible | Toxicological issues due to solvent residue |
| Coacervation | Simple, avoidance of organic solvent | Applicable only to non-pH dependent drugs and alkaline nature lipids |
| Microemulsion | Scale up method, thermodynamically stable formulation | Multiple steps involved, diluted dispersion acquired |
| Double Emulsion | Applicable to hydrophilic drugs | Large particle size, low entrapment efficiency and drug loading |
| Emulsification | High encapsulation efficiency, low energy input, no thermal stress | Removal of organic solvent |
| Thin Film Hydration | MLVs are prepared, good reproducibility | Low encapsulation efficiency |
| Proliposome | High encapsulation efficiency, fast, simple, large quantity production | Poor reproducibility |
| Ether/Ethanol Injection | Simple, large quantity production, reproducible |
Removal of organic solvent, poor encapsulation (in case of ethanol injection method) |
| Solvent Evaporation | Avoidance of heat | Solvent residue may cause toxicological problems, dilute suspension |
| Solvent Injection | Easy and fast, small droplets | Residual solvent, removal of organic solvent |
| Microfluidization | Laboratory and industrial scale method | High amount of solvent residue |
| Self-emulsifying system | Enhanced solubility, prevent the biodegradation of lipophilic drugs | Low drug loading capacity, drug leakage, low stability |
1.1. Methods of Preparation for SLN and NLC
1.1.1. High-Pressure Homogenization (HPH)
High-pressure homogenization (HPH) is a well-established and recognized technique used for producing SLN and NLC. Lippacher et al. prepared nanoemulsions for parenteral administration using the HPH method, with the loaded drug enclosed at 5–10 °C above the melting temperature of the lipid used [23]. In this technique, high pressure (100–2000 bar) is applied to force the liquid through a very narrow gap that eventually produces high velocity (up to 1000 km/h or even more). As a result of elevated shear stress and cavitation force, the particle size is reduced to submicron level [24]. When HPH is performed at high temperatures, it is termed hot homogenization, whereas at lower temperatures, it is known as cold homogenization. Hot HPH is not suitable for thermolabile drugs or materials. In case of cold HPH, an aqueous phase is mixed with lipid phase at 2 °C to 6 °C with constant stirring. The resultant coarse suspension is subjected to HPH maintained at lower temperature. Due to the absence of elevated temperature, cold HPH is a suitable method for heat sensitive materials.
1.1.2. High Shear Homogenization and Ultrasonication
Two of the most familiar dispersing techniques are high shear homogenization and ultrasonication. A warm aqueous phase containing surfactant is combined with the melted lipid under high shear homogenization, then the obtained emulsion is subjected to ultrasonication as a subsequent step to reduce the particle size to submicron level. High speed mixing and ultrasonication at relatively elevated temperature results in a smooth formulation. Intense shear forces during the ultrasonication help to break the particles to nanosize. Moreover, the type and ratio of surfactant and lipids as well as time and speed of sonication play a crucial role to produce a stable nanoformulation [25].
1.1.3. Microemulsion Technique
The microemulsion technique is a multi-step method in which a surfactant solution and melted lipid are gently mixed to produce a kinetically stable dispersion.
In order to prepare a thermodynamically stable o/w microemulsion, the resultant emulsion is dispersed into sufficient amount of cold water upon continuous mixing. The ratio of water to microemulsion usually varies from 10:1 to 50:1. This dilution helps to scale down the micro formulation to nano formulation. Size of the obtained nanoparticles is dependent on two factors; one is temperature difference between cold water and hot microemulsion and the other is primary size of emulsion. NLC and SLN dispersions having micro and nano size range with spherical shapes and narrow size distribution are prepared by this method. The notable drawback associated with this method is the final diluted dispersion [26].
1.1.4. Phase Inversion Temperature (PIT) Technique
The conversion of an o/w emulsion to a w/o emulsion is termed ‘phase inversion’ and depends on a change in temperature. Polyoxyethylated surfactants present different properties at different temperatures; for example, the hydration of the hydrophilic portion of the surfactant at 25 °C and the dehydration of epoxy groups occurs as the temperature increases. The temperature point at which the surfactant acquires equal affinity for both hydrophilic and lipophilic phases is known as phase inversion temperature. As the temperature continues to increase, the surfactant exhibits a greater affinity for the lipid phase, leading to a conversion into a w/o emulsion type. Weerapol prepared SLN by phase-inversion temperature method to incorporate rosemary oil (RMO) [27,28].
1.1.5. Double Emulsion Technique
The most suitable method for the incorporation of peptides and hydrophilic compounds is the double emulsion technique. Initially, the drug is solubilized in an aqueous solution, which is then emulsified with lipid to form a w/o emulsion with the incorporation of suitable surfactants. The obtained w/o emulsion is dispersed in an emulsified aqueous solution to form a double w/o/w emulsion through continuous stirring. After filtration, the nanoparticles are separated. This technique tends to relatively produced large particles [29].
1.1.6. Solvent Injection Technique
A fast and simple method that uses organic solvents (such as methanol and acetone) to dissolve lipids, which are then injected into an emulsified aqueous phase through a needle. The quick and gradual diffusion of lipids into the aqueous phase produces small sized droplets that could be controlled by adjusting the injection speed [30].
1.1.7. Solvent Evaporation Method
Lipophilic contents are dissolved in an organic solvent and incorporated into an aqueous phase containing surface active agents. Upon evaporation, the lipid precipitates, resulting in the formation of nanoparticles with a size range of 25 nm. In the next step, the solution is emulsified by high-pressure homogenization with the removal of the organic solvent under reduced pressure (40–60 bar) [31].
1.1.8. Precipitation Method
An organic solvent is required in the precipitation method; this is a drawback of this technique. In the initial step, an organic solvent (such as chloroform) and glyceride are mixed together with the subsequent addition of emulsified aqueous phase. The resultant mixture is subjected to evaporation, forming nanoparticles with lipid precipitation [25].
1.1.9. Coacervation Method
The coacervation method begins with the heating of the polymer in water. Subsequently, lipids are incorporated into the hot solution while stirring continuously above the Kraft point of the lipid.
At first, the active ingredient solution is added to the resultant mixture, and finally, the coacervating solution is added dropwise to set the desired pH. Once the desired pH is acquired, the solution is cooled down to 15 °C in a water bath while mixing [32].
1.2. Methods of Preparation for Liposomes
1.2.1. Thin-Film Method
One of the most widely used methods for liposomal preparation is the thin film hydration method. A thin film of lipid with active ingredients is formed at the bottom of a rotary evaporator and is subsequently hydrated with buffer or water. To obtain a smoother bilayer, both the lipid film and hydrating solutions should be heated above the transitional temperature of lipids. Sonication helps in peeling off the film from the surface and in the formation of liposomes. This method is used to prepare MLV (multilamellar vesicles) liposomes; it is a reproducible method but has the shortcoming of low encapsulation efficiency [33].
1.2.2. Proliposome Method
This is the simplest method to prepare a larger quantity of liposomes. Compared to the thin film method, it has less reproducibility but offers higher entrapment efficiency. The aqueous solution of lipid and ethanol is heated on stirring for 10 min at 60 °C. Once the smooth lipid melt has cooled down to a lower temperature, the buffer or aqueous phase is incorporated dropwise while stirring. The obtained suspension is further hydrated for an hour to acquire MLV liposomes [34].
1.2.3. Injection Methods
A lipid suspension of organic solvents is injected into an aqueous phase with some variations to prepare liposomes in larger quantities. Ethanol and ether injection methods are types of injection methods that produce SUVs (small unilamellar vesicles) and LUVs (large unilamellar vesicles), respectively. In ethanol injection method, lipids are dissolved in ethanol and injected into aqueous phase upon continuous stirring with subsequent removal of solvent. Ethanol removal from the system can be performed by centrifugation or rotary evaporation. The resultant solution is subjected to hydration afterwards. This method may lead to poor encapsulation of hydrophilic drugs. On the other hand, as ether does not mix with water and due to having high lipid solubility aspect, the ether injection method produces liposomes with increased encapsulation efficiency [35].
1.2.4. Emulsification Method
The emulsification method is somewhat similar to the injection methods. According to this technique, lipids are solubilized in organic solvents and gradually mixed with the aqueous phase. An emulsion of water in oil is formulated with a lipid monolayer around the water droplet. Once the solvent is evaporated, it results in liposome formation. This method ensures higher encapsulation efficiency compared to the injection method [36].
1.3. Methods of Preparation of Nanoemulsion
Various high-energy methods, utilizing significant fluid stress, are employed to break down the dispersed phase into small-sized droplets when formulating nanoemulsions. Ultrasonication, high-shear mixing, high-pressure homogenization, and microfluidics are the techniques to obtain particles with controlled size and stability [37]. Gharibzahedi et al. reported on the effectiveness of high-energy methods in encapsulating nutritional food components while preserving their activation and storage [38].
1.3.1. High-Energy Methods
High-Pressure Homogenization
HPH is responsible for reducing coarse emulsion particles to an extremely low size with the application of turbulence, hydraulic force, and cavitation during the homogenization. Other parameters that define the size of primary emulsion droplets are intensity, time, temperature, and the type of homogenization method. The application of the HPH method has been extended to pharmaceuticals, nutraceuticals, food, and biotechnological active ingredients [39]. HPH technique has been discussed in detail in Section 1.1.1.
Microfluidization
This high-energy mixing technique involves the use of a microfluidizer under high pressure (500~20,000 psi) to homogenize a coarse emulsion. It is a combination of imprinting technology, water jet technology, and the homogenization technique with the utilization of an interaction chamber. The process efficiency is dependent on diameter, pressure, treatment temperature, and design of the flow channel. Coarse emulsion occurs under pneumatc pressure, directed to the interaction chamber of microfludizer where it enters into narrow channels. There, emulsion streams collide with each other at high velocity, resulting in the formulation of a nanoemulsion. At the outlet of the microfluidizer interaction chamber, an in-line heat exchanger is used to cool down the resultant dispersion. Due to the fixed geometry of the microfludizer, this method showed better reproducibility [40].
Ultrasonication
Ultrasonication has gained more recognition compared to other high-energy techniques due to the cleanliness of operation. Ultrasonic waves are responsible for generating cavitation force to break macroemulsion into nanoemulsion. Moreover, the collapsing of microbubbles produces turbulence to break microemulsion into nanoemulsion [41]. Detailed discussion is available in Section 1.1.2.
1.3.2. Low-Energy Methods
Low-energy emulsification methods are believed to be an energy-efficient method as they utilize the internal system energy and gentle stirring to produce emulsions at the nano level.
Phase inversion emulsification and self-emulsification are more common methods for producing food-grade nanoemulsions among low-energy techniques. One important point of consideration is the need for a relatively higher amount of surfactant in case of low-energy methods [42].
A spontaneous change in temperature or composition resulted in a phase change during the emulsification. Namely, phase inversion composition and phase inversion temperature are two types of phase inversion emulsification methods [43].
1.3.3. Self-Nanoemulsification Method
The spontaneous emulsification method is termed the self-emulsification method. Such a system contains more hydrophilic content than lipophilic content. They behave like isotropic mixtures of lipids, drugs, and surfactants. Upon dilution in biological fluids, they form clear o/w emulsions with the help of the agitated movement of the stomach and intestinal motility [44].
1.4. Characterization of LNPs
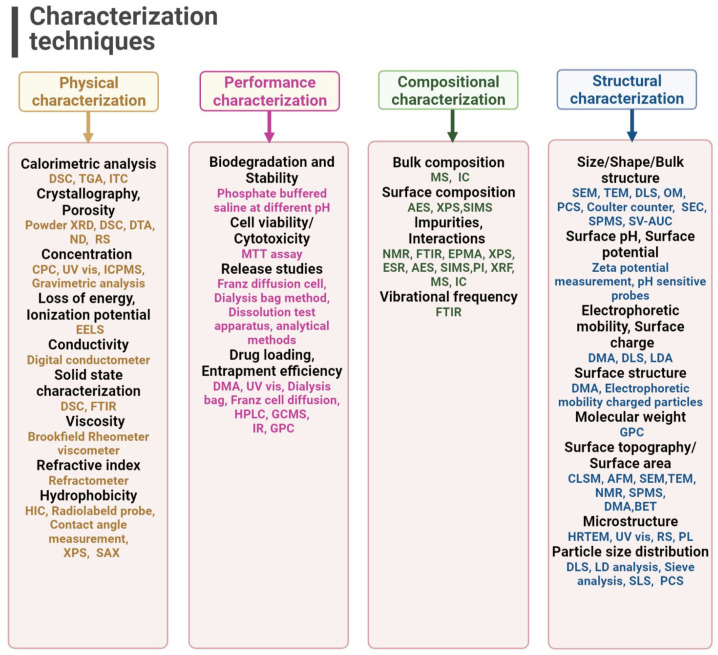
During the formulation and drug development process, the physical and chemical properties of ingredients are required to be investigated. These properties are extensively studied to evaluate the stability, impact, and performance of the product. To characterize lipid nanoparticle formulations, various old and new analytical techniques have been introduced [45] and applied in the pharmaceutical sector. A comprehensive overview of these investigations is available in Figure 2.
Figure 2.
Characterization techniques related to nanoformulations. (Abbreviations: DSC, differential scanning calorimetry; TGA, thermogravimetric analysis; ITC, isothermal titration calorimetry; XRD, X-ray powder diffraction; DTA, differential thermal analysis; ND, neutron diffraction; RS, Raman spectroscopy; CPC, condensation particle counter; UV vis, ultraviolet and visible absorption spectroscopy; ICPMS, inductively coupled plasma mass spectrometry; EELS, electron energy loss spectroscopy; FTIR, Fourier transform infrared spectroscopy; HIC, hydrobhobic interaction chromatography; XPS, X-ray photoelectron spectroscopy; SAX, synchrotron radiation X-ray; MTT assay, (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay; DMA, differential mobility analyzer; HPLC, high performance liquid chromatography; GCMS, gas chromatography mass spectrometry; IR, infrared spectroscopy; GPC, gel permeation chromatography; MS, mass spectroscopy; IC, ion chromatography; AES, atomic emission spectroscopy; SIMS, secondary ion mass spectroscopy; NMR, nuclear magnetic resonance; EPMA, electron probe micro-analyzer; ESR, electron spin resonance; PI, polydispersity index; XRF, X-ray fluorescence spectroscopy; OM, optical microscopy; PCS, photon corelation spectroscopy; SEC, size exclusion chromatography; SEM, scanning electron microscopy; TEM, transmission electron microscopy; DLS, dynamic light scattering; SV-AUC, sedimentation velocity analytical ultracentrifugation; LDA, laser doppler anemometry; CLSM, confocal laser scanning microscopy; AFM, atomic force microscopy; SPMS, single particle mass spectrometry; BET, Brunauer–Emmett–Teller analysis; HRTEM, high resolution transmission electron microscopy; SLS, static light scattering; PL, photoluminescence spectroscopy; LD analysis, laser diffraction analysis.).
2. Phytochemicals
Naturally occurring chemicals in plants that are not required for their growth but protect them from infections and diseases are termed phytochemicals. Countless phytochemicals exist in nature, and most of them have been reported for their medicinal effects, including their antibacterial, anti-inflammatory, hormonal, and enzymatic stimulation properties. According to available and published data, more than 5000 phytochemicals are known so far that are present in vegetables, vegetable oils, fruits, whole grains, and nuts [46].
In this review article, we are describing the beneficial and healthcare roles of phytochemicals in controlling and treating diseases. Moreover, we are highlighting the issues faced in the efficient delivery of phytochemicals along with practical solutions and strategies to overcome these shortcomings. In current times, lipid nanoformulations loaded with phytochemicals are receiving attention as a promising and emerging delivery systems. Most extensively used phytochemicals have been described in the text below, while the recent developments in this field are documented in Table 2.
Table 2.
Miscellaneous phytochemicals loaded in nanocarriers with their documented potential activity between 2019–2023.
| Phytochemicals | Method of Preparation | Potential Activity against | LNPs | References |
|---|---|---|---|---|
| Anacardic acid | Hot homogenization method | Microbial biofilm | SLN | [47] |
| Apigenin | Microemulsion method | Diabetic neuropathy | SLN | [48] |
| Baicalein | Reverse evaporation method | Acute lung injury | Liposomes | [49] |
| Bakuchiol | Hot homogenization method | Psoriasis | SLN | [50] |
| Betulinic acid | Microemulsion method | Retinal oxidative injury | SLN | [51] |
| Caffeic acid | Lipid film hydration | Alzheimer’s disease | Liposomes | [52] |
| Camphor | Emulsification method | Asthma | NE | [53] |
| Carvacrol | High pressure microfludization | Fungal infection | NE | [54] |
| Chrysin | Homogenization and sonication | Pancreatic cancer | SLN | [55] |
| Cinnamaldehyde | Thin film evaporation | Bacterial infection | Liposomes | [56] |
| Citral | High pressure homogenization | Breast cancer | NLC | [57] |
| Coumarin | Cold homogenization method | Drug-resistant bacterial infection | SLN | [58] |
| Eucalyptol | Thin film hydration | Diabetes-associated vascular endothelial injury | Liposomes | [59] |
| Eugenol | High speed shearing | Wound treatment | NE | [60] |
| Ferulic acid | Solvent evaporation | Colon cancer | SLN | [61] |
| Gallic acid | Double emulsion technique | Oxidation in food fortification | SLN | [62] |
| Hesperetin | Phase inversion temperature method | Glioblastoma | NLC | [63] |
| Honokiol | Low energy shaking method | Glioblastoma | NE | [64] |
| Hypericin | Ultrasonication method | Breast cancer | NE | [65] |
| Hyperoside | Thin film hydration | Hepatocellular carcinoma | Liposomes | [66] |
| Juglone | Thin film hydration | Cystic hydatid disease | Liposomes | [67] |
| Kaempferol | Emulsification method | Glioblastoma | NLC | [68] |
| Lapachol | Hot homogenization method | Breast cancer | NE | [69] |
| Licochalcone | High shear homogenization | Schistosomiasis | SLN | [70] |
| Lignin | High pressure homogenization | Hydrophobicity and oxidative stress | NE | [71] |
| Limonene | Ultrasonication method | Fungal infection (tomato preservation) | NLC | [72] |
| Myricetin | Ultrasonication homogenization method | Colorectal cancer | SLN | [73] |
| Oleanolic acid | Thin film hydration | Hepatocellular carcinoma | Liposomes | [74] |
| Paeoniflorin | Thin film hydration | Rheumatoid arthritis | Liposomes | [75] |
| Parthenolide | Thin film hydration | Cervical cancer | Liposomes | [76] |
| Perillyl alcohol | Hot high pressure homogenization | Brain tumor | NLC | [77] |
| Phloretin | Emulsification method | Inflammation | NE | [78] |
| Pinene | Ethanol injection method | Oxidative stress | Liposomes | [79] |
| Pinocembrin | Thin film hydration | Hyperglycemia | Liposomes | [80] |
| Proanthocyanidins | Homogenization method | Parkinson’s disease | SLN | [81] |
| Procyanidin | Thin film hydration | Oxidative stress | Liposomes | [82] |
| Pterostilbene | Ultrasonication method | Breast cancer | SLN | [83] |
| Pulegone | High shear homogenization | Microbial infection | SLN | [84] |
| Quercetin | High shear homogenization | Diabetes mellitus | NE | [85] |
| Thymol | Emulsification method | Oral infections | NE | [86] |
| Thymoquinone | Ultrasonication method | Breast cancer | NE | [87] |
| Umbelliferone | Thin film hydration | Dalton’s ascites lymphoma | Liposomes | [88] |
2.1. Lycopene
Lycopene, a terpenoid present in watermelon, grapefruit, and tomato, is considered one of the most potent naturally occurring antioxidants [89]. It also possesses anti-inflammatory, anti-tumor, and anti-mutagenic properties [90]. In spite of its persuasive medicinal characteristics, the therapeutic role of lycopene is limited due to its low aqueous solubility and poor stability [91].
Riangjanapatee et al. evaluated the behavior of different surfactants on the stability of lycopene-loaded NLCs composed of orange wax (90% w/w) and a lycopene oil solution in rosemary oil (10% w/w). Different surface active agents, C-1216 (sucrose laurate), C-1816 (sucrose stearate), C-1616 (sucrose palmitate), C-1815 (sucrose stearate), and Plantacare 1200 (lauryl glucoside), were investigated for their ability to stabilize the NLCs prepared by hot high-pressure homogenization (HPH). It was found that Plantacare 1200 produced the smallest NLCs of all, attributed to the smaller contact angle of the surfactant with the orange wax. Both the particle size and zeta potential of the lycopene-NLCs remained constant over a period of one month. Furthermore, the chemical stability of lycopene was enhanced many folds in the NLCs (t1/2 = 192.5 h) compared to its solution (t1/2 = 9.6 h) [92].
Riangjanapatee et al. also evaluated the effect of cholesterol on the chemical stability of lycopene in NLCs. The presence of cholesterol resulted in significantly smaller NLCs but also caused the degradation of lycopene [93]. On the other hand, NLCs produced with rice bran oil significantly improved the stability of lycopene within the NLCs, and increased antioxidant activity was observed for the Lycopene-NLCs when compared to blank rice oil NLCs [94].
Zardini et al. used a combined high shear homogenization and ultrasonication technique to formulate lycopene-loaded NLCs and SLNs to evaluate the potential of nanocarriers for food fortification. The size range of nanoparticles was 74.93–183.40 nm. NLCs were found to exhibit higher encapsulation efficiency when compared to SLNs, while particle size was smaller. Nanoencapsulation of lycopene not only precluded the poor solubility but also masked its aftertaste [95]. Nanosized liposomal formulation was loaded with lycopene by Zhao and fellows to improve neuronal protection against ischemic brain injury. Neurological score, oxidative stress markers, neuronal apoptosis, and infarction volume indicated an increase in the amount of lycopene at the site of action due to encapsulation in nano-liposomes [96].
Similarly, a methotrexate-induced kidney dysfunction model was used to test the activity of lycopene in two pharmaceutical forms, one encapsulated in nanoliposomes and the other dissolved in corn oil. During in vivo studies, nanoliposome-encapsulated lycopene reported a reduction in animal kidney dysfunction condition, with a higher degree of recovery [97]. In both in vitro cytotoxicity and in vivo antitumor studies conducted on B16 melanoma-bearing mice, the combination of doxorubicin and lycopene-loaded liposomes exhibited enhanced therapeutic efficacy and reduced cardiotoxicity [98].
Li et al. used medium-chain triglycerides and octenyl succinate anhydride (OSA)-modified starch as stabilizers to create lycopene-loaded oil-in-water NE. Increased lateral packing of the stabilizer at the interface contributed to a reduction in particle size and resulted in a physically stable NE [99]. To acquire stable lycopene NE, different oil phases, such as sesame oil, linseed oil, or walnut oil, were used, with lactoferrin serving as the emulsifying agent to prepare lycopene-loaded NE. The results indicated that lycopene NE formulated with sesame oil exhibited greater stability and a slower degradation rate compared to other formulations, making it an effective choice for lycopene-fortified delivery systems [100].
2.2. β-Carotenoid
Tetraterpenoids are a subclass of carotenoids found in chloroplasts, fungi, and bacteria [101]. They play a meaningful role in photosynthesis by absorbing sunlight and protecting chlorophyll from damage caused by sunlight [102]. β-carotene is a commonly used carotenoid in food and health sciences and is believed to be highly active among the other carotenoids. It is known for its antioxidant, anticancer, and immune-boosting properties due to its ability to scavenge reactive oxygen species (ROS) [103]. Aqueous insolubility and low bioavailability are significant obstacles to the pharmaceutical applications of β-carotene for consumers. Different potential carriers are under investigation for the incorporation of this lipophilic compound [104].
Incorporation of β-carotene in NLCs to enhance β-carotene’s stability was performed by Hentschel et al. It was found that β-carotene degrades quickly on dilution of the dispersion. Protection of β-carotene from degradation seemed to increase after the incorporation of Vitamin E along with β-carotene. The antioxidant property of tocopherol protected the nutraceutical from oxidative, preserving its stability for 11 days in a diluted formulation and 19 days in a pure formulation. The NLCs remained stable without sedimentation and aggregation for a period of 7 months, making them suitable for use in beverages [105].
During the processing of carotenoids, physical and thermal stress decrease the stability and promote oxidative degradation [106]. LNP’s solid core may act as a shelter for carotene, protecting it against oxidation. Helgason et al. investigated the dependence of the stability of bioactive components on surfactant type, lipid crystal structure, and evaluated the activity of lecithin as antioxidant and stabilizer for the system. Crystalline structures of the lipids were achieved during the processing stages with the use of high-melting surfactants. High-melting (HM) surfactants, such as HM-lecithin, were proven to be superior to Tween 60 and Tween 80 in stabilizing the solid lipid carrier system and protecting the payload chemically. The selection of surfactants was identified as a critical factor not only in the development of lipid crystal structures but also in maintaining their integrity [107].
Yi et al. revealed that the type and concentration of the proteins affected the stability, cytotoxicity, and cellular uptake of the β-carotene. Sodium caseinate and whey protein isolate could also produce nanoparticles smaller than 100 nm. SLNs remained stable for 30 days when stored at 4 °C. Sodium caseinate was discovered to establish better physical and chemical stability in SLN systems than soy protein and whey protein isolates. The presence of phosphoserine and the ability of sodium caseinate to form a thick layer at the oil-water interface at higher concentrations contribute to the production of more stable colloidal dispersions. Furthermore, all the proteins boosted the cellular uptake of β-carotene SLNs in Caco-2 cells compared to free β-carotene [108].
Orally administered carotenoid NE stabilized with Tween 40 showed better antioxidative and liver protective effects in Wistar rats than the conventional emulsion, likely due to the smaller droplet size. Tween 40 alone was found to be more toxic to the liver than in its emulsion form, indicating that a careful investigation and analysis are recommended to select a definite amount of surfactant to design an emulsion [109].
Whey protein, gum Arabic, and soy lecithin were tested for the fabrication of a stable oil-in-water NE loaded with carotenoid (paprika oleoresin). Whey protein and soy lecithin showed better results in the context of a smaller droplet size (<150 nm) and interfacial tension, which ultimately contributed to the improved stability of the carotenoid NE [110]. In a recent study, carotenoid NE produced a prominent antiproliferative effect in breast cancer cells (MCF-7) and tumor-bearing mice by virtue of considerable reduction in epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) levels compared to the carotenoid extract [111].
2.3. Eugenol
Eugenol (Eu) is an active phenolic constituent of clove oil. Its antifungal activity was revealed by Chami et al. while performing experiments on rats with candidiasis (oral, vaginal) [112]. After exposure to candidiasis infection, monocytes release interleukins (IL), leading to inflammation. Eu was found to be effective in controlling the production of PG-E2, IL, and tumor necrosis factor (TNF), ultimately reducing the inflammation [113].
Eu loaded SLNs were prepared by Garg et al. to treat oral candidiasis and to improve its antifungal activity. They elaborated the results, highlighting the increased stability and therapeutic effects offered by the SLN system. Two formulations, SLN1 (caprylic triglyceride) and SLN2 (binary lipids: caprylic triglyceride, stearic acid), exhibited identical encapsulation efficiency. An increase in solid lipid concentration resulted in larger particle size. Formulation SLN2, which contains a liquid lipid (stearic acid), exhibited smaller particle size and faster drug release [114].
An NLC-gel formulation containing Eu was developed to achieve sustained drug release into dentinal tubules to treat hypersensitivity problems in gums. Initial burst release followed by slower release of drug was noted. It was claimed to improve the effectiveness of the therapy by providing a rapid relief through the initial release and then maintaining the effect for an extended period of time through the prolonged release of the bioactive from the gel [115].
Loading of Eu inside SLN may lead to increased local supply and decreased systemic absorption of the active ingredient when used for topical applications. However, the lower viscosity of SLNs can be problematic for epidermal application of creams. Garg et al. prepared Eu-SLN and incorporated them into a hydrogel stabilized by Carbopol. In vivo and in vitro testing confirmed the effectiveness of antifungal therapy with augmented occlusive properties [116]. An enhanced antimicrobial effect of the combination of ofloxacin and Eu in chitosan-coated SLNs was reported by Rodenak-Kladniew et al. Controlled drug release and enhanced formulation stability allowed superior inhibition of the growth of Pseudomonas aeruginosa and Staphylococcus aureus [117].
Hyaluronic acid (HA)-coated liposomes loaded with Dacarbazine and Eu were formulated and optimized by the Quality-by-Design (QbD) approach. Migration and proliferation assays confirmed a higher inhibitory effect against metastasis even at reduced doses [118]. Cationic and temperature-sensitive liposomes encapsulating Eu were coated onto negatively charged silk through electrostatic interactions, with the aim of achieving sustained and controlled release of fragrance from the fragrant silk. The purpose of the following study was to lower the release rate and increase the fragrance lasting time. According to release study data, lower than 30% of the fragrance was released from eugenol-loaded liposomes [119].
Eu-loaded liposomes expedited the wound closure and healing process when tested using an in vitro scratch assay wound model of neuronal and microglia origin cell lines. This observation included assessments of ROS production, cell viability, and polarization properties [120]. Chitosan-coated liposomes or chitosomes displayed enhanced Eu incorporation capacity and boosted antioxidant activity when compared to the conventional liposomes [121].
Tanzeem et al. designed a clove oil-based ME loaded with flurbiprofen to utilize the gastroprotective effect of Eu in clove oil and reduce the NSAIDs associated gastric damage. The ME system increased the solubility and dissolution of flurbiprofen, which could increase the drug bioavailability. The stomachs of the rats treated with ME showed fewer lesions and less harm to the gastric mucosa when compared to the pure NSAID in PEG, thus making it a suitable vehicle for delivering NSAIDs that may cause gastritis [122].
Fu et al. developed a stable, Eu-loaded antimicrobial NE by high-speed shearing technique, which was found to be effective against Escherichia coli and Staphylococcus aureus. Severe deformation and membrane rupture were observed in both bacterial strains through SEM, along with measurements of ROS and MDA levels [60].
2.4. Curcumin
The golden spice turmeric, scientifically known as ‘Curcuma longa’, is the natural source of curcumin (Cur; 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) [123]. Cur is frequently used in Central and South Asia as a condiment. It also acts as an iron chelator to control iron accumulation in cardiac and hepatic cells, as well as various parts of the brain [124].
Preclinical studies have been conducted by scientists for many years to investigate the therapeutic potential of Cur, compelling them to proceed with clinical trials for a proper evaluation of its effectiveness [125]. However, while Cur is considered a miracle for mankind, its poor aqueous solubility, low bioavailability, and stability problems hinder its maximum therapeutic potential.
Tiyaboonchai et al. prepared SLN with a mean particle size of approximately 450 nm. They observed the dependence of particle size and Cur encapsulation on the overall percentage of lipids and surfactants used. SLNs were successfully incorporated into a cream formulation and showed a sustained release pattern after topical administration. Stability studies exposed the ability of the SLN to improve the stability of Cur over a period of 6 months [126].
Pliangbangchang et al. studied the anti-aging effects of the Cur-SLN-loaded cream on 33 volunteers for 8 weeks. The cream was found to be safe for the volunteers, with no reports of irritation. Skin wrinkles were markedly reduced, and the hydration level of the skin also improved with the treatment. Additionally, skin elasticity and firmness also improved over the course of the testing period [127].
Kakkar et al. successfully prepared SLNs with an average particle size of 134.6 nm and a drug content loading of 92%. Pharmacokinetic studies were performed with Wistar rat plasma to investigate any changes in the bioavailability (BA) of Cur. A validated LC-MS characterization method confirmed a 39-fold increase in the bioavailability (BA) of curcumin (Cur) at a dose of 50 mg/kg and a 155-fold increase at a dose of 1 mg/kg [128].
Researchers evaluated the ability to mitigate AlCl3-induced neurotoxicity, which is found to be fatal for the central nervous system (CNS) functionality and could lead to Alzheimer’s disease (AD). Cerebral histopathology not only confirmed protection against neurotoxicity but also demonstrated its reversal when treated with Cur-SLN [129]. They demonstrated increased cytotoxicity of Cur-SLN against human alveolar adenocarcinoma (A549 cells), human promyelocytic leukemia (HL-60 cells), and human prostatic small cell carcinoma (PC3 cells) than the Cur solution [130]. Moreover, they also studied the protective role of Cur-SLN in cerebrovascular ischemia-induced rats and found that cognition in rats was almost restored. The Cur-SLN formulation also significantly controlled acetylcholinesterase release while improving the supply of Cur to the brain [131].
Cur also acts as a p300-HAT inhibitor, a subtype of HAT (histone acetyltransferase) enzyme that contributes to its anticancer and anti-inflammatory properties [132].
Puglia et al. conducted in vivo studies in mice, discovering that intraperitoneally administered Precirol 5 ATO and Miglyol-based NLCs, stabilized with Lutrol F68 and Tween 80, effectively improved the anti-tumor effect of Cur in CNS. Western blotting analysis of the spinal cord fluid showed revealed a decrease in the acetylation of histone (H4) at lysine (K12) when compared to the DMSO-Cur solution, which was related to better CNS permeation of the NLCs [133].
Meng et al. probed the effect of Tween 80 (PS 80) on the brain permeation of the Cur-loaded NLCs. PS 80-NLCs remained stable in the plasma and allowed the adsorption of protein that was hypothesized to improve the formulation penetration by promoting the binding of PS 80-NLCs to receptors overexpressed at the blood-brain barrier (BBB). Ex vivo imaging of mouse brain sections confirmed an enhanced BBB penetration by PS 80-NLCs (up to 2-fold) in comparison to ordinary NLCs [134].
Meng et al. designed an NLC carrier system modified with lactoferrin (Lf) to mimic low-density lipoproteins (LDLs) since their receptors (LDLR) are highly expressed in the BBB. This modification enhances the protection provided by Cur against the progression of Alzheimer’s disease in the brain. Lf-NLCs were made with phosphatidylcholine, cholesterol oleate, and glycerol trioleate stabilized with S100-COOH (Carboxylated polyethylene glycol (100) monostearate) to resemble the hydrophobic lipid core of LDL (cholesteryl esters with triacylglycerides, having a surface coat of phospholipids, unesterified cholesterol). Lf was electrostatically adsorbed onto the NLCs to match the presence of the apolipoprotein B100 molecule on natural LDLs. The results indicated that LDL-mimic-NLCs remained stable as they passed through brain endothelial cells. Imaging and histopathological investigations of rat brain sections made it clear that Cur-Lf-mNLCs had better penetration and accumulation in rat brain cells compared to lactoferrin-free NLCs They also conveyed a superior healing effect on the damaged brain cells [135].
Ban et al. used tristearin and PEG as emulsifying agents to formulate Cur-loaded SLNs for enhance oral bioavailability. The digestion of lipids resulted in the phenomenon of micellization, enabling the solubilization of a large amount of Cur. The size and charge of these mixed micelles affected the extent of absorption through the intestinal epithelium which in turn, determined the plasma level of curcumin. PEG-stabilized SLNs improved oral bioavailability in rats due to the permissible size range and neutral charge of the micelles, as well as the extended lipolysis of SLNs [136].
Singh et al. designed a heat-sensitive system by encapsulating Cur into gold-coated liposomes for the stimuli-responsive destabilization of liposomes and controlled release of Cur at the desired site. They used the B16F10 melanoma cell line to study in vitro photothermal effect and intracellular uptake. Upon laser irradiation, curcumin-loaded liposome gold nanoparticles showed enhancement in cancer cell cytotoxicity against B16F10 melanoma cells, confirming the potential of nanoliposome formulation as a potential system for photothermal therapy [137].
Cur-loaded liposomes, formulated using bovine milk phospholipids and krill phospholipids, exhibited high stability with enhanced bioavailability, as well as antioxidative and anti-hyperglycemic properties [138]. To treat infections induced by methicillin-resistant S. aureus (MRSA), Bhatia et al. formulated a berberine-curcumin co-encapsulation liposomal system to evaluate the synergistic antimicrobial effect of both phytochemicals. In comparison with clindamycin, co-encapsulated liposomes showed a 77% reduction in intracellular infection [139]. The co-encapsulation of curcumin and tetrandrine in nano-liposomes (CT–DP–Lip), stabilized with DSPE–MPEG 2000 (DP), yielded formulation of sizes under 100 nm. MTT studies on MDA–MB–231, HepG2, HGC–27, and HCT116 cell lines confirmed the strong anti-tumor effect of the CT–DP–Lip formulation [140].
Paez-Hernandez et al. observed that the selection of oil is crucial in the design of Cur-loaded NEs. Grapeseed oil and olive oil induced recrystallization of the Cur, whereas medium-chain triglycerides produced the most stable NE with the smallest globule size. Time and power of ultrasonication was also negatively correlated to the size of the NE. Similarly, an increase in cycles and pressure in microfluidization led to small-sized NE. However, ultrasonication was superior to the microfluidization technique in obtaining uniformly dispersed smaller formulations [141].
Ahmad et al. used central composite design to prepare Cur NE and evaluate its anti-inflammatory and wound healing effect. Clove oil, Tween-80, and PEG-400 were selected due to their higher Cur solubility. The optimized NE showed negative surface charge with a globule size below 100 nm and spherical morphology, making it appropriate for a non-toxic transdermal delivery system in wound healing applications [142]. Chuacharoen et al. studied the effect of surfactant concentration on the properties of Cur-loaded NE and realized that higher surfactant levels produced small-sized formulations but could also affect the stability under different storage and usage conditions. Nonetheless, too much surfactant caused a prominent color change in fortified milk upon storage; therefore, a balanced approach is needed when selecting the surfactant levels [143].
The antitumor activity of Cur is well-documented in the literature, and several Cur-loaded LNPs for anticancer therapy are listed in Table 3.
Table 3.
Use of various Cur-loaded LNPs against different types of cancers.
| Cancer Type | LNPs | Particle Size (nm) | Preparation Methods | References |
|---|---|---|---|---|
| Lung cancer | SLN | 20–80 | Sol-gel | [144] |
| Liver cancer | NLC | 90 | High-pressure microfluidics | [145] |
| Colon cancer | SLN | <500 | Coacervation | [146] |
| Breast cancer | SLN | 152 | High pressure homogenization | [147] |
| Breast cancer | SLN | 194 | High pressure homogenization | [148] |
| Endometrial cancer | Liposomes | 100–150 | Thin-film hydration | [149] |
| Cervical cancer | Liposome | 350–390 | Thin-film hydration | [150] |
| Hepatocellular cancer | Chitosan coated liposome | 240 | Thin-film hydration | [151] |
| Breast cancer | Surface modified liposomes | 207–297 | Thin-film hydration | [152] |
| Breast cancer | NE | 199 | Nanoemulsification | [153] |
| Lung and liver cancer | SLN | 104.1 | Thin-film ultrasonic hydration method | [154] |
| Breast cancer | SLN | 175–190 | Cold dilution of microemulsion | [155] |
| Liver cancer | NE | 114.7 | Emulsification method | [156] |
| Breast cancer | NE | 40 | Emulsification method | [157] |
| Stomach cancer | NLC | 11–50 | High pressure homogenization | [158] |
| Lung cancer | liposome | 80–100 | Thin-film hydration | [159] |
| Liver cancer | liposome | 90–120 | Thin-film hydration | [160] |
2.5. Resveratrol (Rvt)
Resveratrol (Rvt), abundant in mulberries, peanuts, and grape seeds, is a potent polyphenol that possesses anti-inflammatory, antioxidant, and antibacterial properties [161]. It is also renowned for its cardioprotective and neuroprotective nature. Moreover, its activity against brain tumors, Alzheimer’s disease, and epilepsy has also been reported [162]. The limitations faced by Rvt include its meager solubility, poor stability, and low bioavailability [163]. Pandita et al. prepared stearic acid core SLN to load Rvt for the improvement of its oral bioavailability. The results highlighted an 8-fold improvement in the bioavailability of SLN in comparison to Rvt suspension, approving the suitability of the orally administered lipid-based nanocarrier system [164].
Neves et al. prepared Rvt-loaded SLNs (cetyl palmitate) and NLCs (cetyl palmitate and miglyol-812) and examined them for stability, solubility, and permeability of Rvt across intestinal epithelial cells. They found increased penetration of Rvt loaded LNPs. It was further demonstrated that oral absorption was enhanced during mealtime due to excessive secretions of intestinal juices [165]. Furthermore, SLNs were functionalized with apolipoprotein E (ApoE) to facilitate its passage more effectively through the BBB, as ApoE can bind to the overexpressed LDL receptors present on the BBB. It was discovered that the functionalized formulation successfully penetrated the brain and provided better protection and stability to the loaded entity [166].
Cur and Rvt co-loaded SLNs have also been reported to display excellent anticancer activity against colorectal cancer cells Rvt loaded liposomes were fabricated for mitochondrial-targeted delivery to overcome the narrow plasma half-life of Rvt, which hinders its permeability and retention. Researchers claimed improved targeting of therapeutics and the stimulation of the mitochondrial signaling pathway through Rvt-loaded liposomes. These liposomes were proven to be effective against multidrug-resistant cancer [167].
2.6. Hesperetin
Hesperetin, a flavone, is known for its powerful antioxidant and antitumorigenesis properties [168]. It has inhibitory effect against cardiac diseases and colon cancer [169]. The main obstructions for the use of hesperetin in pharmaceutical field are its poor physicochemical attributes (very low solubility, bitter taste) and erratic pharmacokinetic traits (poor dissolution and low bioavailability) [170].
Hesperetin-loaded NLCs were formulated by Fathi et al. for food fortification of milk. Glycerol monostearate and stearic acid were evaluated as solid lipids, whereas a mixture of oleic acid and estasan were used as an oil phase. Investigations revealed the NLCs of smaller size with greater encapsulation capability were obtained when compared to SLNs. Furthermore, glycerol monostearate produced smaller nanoparticles than stearic acid. Nanoencapsulation in NLCs not only improved the solubility of hesperetin but also masked its unpleasant taste. Burst release and low charge on NLCs’ surfaces were identified as the drawbacks of the formulations that need to be addressed in further research [171].
Wang et al. prepared hesperetin NE using pseudo-ternary phase diagrams and optimized it with response surface methodology. The extent of bioavailability and Cmax showed increases of more than 5-fold and 2-fold, respectively, compared to hesperetin suspension. Hesperetin NE effectively improved lymphatic transport, intestinal permeability, and bioavailability [172].
A tumor-targeting hyaluronic acid (HA)-modified liposome, loaded with cisplatin (CDDP) and hesperetin, was intended to relieve systematic toxicity and anti-metastasis of triple-negative breast cancer. HA modification facilitated cellular uptake in MDA-MB-231 cells. Co-loaded formulation suppressed intracellular signaling pathway PI3K/Akt/mTOR, epithelial-mesenchymal transition, and tumor metastasis, while showing a greater safety profile against normal cells [173].
2.7. Capsaicin
Capsaicin (CAP), a lipophilic pungent tasting constituent of hot pepper, is illustrious for its ability to lower blood pressure, alleviate hyperlipidemia [174], and its widespread use in the treatment of conditions such as arthritis, neuropathy, sciatica, neuralgia, and other inflammatory conditions [175].
Administering CAP is problematic due to its low bioavailability and rapid clearance, requiring the use of organic solvents for solubilization because of its aqueous insolubility. CAP has been encapsulated in SLN gel by Sharma and Arora using the high shear homogenization-ultrasonication technique. Skin permeation studies showed 1.6 times greater penetration in comparison to unprotected CAP. It was summarized that the usage of SLN gel as a carrier potentiated the anti-inflammatory effects of CAP and also gave better and prolonged release pattern in topical administration without using organic solvent [176]. Duangjit and et al. discovered the positive effect of limonene on the topical delivery of CAP-loaded cetyl palmitate-Transcutol P SLNs. This effect was seen as an increase in the permeability and encapsulation efficiency of CAP-SLN. Penetration enhancer effect of the terpene was remarkable but its effect on the stability of the CAP in the SLN was not evaluated [177].
Kunjiappan and coworkers encapsulated capsaicin in SLNs for the treatment of human hepatocellular carcinoma. They used the solvent evaporation-emulsification technique to formulate SLNs. SLNs with an 80 nm diameter showed better aqueous stability. In vivo biodistribution studies confirmed that 15% of SLN formulation was presented in hepatic cells while 0% was found in the brain cells. As the CAP-VEGF receptor (vascular endothelial growth factor) complex in the liver was highly stable, researchers concluded that capsaicin-loaded SLNs are effective against carcinoma due to their comparatively long circulation time (up to 3 days) [178].
2.8. Naringenin (Nar)
Naringenin (Nar; 4′,5,7-trihydroxyflavanone), a naturally occurring flavonoid, is reported to be present in cocoa, tomatoes, fruits such as cherries, and grapefruit [179]. The structure-activity relationship of Nar has been revealed, demonstrating how its hydroxyl groups (OH) are involved in exhibiting antioxidative properties, as OH groups donate hydrogen for the reduction of ROS [180]. Nar has also been also documented for its anti-inflammatory, anticancer, and hepatoprotective properties [181]. The major shortcoming in utilizing Nar is its lower bioavailability when administered orally on account of its insoluble nature in aqueous media [182].
Ji et al. formulated Nar-loaded glycerol monostearate SLNs for pulmonary applications using an emulsification and low-temperature solidification method. They also optimized the formulation by Taguchi orthogonal design. Sustained release of Nar for up to 48 h was observed for Nar-containing SLNs. Additionally, Nar-containing SLNs were also efficiently taken up by A549 cells in a time-dependent manner. Relative pulmonary bioavailability was recorded to be two times greater, with a prolonged half-life for the SLNs when compared with the suspension of Nar [183]. Wang et al. formulated paclitaxel and Nar co-loaded SLNs with Pericol ATO5, Dynasan 114, and Lutrol F188 as the solid lipid, surfactant, and stabilizer, respectively. RGD peptide sequence (cyclic) was added through the chemical conjugation process for surface functionalization.
Drug entrapment efficiency was found to be more than 80%. Pharmacokinetic studies revealed an increased absorption rate of the active substances from the SLNs. U87MG glioma cells were used to perform cytotoxicity studies, and florescence dye uptake measurement confirmed the elevated uptake of the SLNs formulation compared to the blank solution. Co-loaded SLNs modified with cRGD showed improved cellular uptake and anticancer activity [184]. Munir et al. evaluated three types of LNPs, stearic acid (SA), stearic-lauric (SL), and lecithin-chitosan (LC), loaded with Nar in an arthritis model of albino male rats. RA factor, key inflammatory markers, and joint damage inspection showed a tremendous decrease with LNPs in contrast to pure Nar. SL-LNPs showed better anti-inflammatory and therapeutic effect of NAR, followed by LC-LNPs and SA-LNPs [185].
To address the poor solubility and bioavailability issues of Nar, Wang et al. constructed Nar-loaded metal-organic framework (MOF) and further encapsulated this inclusion complex into liposomes that showed sustained release of Nar. The novel formulation was found to be very effective against lung cancer cells (A549 cells) and gastric cancer cells (SGC-7901 cells) [186].
2.9. Baicalin
Baicalin (BA), an active flavonoid, extracted from Scutellaria baicalensis, has been reported for its anti-inflammatory, antiviral, and antioxidant properties [187]. Tu et al. described the neuroprotective activities of baicalin and its effectiveness against cerebral ischemia and reperfusion injury [188].
Liu et al. formulated a BA-loaded pegylated cationic SLN modified with OX26 antibody (OX26-PEG- CSLN) to examine the amino acid levels in rats suffering from ischemia and reperfusion disorder. The levels of excitatory amino acids (EAA) and inhibitory amino acids (IAA) in the extracellular fluids of rats were determined as indicators to monitor the effectiveness of BA-loaded OX26-PEG-CSLN. Enhanced brain penetration of OX26-PEG-CSLN was observed with superior modulation of EAA (glutamate (Glu), aspartic acid (Asp)) and IAA (glycine (Gly), taurine (Tau), and γ-aminobutyric acid (GABA)) in comparison to BA solution [189].
Zhang et al. functionalized BA-loaded liposomes with Borneol (BO) (BO-BA-LP) and evaluated it for pharmacokinetic and pharmacodynamic behavior. A significant increase in the half-life of BA was noted, resulting in a marked improvement in the neurological and pathophysiological conditions of the middle cerebral artery occlusion rat model [190].
BA-loaded lipid-based NE was manufactured to evaluate its absorption by the lymphatic system, using the chylomicron flow blocking model. The lymphatic transport ability was also measured utilizing the lymph node distribution method. Compared to the BA-containing suspension, the Cmax value was 11.5-fold higher for the NE in the rats’ lymph nodes, presenting it as an effective treatment for chronic hepatitis B [191].
2.10. β-Sitosterol
Phytosterols, such as β-sitosterol, are structural components of the plasma membrane that are crucial for the developmental and regulatory processes in plants [192]. For human consumption, they are available in the market in the form of phytosterol-enriched butter, cereals, and margarine [193]. β-sitosterol is considered useful in lowering total cholesterol levels and low-density lipoprotein levels. β-sitosterol has also been found effective against cancers and ROS [194].
Lacatusu et al. designed β-sitosterol and green tea extract containing NLCs to improve antioxidant activity. The results showed that the use of green tea extract along with β-sitosterol was beneficial for attaining smaller NLCs with enhanced scavenging activity. Moreover, the use of natural oils such as grape seed oil helped in attaining a sustained release behavior of the loaded active [195].
β-sitosterol-loaded SLN, formulated by the double emulsion solvent displacement method, was assessed for its anti-arthritic effect against complete Fruend adjuvant (CFA)-induced arthritis. β-sitosterol-SLN reduced the levels of cytokines, cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), VEGF, and nuclear factor kappa light chain enhancer of activated B cells (NF-κB) while increasing the redox status of synovium, superoxide dismutase, glutathione, and catalase, as well as the expression of HO-1 and Nrf2. Therefore, a pronounced anti-arthritic effect from β-sitosterol-SLN was reported [196].
Liposomal-encapsulated β-sitosterol (LS) was administered to mice by oral gavage to study the inhibition of colon tumor invasiveness. Cell viability, protein expression, and invasiveness were determined by MTT assay, western blotting, and invasion assay, respectively. Bioluminescent imaging and ELISA were performed to monitor tumor growth inhibition and determine biomarkers in the intestinal epithelium, respectively. LS exhibited a significant anticancer effect and fewer metastases, which were attributed to the pronounced immune stimulation indicated by elevated levels of interleukins [197].
2.11. Sesamol
Sesame seed or sesame oil contains lignans such as sesamolin and sesamin, which can account for up to 1.5% of the total weight. An antioxidative phenol component known as sesamol (5-hydroxy-1,3-benzodioxole or 3,4-methylenedioxyphenols) is generated through the treatment of sesame oil or the hydrolysis of sesamolin [198]. Sesamol possesses features of a molecule with good oral bioavailability according to the Lipinski rules, but it has erratic pharmacokinetic attributes [199]. Sesamol is a sparingly soluble compound with approximately 35% documented oral bioavailability in Sprague–Dawley (SD) rats [200]. Sesamol undergoes fast elimination within the first 4 h and exhibits low oral bioavailability [201].
VanGilder et al. elaborated the antioxidative properties of sesamol [202]. Additionally, sesamol has been reported to have anti-aging, cardio-, hepato-, and chemoprotective activities [203].
Kakkar et al. designed sesamol-loaded glyceryl behenate SLNs to alleviate the CNS abnormalities associated with menopause, including cognitive loss and anxiety. Sesamol-SLNs with a mean diameter of 122 nm and entrapment efficiency (EE) of about 75% were obtained. Scintigraphic images of a rat model, coupled with biochemical and behavioral assessments, proved the superiority of the oral sesamol SLN formulation as a remedy for neurodegenerative disorders [204]. The progression of liver diseases is mostly associated with the presence of ROS [205].
Sesamol acts as scavenger due to the presence of a benzodioxole group, making it a potential remedy for managing liver disease [206]. Sesamol was encapsulated into SLNs by Singh et al. to evaluate its hepatoprotective ability. Spherical nanocarriers were formulated with the use of Compritol, Tween 80, and soy lecithin via the microemulsification technique. In vivo testing was conducted on rats with CCl4-induced sub-chronic hepatic toxicity. The ultimate levels of liver function markers showed the significant effect of sesamol-SLNs in terms of reducing hepatotoxin levels in the blood at a low dose of 8 mg/kg, as compared to silymarin (25 mg/kg) [207].
Disease induction and recovery effects were assessed through histopathology and by monitoring the levels of liver function biomarkers. Results showed significantly improved hepatic recovery with seasmol-SLNs [208]. Sachdeva et al. administered sesamol-loaded glyceryl behenate SLNs stabilized with Tween 80/soy lecithin in rats with ICV-STZ induced memory deficits. Sesamol was found to be a highly effective neuroprotectant when delivered by SLNs, as it lowered the levels of TNF-α, which is responsible for increased inflammation, neural cell damage, and, ultimately, memory dysfunctions [209].
Skin exposure to ultraviolet light leads to the production of ROS [210] that cause damage to skin in the form of skin aging and, ultimately, skin tumors as a result of DNA mutation [211]. Endogenous antioxidants act as the first line of defense, but they may need backup. To replenish antioxidants, exogenous scavengers are applied to skin [212]. Geetha et al. evaluated the anticarcinogenic effect of sesamol SLNs cream base in mice. Enhanced retention of cream base in the skin was observed. Skin permeation studies conducted by ex vivo experiments also showed a significant retention effect of the formulation. The reversal of cell damage was observed in sesamol SLNs in the mouse models of benzopyrene-induced skin cancer [213].
W/O/W NE containing sesamol and retinol were optimized with the aid of response surface methodology for food fortification applications. A deposited layer of alginate and chitosan surrounding the droplets of NE was formed via electrostatic attraction to control the release of encapsulated components. The sustained release of both sesamol and retinol were reported in simulated gastric and intestinal media, revealing the suitability of the biopolymer functionalized nanosystem for loading multiple payloads and increasing the shelf-life of the system [214].
As a conclusion of the above section, Table 4 presents the summary of potential phytochemicals loaded in lipid nanocarriers.
Table 4.
Phytochemicals possessing anti-inflammatory and antioxidative activities loaded in nanocarriers.
| Phytochemicals | Formulation Technique | Excipients | Indications | Route of Delivery | References |
|---|---|---|---|---|---|
| Sulforaphane, Curcumin |
hot melt emulsion | dichloromethane, stearic acid, poloxamer 188 | pancreatic cancer | oral | [215] |
| Sulforaphane, Curcumin |
hot melt emulsion | stearic acid, poloxamer 188, chitosan | pancreatic cancer | oral | [216] |
| Thymoquinone | high pressure homogenization | olive oil, soy lecithin, phosphatidylcholine, sorbitol, polysorbate 80, thimerosal |
gastroprotective | oral | [217] |
| Rosmarinic acid | hot melt ultrasonication | Witepsol wax, polysorbate 80 | food industry applications | oral | [218] |
| Rosmarinic acid | hot melt ultrasonication | carnauba wax, polysorbate 80 | pharmaceutical/food industry | oral | [219] |
| Rosmarinic acid | hot high pressure homogenization | glycerol monostearate (GMS), polysorbate 80, soy lecithin, hydrogenated soybean phosphatidylcholine (HSPC) | Huntington’s disease | nasal | [220] |
| Astaxanthin | high pressure homogenization | glycerol monostearate, stearic acid, glycerol distearates, polysorbate 20, | food industry/ cosmetics |
oral/topical | [221] |
| Astaxanthin | double emulsion solvent displacement | stearic acid, poloxamer 188, lecithin | neurological disorders | nasal | [222] |
| Epigallocatec-hin gallate (EGCG) |
multiple emulsion (w/o/w) | soybean phosphatidylcholine, Softisan, poloxamer 188, cetyltrimethylammonium bromide (CTAB), dimethyldioctadecylammonium bromide (DDAB) | glaucoma, age-related macular degeneration | ocular | [223] |
| Silibinin | solvent emulsification and evaporation | tristearin, poloxamer 188, polysorbate 80 |
colonic diseases | oral | [224] |
| Lutein | high shear homogenization | glycerol stearate, Tween 80, carnauba wax, fish oil, Tween 80, poloxamer 407 | food industry | oral | [225] |
| Lutein | high pressure homogenization | Plantacare 810, Tween 80, carnauba wax, cetyl palmitate, glyceryl tripalmitate |
photoprotection | topical | [226] |
| Lutein | hot sonication | Tween 80 and Span 60, diethylene glycol monoethyl ether, cyclodextrin | corneal diseases | ocular | [227] |
| Quercetin | high pressure homogenization | caprylic/capric triglyceride, glyceryl monostearate, glycerol monolaurate, polyglyceryl-10 laurate, polyglycerol-6 monostearate, sucrose ester |
functional food industries |
oral | [228] |
| Quercetin | ultrasonication | tripalmitin, lecithin, chitosan, Tween 80 | - | oral | [229] |
| Quercetin | emulsification and solidification |
glyceryl monostearate, Tween-80. PEG 400, soy lecithin | colonic diseases | oral | [230] |
| Quercetin | emulsion evaporation–solidification |
soy lecithin, glyceryl monostearate, stearic acid, medium-chain triglyceride, D-α-tocopheryl polyethylene glycol succinate (TPGS) | dermal infections | topical | [231] |
| Quercetin | hot sonication | glyceryl palmitostearate, Compritol 888, Tween 40 | H2O2-induced oxidative damages | ocular | [232] |
| Quercetin | emulsification and solidification at low temperature |
glycerol monostearate, medium-chain triglycerides, Transcutol, soy lecithin | hepatic, renal, and pulmonary disorders | oral | [233] |
| Luteolin | hot-microemulsion ultrasonic | glycerol monostearate, soybean lecithin, Tween 80 |
neurodegenerative, cancerous disorders | oral | [234] |
| Green tea extract | high shear homogenization | glycerol Stearate, lecithin, n-hexadecyl palmitate, Tween 80, Tween 20 | bacterial infection | oral | [235] |
| Green tea | w/o microemulsion | polyoxyethylene stearate, poloxamer 188, glyceryl monostearate |
topical application | percutaneous | [236] |
| Green tea extract, EGCG | thin-film rehydration | 1,2-dipalmitoyl-sn-glycerol-3-phosphate-rac-(1-glycerol) (DPPG, sodium salt) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol | pulmonary arterial hypertension | pulmonary | [237] |
| Green tea extract, EGCG | high shear homogenization and ultrasonication | Precirol ATO 5, miglyol 812, Tween 80 | infectious and cancer diseases | oral | [238] |
| Mangiferin | homogenization and ultrasonication | Lipoid S75, polysorbate 80, tocopherol, almond oil | skin regeneration | topical | [239] |
| Mangiferin | homogenization and ultrasonication | Lutrol F68, miglyol 812, Compritol 888 ATO | oxidative stress-related ocular diseases | ocular | [240] |
| Genistein | aqueous titration | olive oil, Sefsol 218, Kolliphor RH40, PEG 200 | cancer treatment | - | [241] |
| Chlorogenic Acid | ultrasonic emulsification | Isodecyl neopentanoate, undecyl alcohol, Caprylic/capric triglyceride, DL-alpha-tocopherol acetate, Transcutol, Labrafil, Pluronic F-68 | skin infection | topical | [242] |
| Triptolide | microemulsion | Precirol ATO 5, Compritol 888 ATO, Geleol, Cremophor RH 40, palmitic acid, stearic acid, sodium cholate | antigen-induced arthritis | intra-articular | [243] |
| Triptolide | thin-film hydration | folate was conjugated with polyethylene glycol-distearoyl phosphatidylethanolamine, cholesterol | rheumatoid arthritis | intravenous injection | [244] |
3. Functional Foods
Functional foods are natural substances used in the diet or as part of the daily diet that can have significant positive effects on health and disease management, beyond their nutritional value. Records of various functional foods can be found in this article that were discovered through a comprehensive review of the literature.
3.1. Vitamin B12
Vitamin B12 (Cobalamin), an essential vitamin for living organisms, works as a coenzyme for the synthetic pathway of DNA, where it helps in the synthesis of purine and thymidine and also contributes to the methylation of DNA [245]. Cell propagation inside the body also increases the demand for cobalamin, as this process involves thymidine and methionine for growth [246]. Genetic disorders are also considered to be a consequence of cobalamin deficiency as structural defects in genetic materials occur in the absence of cobalamin, which leads to interruption of DNA methylation. All these abnormalities subsequently result in the development of cancer [247].
Researchers packed cobalamin within the Tween 80 stabilized Compritol SLNs to enhance its anticancer activity. Cobalamin-SLNs were negatively charged and much smaller (200 nm) than free cobalamin (650 nm). Cobalamin-SLNs facilitated the easy passage of cobalamin across the cell membrane with a sustained release profile, thus resulting in a higher death rate of cancerous cells compared to free cobalamin [248]. Singh and colleagues exploited the receptor-mediated transport of the cobalamin through the intestinal epithelium to improve the oral absorption of amphotericin B-loaded SLNs (VBS-AmB-SLNs). Higher stability against the digestive enzymes and increased safety against J774A.1 cells were noted for VBS-AmB-SLNs. Moreover, favorable interaction with mucin was also observed for better absorption of the SLNs. The cobalamin functionalized SLNs were preferentially taken up by macrophages and were superior to the simple drug solution in antileishmanial activity [249].
To treat various neurological disorders, coencapsulation of alpha-lipoic acid (ALA) and cyanocobalamin in NE was performed by Coban et al. Castor oil was found to be suitable for encapsulating both ingredients, and optimum NE in terms of globule size, PDI, and zeta potential were obtained by formulations made by magnetic stirring and fine-tuning of the agitation speed, temperature, and pH. NE was found to be suitable, in comparison to the solution form, for extending the stability of both encapsulated molecules along with a desirable release profile [250].
Transferrin (Tf)-functionalized cobalamin-liposomes were reported to improve the CNS penetration of cobalamin, allowing it to effectively exert its anti-amyloidogenic activity, which is beneficial for treating Alzheimer’s disease (AD). This is particularly advantageous given hydrophilicity and the higher molecular weight of cobalamin. The formulation displayed excellent physical stability, sustained cobalamin release, slowed the formation of Aβ fibrils, and degraded mature fibrils, hence proving its effectiveness against AD [251].
3.2. Vitamin A
Vitamin A (Retinol) is responsible for vision, healthy hair and skin, and mucous membrane production. It can also lead to the peroxidation of lipids present in skin. This fat-soluble vitamin has the ability to moisturize the skin in order to retain its freshness and elasticity. Additionally, it possesses an anti-wrinkle effect that reduces skin degeneration and keratoses. When applied topically, it can also acts as an antioxidant against the aging of the skin [252].
Jenning et al. performed release kinetics studies on retinol-loaded glyceryl behenate SLNs using Franz diffusion cells and correlated release behavior with polymorphic transitions of lipids. After the topical application of SLNs, water evaporated from the formulations and promoted the interaction of SLNs with electrolytes present on the surface of the skin. This interaction resulted in retinol expulsion due to transitions in lipid crystals. The incorporation of surfactant controlled the transition, but only for a very short time period. Using a mixture of surfactants, gelling agents, or humectants could slow down the lipid transition (upon skin contact) to the β form and provide a sustained release effect [253].
Pople et al. discovered that retinol-loaded SLNs incorporated into a gel could easily target and deliver high concentrations of retinol at the application site. Enhanced penetration of the active ingredient was prominent with improved hydration when using the SLNs-loaded gel. The concentration of retinol (determined by penetration studies) was almost double for the SLN-enriched gel compared to the conventional gel [252].
Retinol and ascorbic acid co-loaded NE prepared through microfluidization were evaluated for cytotoxicity and regulation of milk-specific protein synthesis. Changes in αs2-, β-, and κ-casein expressions were higher in NE compared to conventional vitamin administration, revealing that the vitamin-loaded formulation has the potential to influence the expression of milk-related proteins [254].
3.3. Vitamin C
Vitamin C (Ascorbic acid) is famous for its antioxidative properties and apoptotic effects against cancerous cells. Guney et al. incorporated ascorbic acid into SLNs through high-pressure homogenization for efficient and sustained delivery of this potent antioxidant to cancer cells. It was concluded that the SLNs proved to be an effective and potential carrier for protecting the loaded vitamin and enhancing its uptake by rat fibroblast cells compared to free ascorbic acid [255].
Jiao et al. optimized the preparation of ascorbic acid-loaded liposomes using response surface methodology, as the physicochemical properties of the nanocarriers define their biological fate and performance. The optimum liposomes, with a size of 270 nm, a zeta potential of −41 mV, and a loading content of 75%, were obtained at a pressure of 25 MPa, a temperature of 48 °C, and a vitamin to phospholipid feed ratio of 0.25. A complex interplay of independent variables was discovered that needs to be carefully optimized for an efficient delivery system [256].
Hou et al. used ascorbic acid-functionalized liposomes for transfection by loading antimicrobial peptide and cathepsin B (AMP-CatB) mRNA. They suggested that multidrug-resistant bacteria can be treated in immunocompromised mice by adoptive transfer of macrophages containing antimicrobial peptides linked to cathepsin B in the lysosomes (MACs). The results demonstrated that adoptive MAC transfer was effective due to the presence of vitamin C lipid nanoparticles, which helped accumulate AMP-CatB in macrophage lysosomes to eradicate S. aureus and E. coli [257].
A mixture of w/o NEs containing ascorbic acid and collagen, formulated with tocopherol, safflower oil, surfactant, and water, was reported to exhibit pronounced protection against skin damage caused by ultraviolet radiation. This was examined using fibroblast cell lines to assess skin permeability, healing, and cell viability. Ascorbic acid potentiated the production of collagen in the skin, which synergized with the administered collagen and resulted in superior healing properties and protection against ultraviolet B (UVB) irradiation [258].
3.4. Vitamin D
Vitamin D (calciferol) is a fat-soluble vitamin necessary for bone health whose deficiency leads to rickets in children (especially among African-American young people) and osteomalacia in elders [259]. Calciferol deficiency is more prominent among dark-skin people; the increased melanin level in their skin causes the hindrance in calciferol synthesis in the skin [260]. Some other contributing factors responsible for the deficiency of this important fat-soluble vitamin include limited consumption of vitamin-enriched food, clothes type, habitat, reduced exposure to sun light, and excessive use of sunscreen [261]. Naturally, calciferol is synthesized subcutaneously inside living organisms, and the kidneys are responsible for its conversion to the active form. Nonetheless, the ability to produce and activate this vitamin in the body decreases with age, obesity, and malabsorption of fatty foods [262]. Thus, the exogenous fortified supply of calciferol, such as fortified milk and fortified margarine, is crucial [263].
Patel et al. prepared tripalmitin SLNs stabilized with Tween 20 to fortify ergocalciferol (an active form of calciferol). Increasing the concentration of ergocalciferol resulted in smaller SLNs with a gradual decrease in particle size from 120 nm to 65 nm as the proportion of ergocalciferol increased from 0% to 20%. The results indicated a high loading capacity of SLNs for fortifying juices with ergocalciferol [264].
Encapsulation of cholecalciferol in liposomes improved its stability and retention in the skin. Liposomes allowed 1.65 times more retention of the vitamin within the skin than its solution form. Moreover, histopathological observations revealed that the liposomal form of the vitamin augmented the production of collagen fibers, repaired the surface morphology of the skin, and mitigated the ageing phenomenon in a rat photoaging model [265].
3.5. Vitamin E
Vitamin E (Tocopherol), a natural antioxidant with lipophilic nature, is used for protecting exposed skin from UVB radiation. Tocopherol, beneficial for both curing and preventing [266], has a scavenging action against free radicals that promote skin aging and wrinkling [267]. Tocopherol also inherits anticarcinogenic activity, especially against UVB-induced photocarcinogenesis [268].
Dingler et al. evaluated cetyl palmitate SLNs for stabilizing tocopherol, as it is susceptible to chemical degradation. The penetration of tocopherol was enhanced by the SLNs due to the occlusive features of the SLN-based cream. The chemical nature of tocopherol remained intact for 6 months in the formulation. Moreover, SLNs also increased the aesthetic appearance by masking the uneven color [269]. Ma et al. used UVB-irradiated HaCaT keratinocytes to evaluate the photoprotective nature of tocopherol-loaded NLCs (VE-NLCs) made by the HPH technique. The nanosize of the formulation helped to produce occlusive properties, and the MTT assay confirmed photoprotection. Moreover, increased cellular uptake of VE-NLCs by HaCaT keratinocyte was also observed [270].
An ocular formulation of dorzolamide loaded in SLNs stabilized with D- α-Tocopherol polyethylene glycol 1000 succinate (TPGS) was optimized using the Box–Behnken design. In simulated tear fluid, the SLNs exhibited burst release of the drug within the first 2 h, followed by sustained release over a period of 10 h. Compared to the solution form, 2.87 times increase in corneal permeation was noted for the SLNs. Moreover, tocopherol stabilized SLNs were found biocompatible for ocular applications [271].
A tocopherol NE co-loaded with naringenin was tested for the management of Parkinson’s disease through multiple in vivo behavioral studies (akinesia test, narrow beam test, muscular coordination, and grip test) to confirm the effectiveness of the noninvasive intranasal delivery system. A negatively charged formulation with a droplet size of 38 nm was found to achieve higher trans-nasal mucosal flux, that could avoid first-pass metabolism and systemic distribution of the payloads. Significant reversal of Parkinson’s disease was attributed to the raise in SOD and GSH levels, coupled with the reduction of MDA when the co-encapsulated formulation was administered with levodopa [272].
Tocopherol-containing unilamellar liposomes were prepared using the ethanol injection method to study the stability and release of tocopherol, aiming to develop a semen cryopreservation protection system. Release studies revealed that the liposomes retained the tocopherol for 24 h, which was extended to 48 h upon the addition of cholesterol to the system, thereby improving semen motility. Moreover, stability studies confirmed the stability of system for 12 months at 4 °C [273].
3.6. Vitamin K
Vitamin K, a cofactor responsible for wound healing, has anti-hemorrhagic effects and plays a significant role in atherosclerosis by carboxylating proteins [274]. It also promotes hemostasis in nourishing infants [275]. Deficiency of this important cofactor may result in bone demineralization, especially in females. Oral administration of vitamin K causes a greater reduction in the international normalized ratio (INR) compared to subcutaneous administration [276]. Pereira et al. evaluated the variability in vitamin K absorption through the intestinal membrane in young patients with cholestatic diseases and elderly individuals with liver abnormalities [277].
Işcan et al. found SLNs suitable for the topical delivery of mosquito repellent (N, N-diethyl-m-toluamide) and vitamin K in terms of particle size, zeta potential, and short-term stability. The incorporation of vitamin K did not affect the particle size and zeta potential of the SLNs. Co-encapsulated SLNs were proven to be superior in terms of short-term stability, as analyzed by DSC, photon correlation spectroscopy, and zeta analyzer [278].
Liu et al. encapsulated vitamin K in SLNs for oral administration and optimized the formulation for particle size and entrapment efficiency by screening 12 lipids and surfactants with the aid of a design of experiment. About 85% of the vitamin K was entrapped inside SLNs at a feed of 5% and remained stable for more than 50 h in simulated gastric and intestinal conditions (in vitro testing) and also for 4 months on storage at ambient room temperature, thus indicating the suitability of the SLNs for oral delivery of vitamin K [279].
3.7. Omega(ω)-3 Polyunsaturated Fatty Acids
Omega(ω)-3 polyunsaturated fatty acids are considered heart-friendly, as they may reduce the risk of cardiac attacks and associated mortality rates [280]. Foods enriched with ω-3 fatty acids are in high demand nowadays. Companies using fish oils and flaxseeds for this purpose face challenges related to low stability and a high risk of degradation and toxicity during processing and storage. The stability of the solid lipid carrier can be improved with the addition of polyunsaturated fatty acids. Fish oil was reported to decrease the melting point of tripalmitin, inhibit the phase transition, and ultimately enhance the stability of SLNs during storage [281].
Muchow et al. produced fish oil-loaded glyceryl tristearate NLCs with masked aroma and enhanced oral absorption characteristics [282]. High melting (HM) lecithin, when used as a surfactant, was found to prevent the oxidation of the ω-3 fish oil in the SLNs without the use of antioxidants. Salminen et al. deduced that the ability of the saturated layer of HM-lecithin to solidify at interface on cooling, before the solid (high melting) lipid of the carrier, could have induced the crystallization of the carrier lipid. This generated a shell that may prevent the oxidation of ω-3 fish oil by limiting its diffusion to the outside [283].
Extract of the microalga Nannochloropsis gaditana, enriched in ω-3 fatty acids (eicosapentaenoic acid and glycolipids), was loaded onto SLN to protect the labile fatty acids and to control their release. More than 60% of the extract was encapsulated within the SLNs, which remained stable over a period of one month, as indicated by DSC and rheological studies [284].
A nanoliposomal encapsulation of ω-3 fatty acids (using chia oil) and α-lipoic acid (LA) was developed by researchers to protect the payloads from oxidation. Characterization techniques, including FTIR, NMR, and DSC, confirmed the suitability of carrier system with an entrapment efficiency of almost 80%. Cow milk fortified with ω-3 fatty acids and α-lipoic acid co-loaded liposomes was shown to improve its nutritional value [285].
As a conclusion of the above section, Table 5 presents the summary of potential functional food loaded in lipid nanocarriers.
Table 5.
Functional food cargo in lipid nanoparticles (SLNs, NLCs, liposomes, NEs).
| Functional Food | LNPs Type | Pharmacological Activity | Limitations to Address | References |
|---|---|---|---|---|
| Conjugated linoleic acids | NE, NLC | anti-diabetogenic, anti-carcinogenic effects, antioxidant, and anti-inflammatory | oxidative instability | [286,287,288,289] |
| Buckwheat | NE | antioxidant, anti-carcinogenic, and anti-inflammatory | low oral bioavailability, poor systemic absorption | [290,291] |
| Grape seed polyphenols | NE, liposome |
antimicrobial, anti-inflammatory, anti-carcinogenic, antiviral, and antioxidant | light and temperature instability | [292,293,294] |
| Probiotic lactobacilli | NE, liposome |
improve the normal flora, resistance against intrusion of pathogens, including shortening of diarrhea episodes, prevention of antibiotic-related diarrhea, reduction of asthma episodes, and reduction of the incidence of some respiratory infections | processing and storage instability | [295,296,297] |
| β-Glucan | NE, liposome |
serum cholesterol reduction, blood sugar regulation, antioxidant activity, and immunomodulatory activity | higher molecular weight, reduced solubility | [298,299] |
| Phytosterols and phytostanols | NE, SLN |
hypocholesterolemic activity | a high melting point, waxy consistency, easy crystallization | [300,301] |
| Policosanol | NE, liposome |
lipid-lowering/hypocholesterolemic activity, antiplatelet | limited bioavailability (5 to 12%) | [302,303] |
| Yellow Monascus Pigment | NE | anti-mutagenic and anti-carcinogenic properties, antimicrobial activities, potential anti-obesity activities | chemical degradation, instability | [304] |
| γ-Oryzanol | NE | antidiabetic, antioxidant | thermal instability and heat degradation | [305] |
| Monacolin K | liposome | anti-inflammatory, lipid lowering | low intestinal absorption, significant first-pass metabolism | [306] |
| Tocotrienol | NE, liposome |
anti-proliferative, antioxidant, anti-inflammatory, anti-carcinogenic | instability and poor bioavailability | [307,308] |
| Soybean daidzein | NE, liposome |
antioxidant, anti-inflammatory | intense metabolism and insolubility | [309,310] |
| Soybean genistein | SLN | neuroprotective, anti-carcinogenic | oxidation, heat, and light sensitivity, first-pass metabolism | [311] |
| Soybean orobol | SLN, NLC |
anti-aging, antioxidant | light sensitivity, low solubility, first-pass metabolism | [312] |
| Soybean glycitein | NE | anti-aging, antioxidant | bitter taste, low solubility and bioavailability, | [313] |
| Chia mucilage | NE | texturizing agent, film-forming agent, cosmetic ingredient | physical, chemical and biological degradation, instability | [314] |
4. Dietary Supplements
Several enzymes, antioxidants, amino acids, and metabolites that increase the essential nutritional value of a diet are termed as dietary supplements and are considered valuable for maintaining a balanced diet. In this chapter, the most prominent ones are summarized and presented from a nanoformulation point of view.
4.1. Melatonin
Melatonin, also known as N-acetyl-5-methoxytryptamine, is a hormone secreted by the pineal gland. Exogenous melatonin is categorized as a dietary supplement by the FDA that regulates the circadian rhythm. Deficiency of melatonin may contribute to aging, neurological, and cardiovascular disorders [315].
Exogenous melatonin is believed to correct many circadian rhythm disorders, for instance, delayed sleep phase syndrome (DSPS) and desynchronosis [316].
It is a potent antioxidant and neutralizer of nitric oxide (NO), hydrogen peroxide (H2O2), hydroxyl (OH) free radical, singlet oxygen (O2), and peroxynitrite anion (ONOO−) [317].
Some scientists have also documented the antiapoptotic property of melatonin [318]. Melatonin also contributes to reducing cardiotoxicity induced by cyclosporine A (CsA), which has also been reported to produce hepatic and renal disorders. Rezzani and colleagues evaluated the effectiveness of melatonin to improve cardiac health in CsA-treated rats [319]. They found that melatonin-encapsulated SLNs were more effective against CsA-induced cardiomyocyte apoptosis, as SLNs boosted the cellular internalization of melatonin [320].
In order to achieve and maintain a prolonged and sustained melatonin plasma concentration, special carriers other than conventional drug delivery systems are needed [321]. Melatonin undergoes first-pass metabolism when administered orally and also shows rapid plasma clearance, resulting in low bioavailability [322].
In the case of intravenous (i.v) bolus administration, its elimination half-life appears to be 40 min [323]. An in vivo study was conducted by Priano et al. to investigate the transdermal and oral delivery of melatonin-loaded SLNs in healthy human volunteers, with the aim of establishing a sustained release system for melatonin with the desired pharmacokinetic performance. Significantly higher AUC and t1/2 were observed for the oral SLN formulation of melatonin, but there was a 20-min delay in Tmax compared to the melatonin standard solution. However, the transdermal SLN preparation gave a slow absorption and elimination pattern, maintaining steady plasma levels of melatonin for up to 24 h. It was concluded that LNPs can considerably increase the absorption and plasma levels of melatonin, especially in the case of transdermal delivery [324].
Melatonin is a neurohormone which is also secreted in the eyes of mammals [325]. It is stated that the level of melatonin production varies throughout the circadian rhythm; its retinal level increases to maximum at night and decreases during the daytime [326]. Intraocular pressure (IOP) is inversely related to melatonin concentration. Pressure reaches its peak level during the day and gradually lowers to a minimum at night. This fact forms the basis of the hypothesis that melatonin does have an impact on IOP through the involvement of G-protein-coupled receptors (GPCRs) [327].
Alcantara et al. demonstrated in mice that melatonin can reduce IOP when used as medication [328]. The structure of the eye (corneal outer layer) and the presence of protective mechanisms, such as lacrimal secretions, tear production, and eye blinking, contribute to the low bioavailability of administered drugs and cannot be effectively addressed by conventional drug delivery systems [329].
With the aim of increasing the ocular residence time of melatonin, Leonardi et al. prepared bioadhesive SLNs with the help of cationic lipid didecyldimethylammonium bromide (DDAB). The positive charge on the surface of SLNs facilitated electrostatic interactions between the nanoformulation and ocular epithelial cells, which are negatively charged due to mucin. This interaction resulted in prolonged residence time and enhanced penetration of the active ingredient. In rabbits, melatonin-loaded SLNs produced a significant and sustained reduction in IOP that lasted for 24 h [330].
An elastic liposome loaded with melatonin (MLT-EL) was investigated for its efficacy against UV-induced skin photoaging. Permeation studies revealed a 1.5-fold improvement in the penetration of elastic liposomes compared to simple liposomes. Increased skin hydration, coupled with the reinforcement of collagen fibers, indicated the restoration of skin elasticity upon treatment with MLT-EL in photoaging experiments [331].
Chitosan coating was employed to fabricate melatonin-loaded mucoadhesive NE (CS-MLT-NE) to improve the bioavailability of melatonin in the brain through intranasal administration. Pharmacodynamic behavioral analysis of both the NE and suspension, performed using locomotor activity tests and the FST (forced swimming, climbing, and immobility), showed a markedly improved performance of the depressive rats treated with CS-MLT-NE. This indicates its potential as a treatment for depression [332].
4.2. Coenzyme Q10
Coenzyme Q10 (CoQ10), a lipophilic cofactor, is a potent antioxidant consisting of one quinine ring and a side chain of polyisoprenoid [333]. It helps to promote oxidative phosphorylation and ATP synthesis by transferring electrons in the respiratory chain. In its reduced form, CoQ10 shows potential antioxidative properties by reducing oxidative damage to genetic materials, proteins, and lipids [334].
CoQ10 exhibits more efficient free radical removal activity in the epidermal layer of the skin (10-fold higher) compared to the dermal layer [335].
CoQ10 also supports the anti-inflammatory activity of fibroblasts in the dermal layer. In the elderly, levels of CoQ10 in plasma and tissues decrease. This reduction in CoQ10 concentration is believed to be associated with skin aging and exposure to ultraviolet irradiation, necessitating compensation through external sources of CoQ10, such as anti-aging or CoQ10-rich creams. Cutanova Nanorepair Q10 cream was the first NLC cosmetic product launched in 2005 by Dr Rimpler Wedemark and contains CoQ10 for skin hydration and repaire. Pardeike et al. compared Cutanova Nanorepair Q10 cream with an NLC-free cream. In vivo results showed that the NLC contributed to enhanced skin hydration as compared to the NLC-free cream. It was evaluated that the NLC formulation was good in consistency and spreadability, which improved the aesthetic appearance of the formulation [336].
Schwarz et al. reported efficient dermal delivery of CoQ10 using ultrasmall NLCs (cetyl palmitate and dioctyl ether) with a size of 80 nm, which was more effective than the same-sized NE or the conventional NLCs (200 nm). Higher concentrations of the ultra-small NLCs were observed in vitro in the deeper layers of porcine skin [337]. The strongest antioxidant effect of the ultra-small NLCs was also evident in human keratinocyte (HaCaT) cells [338].
Phosphatidylcholine (PtdCho) and apolipoprotein (apo) A-I-containing lipid nanodiscs (NDs) were loaded with CoQ10. Oxygen consumption of the HepG2 cells significantly increased upon exposure to NDs due to the localization of CoQ10 within the mitochondria, which increased oxidative phosphorylation. Hence, the antioxidant potential of the cofactor was improved due to the designed NDs [339].
Chitosan-coated liposomal formulations encapsulating carbamazepine (CBZ) and CoQ10 were fabricated and optimized by the response surface methodology. Spherical nanoparticles with encapsulation efficiencies of over 75% for both payloads and size below 200 nm were obtained. These liposomes remained stable for 3 months and were deemed suitable for the treatment of epilepsy [340].
El-Leithy et al. evaluated the CoQ10-loaded isopropyl myristate NE stabilized with a mixture of Tween 80 and Transcutol HP for anti-aging and anti-wrinkle properties. The NE sustained the payload release (47% release in 24 h) and increased its transdermal flux, improving the smoothness of the skin and significantly decreasing the skin wrinkles [341].
5. Herbs and Spices
Herbs are the flowering or leafy parts of plants that are used for medicine, flavor, and aroma. Examples of herbs with antioxidant properties include oregano, sage, peppermint, lemon balm, Cinnamomi cortex, Scutellariae radix, garlic, nutmeg, cayenne, thyme, clove, and many others. Some herbs and Chinese herbal medicines that have been loaded into SLNs, NLCs, liposome, and nanoemulsion are summarized in Table 6.
Table 6.
Herbs and spices enclosed in nanocarriers with several pharmacological activities.
| Herbs/Spices | Pharmacological Activity | LNP Type | References |
|---|---|---|---|
| Tetrandrine | anti-inflammatory, immunologic, antiallergenic, antiarrhythmic | SLN | [342] |
| Triptolide (TP) | anti-inflammatory, immunosuppressive, antifertility, anti-neoplastic activities | SLN | [343,344] |
| Oridonin | anti-tumour, antibacterial, antioxidative, anti-inflammatory | SLN | [345] |
| Frankincense | antibacterial, antifungal, immuno-stimulating activity, anti-inflammatory, | SLN | [346] |
| Myrrh | antispasmodic, antiseptic, anti-tumour | SLN | [346] |
| Emodin | neuroprotective, anti-carcinogenic | SLN | [347] |
| Rosemary | hepatoprotective, antitumerogenic, antimicrobial, antioxidative | SLN, NLC | [348] |
| Artemisia arborescens L. | antiviral | SLN | [349] |
| Zedoary turmeric oil | antibacterial, anti-tumor, antithrombotic, hepatoprotective | NLC | [350] |
| Sage and Savoury | anti-diarrheal, wound healing activity, anti-inflammatory, antihypertensive | SLN | [351] |
| Breviscapine | antihypertensive, antithrombotic, neuroprotective | SLN | [352,353] |
| Neem oil | anti-inflammatory, antipyretic, antifungal, antibacterial, diuretic | SLN | [354] |
| Oenothera biennis Oil | antihypertensive, anti-inflammatory, sedative, astringent | SLN | [355] |
| Huperzine A | selective inhibitor of acetylcholinesterase | NLC | [356] |
| Carthamus tinctorius | antibacterial, anitoxidant, analgesic, sedative, anticonvulsant, immunosuppressive, anti-inflammatory | NLC | [357] |
| Zingiber Officinale | anti-inflammatory, antibacterial, hepatoprotective, anti-carcinogenic, anti-allergenic | SLN | [358] |
| Anethol | antifungal, antiaflatoxigenic, antioxidant | NE | [359] |
| Allicin | anti-inflammatory, antioxidant, neuroprotective | Liposome | [360] |
| Capsaicin | local anaesthetic | Liposome | [361] |
| 17-hydroxy-jolkinolide B | anti-carcinogenic | Liposome | [362] |
| Kaempferol | anti-carcinogenicr, anti-inflammatory, antioxidant | NLC | [363] |
| Chamomile oil | antibacterial, antifungal, anti-inflammatory, antispasmodic, anti-ulcer, antiviral, sedative effect | SLN | [364] |
| Morin hydrate | anti-carcinogenicr | SLN | [365] |
| Geraniol | anti-carcinogenic | NLC | [366] |
| Eucalyptus oil | antibacterial, antifungal, antiseptic, antihyperglycemic, antioxidant | NLC | [367] |
| Gallic acid | anti-cytotoxic, antiproliferative | SLN | [368] |
| Linalool | antiproliferative agent | SLN, Liposome | [369,370] |
| Ginkgolides A and B | anti-cardiovascular, cerebrovascular | SLN | [371] |
| Hibiscus rosa sinensis | antidepressant, aphrodisiac, abortifacient | SLN | [372] |
| Amaranth oil | anti-tumor, antimicrobial, antifungal | NE | [373] |
| Avocado oil | hypercholesterolemia | NE | [374] |
| Nigella sativa | antioxidative, antimicrobial | SLN | [375] |
| Linseed oil | anti-inflammatory, antitumorigenic | NE, Liposome | [376,377] |
| Neem oil | anti-hemorrhoid, antiprotozoal, antibacterial, antitoxoplasma activity | SLN, NE | [378,379] |
| Glycyrrhizic acid | anti-inflammation, antiviral, detoxification, hepatic protection | modified LNP | [380] |
Allium sativum (garlic) has been used widely all over the world as a food and a medicine for four millennia. It is a potential nutraceutical that has been used to treat cancer, headache, ulcers, and wounds [381]. Garlic contains a number of active constituents, such as PGs, vitamins (B, E, C), fatty acids, phospholipids, amino acids, lectin, and etc. [382]. Most of the biological functions of garlic are due to the presence of organosulphur compounds such as allicin, g-glutamylcysteines, diallyldisulphide (DADS), diallylsulphide (DAS), and numerous others [381].
These compounds are not only responsible for the medicinal properties of garlic but also its distinct flavor and aroma. Ajoene is another important constituent of garlic which remains stable in aqueous medium and can be extracted through chemical processes. Ajoene is considered to be a potent anti-carcinogenic and antithrombotic agent and has also demonstrated antifungal activity [383].
Wencui et al. worked on encapsulating garlic oil in SLNs to address the issue of volatility and low solubility associated with garlic oil. The SLNs remained stable during storage after lyophilization and exhibited minimal loss of oil, despite its volatile nature. However, PK studies in rats revealed rapid clearance of the garlic oil SLNs compared to garlic oil injection. This clearance was attributed to the enhanced phagocytosis of SLNs [384].
Faran et al. utilized the thin film hydration method to prepare nanoemulsomes containing lovastatin, along with ginger oil and garlic oil. These formulations were evaluated for their ability to lower lipid levels in a rat model fed a high-fat diet. The addition of either oil to the nanoparticles boosted the antihyperlipidemic activity of lovastatin and significantly reduced the fatty liver biomarkers. Moreover, liver function tests along with histopathological observations of the liver and kidney revealed the protective effects of these oils against the disease-induced damage [385].
Quach et al. formulated liposomes using lecithin that were functionalized with ginger (Zingiber officinale) oil to improve the stability of the lecithin-based liposomes. Incorporating ginger oil provided pH stability to the liposomes and added intrinsic antibacterial attributes due to its shogaol-6 content. The liposomes showed sufficient loading of the active ingredient and demonstrated pH-dependent release behavior, iterating its suitability as a potential drug delivery system [386].
6. Current Gaps, Challenges, and Future Directions
Nutraceuticals are not merely single natural molecules or ingredients; they consist of a complex mixture of multiple compounds. A number of active ingredients are present in one biological source, and higher doses may be require for daily administration [387]. Isolating and evaluating the biological activities of these complex structures can be challenging compared to chemical drugs with well-defined structures and documented activities. Among these bioactives, many compounds are sensitive to light, oxygen, heat, humidity, and pH. Physical and chemical instability, poor aqueous solubility, degradation, safety, preparation methods, and analytical techniques entail many challenges in the formulation of nutraceutical-based delivery systems. To formulate a stable and effective delivery system for these natural substances, a complete knowledge about their physicochemical properties, interactions with drugs and excipients, suitable formulation and characterization techniques, and storage conditions in harsh environment is needed [388].
Multiple extraction techniques, such as counter-current extraction, microwave-assisted extraction, Soxhlet extraction, maceration, and percolation, are used to obtain plant extracts. Bioactive compounds from plant extracts and concentrates are produced by a biofermentation process. Concentrates may also contain excipients for processing, like spray-dried carriers [389].
Conventional solvent extraction methods are associated with the several shortcomings, involving extensive, multiple steps, time consuming process with a need of a substantial amount of solvent. Furthermore, the most significant drawback of solvent extraction method is the loss or decomposition of thermolabile nutrients from the raw material [390].
Extraction and processing techniques could retain pathogens along with spices and raw herbs. That is why processing technology and extraction methods should be efficient in removing all contaminated materials and microbes from raw herbs and spices. Radiation exposure is practically prevailing in the food industry to disinfect herbs and eliminate contaminated pathogens and hazardous materials. This radiation exposure helps extend the shelf life of herbs by eliminating infectious agents. To professionally isolate potential active compounds, the Supercritical Fluid Extraction (SFE) technique has gained fame. SEF assists in removing pathogens, insects, and other types of contaminants from natural materials; but high maintenance costs and CO2 consumption are major obstacles to overcome [391]. New developments in the methods of extraction, refining, and separation are required to further improve the quality of herbs and spices.
Nutraceuticals are obtained and extracted from different natural sources, for example, food, plants, fungi, and microbes. Nutraceuticals are collected, isolated, purified, and graded through analytical procedures. The entire processing may cause degradation, chemical instability, and a loss of the quality of the active substance [392].
Furthermore, there are numerous challenges that can hinder the recovery of a quality product from natural sources, including contamination and intentional or unintentional adulteration. Unintentional adulteration could arise from several factors, such as harvesting at different plant growth stages, the quantity and quality of fertilizer, microbial attack, contamination with dust, pollens, insects, rodents, parasites, fungi, mold, toxins, harvesting means, and storage conditions. These factors, among others, can be responsible for serious illnesses, infections, and sensitive organ injuries, for instance, hepatic impairment. Standardized quality control and quality assurance procedures are mandatory to ensure the purity of raw materials and finished products. Other than unintentional adulteration, intentional adulteration is performed to gain economic benefits that could result in life-threatening issues. Addition of unlabeled and undeclared synthetic elements alters the quality and pharmacological properties of the finished products [393]. For instance, Panax ginseng, a traditional medicine, was found to be adulterated with the roots of Panax quinquefolius L. and Eleutherococcus senticosusmaxim, which may cause serious health issues [394].
Another example of intentional adulteration is replacing grape seed products with cheap and widely available peanut skin extract. Grape seeds contain a high content of polyphenols that are used to treat neurodegenerative and cardiac disorders. On the contrary, incorporation of peanut skin extract can pose serious allergic reactions to the body. Similarly, the leaf extract of Ginkgo biloba, effective against cerebral vascular insufficiency, was adulterated with free flavonols such as rutin and quercetin. Furthermore, to increase the weight of a product, vegetable oils or several other low-quality oils are added, compromising the final product quality. The presence of undeclared components could also lead to serious health problems. Therefore, comprehensive regulatory measures to control such practices are a pressing priority [395].
Due to the low concentration of active ingredients in plants, many plant sources are needed. Increasing demand will endanger plant species, as seen with Taxus brevifolia, which bears the potential ingredient taxol. Isolating taxol from the bark is tricky, time-consuming, and costly [396]. The need for a huge amount of medicinal plants has put the local ecological environment to serious threats of shortage of this medicinal plant. Various species of Taxus, particularly in China and India, have been listed as critically endangered due to high demand [397].
Hence, to meet the demand, several substandard, adulterated, and inferior quality herbal and plant supplies are hitting the market, compromising the safety and efficacy of nutraceuticals.
Quantification of a sole compound or its constituents can be challenging due to the unavailability of certain validated techniques. Various analytical methods are applied to determine the purity of obtained extracts, notably, thin-layer chromatography, ion exchange chromatography, size exclusion chromatography, high-speed counter-current chromatography, high-performance thin-layer chromatography, high-performance thin-layer chromatography-heated electrospray ionization-high-resolution mass spectroscopy, nuclear magnetic resonance imaging, Fourier-transform infrared spectroscopy, and mass spectroscopy. Issues reported in using these bioanalytical characterization methods include the need for frequent cleaning, throughout sample preparation, sensitivity of the method, accuracy, and potential interference [398].
In addition to the several other analytical issues, noteworthy considerations include the selection of isolation and sampling methods, the absence of regulatory methods, and reliable reference materials [399]. The use of transformative and less destructive sampling techniques is essential to address substandardization issues. Given the complex nature and wide physical diversity of bioactives, it is recommended to utilize a combination of several analytical techniques for ingredient quantification. The combination of methods should be in accordance with the regulatory framework. Spectroscopic methods, voltammetric methods, and chromatographic methods are the main classes of analytical procedures for evaluating nutraceuticals. Furthermore, to ensure risk management and to address safety and toxicity concerns, worldwide acceptable and approved procedures should be implemented [400].
Apart from the issues mentioned above, there are other challenges related to the dosage of nutraceuticals and their interactions with food, drugs, and excipients that must be considered when formulating delivery systems containing nutraceuticals. Figure 3 points out several challenges related to nutraceuticals and nanoformulations. Excessive over-the-counter use of nutraceuticals may cause lethal effects to the body, underscoring the importance of calculating the appropriate dosage based on age and health conditions. Interactions may lead to serious toxicity issues, elevated or diminished response after concomitant administration of nutraceuticals with other functional groups, drugs, or food. Moreover, the limited availability of data and documented information regarding safety, efficacy, and toxicity poses challenges when formulating dosage forms containing phytochemicals. Also, in the case of coadministration of medicine and phytochemicals, the literature does not provide enough in vitro and in vivo release and toxicity data or support evidence on drug–phytochemical interactions [401]. For example, the biocompound allicin, which has antihypertensive properties, can escalate the risk of bleeding when administered with any anticoagulant agent. It can also intensify the hypoglycemic effects of insulin. The consumption of polyphenols with stimulant medications can help mitigate tachycardia [402].
Figure 3.
Challenges associated with nutraceuticals and nanocarriers.
Although several merits and perks of using nanocarrier delivery systems have been documented, an incomplete picture of their toxicity profile remains an issue that requires extensive investigation. The complexation of molecules within the internal body environment raises serious safety concerns, especially in the case of long-term usage. NPs, when passing through membranes and organs, have proteins attaching to their surfaces, which can result in alterations in shape and surface charges. Hence, the NPs reaching the target area may not be the same as the ones administered. Common physical properties, such as size, shape, curvature, and free energy, influence the arrangement of proteins around the NPs. This binding with proteins may cause hindrance in enzymatic activity, unfolding of proteins, and crosslinking of thiol groups, leading to unexpected adverse outcomes [403].
The potential toxicity of NPs is a major concern because of their ability to cross cellular membranes and the blood–brain barrier. Nanocarriers with particle size ranging from 100 nm to 1000 nm can be easily taken up by phagocytes, whereas NPs smaller than 100 nm are more prone to endocytosis. NPs susceptible to phagocytosis and endocytosis need additional screening to protect healthy cells and ensure safe therapy. Although nanoformulations composed of phospholipids are claimed to be safe for body cells, extensive research is still required [404]. For example, cationic liposomes are linked to systemic toxicity, as the positive charge on the liposomal surface interacts with serum proteins and lipoproteins, which can accelerate drug aggregation and release the content even before reaching the target site. That is why high doses can cause serious harm, such as hepatic impairment and spleen toxicity. Much higher doses of lipids have been reported to cause plasma protein depletion and disturbance in the body’s hemostasis. Higher surfactant levels in NE have also been associated with interference in the tight junctions of the gastrointestinal tract and the resulting toxicity [405].
Moreover, NPs also suffer from a short half-life as they are attacked by macrophages and accumulated in the hepatic milieu. Surface coating and PEGylation are employed to facilitate the escape of NPs from the immune system. The pharmacokinetic attributes and distribution of nanomaterials are determined by their physicochemical properties, surface charge, and morphology. Nanoformulations with a particle size greater than 10 nm are primarily found in the spleen, liver, and blood, whereas those smaller than this size range can also be found in the brain, heart, lungs, and kidneys. These physicochemical attributes are sensitive to small changes during the formulation process, and they contribute to quality variations and the reproducibility of the product [406]. Therefore, the major difficulties in nanoscale product development are scaling up and ensuring the reproducibility of nanomedicines. Nanomedicines formulation procedures involve multiple steps, such as melting, homogenization, sonication, milling, emulsification, removal of organic solvents, cooling, etc., that are easier to control at a low scale. At the industrial level, controlling multiple steps with precision is far from challenging and costly. Nanomedicines produced at a large scale may have variable therapeutic effects due to a higher probability of variation and reduced reproducibility [19].
A systematic evaluation and the Quality-by-Design (QbD) approach may provide a way forward to mitigate crucial variations in physicochemical properties during formulation steps and incorporate quality into the process to yield reproducible batches [407]. A summarized overview of future prospects is depicted in Figure 4.
Figure 4.
Future prospects in nanotechnology along with the nutraceutical technology sector.
7. Conclusions
LNPs built with naturally occurring lipids are one of the most reliable nanotechnologies that are being used to load bioactives. They provide a shielding effect to the cargo, ensuring its effectiveness and stability over extended periods of storage. This review article throws light on the intrinsic worth and virtues of natural compounds encapsulated in numerous LNPs. LNPs loaded with effective natural constituents provide a wide range of therapeutic options to combat and prevent ailments. Currently, the absolute necessity is to pay continuous efforts in conducting extensive in vivo experiments and clinical trials on nutraceuticals, as well as in developing a stringent regulatory framework to fully unleash the potential of nutraceutical products.
Author Contributions
Conceptualization, R.A., A.R. and M.B.-S.; writing—original draft preparation, R.A., A.R., S.A., A.K., S.B. and M.B.-S.; writing—review and editing, R.A., A.R., S.A., A.K., S.B. and M.B.-S.; visualization, R.A. and S.A.; supervision, A.R. and M.B.-S.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Project no. TKP2021-EGA-32 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.DeFelice S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995;6:59–61. [Google Scholar]
- 2.Rishi R. Nutraceuticals: Borderline between food and drug. Pharma Rev. 2006;2:51–53. [Google Scholar]
- 3.Gulati O.P., Ottaway P.B. Legislation relating to nutraceuticals in the European Union with a particular focus on botanical-sourced products. Toxicology. 2006;221:75–87. doi: 10.1016/j.tox.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Pandey M., Verma R.K., Saraf S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010;3:11–15. [Google Scholar]
- 5.Rahman A., Uahengo V., Likius D. Mini review on emerging methods of preparation of liposome and its application as Liposome drug delivery systems. Open J. Pharmacol. Pharmacother. 2018;3:5–21. [Google Scholar]
- 6.Huang Q., Given P. Micro/Nano Encapsulation of Active Food Ingredients. American Chemical Society; Washington, DC, USA: 2009. [Google Scholar]
- 7.Huang Q., Yu H., Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010;75:R50–R57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 8.Üner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Die Pharm.-Int. J. Pharm. Sci. 2006;61:375–386. [PubMed] [Google Scholar]
- 9.Cirri M., Bragagni M., Mennini N., Mura P. Development of a new delivery system consisting in “drug–in cyclodextrin–in nanostructured lipid carriers” for ketoprofen topical delivery. Eur. J. Pharm. Biopharm. 2012;80:46–53. doi: 10.1016/j.ejpb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer-Korting M., Mehnert W., Korting H.-C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007;59:427–443. doi: 10.1016/j.addr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Esposito E., Mariani P., Ravani L., Contado C., Volta M., Bido S., Drechsler M., Mazzoni S., Menegatti E., Morari M. Nanoparticulate lipid dispersions for bromocriptine delivery: Characterization and in vivo study. Eur. J. Pharm. Biopharm. 2012;80:306–314. doi: 10.1016/j.ejpb.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Müller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002;54:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 13.Yaghmur A., Glatter O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009;147:333–342. doi: 10.1016/j.cis.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Li M., Du C., Guo N., Teng Y., Meng X., Sun H., Li S., Yu P., Galons H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019;164:640–653. doi: 10.1016/j.ejmech.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Shakeri S., Ashrafizadeh M., Zarrabi A., Roghanian R., Afshar E.G., Pardakhty A., Mohammadinejad R., Kumar A., Thakur V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines. 2020;8:13. doi: 10.3390/biomedicines8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antimisiaris S., Marazioti A., Kannavou M., Natsaridis E., Gkartziou F., Kogkos G., Mourtas S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021;174:53–86. doi: 10.1016/j.addr.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa I.F., Hussein M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials. 2020;10:1608. doi: 10.3390/nano10081608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarhan O., Spotti M.J. Nutraceutical delivery through nano-emulsions: General aspects, recent applications and patented inventions. Colloids Surf. B Biointerfaces. 2021;200:111526. doi: 10.1016/j.colsurfb.2020.111526. [DOI] [PubMed] [Google Scholar]
- 19.Soares S., Sousa J., Pais A., Vitorino C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018;6:360. doi: 10.3389/fchem.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Research P. Nutraceuticals Market Size, Growth, Trends, Report by 2030. Precedence Research; Ottawa, ON, Canada: 2021. [Google Scholar]
- 21.Research G.V. Nanomedicine Market Size, Share & Trends Analysis Report by Application (Drug Delivery), by Indication (Clinical Oncology, Infectious Diseases), by Molecule Type, by Region, and Segment Forecasts, 2023–2030. Grand View Research; San Francisco, CA, USA: 2023. p. 150. 978-1-68038-942-5. [Google Scholar]
- 22.Insights G.M. Nutraceutical Market-By Product (Functional Foods {Baby Food, Cereals}, Dietary Supplements), Form (Capsules, Tablets, Gummies), Condition (Weight Management, Joint Health, Skin, Hair), Distribution Channel (Pharmacies), Global Forecast, 2023–2032. Global Market Insights; Selbyville, DE, USA: 2023. p. 270. GMI5297. [Google Scholar]
- 23.Lippacher A., Müller R., Mäder K. Investigation on the viscoelastic properties of lipid based colloidal drug carriers. Int. J. Pharm. 2000;196:227–230. doi: 10.1016/S0378-5173(99)00428-7. [DOI] [PubMed] [Google Scholar]
- 24.Müller R.H., Mäder K., Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–A review of the state of the art. Eur. J. Pharm. Biopharm. 2000;50:161–177. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 25.Basha S.K., Dhandayuthabani R., Muzammil M.S., Kumari V.S. Solid lipid nanoparticles for oral drug delivery. Mater. Today Proc. 2021;36:313–324. doi: 10.1016/j.matpr.2020.04.109. [DOI] [Google Scholar]
- 26.Svilenov H., Tzachev C. Solid lipid nanoparticles–apromising drug delivery system. Nanomedicine. 2014;8:188–237. [Google Scholar]
- 27.Ren G., Sun Z., Wang Z., Zheng X., Xu Z., Sun D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019;540:177–184. doi: 10.1016/j.jcis.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Weerapol Y., Manmuan S., Chaothanaphat N., Limmatvapirat S., Sirirak J., Tamdee P., Tubtimsri S. New Approach for Preparing Solid Lipid Nanoparticles with Volatile Oil-Loaded Quercetin Using the Phase-Inversion Temperature Method. Pharmaceutics. 2022;14:1984. doi: 10.3390/pharmaceutics14101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L., Wang X., Liu Y., Yang G., Falconer R.J., Zhao C.-X. Lipid nanoparticles for drug delivery. Adv. NanoBiomed Res. 2022;2:2100109. doi: 10.1002/anbr.202100109. [DOI] [Google Scholar]
- 30.Gomaa E., Fathi H.A., Eissa N.G., Elsabahy M. Methods for preparation of nanostructured lipid carriers. Methods. 2022;199:3–8. doi: 10.1016/j.ymeth.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Chen D.-B., Yang T.-Z., Lu W.-L., Zhang Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem. Pharm. Bull. 2001;49:1444–1447. doi: 10.1248/cpb.49.1444. [DOI] [PubMed] [Google Scholar]
- 32.Kaviyarasu K., Matinise N., Mayedwa N., Mongwaketsi N., Letsholathebe D., Mola G., AbdullahAl-Dhabi N., Valan Arasu M., Henini M., Kennedy J. Evaluation on La2O3 garlanded ceria heterostructured binary metal oxide nanoplates for UV/visible light induced removal of organic dye from urban wastewater. S. Afr. J. Chem. Eng. 2018;26:49–60. [Google Scholar]
- 33.Jovanović A.A., Balanč B.D., Ota A., Ahlin Grabnar P., Djordjević V.B., Šavikin K.P., Bugarski B.M., Nedović V.A., Poklar Ulrih N. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur. J. Lipid Sci. Technol. 2018;120:1800039. doi: 10.1002/ejlt.201800039. [DOI] [Google Scholar]
- 34.Istenič K., Cerc Korošec R., Poklar Ulrih N. Encapsulation of (−)-epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. J. Sci. Food Agric. 2016;96:4623–4632. doi: 10.1002/jsfa.7691. [DOI] [PubMed] [Google Scholar]
- 35.Gouda A., Sakr O.S., Nasr M., Sammour O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021;61:102174. doi: 10.1016/j.jddst.2020.102174. [DOI] [Google Scholar]
- 36.Laloy E., Vuillemard J.-C., Ackermann H.-W., Robin O. Preparation of liposomes by a simple emulsification technique. Biotechnol. Tech. 1994;8:717–722. doi: 10.1007/BF00151475. [DOI] [Google Scholar]
- 37.Kumar M., Bishnoi R.S., Shukla A.K., Jain C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019;24:225. doi: 10.3746/pnf.2019.24.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gharibzahedi S.M.T., Hernández-Ortega C., Welti-Chanes J., Putnik P., Barba F.J., Mallikarjunan K., Escobedo-Avellaneda Z., Roohinejad S. High pressure processing of food-grade emulsion systems: Antimicrobial activity, and effect on the physicochemical properties. Food Hydrocoll. 2019;87:307–320. doi: 10.1016/j.foodhyd.2018.08.012. [DOI] [Google Scholar]
- 39.Zaaboul F., Raza H., Cao C., Yuanfa L. The impact of roasting, high pressure homogenization and sterilization on peanut milk and its oil bodies. Food Chem. 2019;280:270–277. doi: 10.1016/j.foodchem.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 40.García-Márquez E., Higuera-Ciapara I., Espinosa-Andrews H. Design of fish oil-in-water nanoemulsion by microfluidization. Innov. Food Sci. Emerg. Technol. 2017;40:87–91. doi: 10.1016/j.ifset.2016.11.007. [DOI] [Google Scholar]
- 41.Akbas E., Soyler B., Oztop M.H. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT. 2018;96:266–273. doi: 10.1016/j.lwt.2018.05.043. [DOI] [Google Scholar]
- 42.Sneha K., Kumar A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022;76:102914. [Google Scholar]
- 43.Chuesiang P., Siripatrawan U., Sanguandeekul R., McLandsborough L., McClements D.J. Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: Impact of oil phase composition and surfactant concentration. J. Colloid Interface Sci. 2018;514:208–216. doi: 10.1016/j.jcis.2017.11.084. [DOI] [PubMed] [Google Scholar]
- 44.Rehman M., Khan M.Z., Tayyab M., Madni A., Khalid Q. Self-Nanoemulsification of Healthy Oils to Enhance the Solubility of Lipophilic Drugs. JoVE. 2022;185:e63995. doi: 10.3791/63995. [DOI] [PubMed] [Google Scholar]
- 45.Thaller A., Schmauder L., Frieß W., Winter G., Menzen T., Hawe A., Richter K. SV-AUC as a stability-indicating method for the characterization of mRNA-LNPs. Eur. J. Pharm. Biopharm. 2023;182:152–156. doi: 10.1016/j.ejpb.2022.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan R., Van Le Q., Yang H., Zhang D., Gu H., Yang Y., Sonne C., Lam S.S., Zhong J., Jianguang Z. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere. 2021;271:129499. doi: 10.1016/j.chemosphere.2020.129499. [DOI] [PubMed] [Google Scholar]
- 47.Anjum M.M., Patel K.K., Dehari D., Pandey N., Tilak R., Agrawal A.K., Singh S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improves antimicrobial activity. Drug Deliv. Transl. Res. 2021;11:305–317. doi: 10.1007/s13346-020-00795-4. [DOI] [PubMed] [Google Scholar]
- 48.Li P., Bukhari S.N.A., Khan T., Chitti R., Bevoor D.B., Hiremath A.R., SreeHarsha N., Singh Y., Gubbiyappa K.S. Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/HO-1/NF-kB Signalling Pathway. Int. J. Nanomed. 2020;15:9115–9124. doi: 10.2147/IJN.S256494. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Long Y., Xiang Y., Liu S., Zhang Y., Wan J., Yang Q., Cui M., Ci Z., Li N., Peng W. Baicalin Liposome Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice via Inhibiting TLR4/JNK/ERK/NF-κB Pathway. Mediat. Inflamm. 2020;2020:8414062. doi: 10.1155/2020/8414062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attri S., Kumar A., Kaur K., Kaur P., Punj S., Bedi N., Tuli H.S., Arora S. Assessment of anti-psoriatic activity of bakuchiol-loaded solid lipid nanoparticles-based gel: Design, characterization, and mechanistic insight via NF-kB signaling pathway. Naunyn-Schmiedeberg's Arch. Pharmacol. 2023;396:2105–2125. doi: 10.1007/s00210-023-02445-1. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Z., Li Y., Wang K., Zhu X., Tharkar P., Shu W., Zhang T., Zeng S., Zhu L., Murray M., et al. Compritol solid lipid nanoparticle formulations enhance the protective effect of betulinic acid derivatives in human Müller cells against oxidative injury. Exp. Eye Res. 2022;215:108906. doi: 10.1016/j.exer.2021.108906. [DOI] [PubMed] [Google Scholar]
- 52.Andrade S., Pereira M.C., Loureiro J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer's disease therapy. Colloids Surf. B Biointerfaces. 2023;225:113270. doi: 10.1016/j.colsurfb.2023.113270. [DOI] [PubMed] [Google Scholar]
- 53.Freitas M.M., Cavalcante P.M., Duarte-Filho L.A.M.S., Macedo C.A.F., Brito M.C., Menezes P.M.N., Ribeiro T.F., Costa S.M., Carvalho B.A.G., Ribeiro F.P.R.A., et al. Investigation of the relaxing effect of a camphor nanoemulsion on rat isolated trachea. Chem.-Biol. Interact. 2021;348:109656. doi: 10.1016/j.cbi.2021.109656. [DOI] [PubMed] [Google Scholar]
- 54.Yang R., Miao J., Shen Y., Cai N., Wan C., Zou L., Chen C., Chen J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. LWT. 2021;141:110924. doi: 10.1016/j.lwt.2021.110924. [DOI] [Google Scholar]
- 55.Farhadi A., Homayouni Tabrizi M., Sadeghi S., Vala D., Khosravi T. Targeted delivery and anticancer effects of Chrysin-loaded chitosan-folic acid coated solid lipid nanoparticles in pancreatic malignant cells. J. Biomater. Sci. Polym. Ed. 2023;34:315–333. doi: 10.1080/09205063.2022.2121589. [DOI] [PubMed] [Google Scholar]
- 56.Sang N., Jiang L., Wang Z., Zhu Y., Lin G., Li R., Zhang J. Bacteria-targeting liposomes for enhanced delivery of cinnamaldehyde and infection management. Int. J. Pharm. 2022;612:121356. doi: 10.1016/j.ijpharm.2021.121356. [DOI] [PubMed] [Google Scholar]
- 57.Nordin N., Yeap S.K., Rahman H.S., Zamberi N.R., Abu N., Mohamad N.E., How C.W., Masarudin M.J., Abdullah R., Alitheen N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019;9:1614. doi: 10.1038/s41598-018-38214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alqarni M.H., Foudah A.I., Alam A., Salkini M.A., Muharram M.M., Labrou N.E., Rawat P. Coumarin-Encapsulated Solid Lipid Nanoparticles as an Effective Therapy against Methicillin-Resistant Staphylococcus aureus. Bioengineering. 2022;9:484. doi: 10.3390/bioengineering9100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng J., Jiang Z., Wu G., Cai Z., Du Q., Tao L., Zhang Y., Chen Y., Shen X. Improving protection effects of eucalyptol via carboxymethyl chitosan-coated lipid nanoparticles on hyperglycaemia-induced vascular endothelial injury in rats. J. Drug Targeting. 2021;29:520–530. doi: 10.1080/1061186X.2020.1859514. [DOI] [PubMed] [Google Scholar]
- 60.Fu X., Gao Y., Yan W., Zhang Z., Sarker S., Yin Y., Liu Q., Feng J., Chen J. Preparation of eugenol nanoemulsions for antibacterial activities. RSC Adv. 2022;12:3180–3190. doi: 10.1039/D1RA08184E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senthil Kumar C., Thangam R., Mary S.A., Kannan P.R., Arun G., Madhan B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020;231:115682. doi: 10.1016/j.carbpol.2019.115682. [DOI] [PubMed] [Google Scholar]
- 62.Subroto E., Andoyo R., Indiarto R., Lembong E., Rahmani F. Physicochemical properties, sensory acceptability, and antioxidant activity of chocolate bar fortified by solid lipid nanoparticles of gallic acid. Int. J. Food Prop. 2022;25:1907–1919. doi: 10.1080/10942912.2022.2115066. [DOI] [Google Scholar]
- 63.Simão D.O., Honorato T.D., Gobo G.G., Piva H.L., Goto P.L., Rolim L.A., Turrin C.-O., Blanzat M., Tedesco A.C., Siqueira-Moura M.P. Preparation and cytotoxicity of lipid nanocarriers containing a hydrophobic flavanone. Colloids Surf. Physicochem. Eng. Asp. 2020;601:124982. doi: 10.1016/j.colsurfa.2020.124982. [DOI] [Google Scholar]
- 64.Gostyńska A., Czerniel J., Kuźmińska J., Brzozowski J., Majchrzak-Celińska A., Krajka-Kuźniak V., Stawny M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics. 2023;15:448. doi: 10.3390/pharmaceutics15020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma H.L., Varanda L.C., Perussi J.R., Carrilho E. Hypericin-loaded oil-in-water nanoemulsion synthesized by ultrasonication process enhances photodynamic therapy efficiency. J. Photochem. Photobiol. B Biol. 2021;223:112303. doi: 10.1016/j.jphotobiol.2021.112303. [DOI] [PubMed] [Google Scholar]
- 66.Feng Y., Qin G., Chang S., Jing Z., Zhang Y., Wang Y. Antitumor Effect of Hyperoside Loaded in Charge Reversed and Mitochondria-Targeted Liposomes. Int. J. Nanomed. 2021;16:3073–3089. doi: 10.2147/IJN.S297716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moghadaszadeh M., Khayyati M., Spotin A., Norouzi R., Pagheh A.S., Oliveira S.M.R., Pereira M.d.L., Ahmadpour E. Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces. Pharmaceuticals. 2021;14:623. doi: 10.3390/ph14070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicoleti L.R., Di Filippo L.D., Duarte J.L., Luiz M.T., Sábio R.M., Chorilli M. Development, characterization and in vitro cytotoxicity of kaempferol-loaded nanostructured lipid carriers in glioblastoma multiforme cells. Colloids Surf. B Biointerfaces. 2023;226:113309. doi: 10.1016/j.colsurfb.2023.113309. [DOI] [PubMed] [Google Scholar]
- 69.Mendes Miranda S.E., Alcântara Lemos J.d., Fernandes R.S., Silva J.d.O., Ottoni F.M., Townsend D.M., Rubello D., Alves R.J., Cassali G.D., Ferreira L.A.M., et al. Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model. Biomed. Pharmacother. 2021;133:110936. doi: 10.1016/j.biopha.2020.110936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva L.M., Marconato D.G., Silva M.P.N.d., Raposo N.R.B., Facchini G.d.F.S., Macedo G.C., de Sá Teixeira F., Salvadori M.C.B.d.S., Pinto P.d.F., Moraes J.d., et al. Licochalcone A-loaded solid lipid nanoparticles improve antischistosomal activity in vitro and in vivo. Nanomedicine. 2021;16:1641–1655. doi: 10.2217/nnm-2021-0146. [DOI] [PubMed] [Google Scholar]
- 71.Brenelli L.B., Mariutti L.R.B., Villares Portugal R., de Farias M.A., Bragagnolo N., Mercadante A.Z., Franco T.T., Rabelo S.C., Squina F.M. Modified lignin from sugarcane bagasse as an emulsifier in oil-in-water nanoemulsions. Ind. Crops Prod. 2021;167:113532. doi: 10.1016/j.indcrop.2021.113532. [DOI] [Google Scholar]
- 72.Wang X., Sun J., Zhao S., Zhang F., Meng X., Liu B. Highly stable nanostructured lipid carriers containing candelilla wax for d-limonene encapsulation: Preparation, characterization and antifungal activity. Food Hydrocoll. 2023;145:109101. doi: 10.1016/j.foodhyd.2023.109101. [DOI] [Google Scholar]
- 73.Alidadi H., Ashtari A., Samimi A., Karami M.A., Khorsandi L. Myricetin loaded in solid lipid nanoparticles induces apoptosis in the HT-29 colorectal cancer cells via mitochondrial dysfunction. Mol. Biol. Rep. 2022;49:8537–8545. doi: 10.1007/s11033-022-07683-9. [DOI] [PubMed] [Google Scholar]
- 74.Wei X., Yang D., Xing Z., Cai J., Wang L., Zhao C., Wei X., Jiang M., Sun H., Zhou L., et al. Hepatocyte-targeted delivery using oleanolic acid-loaded liposomes for enhanced hepatocellular carcinoma therapy. Biomater. Sci. 2023;11:3952–3964. doi: 10.1039/D3BM00261F. [DOI] [PubMed] [Google Scholar]
- 75.Wang D., Yang F., Shang W., Zhao Z., Shen J., Cai H. Paeoniflorin-loaded pH-sensitive liposomes alleviate synovial inflammation by altering macrophage polarity via STAT signaling. Int. Immunopharmacol. 2021;101:108310. doi: 10.1016/j.intimp.2021.108310. [DOI] [PubMed] [Google Scholar]
- 76.Gao W., Wei S., Li Z., Li L., Zhang X., Li C., Gao D. Nano magnetic liposomes-encapsulated parthenolide and glucose oxidase for ultra-efficient synergistic antitumor therapy. Nanotechnology. 2020;31:355104. doi: 10.1088/1361-6528/ab92c8. [DOI] [PubMed] [Google Scholar]
- 77.Peczek S.H., Tartari A.P.S., Zittlau I.C., Diedrich C., Machado C.S., Mainardes R.M. Enhancing Oral Bioavailability and Brain Biodistribution of Perillyl Alcohol Using Nanostructured Lipid Carriers. Pharmaceuticals. 2023;16:1055. doi: 10.3390/ph16081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y., Li D., Lin H., Jiang S., Han L., Hou S., Lin S., Cheng Z., Bian W., Zhang X., et al. Enhanced oral bioavailability and bioefficacy of phloretin using mixed polymeric modified self-nanoemulsions. Food Sci. Nutr. 2020;8:3545–3558. doi: 10.1002/fsn3.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hammoud Z., Kayouka M., Trifan A., Sieniawska E., Jemâa J.M.B., Elaissari A., Greige-Gerges H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules. 2021;26:6840. doi: 10.3390/molecules26226840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen X., Rong W., Adu-Frimpong M., He Q., Li X., Shi F., Ji H., Toreniyazov E., Xia X., Zhang J., et al. Preparation, in vitro and in vivo evaluation of pinocembrin-loaded TPGS modified liposomes with enhanced bioavailability and antihyperglycemic activity. Drug Dev. Ind. Pharm. 2022;48:623–634. doi: 10.1080/03639045.2022.2151616. [DOI] [PubMed] [Google Scholar]
- 81.Trapani A., Guerra L., Corbo F., Castellani S., Sanna E., Capobianco L., Monteduro A.G., Manno D.E., Mandracchia D., Di Gioia S., et al. Cyto/Biocompatibility of Dopamine Combined with the Antioxidant Grape Seed-Derived Polyphenol Compounds in Solid Lipid Nanoparticles. Molecules. 2021;26:916. doi: 10.3390/molecules26040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo M., Zhang R., Liu L., Chi J., Huang F., Dong L., Ma Q., Jia X., Zhang M. Preparation, stability and antioxidant capacity of nano liposomes loaded with procyandins from lychee pericarp. J. Food Eng. 2020;284:110065. doi: 10.1016/j.jfoodeng.2020.110065. [DOI] [Google Scholar]
- 83.Aly S., El-Kamel A.H., Sheta E., El-Habashy S.E. Chondroitin/Lactoferrin-dual functionalized pterostilbene-solid lipid nanoparticles as targeted breast cancer therapy. Int. J. Pharm. 2023;642:123163. doi: 10.1016/j.ijpharm.2023.123163. [DOI] [PubMed] [Google Scholar]
- 84.Hosseinpour Jajarm F., Moravvej G., Modarres Awal M., Golmohammadzadeh S. Insecticidal activity of solid lipid nanoparticle loaded by Ziziphora clinopodioides Lam. against Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae) Int. J. Pest Manag. 2021;67:147–154. doi: 10.1080/09670874.2020.1713420. [DOI] [Google Scholar]
- 85.Mahadev M., Nandini H.S., Ramu R., Gowda D.V., Almarhoon Z.M., Al-Ghorbani M., Mabkhot Y.N. Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus. Pharmaceuticals. 2022;15:70. doi: 10.3390/ph15010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saatkamp R.H., Sanches M.P., Gambin J.P.D., Amaral B.R., Farias N.S.d., Caon T., Müller C.M.O., Parize A.L. Development of thymol nanoemulsions with potential application in oral infections. J. Drug Deliv. Sci. Technol. 2023;87:104855. doi: 10.1016/j.jddst.2023.104855. [DOI] [Google Scholar]
- 87.Alkhatib M.H., Bawadud R.S., Gashlan H.M. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci. Rep. 2020;10:18124. doi: 10.1038/s41598-020-75017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abeesh P., Guruvayoorappan C. Umbelliferone loaded PEGylated liposomes: Preparation, characterization and its mitigatory effects on Dalton’s ascites lymphoma development. 3 Biotech. 2023;13:216. doi: 10.1007/s13205-023-03615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stahl W., Heinrich U., Aust O., Tronnier H., Sies H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006;5:238–242. doi: 10.1039/b505312a. [DOI] [PubMed] [Google Scholar]
- 90.Shi J., Maguer M., Bryan M., Kakuda Y. Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J. Food Process Eng. 2003;25:485–498. doi: 10.1111/j.1745-4530.2003.tb00647.x. [DOI] [Google Scholar]
- 91.Sharma S.K., Le Maguer M. Kinetics of lycopene degradation in tomato pulp solids under different processing and storage conditions. Food Res. Int. 1996;29:309–315. doi: 10.1016/0963-9969(96)00029-4. [DOI] [Google Scholar]
- 92.Riangjanapatee P., Okonogi S. Effect of surfactant on lycopene-loaded nanostructured lipid carriers. Drug Discov. Ther. 2012;6:163–168. doi: 10.5582/ddt.2012.v6.3.163. [DOI] [PubMed] [Google Scholar]
- 93.Riangjanapatee P., Müller R., Keck C., Okonogi S. Development of lycopene-loaded nanostructured lipid carriers: Effect of rice oil and cholesterol. Die Pharm.-Int. J. Pharm. Sci. 2013;68:723–731. [PubMed] [Google Scholar]
- 94.Okonogi S., Riangjanapatee P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015;478:726–735. doi: 10.1016/j.ijpharm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Akhoond Zardini A., Mohebbi M., Farhoosh R., Bolurian S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018;55:287–298. doi: 10.1007/s13197-017-2937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y., Xin Z., Li N., Chang S., Chen Y., Geng L., Chang H., Shi H., Chang Y.-Z. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic. Biol. Med. 2018;124:1–11. doi: 10.1016/j.freeradbiomed.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 97.Stojiljkovic N., Ilic S., Jakovljevic V., Stojanovic N., Stojnev S., Kocic H., Stojanovic M., Kocic G. The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxidative Med. Cell. Longev. 2018;2018:2627917. doi: 10.1155/2018/2627917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu J., Hu Q., Shen S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J. Liposome Res. 2020;30:37–44. doi: 10.1080/08982104.2019.1580720. [DOI] [PubMed] [Google Scholar]
- 99.Li D., Li L., Xiao N., Li M., Xie X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. Physicochem. Eng. Asp. 2018;552:59–66. doi: 10.1016/j.colsurfa.2018.04.055. [DOI] [Google Scholar]
- 100.Zhao C., Wei L., Yin B., Liu F., Li J., Liu X., Wang J., Wang Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020;153:912–920. doi: 10.1016/j.ijbiomac.2020.03.063. [DOI] [PubMed] [Google Scholar]
- 101.Altincicek B., Kovacs J.L., Gerardo N.M. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 2012;8:253–257. doi: 10.1098/rsbl.2011.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Armstrong G.A., Hearst J.E. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996;10:228–237. doi: 10.1096/fasebj.10.2.8641556. [DOI] [PubMed] [Google Scholar]
- 103.Bendich A., Olson J.A. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. doi: 10.1096/fasebj.3.8.2656356. [DOI] [PubMed] [Google Scholar]
- 104.Horn D., Lüddecke E. Fine Particles Science and Technology. Springer; Berlin/Heidelberg, Germany: 1996. Preparation and characterization of nano-sized carotenoid hydrosols; pp. 761–775. [Google Scholar]
- 105.Hentschel A., Gramdorf S., Müller R., Kurz T. β-Carotene-loaded nanostructured lipid carriers. J. Food Sci. 2008;73:N1–N6. doi: 10.1111/j.1750-3841.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 106.Ryan L., O’Connell O., O’Sullivan L., Aherne S., O’Brien N. Micellarisation of carotenoids from raw and cooked vegetables. Plant Foods Hum. Nutr. 2008;63:127–133. doi: 10.1007/s11130-008-0081-0. [DOI] [PubMed] [Google Scholar]
- 107.Helgason T., Awad T.S., Kristbergsson K., Decker E.A., McClements D.J., Weiss J. Impact of surfactant properties on oxidative stability of β-carotene encapsulated within solid lipid nanoparticles. J. Agric. Food Chem. 2009;57:8033–8040. doi: 10.1021/jf901682m. [DOI] [PubMed] [Google Scholar]
- 108.Yi J., Lam T.I., Yokoyama W., Cheng L.W., Zhong F. Cellular uptake of β-carotene from protein stabilized solid lipid nanoparticles prepared by homogenization–evaporation method. J. Agric. Food Chem. 2014;62:1096–1104. doi: 10.1021/jf404073c. [DOI] [PubMed] [Google Scholar]
- 109.Jimenez-Escobar M., Pascual-Mathey L., Beristain C., Flores-Andrade E., Jiménez M., Pascual-Pineda L. In vitro and In vivo antioxidant properties of paprika carotenoids nanoemulsions. LWT. 2020;118:108694. doi: 10.1016/j.lwt.2019.108694. [DOI] [Google Scholar]
- 110.Flores-Andrade E., Allende-Baltazar Z., Sandoval-González P.E., Jiménez-Fernández M., Beristain C.I., Pascual-Pineda L.A. Carotenoid nanoemulsions stabilized by natural emulsifiers: Whey protein, gum Arabic, and soy lecithin. J. Food Eng. 2021;290:110208. doi: 10.1016/j.jfoodeng.2020.110208. [DOI] [Google Scholar]
- 111.Hsu H.-Y., Chen B.-H. A comparative study on inhibition of breast cancer cells and tumors in mice by carotenoid extract and nanoemulsion prepared from sweet potato (Ipomoea batatas L.) peel. Pharmaceutics. 2022;14:980. doi: 10.3390/pharmaceutics14050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chami F., Chami N., Bennis S., Trouillas J., Remmal A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J. Antimicrob. Chemother. 2004;54:909–914. doi: 10.1093/jac/dkh436. [DOI] [PubMed] [Google Scholar]
- 113.He M., Du M., Fan M., Bian Z. In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia. 2007;163:137–143. doi: 10.1007/s11046-007-0097-2. [DOI] [PubMed] [Google Scholar]
- 114.Garg A., Singh S. Enhancement in antifungal activity of eugenol in immunosuppressed rats through lipid nanocarriers. Colloids Surf. B Biointerfaces. 2011;87:280–288. doi: 10.1016/j.colsurfb.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 115.Pokharkar V.B., Shekhawat P.B., Dhapte V.V., Mandpe L.P. Development and optimization of eugenol loaded nanostructured lipid carriers for periodontal delivery. Int. J. Pharm. Pharm. Sci. 2011;3:138–143. [Google Scholar]
- 116.Garg A., Singh S. Targeting of eugenol-loaded solid lipid nanoparticles to the epidermal layer of human skin. Nanomedicine. 2014;9:1223–1238. doi: 10.2217/nnm.13.33. [DOI] [PubMed] [Google Scholar]
- 117.Rodenak-Kladniew B., Montoto S.S., Sbaraglini M.L., Di Ianni M., Ruiz M.E., Talevi A., Alvarez V.A., Durán N., Castro G.R., Islan G.A. Hybrid Ofloxacin/eugenol co-loaded solid lipid nanoparticles with enhanced and targetable antimicrobial properties. Int. J. Pharm. 2019;569:118575. doi: 10.1016/j.ijpharm.2019.118575. [DOI] [PubMed] [Google Scholar]
- 118.Mishra H., Mishra P.K., Iqbal Z., Jaggi M., Madaan A., Bhuyan K., Gupta N., Gupta N., Vats K., Verma R. Co-delivery of eugenol and dacarbazine by hyaluronic acid-coated liposomes for targeted inhibition of survivin in treatment of resistant metastatic melanoma. Pharmaceutics. 2019;11:163. doi: 10.3390/pharmaceutics11040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu Z., Wang X., Zhang T., Zhang L., Yang J., Li Y., Shen J., Wang J., Niu Y., Xiao Z. Cationic and temperature-sensitive liposomes loaded with eugenol for the application to silk. Chin. Chem. Lett. 2020;31:3139–3142. doi: 10.1016/j.cclet.2020.07.013. [DOI] [Google Scholar]
- 120.Revi N., Sankaranarayanan S.A., Rengan A.K. A study on the role of eugenol encapsulated liposomes in facilitating neuron-microglia mediated wound recovery. Materialia. 2022;23:101454. doi: 10.1016/j.mtla.2022.101454. [DOI] [Google Scholar]
- 121.Sebaaly C., Haydar S., Greige-Gerges H. Eugenol encapsulation into conventional liposomes and chitosan-coated liposomes: A comparative study. J. Drug Deliv. Sci. Technol. 2022;67:102942. doi: 10.1016/j.jddst.2021.102942. [DOI] [Google Scholar]
- 122.Tanzeem M.U., Asghar S., Khalid S.H., Asif M., Ullah M.S., Khan I.U., Khalid I., Faran S.A., Rehman A., Gohar U.F. Clove oil based co-surfactant free microemulsion of flurbiprofen: Improved solubility with ameliorated drug-induced gastritis. Pak. J. Pharm. Sci. 2019;32:2787. [PubMed] [Google Scholar]
- 123.Khanna N. Turmeric-Nature’s precious gift. Curr. Sci. 1999;76:1351–1356. [Google Scholar]
- 124.Rainey N.E., Moustapha A., Saric A., Nicolas G., Sureau F., Petit P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019;5:150. doi: 10.1038/s41420-019-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tiyaboonchai W., Tungpradit W., Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007;337:299–306. doi: 10.1016/j.ijpharm.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 127.Plianbangchang P., Tungpradit W., Tiyaboonchai W. Efficacy and safety of curcuminoids loaded solid lipid nanoparticles facial cream as an anti-aging agent. Naresuan Univ. J. 2007;15:73–81. [Google Scholar]
- 128.Kakkar V., Singh S., Singla D., Kaur I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011;55:495–503. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- 129.Kakkar V., Kaur I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011;49:2906–2913. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 130.Kakkar V., Bhushan S., Guru S.K., Kaur I.P. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol. Pharm. 2012;9:3411–3421. doi: 10.1021/mp300209k. [DOI] [PubMed] [Google Scholar]
- 131.Kakkar V., Muppu S.K., Chopra K., Kaur I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013;85:339–345. doi: 10.1016/j.ejpb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 132.Balasubramanyam K., Varier R.A., Altaf M., Swaminathan V., Siddappa N.B., Ranga U., Kundu T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 133.Puglia C., Frasca G., Musumeci T., Rizza L., Puglisi G., Bonina F., Chiechio S. Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice. Eur. J. Pharm. Biopharm. 2012;81:288–293. doi: 10.1016/j.ejpb.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 134.Meng F., Asghar S., Xu Y., Wang J., Jin X., Wang Z., Wang J., Ping Q., Zhou J., Xiao Y. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carrier for brain targeting. Int. J. Pharm. 2016;506:46–56. doi: 10.1016/j.ijpharm.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 135.Meng F., Asghar S., Gao S., Su Z., Song J., Huo M., Meng W., Ping Q., Xiao Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer's disease. Colloids Surf. B Biointerfaces. 2015;134:88–97. doi: 10.1016/j.colsurfb.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 136.Ban C., Jo M., Park Y.H., Kim J.H., Han J.Y., Lee K.W., Kweon D.-H., Choi Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020;302:125328. doi: 10.1016/j.foodchem.2019.125328. [DOI] [PubMed] [Google Scholar]
- 137.Singh S.P., Alvi S.B., Pemmaraju D.B., Singh A.D., Manda S.V., Srivastava R., Rengan A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018;110:375–382. doi: 10.1016/j.ijbiomac.2017.11.163. [DOI] [PubMed] [Google Scholar]
- 138.Wu Y., Mou B., Song S., Tan C.-P., Lai O.-M., Shen C., Cheong L.-Z. Curcumin-loaded liposomes prepared from bovine milk and krill phospholipids: Effects of chemical composition on storage stability, in-vitro digestibility and anti-hyperglycemic properties. Food Res. Int. 2020;136:109301. doi: 10.1016/j.foodres.2020.109301. [DOI] [PubMed] [Google Scholar]
- 139.Bhatia E., Sharma S., Jadhav K., Banerjee R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B. 2021;9:864–875. doi: 10.1039/D0TB02036B. [DOI] [PubMed] [Google Scholar]
- 140.Song J.-W., Liu Y.-S., Guo Y.-R., Zhong W.-X., Guo Y.-P., Guo L. Nano–liposomes double loaded with curcumin and tetrandrine: Preparation, characterization, hepatotoxicity and anti–tumor effects. Int. J. Mol. Sci. 2022;23:6858. doi: 10.3390/ijms23126858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Páez-Hernández G., Mondragón-Cortez P., Espinosa-Andrews H. Developing curcumin nanoemulsions by high-intensity methods: Impact of ultrasonication and microfluidization parameters. LWT. 2019;111:291–300. doi: 10.1016/j.lwt.2019.05.012. [DOI] [Google Scholar]
- 142.Ahmad N., Ahmad R., Al-Qudaihi A., Alaseel S.E., Fita I.Z., Khalid M.S., Pottoo F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019;9:20192–20206. doi: 10.1039/C9RA03102B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chuacharoen T., Prasongsuk S., Sabliov C.M. Effect of surfactant concentrations on physicochemical properties and functionality of curcumin nanoemulsions under conditions relevant to commercial utilization. Molecules. 2019;24:2744. doi: 10.3390/molecules24152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang P., Zhang L., Peng H., Li Y., Xiong J., Xu Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. C. 2013;33:4802–4808. doi: 10.1016/j.msec.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 145.Zhao X., Chen Q., Liu W., Li Y., Tang H., Liu X., Yang X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int. J. Nanomed. 2015;10:257. doi: 10.2147/IJN.S73322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chirio D., Gallarate M., Peira E., Battaglia L., Serpe L., Trotta M. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencaps. 2011;28:537–548. doi: 10.3109/02652048.2011.590615. [DOI] [PubMed] [Google Scholar]
- 147.Sun J., Bi C., Chan H.M., Sun S., Zhang Q., Zheng Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B Biointerfaces. 2013;111:367–375. doi: 10.1016/j.colsurfb.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 148.Mulik R.S., Mönkkönen J., Juvonen R.O., Mahadik K.R., Paradkar A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: Enhanced in vitro anticancer activity by induction of apoptosis. Int. J. Pharm. 2010;398:190–203. doi: 10.1016/j.ijpharm.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 149.Xu H., Gong Z., Zhou S., Yang S., Wang D., Chen X., Wu J., Liu L., Zhong S., Zhao J. Liposomal curcumin targeting endometrial Cancer through the NF-κB pathway. Cell. Physiol. Biochem. 2018;48:569–582. doi: 10.1159/000491886. [DOI] [PubMed] [Google Scholar]
- 150.Lakshmi B.A., Reddy A.S., Sangubotla R., Hong J.W., Kim S. Ruthenium (II)-curcumin liposome nanoparticles: Synthesis, characterization, and their effects against cervical cancer. Colloids Surf. B Biointerfaces. 2021;204:111773. doi: 10.1016/j.colsurfb.2021.111773. [DOI] [PubMed] [Google Scholar]
- 151.Hanafy N.A., Sheashaa R.F., Moussa E.A., Mahfouz M.E. Potential of curcumin and niacin-loaded targeted chitosan coated liposomes to activate autophagy in hepatocellular carcinoma cells: An in vitro evaluation in HePG2 cell line. Int. J. Biol. Macromol. 2023;245:125572. doi: 10.1016/j.ijbiomac.2023.125572. [DOI] [PubMed] [Google Scholar]
- 152.Othman A.K., El Kurdi R., Badran A., Mesmar J., Baydoun E., Patra D. Liposome-based nanocapsules for the controlled release of dietary curcumin: PDDA and silica nanoparticle-coated DMPC liposomes enhance the fluorescence efficiency and anticancer activity of curcumin. RSC Adv. 2022;12:11282–11292. doi: 10.1039/D2RA00071G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Machado F.C., de Matos R.P.A., Primo F.L., Tedesco A.C., Rahal P., Calmon M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Biorg. Med. Chem. 2019;27:1882–1890. doi: 10.1016/j.bmc.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 154.Li K., Pi C., Wen J., He Y., Yuan J., Shen H., Zhao W., Zeng M., Song X., Lee R.J. Formulation of the novel structure curcumin derivative–loaded solid lipid nanoparticles: Synthesis, optimization, characterization and anti-tumor activity screening in vitro. Drug Deliv. 2022;29:2044–2057. doi: 10.1080/10717544.2022.2092235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fathy Abd-Ellatef G.-E., Gazzano E., Chirio D., Ragab Hamed A., Belisario D.C., Zuddas C., Peira E., Rolando B., Kopecka J., Assem Said Marie M. Curcumin-loaded solid lipid nanoparticles bypass p-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics. 2020;12:96. doi: 10.3390/pharmaceutics12020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo P., Pi C., Zhao S., Fu S., Yang H., Zheng X., Zhang X., Zhao L., Wei Y. Oral co-delivery nanoemulsion of 5-fluorouracil and curcumin for synergistic effects against liver cancer. Expert Opin. Drug Deliv. 2020;17:1473–1484. doi: 10.1080/17425247.2020.1796629. [DOI] [PubMed] [Google Scholar]
- 157.Youssry S.A., El-Sheredy H.G., Shalaby T.I. In vitro evaluation of antitumor and immunomodulatory potential of curcumin nano-emulsion on breast cancer. BioNanoScience. 2022;12:841–850. doi: 10.1007/s12668-022-00981-3. [DOI] [Google Scholar]
- 158.Angeline N., Suhito I.R., Kim C.-H., Hong G.-P., Park C.G., Bhang S.H., Luo Z., Kim T.-H. A fibronectin-coated gold nanostructure composite for electrochemical detection of effects of curcumin-carrying nanoliposomes on human stomach cancer cells. Analyst. 2020;145:675–684. doi: 10.1039/C9AN01553A. [DOI] [PubMed] [Google Scholar]
- 159.Sheikhpour M., Sadeghizadeh M., Yazdian F., Mansoori A., Asadi H., Movafagh A., Shahraeini S.S. Co-administration of curcumin and bromocriptine nano-liposomes for induction of apoptosis in lung cancer cells. Iran. Biomed. J. 2020;24:24. doi: 10.29252/ibj.24.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wei Y., Wei Y., Sheng L., Ma J., Su Z., Wen J., Li L., Jia Q., Liu H., Si H. Construction of Curcumin and Paclitaxel Co-Loaded Lipid Nano Platform and Evaluation of Its Anti-Hepatoma Activity in vitro and Pharmacokinetics in vivo. Int. J. Nanomed. 2023;18:2087–2107. doi: 10.2147/IJN.S399289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu C., Yang J., Wang F., Wang X. Resveratrol: Botanical origin, pharmacological activity and applications. Chin. J. Nat. Med. 2013;11:1–15. doi: 10.3724/SP.J.1009.2013.00001. [DOI] [Google Scholar]
- 162.Ge J.-F., Qiao J.-P., Qi C.-C., Wang C.-W., Zhou J.-N. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem. Int. 2012;61:1192–1201. doi: 10.1016/j.neuint.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 163.Amri A., Chaumeil J., Sfar S., Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release. 2012;158:182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 164.Pandita D., Kumar S., Poonia N., Lather V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014;62:1165–1174. doi: 10.1016/j.foodres.2014.05.059. [DOI] [Google Scholar]
- 165.Neves A.R., Martins S., Segundo M.A., Reis S. Nanoscale delivery of resveratrol towards enhancement of supplements and nutraceuticals. Nutrients. 2016;8:131. doi: 10.3390/nu8030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neves A.R., Queiroz J.F., Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016;14:27. doi: 10.1186/s12951-016-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kang J.H., Ko Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics. 2019;11:423. doi: 10.3390/pharmaceutics11080423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tomás-Barberán F.A., Clifford M.N. Flavanones, chalcones and dihydrochalcones–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000;80:1073–1080. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1073::AID-JSFA568>3.0.CO;2-B. [DOI] [Google Scholar]
- 169.Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004;24:851–874. doi: 10.1016/j.nutres.2004.07.005. [DOI] [Google Scholar]
- 170.Sansone F., Rossi A., Del Gaudio P., De Simone F., Aquino R.P., Lauro M.R. Hesperidin gastroresistant microparticles by spray-drying: Preparation, characterization, and dissolution profiles. AAPS PharmSciTech. 2009;10:391–401. doi: 10.1208/s12249-009-9219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fathi M., Varshosaz J., Mohebbi M., Shahidi F. hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, characterization, and modeling. Food Bioprocess Technol. 2013;6:1464–1475. doi: 10.1007/s11947-012-0845-2. [DOI] [Google Scholar]
- 172.Zeng F., Wang D., Tian Y., Wang M., Liu R., Xia Z., Huang Y. Nanoemulsion for Improving the Oral Bioavailability of Hesperetin: Formulation Optimization and Absorption Mechanism. J. Pharm. Sci. 2021;110:2555–2561. doi: 10.1016/j.xphs.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 173.Wang X., Song Y., Yu L., Xue X., Pang M., Li Y., Luo X., Hua Z., Lu C., Lu A. Co-Delivery of Hesperetin and Cisplatin via Hyaluronic Acid-Modified Liposome for Targeted Inhibition of Aggression and Metastasis of Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces. 2023;15:29. doi: 10.1021/acsami.3c03233. [DOI] [PubMed] [Google Scholar]
- 174.Clozel J., Roberts A., Hoffman J., Coleridge H., Coleridge J. Vagal chemoreflex coronary vasodilation evoked by stimulating pulmonary C-fibers in dogs. Circ. Res. 1985;57:450–460. doi: 10.1161/01.RES.57.3.450. [DOI] [PubMed] [Google Scholar]
- 175.Fusco B.M., Giacovazzo M. Peppers and pain. Drugs. 1997;53:909–914. doi: 10.2165/00003495-199753060-00001. [DOI] [PubMed] [Google Scholar]
- 176.Sharma A., Arora S. Development of Topical Gel of Capsaicin Loaded Solid Lipid Nanoparticles (SLNs): In vitro and in vivo Evaluation. Indian J. Pharm. 2011;2:29–41. [Google Scholar]
- 177.Duangjit S., Pucharean C., Yoddee P., Kanlayawuttipong K., Ngawhirunpat T. Effect of Terpene on Physicochemical Properties and Skin Permeability of Capsaicin Loaded Solid Lipid Nanoparticles. Isan J. Pharm. Sci. 2016;11:124–134. [Google Scholar]
- 178.Kunjiappan S., Sankaranarayanan M., Kumar B.K., Pavadai P., Babkiewicz E., Maszczyk P., Glodkowska-Mrowka E., Arunachalam S., Pandian S.R.K., Ravishankar V. Capsaicin-loaded solid lipid nanoparticles: Design, biodistribution, in silico modeling and in vitro cytotoxicity evaluation. Nanotechnology. 2020;32:095101. doi: 10.1088/1361-6528/abc57e. [DOI] [PubMed] [Google Scholar]
- 179.Stark T., Bareuther S., Hofmann T. Sensory-guided decomposition of roasted cocoa nibs (Theobroma cacao) and structure determination of taste-active polyphenols. J. Agric. Food Chem. 2005;53:5407–5418. doi: 10.1021/jf050457y. [DOI] [PubMed] [Google Scholar]
- 180.Shen S.-C., Ko C.H., Tseng S.-W., Tsai S.-H., Chen Y.-C. Structurally related antitumor effects of flavanones in vitro and in vivo: Involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004;197:84–95. doi: 10.1016/j.taap.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 181.Kanno S.-I., Tomizawa A., Hiura T., Osanai Y., Shouji A., Ujibe M., Ohtake T., Kimura K., Ishikawa M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol. Pharm. Bull. 2005;28:527–530. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 182.Ratnam D.V., Ankola D., Bhardwaj V., Sahana D., Kumar M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 183.Ji P., Yu T., Liu Y., Jiang J., Xu J., Zhao Y., Hao Y., Qiu Y., Zhao W., Wu C. naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016;10:911. doi: 10.2147/DDDT.S97738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang L., Wang X., Shen L., Alrobaian M., Panda S.K., Almasmoum H.A., Ghaith M.M., Almaimani R.A., Ibrahim I.A.A., Singh T. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed. Pharmacother. 2021;138:111461. doi: 10.1016/j.biopha.2021.111461. [DOI] [PubMed] [Google Scholar]
- 185.Munir A., Muhammad F., Zaheer Y., Ali M.A., Iqbal M., Rehman M., Munir M.U., Akhtar B., Webster T.J., Sharif A. Synthesis of naringenin loaded lipid based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J. Drug Deliv. Sci. Technol. 2021;66:102854. doi: 10.1016/j.jddst.2021.102854. [DOI] [Google Scholar]
- 186.Wang Z., Liu L., Yin W., Liu Z., Shi L., Tang M. A novel drug delivery system: The encapsulation of naringenin in metal-organic frameworks into liposomes. AAPS PharmSciTech. 2021;22:61. doi: 10.1208/s12249-021-01927-w. [DOI] [PubMed] [Google Scholar]
- 187.Kong F., Luan Y., Zhang Z.H., Cheng G.H., Qi T.G., Sun C. Baicalin protects the myocardium from reperfusion-induced damage in isolated rat hearts via the antioxidant and paracrine effect. Exp. Ther. Med. 2014;7:254–259. doi: 10.3892/etm.2013.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tu X.-K., Yang W.-Z., Shi S.-S., Wang C.-H., Chen C.-M. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem. Res. 2009;34:1626–1634. doi: 10.1007/s11064-009-9953-4. [DOI] [PubMed] [Google Scholar]
- 189.Liu Z., Zhang L., He Q., Liu X., Ikechukwu O.C., Tong L., Guo L., Yang H., Zhang Q., Zhao H. Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia–reperfusion in rats. Int. J. Pharm. 2015;489:131–138. doi: 10.1016/j.ijpharm.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 190.Zhang Y., Liu S., Wan J., Yang Q., Xiang Y., Ni L., Long Y., Cui M., Ci Z., Tang D., et al. Preparation, Characterization and in vivo Study of Borneol-Baicalin-Liposomes for Treatment of Cerebral Ischemia-Reperfusion Injury. Int. J. Nanomed. 2020;15:5977–5989. doi: 10.2147/IJN.S259938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xu Q., Zhou A., Wu H., Bi Y. Development and in vivo evaluation of baicalin-loaded W/O nanoemulsion for lymphatic absorption. Pharm. Dev. Technol. 2019;24:1155–1163. doi: 10.1080/10837450.2019.1646757. [DOI] [PubMed] [Google Scholar]
- 192.Lindsey K., Pullen M.L., Topping J.F. Importance of plant sterols in pattern formation and hormone signalling. Trends Plant Sci. 2003;8:521–525. doi: 10.1016/j.tplants.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 193.Ostlund R.E., Jr. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- 194.Racette S.B., Lin X., Lefevre M., Spearie C.A., Most M.M., Ma L., Ostlund R.E. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010;91:32–38. doi: 10.3945/ajcn.2009.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lacatusu I., Badea N., Stan R., Meghea A. Novel bio-active lipid nanocarriers for the stabilization and sustained release of sitosterol. Nanotechnology. 2012;23:455702. doi: 10.1088/0957-4484/23/45/455702. [DOI] [PubMed] [Google Scholar]
- 196.Zhang F., Liu Z., He X., Li Z., Shi B., Cai F. β-Sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund’s adjuvant-induced arthritis in rats: Involvement of NF-кB and HO-1/Nrf-2 pathway. Drug Deliv. 2020;27:1329–1341. doi: 10.1080/10717544.2020.1818883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shen C.-Y., Lee C.-F., Chou W.-T., Hwang J.-J., Tyan Y.-S., Chuang H.-Y. Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics. 2022;14:1214. doi: 10.3390/pharmaceutics14061214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chennuru A., Saleem M.T. Antioxidant, lipid lowering, and membrane stabilization effect of sesamol against doxorubicin-induced cardiomyopathy in experimental rats. BioMed Res. Int. 2013;2013:934239. doi: 10.1155/2013/934239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Geetha T., Singh N., Deol P.K., Kaur I.P. Biopharmaceutical profiling of sesamol: Physiochemical characterization, gastrointestinal permeability and pharmacokinetic evaluation. RSC Adv. 2015;5:4083–4091. doi: 10.1039/C4RA10926K. [DOI] [Google Scholar]
- 200.Wenzel E., Soldo T., Erbersdobler H., Somoza V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005;49:482–494. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- 201.Jan K.C., Ho C.T., Hwang L.S. Elimination and metabolism of sesamol, a bioactive compound in sesame oil, in rats. Mol. Nutr. Food Res. 2009;53:S36–S43. doi: 10.1002/mnfr.200800214. [DOI] [PubMed] [Google Scholar]
- 202.VanGilder R., Kelly K., Chua M., Ptachcinski R., Huber J.D. Administration of sesamol improved blood–brain barrier function in streptozotocin-induced diabetic rats. Exp. Brain Res. 2009;197:23–34. doi: 10.1007/s00221-009-1866-6. [DOI] [PubMed] [Google Scholar]
- 203.Geetha T., Rohit B., Pal K.I. Sesamol: An efficient antioxidant with potential therapeutic benefits. Med. Chem. 2009;5:367–371. doi: 10.2174/157340609788681476. [DOI] [PubMed] [Google Scholar]
- 204.Kakkar V., Mishra A.K., Chuttani K., Chopra K., Kaur I.P. Delivery of sesamol-loaded solid lipid nanoparticles to the brain for menopause-related emotional and cognitive central nervous system derangements. Rejuvenation Res. 2011;14:597–604. doi: 10.1089/rej.2011.1193. [DOI] [PubMed] [Google Scholar]
- 205.Morisco F., Vitaglione P., Amoruso D., Russo B., Fogliano V., Caporaso N. Foods and liver health. Mol. Asp. Med. 2008;29:144–150. doi: 10.1016/j.mam.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 206.Kumagai Y., Lin L.Y., Schmitz D.A., Cho A.K. Hydroxyl radical mediated demethylenation of (methylenedioxy)phenyl compounds. Chem. Res. Toxicol. 1991;4:330–334. doi: 10.1021/tx00021a012. [DOI] [PubMed] [Google Scholar]
- 207.Singh N., Khullar N., Kakkar V., Kaur I.P. Hepatoprotective effects of sesamol loaded solid lipid nanoparticles in carbon tetrachloride induced sub-chronic hepatotoxicity in rats. Environ. Toxicol. 2014;31:520–532. doi: 10.1002/tox.22064. [DOI] [PubMed] [Google Scholar]
- 208.Singh N., Khullar N., Kakkar V., Kaur I.P. Sesamol loaded solid lipid nanoparticles: A promising intervention for control of carbon tetrachloride induced hepatotoxicity. BMC Complement. Altern. Med. 2015;15:142. doi: 10.1186/s12906-015-0655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sachdeva A.K., Misra S., Kaur I.P., Chopra K. Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: Behavioral and biochemical evidence. Eur. J. Pharmacol. 2015;747:132–140. doi: 10.1016/j.ejphar.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 210.Yaar M., Gilchrest B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 211.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 212.Ames B.N., Durston W.E., Yamasaki E., Lee F.D. Carcinogens are mutagens: A simple test system combining liver homogenates for activation and bacteria for detection. Proc. Natl. Acad. Sci. USA. 1973;70:2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Geetha T., Kapila M., Prakash O., Deol P.K., Kakkar V., Kaur I.P. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J. Drug Target. 2015;23:159–169. doi: 10.3109/1061186X.2014.965717. [DOI] [PubMed] [Google Scholar]
- 214.Yousefi S., Rajaei P., Nateghi L., Nodeh H.R., Rashidi L. Encapsulation of sesamol and retinol using alginate and chitosan-coated W/O/W multiple emulsions containing Tween 80 and Span 80. Int. J. Biol. Macromol. 2023;242:124766. doi: 10.1016/j.ijbiomac.2023.124766. [DOI] [PubMed] [Google Scholar]
- 215.Sutaria D., Grandhi B.K., Thakkar A., Wang J., Prabhu S. Chemoprevention of pancreatic cancer using solid-lipid nanoparticulate delivery of a novel aspirin, curcumin and sulforaphane drug combination regimen. Int. J. Oncol. 2012;41:2260–2268. doi: 10.3892/ijo.2012.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Thakkar A., Chenreddy S., Wang J., Prabhu S. Preclinical systemic toxicity evaluation of chitosan-solid lipid nanoparticles encapsulated aspirin, curcumin and free sulforaphane (ACS) combinations in BALB/c mice. Cancer Res. 2015;75:2621. doi: 10.1158/1538-7445.AM2015-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Abdelwahab S.I., Sheikh B.Y., Taha M.M.E., How C.W., Abdullah R., Yagoub U., El-Sunousi R., Eid E. Thymoquinone-loaded nanostructured lipid carriers: Preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013;8:2163–2172. doi: 10.2147/IJN.S44108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Campos D.A., Madureira A.R., Gomes A.M., Sarmento B., Pintado M.M. Optimization of the production of solid Witepsol nanoparticles loaded with rosmarinic acid. Colloids Surf. B Biointerfaces. 2014;115:109–117. doi: 10.1016/j.colsurfb.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 219.Madureira A.R., Campos D.A., Fonte P., Nunes S., Reis F., Gomes A.M., Sarmento B., Pintado M.M. Characterization of solid lipid nanoparticles produced with carnauba wax for rosmarinic acid oral delivery. RSC Adv. 2015;5:22665–22673. doi: 10.1039/C4RA15802D. [DOI] [Google Scholar]
- 220.Bhatt R., Singh D., Prakash A., Mishra N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015;22:931–939. doi: 10.3109/10717544.2014.880860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li M., Zahi M.R., Yuan Q., Tian F., Liang H. Preparation and stability of astaxanthin solid lipid nanoparticles based on stearic acid. Eur. J. Lipid Sci. Technol. 2015;118:592–602. doi: 10.1002/ejlt.201400650. [DOI] [Google Scholar]
- 222.Bhatt P.C., Srivastava P., Pandey P., Khan W., Panda B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016;6:10001–10010. doi: 10.1039/C5RA19113K. [DOI] [Google Scholar]
- 223.Fangueiro J.F., Andreani T., Fernandes L., Garcia M.L., Egea M.A., Silva A.M., Souto E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces. 2014;123:452–460. doi: 10.1016/j.colsurfb.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 224.Sadiq A.A., Abdul Rassol A. Formulation and evaluation of silibinin loaded solid lipid nanoparticles for peroral use targeting lower part of gastrointestinal tract. Int. J. Pharm. Pharm. Sci. 2014;6:55–67. [Google Scholar]
- 225.Lacatusu I., Mitrea E., Badea N., Stan R., Oprea O., Meghea A. Lipid nanoparticles based on omega-3 fatty acids as effective carriers for lutein delivery. Preparation and in vitro characterization studies. J. Funct. Foods. 2013;5:1260–1269. doi: 10.1016/j.jff.2013.04.010. [DOI] [Google Scholar]
- 226.Mitri K., Shegokar R., Gohla S., Anselmi C., Müller R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011;414:267–275. doi: 10.1016/j.ijpharm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 227.Liu C.-H., Chiu H.-C., Wu W.-C., Sahoo S.L., Hsu C.-Y. Novel lutein loaded lipid nanoparticles on porcine corneal distribution. J. Ophthalmol. 2014;2014:304694. doi: 10.1155/2014/304694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ni S., Sun R., Zhao G., Xia Q. Quercetin loaded nanostructured lipid carrier for food fortification: Preparation, characterization and in vitro study. J. Food Process Eng. 2015;38:93–106. doi: 10.1111/jfpe.12130. [DOI] [Google Scholar]
- 229.Vijayakumar A., Baskaran R., Jang Y.S., Oh S.H., Yoo B.K. Quercetin-Loaded Solid Lipid Nanoparticle Dispersion with Improved Physicochemical Properties and Cellular Uptake. AAPS PharmSciTech. 2016;18:875–883. doi: 10.1208/s12249-016-0573-4. [DOI] [PubMed] [Google Scholar]
- 230.Li H., Zhao X., Ma Y., Zhai G., Li L., Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release. 2009;133:238–244. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 231.Chen-yu G., Chun-fen Y., Qi-lu L., Qi T., Yan-wei X., Wei-na L., Guang-xi Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012;430:292–298. doi: 10.1016/j.ijpharm.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 232.Liu C.-H., Huang Y.-C., Jhang J.-W., Liu Y.-H., Wu W.-C. Quercetin delivery to porcine cornea and sclera by solid lipid nanoparticles and nanoemulsion. RSC Adv. 2015;5:100923–100933. doi: 10.1039/C5RA17423F. [DOI] [Google Scholar]
- 233.Liu L., Tang Y., Gao C., Li Y., Chen S., Xiong T., Li J., Du M., Gong Z., Chen H. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf. B Biointerfaces. 2014;115:125–131. doi: 10.1016/j.colsurfb.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 234.Dang H., Meng M.H.W., Zhao H., Iqbal J., Dai R., Deng Y., Lv F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanopart. Res. 2014;16:2347. [Google Scholar]
- 235.Manea A.-M., Andronescu C., Meghea A. Green tea extract loaded into solid lipid nanoparticles. UPB Sci. Bull. Ser. B. 2014;76:125–136. [Google Scholar]
- 236.Ma Q.H., Xia Q., Lu Y.Y., Hao X.Z., Gu N., Lin X.F., Luo D. Preparation of tea polyphenols-loaded solid lipid nanoparticles based on the phase behaviors of hot microemulsions. Solid State Phenom. 2007;121–123:705–708. doi: 10.4028/www.scientific.net/SSP.121-123.705. [DOI] [Google Scholar]
- 237.Haddad F., Mohammed N., Gopalan R., Ayoub Y.A., Nasim M.T., Assi K. Development and Optimisation of Inhalable EGCG Nano-Liposomes as a Potential Treatment for Pulmonary Arterial Hypertension by Implementation of the Design of Experiments Approach. Pharmaceutics. 2023;15:539. doi: 10.3390/pharmaceutics15020539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Granja A., Neves A.R., Sousa C.T., Pinheiro M., Reis S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon. 2019;5:e02020. doi: 10.1016/j.heliyon.2019.e02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pleguezuelos-Villa M., Nácher A., Hernández M., Buso M.O.V., Sauri A.R., Díez-Sales O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019;564:299–307. doi: 10.1016/j.ijpharm.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 240.Santonocito D., Barbagallo I., Distefano A., Sferrazzo G., Vivero-Lopez M., Sarpietro M.G., Puglia C. Nanostructured Lipid Carriers Aimed to the Ocular Delivery of Mangiferin: In Vitro Evidence. Pharmaceutics. 2023;15:951. doi: 10.3390/pharmaceutics15030951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jaiswal N., Akhtar J., Khan M.I., Ali A., Khan M.F., Alahmari A.K. Fabrication and Optimization of Genistein Nanoemulsion. Biochem. Cell. Arch. 2022;22:1499–1508. [Google Scholar]
- 242.Gok B., Kilinc Y.B. Chlorogenic acid nanoemulsion for staphylococcus aureus causing skin infection: Synthesis, characterization and evaluation of antibacterial efficacy. Sigma J. Eng. Nat. Sci. 2022;41:322–330. [Google Scholar]
- 243.Li S., Su L., Lv G., Luo W., Kang Y. Ultrasound Guided Intra-Articular Injection of Triptolide-loaded Solid Lipid Nanoparticle for Treatment of Antigen-Induced Arthritis in Rabbits. Front. Pharmacol. 2022;13:824015. doi: 10.3389/fphar.2022.824015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Guo R.-B., Zhang X.-Y., Yan D.-K., Yu Y.-J., Wang Y.-J., Geng H.-X., Wu Y.-N., Liu Y., Kong L., Li X.-T. Folate-modified triptolide liposomes target activated macrophages for safe rheumatoid arthritis therapy. Biomater. Sci. 2022;10:499–513. doi: 10.1039/D1BM01520F. [DOI] [PubMed] [Google Scholar]
- 245.Obeid R., Kuhlmann M., Kirsch C.-M., Herrmann W. Cellular uptake of vitamin B12 in patients with chronic renal failure. Nephron Clin. Pract. 2005;99:c42–c48. doi: 10.1159/000083132. [DOI] [PubMed] [Google Scholar]
- 246.Waibel R., Treichler H., Schaefer N.G., van Staveren D.R., Mundwiler S., Kunze S., Küenzi M., Alberto R., Nüesch J., Knuth A. New derivatives of vitamin B12 show preferential targeting of tumors. Cancer Res. 2008;68:2904–2911. doi: 10.1158/0008-5472.CAN-07-6771. [DOI] [PubMed] [Google Scholar]
- 247.Lee I.-M., Cook N., Selhub J., Manson J., Buring J., Zhang S. Plasma folate, vitamin B6, vitamin B12 and risk of breast cancer in women. Cancer Res. 2007;67:860. [Google Scholar]
- 248.Genç L., Kutlu H.M., Güney G. Vitamin B12-loaded solid lipid nanoparticles as a drug carrier in cancer therapy. Pharm. Dev. Technol. 2015;20:337–344. doi: 10.3109/10837450.2013.867447. [DOI] [PubMed] [Google Scholar]
- 249.Singh A., Yadagiri G., Parvez S., Singh O.P., Verma A., Sundar S., Mudavath S.L. Formulation, characterization and in vitro anti-leishmanial evaluation of amphotericin B loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate. Mater. Sci. Eng. C. 2020;117:111279. doi: 10.1016/j.msec.2020.111279. [DOI] [PubMed] [Google Scholar]
- 250.Çoban Ö., Yıldırım S., Bakır T. Alpha-Lipoic Acid and Cyanocobalamin Co-Loaded Nanoemulsions: Development, Characterization, and Evaluation of Stability. J. Pharm. Innov. 2022;17:510–520. doi: 10.1007/s12247-020-09531-4. [DOI] [Google Scholar]
- 251.Andrade S., Ramalho M.J., Loureiro J.A., Pereira M.C. Transferrin-functionalized liposomes loaded with vitamin VB12 for Alzheimer's disease therapy. Int. J. Pharm. 2022;626:122167. doi: 10.1016/j.ijpharm.2022.122167. [DOI] [PubMed] [Google Scholar]
- 252.Pople P.V., Singh K.K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech. 2006;7:E63–E69. doi: 10.1208/pt070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jenning V., Gysler A., Schäfer-Korting M., Gohla S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000;49:211–218. doi: 10.1016/S0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 254.Kim T.-I., Kim T.-G., Lim D.-H., Kim S.-B., Park S.-M., Hur T.-Y., Ki K.-S., Kwon E.-G., Vijayakumar M., Kim Y.-J. Preparation of Nanoemulsions of Vitamin A and C by Microfluidization: Efficacy on the Expression Pattern of Milk-Specific Proteins in MAC-T Cells. Molecules. 2019;24:2566. doi: 10.3390/molecules24142566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Güney G., Kutlu H.M., Genç L. Preparation and characterization of ascorbic acid loaded solid lipid nanoparticles and investigation of their apoptotic effects. Colloids Surf. B Biointerfaces. 2014;121:270–280. doi: 10.1016/j.colsurfb.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 256.Jiao Z., Wang X., Han S., Zha X., Xia J. Preparation of vitamin C liposomes by rapid expansion of supercritical solution process: Experiments and optimization. J. Drug Deliv. Sci. Technol. 2019;51:1–6. doi: 10.1016/j.jddst.2019.02.015. [DOI] [Google Scholar]
- 257.Hou X., Zhang X., Zhao W., Zeng C., Deng B., McComb D.W., Du S., Zhang C., Li W., Dong Y. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 2020;15:41–46. doi: 10.1038/s41565-019-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yousry C., Saber M.M., Abd-Elsalam W.H. A Cosmeceutical Topical Water-in-Oil Nanoemulsion of Natural Bioactives: Design of Experiment, in vitro Characterization, and in vivo Skin Performance Against UVB Irradiation-Induced Skin Damages. Int. J. Nanomed. 2022;17:2995–3012. doi: 10.2147/IJN.S363779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Beers M.H., Jones T.V. The Merck Manual of Health & Aging. Random House Digital, Inc.; New York, NY, USA: 2005. [Google Scholar]
- 260.Nesby-O’Dell S., Scanlon K.S., Cogswell M.E., Gillespie C., Hollis B.W., Looker A.C., Allen C., Doughertly C., Gunter E.W., Bowman B.A. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 261.Ward L.M., Gaboury I., Ladhani M., Zlotkin S. Vitamin D–deficiency rickets among children in Canada. Can. Med. Assoc. J. 2007;177:161–166. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 263.Brody T. Food and Dietary Supplement Package Labeling—Guidance from FDA's Warning Letters and Title 21 of the Code of Federal Regulations. Compr. Rev. Food Sci. Food Saf. 2016;15:92–129. doi: 10.1111/1541-4337.12172. [DOI] [PubMed] [Google Scholar]
- 264.Patel M.R., Martin-Gonzalez S., Fernanda M. Characterization of ergocalciferol loaded solid lipid nanoparticles. J. Food Sci. 2012;77:N8–N13. doi: 10.1111/j.1750-3841.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 265.Bi Y., Xia H., Li L., Lee R.J., Xie J., Liu Z., Qiu Z., Teng L. Liposomal Vitamin D3 as an Anti-aging Agent for the Skin. Pharmaceutics. 2019;11:311. doi: 10.3390/pharmaceutics11070311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Maalouf S., El-Sabban M., Darwiche N., Gali-Muhtasib H. Protective effect of vitamin E on ultraviolet B light-induced damage in keratinocytes. Mol. Carcinog. 2002;34:121–130. doi: 10.1002/mc.10055. [DOI] [PubMed] [Google Scholar]
- 267.Uemura M., Manabe H., Yoshida N., Fujita N., Ochiai J., Matsumoto N., Takagi T., Naito Y., Yoshikawa T. α-Tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 2002;456:29–37. doi: 10.1016/S0014-2999(02)02639-0. [DOI] [PubMed] [Google Scholar]
- 268.McVean M., Liebler D.C. Prevention of DNA photodamage by vitamin E compounds and sunscreens: Roles of ultraviolet absorbance and cellular uptake. Mol. Carcinog. 1999;24:169–176. doi: 10.1002/(SICI)1098-2744(199903)24:3<169::AID-MC3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 269.Dingler A., Blum R., Niehus H., Muller R., Gohla S. Solid lipid nanoparticles (SLNTM/LipopearlsTM) a pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J. Microencaps. 1999;16:751–767. doi: 10.1080/026520499288690. [DOI] [PubMed] [Google Scholar]
- 270.Ma Q., Wang Y., Lin X., Luo D., Gu N. Preparation, characterization and photoprotection of tocopherol loaded nanostructured lipid carriers; Proceedings of the 2007 IEEE/ICME International Conference on Complex Medical Engineering; Beijing, China. 23–27 May 2007; pp. 203–208. [Google Scholar]
- 271.Shahab M.S., Rizwanullah M., Imam S.S. Formulation, optimization and evaluation of vitamin E TPGS emulsified dorzolamide solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2022;68:103062. doi: 10.1016/j.jddst.2021.103062. [DOI] [Google Scholar]
- 272.Gaba B., Khan T., Haider M.F., Alam T., Baboota S., Parvez S., Ali J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019;2019:2382563. doi: 10.1155/2019/2382563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Taouzinet L., Fatmi S., Khellouf A., Lahiani-Skiba M., Skiba M., Iguer-Ouada M. Drug Release, Stability And Efficiency of Vitamin E Loaded in Liposomes for Bovine Sperm Protection in Cryopreservation Medium. Cryoletters. 2022;43:50–57. doi: 10.54680/fr22110110612. [DOI] [PubMed] [Google Scholar]
- 274.Berkner K., Runge K. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J. Thromb. Haemost. 2004;2:2118–2132. doi: 10.1111/j.1538-7836.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 275.IJland M.M., Pereira R.R., Cornelissen E.A. Incidence of late vitamin K deficiency bleeding in newborns in The Netherlands in 2005: Evaluation of the current guideline. Eur. J. Pediatr. 2008;167:165–169. doi: 10.1007/s00431-007-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Crowther M.A., Douketis J.D., Schnurr T., Steidl L., Mera V., Ultori C., Venco A., Ageno W. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy: A randomized, controlled trial. Ann. Intern. Med. 2002;137:251–254. doi: 10.7326/0003-4819-137-4-200208200-00009. [DOI] [PubMed] [Google Scholar]
- 277.Pereira S., Shearer M., Williams R., Mieli-Vergani G. Intestinal absorption of mixed micellar phylloquinone (vitamin K1) is unreliable in infants with conjugated hyperbilirubinaemia: Implications for oral prophylaxis of vitamin K deficiency bleeding. Arch. Dis. Child.-Fetal Neonatal Ed. 2003;88:F113–F118. doi: 10.1136/fn.88.2.F113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Işcan Y., Wissing S., Müller R. Solid Lipid Nanoparticles (SLNTM) for topical drug delivery: Incorporation of the lipophilic drugs N, N-diethyl-m-toluamide and vitamin K. Die Pharm. 2005;60:905–909. [PubMed] [Google Scholar]
- 279.Liu C.-H., Wu C.-T., Fang J.-Y. Characterization and formulation optimization of solid lipid nanoparticles in vitamin K1 delivery. Drug Dev. Ind. Pharm. 2010;36:751–761. doi: 10.3109/03639040903460453. [DOI] [PubMed] [Google Scholar]
- 280.König A., Bouzan C., Cohen J.T., Connor W.E., Kris-Etherton P.M., Gray G.M., Lawrence R.S., Savitz D.A., Teutsch S.M. A quantitative analysis of fish consumption and coronary heart disease mortality. Am. J. Prev. Med. 2005;29:335–346. doi: 10.1016/j.amepre.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 281.Awad T.S., Helgason T., Weiss J., Decker E.A., McClements D.J. Effect of omega-3 fatty acids on crystallization, polymorphic transformation and stability of tripalmitin solid lipid nanoparticle suspensions. Cryst. Growth Des. 2009;9:3405–3411. doi: 10.1021/cg8011684. [DOI] [Google Scholar]
- 282.Muchow M., Schmitz E., Despatova N., Maincent P., Müller R. Omega-3 fatty acids-loaded lipid nanoparticles for patient-convenient oral bioavailability enhancement. Die Pharm. 2009;64:499–504. [PubMed] [Google Scholar]
- 283.Salminen H., Helgason T., Kristinsson B., Kristbergsson K., Weiss J. Formation of solid shell nanoparticles with liquid ω-3 fatty acid core. Food Chem. 2013;141:2934–2943. doi: 10.1016/j.foodchem.2013.05.120. [DOI] [PubMed] [Google Scholar]
- 284.Blanco-Llamero C., Galindo-Camacho R.M., Fonseca J., Santini A., Señoráns F.J., Souto E.B. Development of Lipid Nanoparticles Containing Omega-3-Rich Extract of Microalga Nannochlorpsis gaditana. Foods. 2022;11:3749. doi: 10.3390/foods11233749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Choudhary P., Dutta S., Moses J., Anandharamakrishnan C. Liposomal encapsulation of omega-3 fatty acid and α-lipoic acid conjugate for cow milk fortification. J. Food Process. Preserv. 2022;46:e16082. doi: 10.1111/jfpp.16082. [DOI] [Google Scholar]
- 286.Katsouli M., Tzia C. Effect of lipid type, dispersed phase volume fraction and emulsifier on the physicochemical properties of nanoemulsions fortified with conjugated linoleic acid (CLA): Process optimization and stability assessment during storage conditions. J. Mol. Liq. 2019;292:111397. doi: 10.1016/j.molliq.2019.111397. [DOI] [Google Scholar]
- 287.Vélez M.A., Perotti M.C., Hynes E.R., Gennaro A.M. Effect of lyophilization on food grade liposomes loaded with conjugated linoleic acid. J. Food Eng. 2019;240:199–206. doi: 10.1016/j.jfoodeng.2018.07.033. [DOI] [Google Scholar]
- 288.Pucek A., Niezgoda N., Kulbacka J., Wawrzeńczyk C., Wilk K.A. Phosphatidylcholine with conjugated linoleic acid in fabrication of novel lipid nanocarriers. Colloids Surf. Physicochem. Eng. Asp. 2017;532:377–388. doi: 10.1016/j.colsurfa.2017.04.061. [DOI] [Google Scholar]
- 289.Hashemi F.S., Farzadnia F., Aghajani A., Ahmadzadeh NobariAzar F., Pezeshki A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020;8:4185–4195. doi: 10.1002/fsn3.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhao Z., Cui X., Ma X., Wang Z. Preparation, characterization, and evaluation of antioxidant activity and bioavailability of a self-nanoemulsifying drug delivery system (SNEDDS) for buckwheat flavonoids. Acta Biochim. Biophys. Sin. 2020;52:1265–1274. doi: 10.1093/abbs/gmaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gharekhani M., Alirezalu K., Roufegarinejad L., Azadmard_damirchi S. Enrichment of buckwheat and lentil based fermented beverages with vitamin E and vitamin C Nano liposomes. J. Food Sci. Technol. 2022;19:193–205. [Google Scholar]
- 292.El-Sayed S.M., El-Sayed H.S., Elgamily H.M., Youssef A.M. Preparation and evaluation of yogurt fortified with probiotics jelly candy enriched with grape seeds extract nanoemulsion. J. Food Process. Preserv. 2022;46:e16713. doi: 10.1111/jfpp.16713. [DOI] [Google Scholar]
- 293.Montagner G.E., Ribeiro M.F., Cadoná F.C., Franco C., Gomes P. Liposomes loading grape seed extract: A nanotechnological solution to reduce wine-making waste and obtain health-promoting products. Future Foods. 2022;5:100144. doi: 10.1016/j.fufo.2022.100144. [DOI] [Google Scholar]
- 294.Trapani A., Esteban M.Á., Curci F., Manno D.E., Serra A., Fracchiolla G., Espinosa-Ruiz C., Castellani S., Conese M. Solid lipid nanoparticles administering antioxidant grape seed-derived polyphenol compounds: A potential application in aquaculture. Molecules. 2022;27:344. doi: 10.3390/molecules27020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Vaishanavi S., Preetha R. Soy protein incorporated nanoemulsion for enhanced stability of probiotic (Lactobacillus delbrueckii subsp. bulgaricus) and its characterization. Mater. Today Proc. 2021;40:S148–S153. doi: 10.1016/j.matpr.2020.05.008. [DOI] [Google Scholar]
- 296.Hussein J., El-Bana M.A., El-Naggar M.E., Abdel-Latif Y., El-Sayed S.M., Medhat D. Prophylactic effect of probiotics fortified with Aloe vera pulp nanoemulsion against ethanol-induced gastric ulcer. Toxicol. Mech. Methods. 2021;31:699–710. doi: 10.1080/15376516.2021.1958112. [DOI] [PubMed] [Google Scholar]
- 297.Hosseini S.F., Ansari B., Gharsallaoui A. Polyelectrolytes-stabilized liposomes for efficient encapsulation of Lactobacillus rhamnosus and improvement of its survivability under adverse conditions. Food Chem. 2022;372:131358. doi: 10.1016/j.foodchem.2021.131358. [DOI] [PubMed] [Google Scholar]
- 298.Gani A., Benjakul S. Effect of β-glucan stabilized virgin coconut oil nanoemulsion on properties of croaker surimi gel. J. Aquat. Food Prod. Technol. 2019;28:194–209. doi: 10.1080/10498850.2019.1571552. [DOI] [Google Scholar]
- 299.Yanagihara S., Kasho N., Sasaki K., Shironaka N., Kitayama Y., Yuba E., Harada A. pH-Sensitive branched β-glucan-modified liposomes for activation of antigen presenting cells and induction of antitumor immunity. J. Mater. Chem. B. 2021;9:7713–7724. doi: 10.1039/D1TB00786F. [DOI] [PubMed] [Google Scholar]
- 300.Acevedo-Estupiñan M.V., Gutierrez-Lopez G.F., Cano-Sarmiento C., Parra-Escudero C.O., Rodriguez-Estrada M.T., Garcia-Varela R., García H.S. Stability and characterization of O/W free phytosterols nanoemulsions formulated with an enzymatically modified emulsifier. LWT. 2019;107:151–157. doi: 10.1016/j.lwt.2019.03.004. [DOI] [Google Scholar]
- 301.Shrestha S.C., Ghebremeskel K., White K., Minelli C., Tewfik I., Thapa P., Tewfik S. Formulation and characterization of phytostanol ester solid lipid nanoparticles for the management of hypercholesterolemia: An ex vivo study. Int. J. Nanomed. 2021;16:1977–1992. doi: 10.2147/IJN.S276301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ishaka A., Imam M.U., Ismail M. Nanoemulsification of Rice Bran Wax Policosanol Enhances Its Cardio-protective Effects via Modulation of Hepatic Peroxisome Proliferator-activated Receptor gamma in Hyperlipidemic Rats. J. Oleo Sci. 2020;69:1287–1295. doi: 10.5650/jos.ess20098. [DOI] [PubMed] [Google Scholar]
- 303.Amante C., Esposito T., Luccheo G., Luccheo L., Russo P., Del Gaudio P. Recapsoma®: A Novel Mixture Based on Bergamot, Ipomoea Batatas, Policosanol Extracts and Liposomal Berberine for the Treatment of Hypercholesterolemia. Life. 2022;12:1162. doi: 10.3390/life12081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li K., Guo Z., Li H., Ren X., Sun C., Feng Q., Kou S., Li Q. Nanoemulsion containing Yellow Monascus pigment: Fabrication, characterization, storage stability, and lipase hydrolytic activity in vitro digestion. Colloids Surf. B Biointerfaces. 2023;224:113199. doi: 10.1016/j.colsurfb.2023.113199. [DOI] [PubMed] [Google Scholar]
- 305.Zhong J., Liu X., Wang Y., Qin X., Li Z. γ-Oryzanol nanoemulsions produced by a low-energy emulsification method: An evaluation of process parameters and physicochemical stability. Food Funct. 2017;8:2202–2211. doi: 10.1039/C7FO00023E. [DOI] [PubMed] [Google Scholar]
- 306.Biagi M., Minoretti P., Bertona M., Emanuele E. Effects of a nutraceutical combination of fermented red rice, liposomal berberine, and curcumin on lipid and inflammatory parameters in patients with mild-to-moderate hypercholesterolemia: An 8-week, open-label, single-arm pilot study. Arch. Med. Sci.-Atheroscler. Dis. 2018;3:137–141. doi: 10.5114/amsad.2018.79597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Raviadaran R., Ng M.H., Manickam S., Chandran D. Ultrasound-assisted production of palm oil-based isotonic W/O/W multiple nanoemulsion encapsulating both hydrophobic tocotrienols and hydrophilic caffeic acid with enhanced stability using oil-based Sucragel. Ultrason. Sonochem. 2020;64:104995. doi: 10.1016/j.ultsonch.2020.104995. [DOI] [PubMed] [Google Scholar]
- 308.Lee S.-g., Kalidindi T.M., Lou H., Gangangari K., Punzalan B., Bitton A., Lee C.J., Vargas H.A., Park S., Bodei L. γ-Tocotrienol–Loaded Liposomes for Radioprotection from Hematopoietic Side Effects Caused by Radiotherapeutic Drugs. J. Nucl. Med. 2021;62:584–590. doi: 10.2967/jnumed.120.244681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kaplan A.B.U., Cetin M., Orgul D., Taghizadehghalehjoughi A., Hacımuftuoglu A., Hekimoglu S. Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Deliv. Sci. Technol. 2019;52:189–203. doi: 10.1016/j.jddst.2019.04.027. [DOI] [Google Scholar]
- 310.Wang Q., Liu W., Wang J., Liu H., Chen Y. Preparation and pharmacokinetic study of Daidzein Long-circulating liposomes. Nanoscale Res. Lett. 2019;14:321. doi: 10.1186/s11671-019-3164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Obinu A., Burrai G.P., Cavalli R., Galleri G., Migheli R., Antuofermo E., Rassu G., Gavini E., Giunchedi P. Transmucosal solid lipid nanoparticles to improve genistein absorption via intestinal lymphatic transport. Pharmaceutics. 2021;13:267. doi: 10.3390/pharmaceutics13020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kim M.-H., Jeon Y.-E., Kang S., Lee J.-Y., Lee K.W., Kim K.-T., Kim D.-D. Lipid nanoparticles for enhancing the physicochemical stability and topical skin delivery of orobol. Pharmaceutics. 2020;12:845. doi: 10.3390/pharmaceutics12090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nemitz M.C., von Poser G.L., Teixeira H.F. In vitro skin permeation/retention of daidzein, genistein and glycitein from a soybean isoflavone rich fraction-loaded nanoemulsions and derived hydrogels. J. Drug Deliv. Sci. Technol. 2019;51:63–69. doi: 10.1016/j.jddst.2019.02.034. [DOI] [Google Scholar]
- 314.Fernandes S.S., Egea M.B., Salas-Mellado M.d.l.M., Segura-Campos M.R. Chia Oil and Mucilage Nanoemulsion: Potential Strategy to Protect a Functional Ingredient. Int. J. Mol. Sci. 2023;24:7384. doi: 10.3390/ijms24087384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Altun A., Ugur-Altun B. Melatonin: Therapeutic and clinical utilization. Int. J. Clin. Pract. 2007;61:835–845. doi: 10.1111/j.1742-1241.2006.01191.x. [DOI] [PubMed] [Google Scholar]
- 316.Wasdell M.B., Jan J.E., Bomben M.M., Freeman R.D., Rietveld W.J., Tai J., Hamilton D., Weiss M.D. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J. Pineal Res. 2008;44:57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 317.Jou M.J., Peng T.I., Yu P.Z., Jou S.B., Reiter R.J., Chen J.Y., Wu H.Y., Chen C.C., Hsu L.F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2007;43:389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 318.Pieper G.M., Nilakantan V., Nguyen T.K., Hilton G., Roza A.M., Johnson C.P. Reactive oxygen and reactive nitrogen as signaling molecules for caspase 3 activation in acute cardiac transplant rejection. Antioxid. Redox Signal. 2008;10:1031–1039. doi: 10.1089/ars.2007.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tengattini S., Reiter R.J., Tan D.X., Terron M.P., Rodella L.F., Rezzani R. Cardiovascular diseases: Protective effects of melatonin. J. Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 320.Rezzani R., Rodella L.F., Fraschini F., Gasco M.R., Demartini G., Musicanti C., Reiter R.J. Melatonin delivery in solid lipid nanoparticles: Prevention of cyclosporine A induced cardiac damage. J. Pineal Res. 2009;46:255–261. doi: 10.1111/j.1600-079X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 321.Kanikkannan N., Andega S., Burton S., Babu R., Singh M. Formulation and in vitro evaluation of transdermal patches of melatonin. Drug Dev. Ind. Pharm. 2004;30:205–212. doi: 10.1081/DDC-120028716. [DOI] [PubMed] [Google Scholar]
- 322.DeMuro R.L., Nafziger A.N., Blask D.E., Menhinick A.M., Bertino J.S. The absolute bioavailability of oral melatonin. J. Clin. Pharmacol. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- 323.Mallo C., Zaidan R., Galy G., Vermeulen E., Brun J., Chazot G., Claustrat B. Pharmacokinetics of melatonin in man after intravenous infusion and bolus injection. Eur. J. Clin. Pharmacol. 1990;38:297–301. doi: 10.1007/BF00315035. [DOI] [PubMed] [Google Scholar]
- 324.Priano L., Esposti D., Esposti R., Castagna G., De Medici C., Fraschini F., Gasco M.R., Mauro A. Solid lipid nanoparticles incorporating melatonin as new model for sustained oral and transdermal delivery systems. J. Nanosci. Nanotechnol. 2007;7:3596–3601. doi: 10.1166/jnn.2007.809. [DOI] [PubMed] [Google Scholar]
- 325.Lundmark P.O., Pandi-Perumal S., Srinivasan V., Cardinali D., Rosenstein R. Melatonin in the eye: Implications for glaucoma. Exp. Eye Res. 2007;84:1021–1030. doi: 10.1016/j.exer.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 326.Wiechmann A.F., Summers J.A. Circadian rhythms in the eye: The physiological significance of melatonin receptors in ocular tissues. Prog. Retin. Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 327.Jockers R., Maurice P., Boutin J., Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Alcantara-Contreras S., Baba K., Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci. Lett. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Bucolo C., Salomone S., Drago F., Reibaldi M., Longo A., Uva M.G. Pharmacological management of ocular hypertension: Current approaches and future prospective. Curr. Opin. Pharm. 2013;13:50–55. doi: 10.1016/j.coph.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 330.Leonardi A., Bucolo C., Drago F., Salomone S., Pignatello R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015;478:180–186. doi: 10.1016/j.ijpharm.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 331.Hou X., Qiu X., Wang Y., Song S., Cong Y., Hao J. Application and Efficacy of Melatonin Elastic Liposomes in Photoaging Mice. Oxidative Med. Cell. Longev. 2022;2022:7135125. doi: 10.1155/2022/7135125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ahmad N., Khalid M.S., Al Ramadhan A.M., Alaradi M.Z., Al Hammad M.R., Ansari K., Alqurashi Y.D., Khan M.F., Albassam A.A., Ansari M.J., et al. Preparation of melatonin novel-mucoadhesive nanoemulsion used in the treatment of depression. Polym. Bull. 2023;80:8093–8132. doi: 10.1007/s00289-022-04436-3. [DOI] [Google Scholar]
- 333.Bentinger M., Brismar K., Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7:S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 334.Beyer R.E. Inhibition by coenzyme Q of ethanol-and carbon tetrachloride-stimulated lipid peroxidation in vivo and catalyzed by microsomal and mitochondrial systems. Free Radic. Biol. Med. 1988;5:297–303. doi: 10.1016/0891-5849(88)90100-1. [DOI] [PubMed] [Google Scholar]
- 335.Shindo Y., Witt E., Han D., Epstein W., Packer L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994;102:122–124. doi: 10.1111/1523-1747.ep12371744. [DOI] [PubMed] [Google Scholar]
- 336.Pardeike J., Schwabe K., Müller R.H. Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effect. Int. J. Pharm. 2010;396:166–173. doi: 10.1016/j.ijpharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 337.Schwarz J.C., Baisaeng N., Hoppel M., Löw M., Keck C.M., Valenta C. Ultra-small NLC for improved dermal delivery of coenyzme Q10. Int. J. Pharm. 2013;447:213–217. doi: 10.1016/j.ijpharm.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 338.Lohan S.B., Bauersachs S., Ahlberg S., Baisaeng N., Keck C.M., Müller R.H., Witte E., Wolk K., Hackbarth S., Röder B., et al. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur. J. Pharm. Biopharm. 2015;89:201–207. doi: 10.1016/j.ejpb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 339.Moschetti A., Vine L.N., Lethcoe K., Dagda R.K., Ellison P., Ryan R.O. Assembly and Characterization of Biocompatible Coenzyme Q10-Enriched Lipid Nanoparticles. Lipids. 2020;55:141–149. doi: 10.1002/lipd.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sağıroğlu A.A. Chitosan-coated liposome-containing carbamazepine and coenzyme Q10: Design, optimization and evaluation. J. Liposome Res. 2021;31:389–398. doi: 10.1080/08982104.2020.1849280. [DOI] [PubMed] [Google Scholar]
- 341.El-Leithy E.S., Makky A.M., Khattab A.M., Hussein D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018;44:316–328. doi: 10.1080/03639045.2017.1391836. [DOI] [PubMed] [Google Scholar]
- 342.Li Y., Dong L., Jia A., Chang X., Xue H. Preparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicine. Int. J. Biol. Macromol. 2006;38:296–299. doi: 10.1016/j.ijbiomac.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 343.Mei Z., Chen H., Weng T., Yang Y., Yang X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur. J. Pharm. Biopharm. 2003;56:189–196. doi: 10.1016/S0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 344.Mei Z., Li X., Wu Q., Hu S., Yang X. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol. Res. 2005;51:345–351. doi: 10.1016/j.phrs.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 345.Zhang D., Tan T., Gao L. Preparation of oridonin-loaded solid lipid nanoparticles and studies on them in vitro and in vivo. Nanotechnology. 2006;17:5821. doi: 10.1088/0957-4484/17/23/018. [DOI] [Google Scholar]
- 346.Shi F., Zhao J.-H., Liu Y., Wang Z., Zhang Y.-T., Feng N.-P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012;7:2033–2043. doi: 10.2147/IJN.S30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wang S., Chen T., Chen R., Hu Y., Chen M., Wang Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012;430:238–246. doi: 10.1016/j.ijpharm.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 348.Lacatusu I., Badea N., Murariu A., Nichita C., Bojin D., Meghea A. Antioxidant capacity of lipid nanoparticles loaded with rosemary extract. Mol. Cryst. Liq. Cryst. 2010;523:260/[832]–272/[844]. doi: 10.1080/15421401003719886. [DOI] [Google Scholar]
- 349.Lai F., Wissing S.A., Müller R.H., Fadda A.M. Artemisia arborescens L essential oil-loaded solid lipid nanoparticles for potential agricultural application: Preparation and characterization. AAPS PharmSciTech. 2006;7:E10–E18. doi: 10.1208/pt070102. [DOI] [PubMed] [Google Scholar]
- 350.Zhao X.-L., Yang C.-R., Yang K.-L., Li K.-X., Hu H.-Y., Chen D.-W. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev. Ind. Pharm. 2010;36:773–780. doi: 10.3109/03639040903485716. [DOI] [PubMed] [Google Scholar]
- 351.Campos D.A., Madureira A.R., Sarmento B., Gomes A.M., Pintado M.M. Stability of bioactive solid lipid nanoparticles loaded with herbal extracts when exposed to simulated gastrointestinal tract conditions. Food Res. Int. 2015;78:131–140. doi: 10.1016/j.foodres.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 352.Tang S., Tan S., Yao L., Li F., Li L., Guo X., Liu Y., Hao X., Li Y., Ding X. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: Retrospective multi-center investigation. PLoS ONE. 2013;8:e82943. doi: 10.1371/journal.pone.0082943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Liu Z., Okeke C.I., Zhang L., Zhao H., Li J., Aggrey M.O., Li N., Guo X., Pang X., Fan L. Mixed polyethylene glycol-modified breviscapine-loaded solid lipid nanoparticles for improved brain bioavailability: Preparation, characterization, and in vivo cerebral microdialysis evaluation in adult Sprague Dawley rats. AAPS PharmSciTech. 2014;15:483–496. doi: 10.1208/s12249-014-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Vijayan V., Aafreen S., Sakthivel S., Reddy K.R. Formulation and characterization of solid lipid nanoparticles loaded Neem oil for topical treatment of acne. J. Acute Dis. 2013;2:282–286. doi: 10.1016/S2221-6189(13)60144-4. [DOI] [Google Scholar]
- 355.Piao L., Jin Y., Cui Y., Yin S. Preparation of Oenothera biennis Oil Solid Lipid Nanoparticles Based on Microemulsion Technique. J. Chin. Med. Mater. 2015;38:1290–1294. [PubMed] [Google Scholar]
- 356.Yang C.-R., Zhao X.-L., Hu H.-Y., Li K.-X., Sun X., Li L., Chen D.-W. Preparation, optimization and characteristic of huperzine a loaded nanostructured lipid carriers. Chem. Pharm. Bull. 2010;58:656–661. doi: 10.1248/cpb.58.656. [DOI] [PubMed] [Google Scholar]
- 357.Kumar N., Chaiyasut C. Hair Growth Promoting Activity of Carthamus Tinctorius Florets Extract-Loaded Nanostructured Lipid Carriers. Int. J. Pharm. Pharm. Sci. 2014;7:252–257. [Google Scholar]
- 358.Ratcharin N., Wongtrakul P., Indranupakorn R. Preparation of Zingiber officinale Extract Loaded Solid Lipid Nanoparticles. Adv. Mater. Res. 2012;506:389–392. doi: 10.4028/www.scientific.net/AMR.506.389. [DOI] [Google Scholar]
- 359.Chaudhari A.K., Singh V.K., Das S., Deepika, Singh B.K., Dubey N.K. Antimicrobial, Aflatoxin B1 Inhibitory and Lipid Oxidation Suppressing Potential of Anethole-Based Chitosan Nanoemulsion as Novel Preservative for Protection of Stored Maize. Food Bioprocess Technol. 2020;13:1462–1477. doi: 10.1007/s11947-020-02479-w. [DOI] [Google Scholar]
- 360.Mohammad Gholinezhad R., Gholipour Zanjani N., Kamran Pirzaman A., Zahed B. Evaluation of drug delivery of allicin liposome based on phosphatidylethanolamine and salep-based hydrogel. J. Appl. Chem. 2023;18:107–124. doi: 10.22075/chem.2022.26440.2056. [DOI] [Google Scholar]
- 361.Shomorony A., Santamaria C.M., Zhao C., Rwei A.Y., Mehta M., Zurakowski D., Kohane D.S. Prolonged Duration Local Anesthesia by Combined Delivery of Capsaicin- and Tetrodotoxin-Loaded Liposomes. Anesth. Analg. 2019;129:709–717. doi: 10.1213/ANE.0000000000004108. [DOI] [PubMed] [Google Scholar]
- 362.Liu D., Zhang Q., Wang J., Guan S., Cai D., Liu J. Inhibition of growth and metastasis of breast cancer by targeted delivery of 17-hydroxy-jolkinolide B via hyaluronic acid-coated liposomes. Carbohydr. Polym. 2021;257:117572. doi: 10.1016/j.carbpol.2020.117572. [DOI] [PubMed] [Google Scholar]
- 363.Aghazadeh T., Bakhtiari N., Rad I.A., Ramezani F.J.B.R.A.C. Formulation of kaempferol in nanostructured lipid carriers (NLCs): A delivery platform to sensitization of MDA-MB468 breast cancer cells to paclitaxel. Biointerface Res. Appl. Chem. 2021;11:14591–14601. [Google Scholar]
- 364.Gad H.A., Abd El-Rahman F.A.A., Hamdy G.M. Chamomile oil loaded solid lipid nanoparticles: A naturally formulated remedy to enhance the wound healing. J. Drug Deliv. Sci. Technol. 2019;50:329–338. doi: 10.1016/j.jddst.2019.01.008. [DOI] [Google Scholar]
- 365.Karamchedu S., Tunki L., Kulhari H., Pooja D. Morin hydrate loaded solid lipid nanoparticles: Characterization, stability, anticancer activity, and bioavailability. Chem. Phys. Lipids. 2020;233:104988. doi: 10.1016/j.chemphyslip.2020.104988. [DOI] [PubMed] [Google Scholar]
- 366.Rodenak-Kladniew B., Gambaro R., Cisneros J.S., Huck-Iriart C., Padula G., Castro G.R., Chain C.Y., Islan G.A. Enhanced anticancer activity of encapsulated geraniol into biocompatible lipid nanoparticles against A549 human lung cancer cells. J. Drug Deliv. Sci. Technol. 2023;80:104159. doi: 10.1016/j.jddst.2023.104159. [DOI] [Google Scholar]
- 367.Saporito F., Sandri G., Bonferoni M.C., Rossi S., Boselli C., Icaro Cornaglia A., Mannucci B., Grisoli P., Vigani B., Ferrari F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018;13:175–186. doi: 10.2147/IJN.S152529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Cordova C.A.S., Locatelli C., Winter E., Silva A.H., Zanetti-Ramos B.G., Jasper R., Mascarello A., Yunes R.A., Nunes R.J., Creczynski-Pasa T.B. Solid lipid nanoparticles improve octyl gallate antimetastatic activity and ameliorate its renal and hepatic toxic effects. Anticancer Drugs. 2017;28:977–988. doi: 10.1097/CAD.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 369.Rodenak-Kladniew B., Islan G.A., de Bravo M.G., Durán N., Castro G.R. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf. B Biointerfaces. 2017;154:123–132. doi: 10.1016/j.colsurfb.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 370.Wi T.I., Won J.E., Lee C.M., Lee J.W., Kang T.H., Shin B.C., Han H.D., Park Y.M. Efficacy of Combination Therapy with Linalool and Doxorubicin Encapsulated by Liposomes as a Two-in-One Hybrid Carrier System for Epithelial Ovarian Carcinoma. Int. J. Nanomed. 2020;15:8427–8436. doi: 10.2147/IJN.S272319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Liu M., Chen J.H., Dong F.R., Liu Y. Optimized preparation of ginkgolides A and B long-circulating solid lipid nanoparticles by central composite design and response surface method. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:700–703. [PubMed] [Google Scholar]
- 372.Vijayanand P., Jyothi V., Aditya N., Mounika A. Development and Characterization of Solid Lipid Nanoparticles Containing Herbal Extract: In Vivo Antidepressant Activity. J. Drug Deliv. 2018;2018:2908626. doi: 10.1155/2018/2908626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ivanova Y.O., Kostromicheva M.M., Ofitserov E.N., Koroleva M.Y. Nanoemulsions with Amaranth and Sea Buckthorn Oils. Colloid J. 2022;84:31–38. doi: 10.1134/S1061933X22010045. [DOI] [Google Scholar]
- 374.Riquelme N., Sepúlveda C., Arancibia C. Influence of Ternary Emulsifier Mixtures on Oxidative Stability of Nanoemulsions Based on Avocado Oil. Foods. 2020;9:42. doi: 10.3390/foods9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Suksuwan A., Arour Z., Santiworakun N., Dahlan W. Preparation and characterization of black seed oil loaded solid lipid nanoparticles for topical formulations: A preliminary study. AIP Conf. Proc. 2021;2353:020003. doi: 10.1063/5.0052706. [DOI] [Google Scholar]
- 376.Kildaci I., Budama-Kilinc Y., Kecel-Gunduz S., Altuntas E. Linseed Oil Nanoemulsions for treatment of Atopic Dermatitis disease: Formulation, characterization, in vitro and in silico evaluations. J. Drug Deliv. Sci. Technol. 2021;64:102652. doi: 10.1016/j.jddst.2021.102652. [DOI] [Google Scholar]
- 377.Huang J., Wang Q., Chu L., Xia Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT. 2020;117:108615. doi: 10.1016/j.lwt.2019.108615. [DOI] [Google Scholar]
- 378.Nemati S., Mohammad Rahimi H., Hesari Z., Sharifdini M., Jalilzadeh Aghdam N., Mirjalali H., Zali M.R. Formulation of Neem oil-loaded solid lipid nanoparticles and evaluation of its anti-Toxoplasma activity. BMC Complement. Med. Ther. 2022;22:122. doi: 10.1186/s12906-022-03607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kumar S., Singh N., Devi L.S., Kumar S., Kamle M., Kumar P., Mukherjee A. Neem oil and its nanoemulsion in sustainable food preservation and packaging: Current status and future prospects. J. Agric. Food Res. 2022;7:100254. doi: 10.1016/j.jafr.2021.100254. [DOI] [Google Scholar]
- 380.Yin Q., Song X., Yang P., Yang W., Li X., Wang X., Wang S. Incorporation of glycyrrhizic acid and polyene phosphatidylcholine in lipid nanoparticles ameliorates acute liver injury via delivering p65 siRNA. Nanomedicine. 2023;48:102649. doi: 10.1016/j.nano.2022.102649. [DOI] [PubMed] [Google Scholar]
- 381.Corzo-Martínez M., Corzo N., Villamiel M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007;18:609–625. doi: 10.1016/j.tifs.2007.07.011. [DOI] [Google Scholar]
- 382.Fenwick G.R., Hanley A.B., Whitaker J.R. The genus allium. Part 2. Crit. Rev. Food Sci. Nutr. 1985;22:273–377. doi: 10.1080/10408398509527417. [DOI] [PubMed] [Google Scholar]
- 383.Ledezma E., Apitz-Castro R. Ajoene the main active compound of garlic (Allium sativum): A new antifungal agent] Rev. Iberoam. Micol. 2006;23:75–80. doi: 10.1016/S1130-1406(06)70017-1. [DOI] [PubMed] [Google Scholar]
- 384.Wencui Z., Qi Z., Ying W., Di W. Preparation of solid lipid nanoparticles loaded with garlic oil and evaluation of their in vitro and in vivo characteristics. Eur. Rev. Med. Pharmacol. Sci. 2015;19:3742–3750. [PubMed] [Google Scholar]
- 385.Faran S.A., Asghar S., Khalid S.H., Khan I.U., Asif M., Khalid I., Gohar U.F., Hussain T. Hepatoprotective and Renoprotective Properties of Lovastatin-Loaded Ginger and Garlic Oil Nanoemulsomes: Insights into Serum Biological Parameters. Medicina. 2019;55:579. doi: 10.3390/medicina55090579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Quach H., Le T.V., Nguyen T.T., Nguyen P., Nguyen C.K., Dang L.H. Nano-Lipids Based on Ginger Oil and Lecithin as a Potential Drug Delivery System. Pharmaceutics. 2022;14:1654. doi: 10.3390/pharmaceutics14081654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Mahato N., Sinha M., Sharma K., Koteswararao R., Cho M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods. 2019;8:523. doi: 10.3390/foods8110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Assadpour E., Mahdi Jafari S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019;59:3129–3151. doi: 10.1080/10408398.2018.1484687. [DOI] [PubMed] [Google Scholar]
- 389.Muala W.C.B., Desobgo Z.S.C., Jong N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon. 2021;7:e06744. doi: 10.1016/j.heliyon.2021.e06744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.El-Sayed S.M., Youssef A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon. 2019;5:e01989. doi: 10.1016/j.heliyon.2019.e01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Gan Y., Xu D., Zhang J., Wang Z., Wang S., Guo H., Zhang K., Li Y., Wang Y. Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis. Molecules. 2020;25:4170. doi: 10.3390/molecules25184170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Piccolella S., Crescente G., Candela L., Pacifico S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019;175:112774. doi: 10.1016/j.jpba.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 393.Gopi S., Balakrishnan P. Advances in Nutraceuticals and Functional Foods: Concepts and Applications. CRC Press; Boca Raton, FL, USA: 2022. [Google Scholar]
- 394.Kesanakurti P., Ragupathy S., Faller A.C., Shanmughanandhan D., Buongiorno F., Della Noce I., Lu Z., Zhang Y., Newmaster S.G. Development of Hydrolysis Probe-Based qPCR Assays for Panax ginseng and Panax quinquefolius for Detection of Adulteration in Ginseng Herbal Products. Foods. 2021;10:2705. doi: 10.3390/foods10112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chen Y., Huang C., Jin Z., Xu X., Cai Y., Bai Y. HPTLC-bioautography/SERS screening nifedipine adulteration in food supplement based on Ginkgo biloba. Microchem. J. 2020;154:104647. doi: 10.1016/j.microc.2020.104647. [DOI] [Google Scholar]
- 396.Moo-Young M. Comprehensive Biotechnology. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 397.Ram M., Misra H. Medicinal and Aromatic Plants. Elsevier; Amsterdam, The Netherlands: 2021. Metabolic engineering for the production of plant therapeutic compounds; pp. 169–207. [Google Scholar]
- 398.Pai S., Hebbar A., Selvaraj S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. Int. 2022;29:35518–35541. doi: 10.1007/s11356-022-19423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Antal D.S., Ardelean F. Chapter 16-Challenges in the nanoscale delivery systems development in the pharmaceutical and nutraceutical markets. In: de Oliveira M.R., editor. Mitochondrial Dysfunction and Nanotherapeutics. Academic Press; Cambridge, MA, USA: 2021. pp. 441–458. [Google Scholar]
- 400.Vidal-Casanella O., Núñez O., Granados M., Saurina J., Sentellas S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021;11:8276. doi: 10.3390/app11188276. [DOI] [Google Scholar]
- 401.Chavda V.P., Vihol D., Mehta B., Shah D., Patel M., Vora L.K., Pereira-Silva M., Paiva-Santos A.C. Phytochemical-loaded liposomes for anticancer therapy: An updated review. Nanomedicine. 2022;17:547–568. doi: 10.2217/nnm-2021-0463. [DOI] [PubMed] [Google Scholar]
- 402.Choi M.K., Song I.S. Pharmacokinetic Drug-Drug Interactions and Herb-Drug Interactions. Pharmaceutics. 2021;13:610. doi: 10.3390/pharmaceutics13050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 404.Watkins R., Wu L., Zhang C., Davis R.M., Xu B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015;10:6055–6074. doi: 10.2147/ijn.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Milewska S., Niemirowicz-Laskowska K., Siemiaszko G., Nowicki P., Wilczewska A.Z., Car H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021;16:6593–6644. doi: 10.2147/IJN.S323831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Agency E.M. ICH Q8 (R2) Pharmaceutical Development. European Medicines Agency; London, UK: 2017. [Google Scholar]
- 407.Agrahari V., Hiremath P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine. 2017;12:819–823. doi: 10.2217/nnm-2017-0039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.