Abstract
The prediction of severity in acute calculous cholecystitis (AC) is important in therapeutic management to ensure an early recovery and prevent adverse postoperative events. We analyzed the value of the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic inflammatory index (SII) to predict advanced inflammation, the risk for conversion, and postoperative complications in AC. Advanced AC was considered the cases with empyema, gangrene, perforation of the gallbladder, abscesses, or difficulties in achieving the critical view of safety. A 3-year retrospective was performed on 235 patients admitted in emergency care for AC. The NLR was superior to the PLR and SII in predicting advanced inflammation and risk for conversion. The best predictive value was found to be at an NLR “cut-off” value of >4.19, with a sensitivity of 85.5% and a specificity of 66.9% (AUC = 0.824). The NLR, SII, and TG 13/18 correlate well with postoperative complications of Clavien–Dindo grade IV (p < 0.001 for all variables) and sepsis. For predicting early postoperative sepsis, TG 13/18 grading >2 and NLR > 8.54 show the best predicting power (AUC = 0.931; AUC = 0.888, respectively), although not significantly higher than that of the PLR and SII. The NLR is a useful biomarker in assessing the severity of inflammation in AC. The SII and PLR may be useful in the prediction of systemic inflammatory response.
Keywords: acute cholecystitis, systemic inflammatory biomarkers, NLR, PLR, SII, postoperative outcome
1. Introduction
Acute calculous cholecystitis (ACC) is a common cause of abdominal pain in emergencies. In a multicentric study designed by the World Society of Emergency Surgery in 2015, ACC ranked as the second cause of complicated intra-abdominal infections, accounting for 18.5% of the total number of cases [1,2]. Early laparoscopic cholecystectomy (LC) is the gold standard in the current therapeutical approach, with favorable outcomes in most cases. However, recent studies found a 0.1–1% mortality risk and a 6–9% risk of major complications [3], such as main common bile duct lesions, myocardial infarction, and pulmonary complications, and this risk is highly increased in emergency LC performed in cases with severe inflammation. A recent study by Lucocq et al. [4] found that 36.7% of LCs performed in emergency had a non-standard outcome, including conversion, subtotal cholecystectomy, bile leak, and prolonged postoperative stay [4]. Most of these cases were related to the severity of local inflammation, intraoperative findings showing gangrenous cholecystitis, empyema, perforation of the gallbladder, and difficult dissection of the Calot Triangle, all these conditions being generally referred to as advanced acute cholecystitis [5] or severe cholecystitis [6,7] by different authors. Preoperative identification of these cases is important to optimize the therapeutic approach and improve the clinical outcome [5].
Currently, the role of different biomarkers with predictive value in acute cholecystitis is still a subject of research. TG13/18 guidelines propose a grading scale for evaluating local inflammation and its systemic involvement based on clinical evaluation, leukocytes, and CRP as well as the presence or not of the alterations of the vital functions related to the septic process [8,9].
However, a systematic review of Tufo et al. [3] found that while grade III TG 13/18 may be associated with higher mortality when compared with grade I, there is no consensus regarding the preoperatory predictive risk evaluation in patients with acute calculous cholecystitis.
Several studies found CRP to be a good predictive factor for conversion; however, the cut-off values varied widely from 76 mg/L to 220 mg/L [5,10,11,12,13]. Together with the valuable findings provided by ultrasound and CT exam, specific biomarkers were analyzed for the possible predictive role for the severity of local inflammation, such as the YKL-40 protein level [14], serum level of visfatin [15], procalcitonin [16], human neutrophil lipocalin [17], chitotriosidase, and neopterin [18]. However, their availability in emergencies is limited in many surgical departments.
Recently, the systemic inflammatory biomarkers, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), were investigated for their predictive value in many inflammatory and septic conditions [18,19,20,21,22], such as septic shock, diabetic foot ulcer, acute appendicitis, and spontaneous bacterial peritonitis. They are cheap and inexpensive biomarkers, easy to calculate based on the complete blood count (CBC). Several studies found a good correlation between the NLR and PLR and the severity of inflammation in acute calculous cholecystitis as well as the length of postoperative stay. However, there is still conflicting evidence regarding the clinical significance of these biomarkers and their cut-off value that could be used in therapeutic management.
In the present study, we aimed to analyze the value of the NLR, PLR, and SII in predicting severe forms of acute cholecystitis, conversion to open surgery, and adverse postoperative outcomes.
2. Materials and Methods
2.1. Patient Selection
A 3-year retrospective study was carried out between January 2020 and December 2022 on the patients admitted for acute cholecystitis in the 4th Department of Surgery, Emergency University Hospital Bucharest. Data were collected from electronic patient records and operatory protocols. All patients admitted in emergency care, aged over 18 years, for whom the diagnosis of acute cholecystitis could be confirmed based on intraoperative findings were included in the statistical analysis. Along with local and systemic inflammatory signs, ultrasonography and/or abdominal CT were used to document the presence of calculi, the thickness of the gallbladder walls, common biliary duct (CBD) diameter, and the potential signs of pericholecystitis. For all patients, age, associated comorbidities, time elapsed from the onset of symptoms to presentation, and clinical signs were assessed at admission. Biological tests at admission included a complete blood count with differentials of fibrinogen, bilirubin, hepatic transaminases, INR, urea, and creatinine. Systemic inflammatory biomarkers were calculated based on the counts for neutrophils, platelets, and neutrophils measured from the same sample and expressed as their value in cells/L. SII was calculated using the formula SII = P × N/L, where P, N, and L are the counts of platelets, neutrophils, and lymphocytes, respectively [23].
C-reactive protein (CRP) was not available in an emergency in our hospital but was determined the next day, in cases in which surgical intervention was postponed due to local or general conditions. For this reason, CRP was not included in the statistical analysis.
Patients with associated malignancies as well as hematological and autoimmune diseases were excluded due to their previously documented impact on the blood cells and derivate systemic inflammatory indices.
2.2. Study Design
The patients included in the study were classified according to the intraoperative findings into mild and advanced acute cholecystitis, according to the intensity of local inflammation. Advanced forms were considered the cases with empyema, gangrene, perforation of the gallbladder, abscesses, adhesions, or difficulty in dissecting Calot’s triangle, likely to be associated with increased operative difficulty [9,24].
The patients were classified according to TG 13/TG18 Tokyo guidelines for acute cholecystitis as grade I (mild) acute cholecystitis, grade II (moderate), and grade III (severe) if associated with organ dysfunction [9]. Systemic inflammatory biomarkers NLR, PLR, and SII were calculated based on the complete blood cell count at admission. The prediction values of TG 13/TG 18 severity grading, NLR, PLR, and SII were analyzed for advanced AC, postoperative complications, and hospital stay.
2.3. Statistical Analysis
Microsoft Excel and Med Calc® Statistical Software (version 22.006 Med Calc Software Ltd., Ostend, Belgium; https://www.medcalc.org; accessed on 10 August 2023) were used for data analysis. Pearson’s Chi-squared test was used to evaluate the association between discrete variables, while ANOVA was used for continuous variables. For the statistically significant results, a post hoc analysis was performed to establish the differences within groups by using the Scheffe test for all pairwise comparisons.
The specificity and sensitivity of NLR, PLR, and SII in predicting the severity of inflammation, and local and systemic complications were analyzed by ROC curves. According to the widely accepted classification scale described by Safari et al. [25], the AUC values were categorized as 90–100 = excellent; 80–90 = good; 70–80 = fair; 60–70 = poor; and 50–60 = fail.
3. Results
3.1. General Data of the Patients Included in the Study Group
A total of 235 patients with acute cholecystitis were included in the study, with a mean age of 54.6 ± 16.3. Most of the cases were mild (70.6%) and of female patients (71.4%). In the advanced AC group, the mean age was significantly higher (61 ± 15.6 vs. 52 ± 15.9, <0.001), and there were significantly more male patients (p = 0.008) when compared to mild cases (Table 1).
Table 1.
General data of the patients included in the study group.
| Parameter | Total | Mild AC | Advanced AC | p Value |
| No. of patients | 235 | 166 (70.6%) | 69 (29.4%) | |
| Females | 168 (71.4%) | 127 (76.5%) | 41 (59.4%) | 0.008 1 |
| Age | 54.6 ± 16.3 | 52 ± 15.9 | 61 ± 15.6 | <0.001 1 |
| Comorbidities (No.): Obesity Arterial hypertension Cardiac ischemic disease Chronic hepatic diseases Chronic respiratory diseases Chronic renal diseases Cardiac failure/shock Diabetes Others |
2 ± 1.4 | 1.8 ± 1.3 | 2.6 ± 1.6 | <0.001 2 |
| 116 (49.4%) | 75 (45.1%) | 41 (59.4%) | 0.047 2 | |
| 107 (45.5%) | 57 (53.3%) | 50 (46.7%) | 0.093 2 | |
| 28 (11.9%) | 14 (8.4%) | 14 (20.2%) | 0.01 2 | |
| 67 (28.5%) | 40 (24%) | 27 (39.1%) | 0.02 2 | |
| 31 (13.2%) | 18 (10.8%) | 13 (18.8%) | 0.099 2 | |
| 39 (16.6%) | 25 (15%) | 14 (20.2%) | 0.327 2 | |
| 11 (4.7%) | 4 (2.4%) | 7 (10.1%) | 0.01 2 | |
| 32 (13.6%) | 15 (9%) | 17 (24.6%) | 0.001 2 | |
| 84 (35.7%) | 62 (37.3%) | 22 (31.8%) | 0.426 2 | |
| ASA PS risk scale I II III IV V |
0.008 2 (0.0003 3 for trend) |
|||
| 16 (6.8%) | 14 (8.4%) | 2 (2.8%) | ||
| 124 (52.8%) | 96 (57.8%) | 28 (40.5%) | ||
| 78 (33.2%) | 48 (28.9%) | 30 (43.4%) | ||
| 16 (6.8%) | 8 (4.8%) | 8 (11.5%) | ||
| 1 (0.4%) | 0 | 1 (1.4%) | ||
| TG 13/18 severity grading I II III |
<0.0001 2 (<0.0001 3 for trend) | |||
| 145 (61.7%) | 121 (72.9%) | 24 (37.4%) | ||
| 73 (31.1%) | 40 (24%) | 33 (47.8%) | ||
| 17 (7.2%) | 5 (3%) | 12 (17.4%) | ||
| Angiocholitis/CBD stones | 18 (7.6%) | 7 (4.2%) | 11 (15.9%) | 0.013 2 |
| Leukocytes (/μL) | 10,441 ± 4895.3 | 9187.6 ± 3787.4 | 13,456.2 ± 5882 | <0.0001 1 |
| Neutrophils (/μL) | 7796 ± 4867.5 | 6413.4 ± 3728.6 | 11,124.9 ± 5646.6 | 0.001 1 |
| Platelets (/μL) | 239,767.1 ± 82,016.7 | 245,341.4 ± 77,532.9 | 226,356.5 ± 91,122 | 0.053 1 |
| Fibrinogen (mg/dL) | 450.1 ± 186.2 | 389.1 ± 119.1 | 596.8 ± 232.3 | <0.001 1 |
| INR | 1.3 ± 1.1 | 1.2 ± 0.9 | 1.3 ± 1.2 | 0.327 1 |
| Bilirubin | 1.3 ± 2.0 | 0.95 ± 1.1 | 2.3 ± 3.1 | <0.001 1 |
| AST | 68.9 ± 116 | 63.2 ± 116.2 | 125 ± 297.1 | 0.063 1 |
| ALT | 107.4 ± 165.9 | 85.8 ± 136.9 | 120 ± 179 | 0.056 1 |
| Creatinine | 1.3 ± 0.5 | 1.2 ± 0.3 | 1.5 ± 1.4 | 0.341 1 |
| NLR | 7.29 ± 12.2 | 4.3 ± 5.2 | 14.3 ± 19.4 | <0.001 1 |
| PLR | 181.2 ± 229.4 | 143.8 ± 68.7 | 273.5 ± 397 | <0.001 1 |
| SII | 1701.6 ± 3416.4 | 1009.5 ± 993.8 | 3366.8 ± 5812.4 | <0.001 1 |
Footnote: 1 ANOVA; 2 Chi-squared test; 3 test of linear-by-linear association; ASA PS: American Society of Anesthesiologists Physical Status Classification; TG13/18: Tokyo Guidelines classification risk; AST: aspartate aminotransferase; ALT: Alanyl aminotransferase; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SII: systemic inflammatory index.
Most patients included in the study group presented with two or more comorbidities. The subjects included in the advanced AC group had significantly more comorbidities than those admitted with mild AC (p < 0.001). Older age (p < 0.001), obesity (p = 0.047), diabetes (p = 0.001), ischemic cardiac disease (p = 0.01), chronic hepatic diseases (p = 0.02), and cardiac failure/shock at admission (p = 0.01) were correlated with advanced AC in the study group. According to the ASA risk scale, most patients were graded as grade II or III in both groups. However, there was an upward trend of distribution towards higher grades in the advanced AC group, confirmed by the linear-by-linear association test (p = 0.0003). A similar upward trend was observed for the TG 13/18 severity scale, with more grade II and III cases in the advanced AC group (<0.0001).
Statistical analysis showed significantly higher values for leukocytes (p < 0.0001), neutrophils (p = 0.001), the NLR (p < 0.001), the PLR (p < 0.001), the SII (p < 0.001), fibrinogen (p < 0.001), and bilirubin (p < 0.001) with no significant difference for platelets, INR, transaminases, and creatinine levels.
3.2. Comparative Analysis of NLR, PLR, and SII Values with TG 13/18 Grading in the Study Group
Furthermore, we investigated how the TG13/18 severity grading scale for AC correlates with the values of NLR, PLR, and SII by using the Chi-squared test and Scheffe test for pairwise comparison. The statistical analysis found a significant positive correlation in all cases, with the mean values of the investigated systemic inflammatory biomarkers rising from the grade I to grade III groups. However, there are differences in the Scheffe test results, which may suggest that each of the biomarkers characterizes specific changes in the inflammation process (Table 2).
Table 2.
Correlations between NLR, PLR, and SII with TG 13/18 grading in the study group.
| TG13/18 Grade I (1) | TG13/18 Grade II (2) | TG13/18 Grade III (3) | p Value (Chi-Squared Test) | Scheffe Test for Pairwise Comparison | |
|---|---|---|---|---|---|
| NLR | 3.6 ± 3 | 11.7 ± 14.6 | 18.8 ± 28 | <0.001 | (1) differs from (2) and (3) |
| PLR | 147.8 ± 80.3 | 191.2 ± 123.6 | 432.1 ± 752 | <0.001 | (1) and (2) differ from (3) |
| SII | 879.9 ± 726.5 | 2393.6 ± 2477 | 5738.6 ± 10,617 | <0.001 | Each group differs significantly from the others. |
While the NLR is an early inflammatory biomarker, which significantly raises between mild and moderate forms, the PLR seems to be significantly elevated in advanced stages, when local inflammation of the gallbladder and surrounding tissues reaches systemic involvement. SII values, combining in their formula both the number of neutrophils and platelets, discriminate best among the three stages defined by the TG 13/18 scale.
3.3. Prediction Value of NLR, PLR, SII, and TG 13/18 Grading Scale for Advanced Acute Cholecystitis
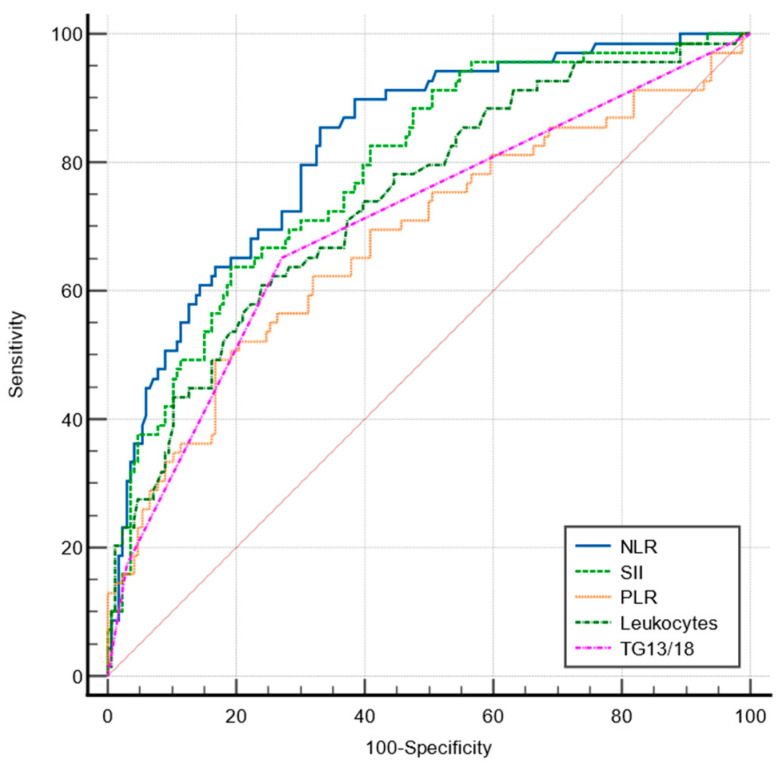
The sensitivity and specificity of the NLR, PLR, SII, total leukocytes, and TG 13/18 grading scale for predicting advanced forms of AC were analyzed by the ROC curves (Figure 1).
Figure 1.
Comparative ROC curves for NLR, PLR, SII, TG13/18, and total leukocytes in predicting advanced AC.
Only the NLR showed a good predictive value (AUC = 0.824). The pairwise comparison of ROC curves for predicting advanced AC found that the predictive value of the NLR was significantly superior to that of the SII (p = 0.0065), PLR (p < 0.0001), total leukocytes (p = 0.0103), and TG 13/18 grading (p = 0.0006). The best predictive value was found to be at a cut-off value of >4.19, with a sensitivity of 85.5% and a specificity of 66.9% (Table 3).
Table 3.
Sensitivity and specificity at the “cut-off” value predicting advanced forms.
| Sensitivity | Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 85.5 | 66.9 | >4.19 | 0.824 | <0.001 |
| PLR | 49.3 | 83.1 | >189.3 | 0.679 | <0.001 |
| SII | 63.8 | 80.7 | >1442.4 | 0.787 | <0.001 |
| TG13/18 | 65.22 | 72.8 | >1(mild) | 0.704 | <0.001 |
| Leukocytes | 60.87 | 75.9 | >11,300 | 0.741 | <0.001 |
3.4. Surgical Approach and Postoperative Outcomes
Laparoscopic cholecystectomy was the most common procedure in both groups. However, the number of cases that required conversion, open surgical procedures, Kehr drainage, or perioperative ERCP was significantly higher in the advanced AC group (Table 4).
Table 4.
Surgical treatment and outcomes.
| Total (n = 235) |
Mild AC (n = 166) | Advanced AC (n = 69) | p Value | |
|---|---|---|---|---|
| Types of surgery: LC LC-conversion CC Cholecystostomy |
<0.0001 1 (<0.0001 2 for trend) | |||
| 208 (88.6%) | 162 (97.6%) | 46 (66.6%) | ||
| 16 (6.8%) | 1 (0.6%) | 15 (21.7%) | ||
| 10 (4.2%) | 3 (1.8%) | 7 (10.2%) | ||
| 1 (0.4%) | 0 | 1 (1.5%) | ||
| Kehr drainage | 5 (2.1%) | 1 (0.6%) | 4 (5.8%) | 0.012 1 |
| ERCP + calculi removal (pre or postop) | 17 (7.2%) | 7 (4.2%) | 10 (14.5%) | 0.005 1 |
| Postoperative hospital stay (days) | 3.6 ± 3.4 | 2.9 ± 2.8 | 5.1 ± 4 | <0.001 3 |
| Length of stay (days) | 7.1 ± 4.5 | 6.1 ± 3.9 | 9.3 ± 5.2 | <0.001 3 |
Footnote: LC = laparoscopic cholecystectomy; CC = classic (open) cholecystectomy; ERCP = endoscopic retrograde cholecysto-pancreatography; 1—Chi-squared test; 2—Scheffe test for pairwise comparison; 3—ANOVA.
The reason for conversion to open surgery in the mild AC group was the unclear anatomy of the Calot triangle due to extensive fibrosis. In the advanced AC group, most cases were converted due to a friable hemorrhagic gangrenous gallbladder wall (five cases) and the impossibility of achieving the critical view of safety (CVS) due to inflammation and adherences (six cases). Other causes of conversion included biliary fistula (one case), Mirizzi Syndrome (one case), biliary peritonitis due to a perforated gallbladder abscess (one case), and a pericholecystic abscess (one case).
Open surgery as the first choice was mainly dictated by the general status and associated comorbidities in patients graded as ASA IV or V (seven cases, including the three cases in the mild AC group), for whom the laparoscopic approach was not considered safe to be performed by the intensive care team. In three cases, the decision was made based on the clinical and imagistic data: pseudo-tumoral pericholecystic mass (one case) and gallbladder abscess (two cases). There was one case treated by cholecystostoma, an 85-year-old patient with piocholecystitis and septic shock at admission, who died 3 days after surgery in the intensive care unit due to sepsis and acute limb ischemia.
In the study group, there were 16 patients who were COVID-19-positive at the moment of admission. Out of these, 14 were treated safely by laparoscopic cholecystectomy, after all the required safety measures were taken to prevent the contamination of the operatory team. In the remaining cases, open surgery was performed due to associated septic shock (one case) and COVID-19 severe pneumonia (one case).
Furthermore, we analyzed the postoperative complications encountered in the study group, registered after Clavien–Dindo classification (Table 5).
Table 5.
Postoperative complications according to Clavien–Dindo Classification.
| Total (n = 235) |
Mild AC (n = 166) |
Advanced AC (n = 69) |
p-Value * | |
|---|---|---|---|---|
| I (surgical site infections) | 4 (1.7%) | 1 (0.6%) | 3 (4.3%) | 0.043 |
| II (requiring pharmacological treatment) surgical-related complications, treated conservatory Nosocomial infections |
||||
| 11 (4.6%) | 5 (3%) | 6 (8.6%) | 0.064 | |
| 15 (6.4%) | 6 (3.6%) | 9 (13%) | 0.007 | |
| III (surgical-related complications requiring endoscopic/surgical/Rx approach) | 2 (0.8%) | 1 (0.6%) | 1 (1.4%) | 1.00 |
| IV (general complications requiring intensive care) Malign hypertension Hemodynamic instability Sepsis Pulmonary edema/pleurisy |
16 (6.8%) | 4 (2.4%) | 12 (17.3%) | 0.002 |
| 4 (1.7%) | 1 (0.6%) | 3 (4.3%) | 0.043 | |
| 1 (0.4%) | 0 | 1 (1.4%) | 0.12 | |
| 8 (3.4%) | 2 (1.2%) | 6 (8.6%) | 0.004 | |
| 3 (1.3%) | 1 (0.6%) | 2 (2.8%) | 0.15 | |
| V (deceased) | 5 (2.1%) | 2 (1.2%) | 3 (4.3%) | 0.129 |
Footnote: * p-value was calculated by Chi-squared test.
Statistically significant differences observed between the mild and advanced AC groups for surgical site infections (p = 0.043) and nosocomial infections (p = 0.007) could be correlated with higher numbers of open surgeries and conversions, as well as with increased hospital stays in the advanced AC group. General complications requiring intensive care were more frequent in the advanced AC group (p = 0.002), including sepsis (p = 0.004) and postoperative malign hypertension (p = 0.043).
3.5. Correlations between Inflammatory Parameters and Types of Surgery in the Study Group
NLR and TG13/18 grading correlated well with the type of surgery performed (p = 0.001; and p < 0.0001, respectively), while the PLR and SII mean values were higher in the conversion and open surgery groups but not statistically significant (Table 6).
Table 6.
Correlations between the types of surgery and NLR, PLR, SII, and TG13/18 in the study group.
| LC (1) | LC-Conversion (2) | CC/Cholecystostomy (3) | p-Value | Scheffe Test for Pairwise Comparison |
|
|---|---|---|---|---|---|
| NLR | 6.2 ± 11.8 | 12.1 ± 7 | 20.2 ± 17.3 | 0.001 1 | (1) differs from (3) |
| PLR | 178.5 ± 241.9 | 182 ± 82.7 | 248.1 ± 73.4 | 0.823 1 | NS |
| SII | 1583.8 ± 3572.4 | 2323.2 ± 1474.2 | 3014.5 ± 1779.5 | 0.49 1 | NS |
| TG 13/18 grading | <0.0001 2 | Each group differs significantly from the others |
|||
| I | 139 (66.9%) | 4 (25%) | 2 (18.2%) | ||
| II | 58 (27.9%) | 10 (62.5%) | 5 (45.4%) | ||
| III | 11 (5.2%) | 2 (12.5%) | 4 (36.4%) |
1 ANOVA; 2 Chi-squared test; NS: not significant.
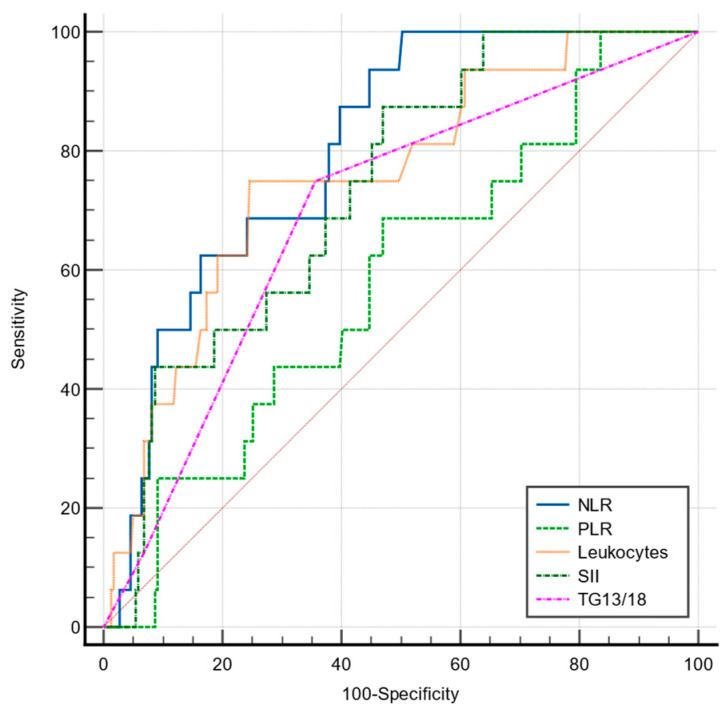
The predictive value for conversion for the NLR, PLR, SII, TG 13/18, and total leukocytes was analyzed by ROC curves (Figure 2).
Figure 2.
Comparative ROC curves for NLR, PLR, SII, TG13/18, and total leukocytes in predicting conversion to open cholecystectomy.
Out of the studied parameters, only the NLR showed a good predictive value for conversion, with a cut-off value of 4.24 (AUC = 0.802, p < 0.001), significantly higher compared to that of leukocytes (AUC = 0.755), SII (AUC = 0.734), and TG13/18 (AUC = 0.690) (Table 7).
Table 7.
Prediction value of NLR, PLR, SII, and TG 13/18 for conversion to open surgery.
| PDR Sensitivity | PDR Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 93.7 | 55.2 | >4.24 | 0.802 | <0.001 |
| PLR | 68.7 | 53 | >141.8 | 0.582 | 0.246 |
| SII | 87.5 | 53 | >949.6 | 0.734 | <0.001 |
| TG13/18 | 75 | 64.3 | >1 | 0.690 | 0.001 |
| Leukocytes | 75 | 75.3 | >12,200 | 0.755 | <0.001 |
3.6. Correlations between Inflammatory Parameters and Postoperative Outcomes in the Study Group
ANOVA showed a good correlation between the NLR, PLR, SII, and TG 13/18 grading scale and the postoperative hospital stay (p < 0.001; p < 0.001; p < 0.001; and p = 0.008, respectively) and total hospital stay (p = 0.002; p < 0.001; p < 0.001; and p = 0.001, respectively).
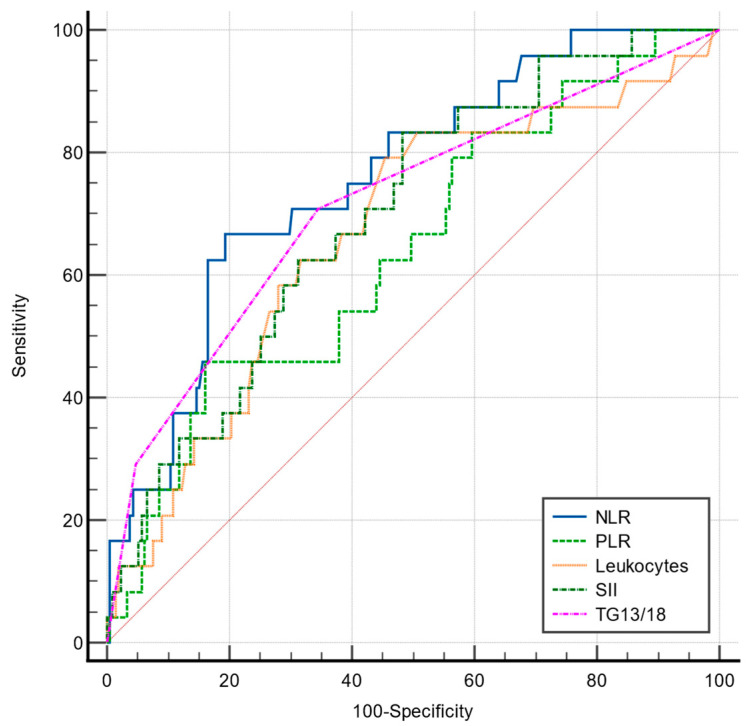
In the present study, the NLR, PLR, SII, and TG 13/18 grading scales had a poor prognostic value for predicting local postoperative complications, almost equal to a coin toss (Table 8, Figure 3), and did not correlate well with the postoperative complications related to surgery, Clavien–Dindo grades II and III (p = 0.83; p= 0.843; and p = 0.898, respectively).
Table 8.
Prediction value of NLR, PLR, SII, and TG 13/18 for surgical-related postoperative complications.
| PDR Sensitivity | PDR Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 45.45 | 80.8 | >8.88 | 0.595 | 0.33 |
| PLR | 45.45 | 75.45 | >194.6 | 0.528 | 0.776 |
| SII | 45.45 | 70.54 | >1525.9 | 0.530 | 0.3 |
| TG13/18 | 54.5 | 62.5 | >1 | 0.583 | 0.31 |
| Leukocytes | 72.73 | 5 | <17,800 | 0.510 | 0.935 |
Figure 3.
Comparative ROC curves for NLR, PLR, SII, TG13/18, and total leukocytes in predicting postoperative local complications.
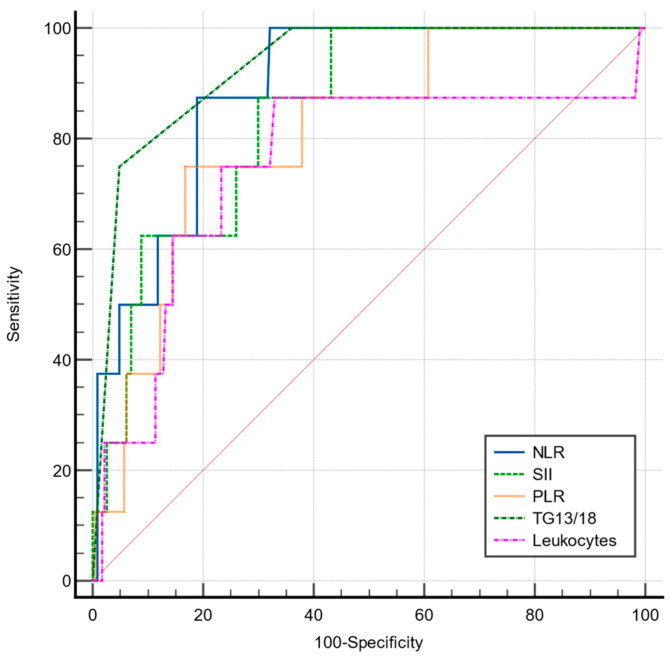
However, the NLR, SII, and TG 13/18 correlated well with postoperative complications of Clavien–Dindo grade IV (p < 0.001 for all variables), while the values were not statistically significant for the PLR (p = 0.113). However, their predictive power evaluated by ROC curves varied from poor (PLR and SII) to fair (TG 13/18 grading and NLR), as shown in Table 9, Figure 4.
Table 9.
Prediction value of NLR, PLR, SII, and TG 13/18 for general postoperative complications requiring intensive care (Clavien–Dindo IV).
| Sensitivity | Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 66.7 | 80.6 | >7.67 | 0.758 | <0.001 |
| PLR | 45.8 | 83.9 | >221.3 | 0.640 | 0.02 |
| SII | 83.3 | 51.7 | >858.3 | 0.697 | 0.001 |
| TG13/18 | 70.83 | 65.4 | >1 | 0.715 | <0.001 |
| Leukocytes | 79.2 | 54.4 | >9100 | 0.668 | 0.006 |
Figure 4.
Comparative ROC curves for NLR, PLR, SII, TG13/18, and total leukocytes in predicting general complications requiring intensive care support (Clavien–Dindo grade IV).
For predicting early postoperative sepsis, a TG 13/18 grading > 2 and NLR > 8.54 showed the best predicting power (AUC = 0.931; AUC = 0.888, respectively), though not significantly higher than that of the PLR and SII (Table 10, Figure 5).
Table 10.
Prediction value of NLR, PLR, SII, and TG 13/18 for postoperative sepsis.
| PDR Sensitivity | PDR Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 87.5 | 81 | >8.54 | 0.888 | <0.001 |
| PLR | 75 | 83.2 | >222.46 | 0.807 | <0.001 |
| SII | 87.5 | 70.04 | >1447.68 | 0.845 | <0.001 |
| TG13/18 | 75 | 92.1 | >2 | 0.931 | <0.0001 |
| Leukocytes | 87.5 | 66.9 | >11,300 | 0.753 | 0.025 |
Figure 5.
Comparison of ROC curve for NLR, SII, PLR, TG13/18, and total leukocytes in predicting sepsis in the early postoperative period.
4. Discussion
Predicting the severity of acute cholecystitis is important to achieve the best therapeutic outcomes and prevent adverse postoperative events [2,26,27,28,29]. Local inflammation and surgical trauma induce metabolic and systemic inflammatory responses, which may lead to systemic complications [30]. Understanding and addressing inflammation and the possible systemic imbalances that it may cause is important to prevent adverse outcomes and unnecessarily prolonged hospital stays in cases with AC.
Commonly used for diagnosis, CT and ultrasound examination may not accurately predict advanced AC [30,31]. In a study on 1115 patients who underwent surgery for acute calculous cholecystitis, Goiayev et al. [31] found that even in cases with a gallbladder wall of ≤ 4.85 mm, if the NLR > 5.65 and the total leukocytes exceed 8100/mm3, there is a 92% probability of complicated AC, including gangrenous, perforated, emphysematous, or necrotizing AC. NLR is a cheap, easy-to-calculate inflammatory biomarker that combines the relative ratio of neutrophils—the first line of cellular defense in acute inflammation—and the lymphocytes, with an immunomodulatory role [30].
Although several studies have found a significant correlation between the NLR and the severity of inflammation in AC [30,32,33,34], there is limited evidence regarding the specific cut-off value, with possible clinical use.
In the present study, we comparatively examined NLR, PLR, SII, total leukocytes, and TG13/18 grading scale for predicting severe inflammation in acute cholecystitis, risk for conversion, and adverse outcomes. We found that the NLR performed best for predicting advanced AC, with an AUC of 0.824 at a cut-off value >4.19. The NLR also has a good predictive value for conversion (AUC = 0.804, cut-off value of 4.24), with high sensitivity (93.7%) but low sensitivity (55.7%).
A previous study by Micic et al. [24] on 136 patients who underwent LC for acute cholecystitis found a similar cut-off value of 4.18 for predicting advanced AC with a 78.3% sensitivity and 74.3% specificity [24], while another recent study found a cut-off value of 4.17 for moderate to severe AC [35], with a predictive value similar to that of CRP.
A higher “cut-off” value of 5.5 for the NLR was found by Turhan et al. [36] with a good predictive value, 80.8% sensitivity, and 80.1% specificity. This may be explained, however, by the selection criteria the authors defined for the complicated AC group in their study, which included very advanced changes of the gallbladder wall, such as perforation, gangrenous cholecystitis, and emphysematous cholecystitis. The definition of “difficult cholecystectomy” is still a challenging subject, with no international consensus being reached. In the present study, we followed the recommendations of Manuel Velasques et al. [37], so we also included severe local inflammation which led to the impossibility of achieving the critical view of safety. On the other hand, Turhan et al. [37] also found that the PLR correlated with inflammation, but with a lower predictive value when compared to that of the NLR for complicated AC (AUC = 0.704 vs. 0.873, respectively), which is consistent with our findings. Diez Ares et al., in a study on 130 patients operated on for AC, found that an NLR value of >5 and a CRP value of >100 mg/dL were independent risk factors for gangrenous cholecystitis, with good predictive value estimated by ROC curves (AUC = 0.75 vs. AUC = 0.80, respectively), and should be taken into account in the therapeutic decision, considering that early laparoscopic cholecystectomy provides the best outcomes in gangrenous AC [38].
A different approach was used by Unal et al. [34], who analyzed the correlation between the NLR and the TG 13/18 grading scale. He found that an NLR cut-off value of 5.2 may discriminate well between TG13/18 grade 1 vs. grades 2 and 3 with a sensitivity of 76.76% and specificity of 76.17% (AUC of 0.817), while a NRL > 8.5 is a good predictor for TG 13/18 grade 3, which associates with systemic imbalance due to inflammation [34]. In our study, we also found a cut-off value of >8.54 to be a good predictor for early postoperative sepsis.
Kartal and Kalayci [38] found no correlation between the NLR and postoperative overall morbidity in the elderly with AC [38]. In the present study, we found no correlation between the systemic inflammatory biomarkers and Clavien–Dindo complications grades II and III. This finding may support the current recommendation that early cholecystectomy may be performed safely in all cases of acute cholecystitis, even those with severe inflammation. However, inflammatory biomarkers were well-correlated with the grade IV Clavien–Dindo complications, requiring intensive care. In the present study, we found that an NLR value of >8.54 has 87.5% sensitivity and 81% specificity for early postoperative sepsis. Although postoperative complications are more frequent in the severe cholecystitis group, there is no correlation between postoperative surgical complications and the values of the NLR, SII, PLR, or TG 13/18 grading scale. This supports, on the one hand, the idea that choosing the appropriate technique for each case allows for a successful solution regardless of the severity of the local inflammation [1,39,40,41]. On the other hand, repeated inflammatory relapses that lead to local fibrous rearrangements, but also the involvement of the human factor (perception errors of the struts during dissection), can generate vascular-biliary lesions [42,43].
In our study, we found that the PLR is an important biomarker in predicting sepsis in patients with AC admitted in emergency care. Part of the complicated underlying pathophysiology of sepsis syndrome is clot formation and bleeding diathesis associated with platelet disfunction, endothelial activation, and disseminated intravascular coagulation [44]. Prompt identification of these patients is essential for improving the survival rate in these patients [44,45,46].
Mitigation of perioperative inflammation and pain is important for enhanced recovery after surgery (ERAS) and preventing postoperative complications [47,48,49]. Postoperative analgesia is a very important part of perioperative management in patients with AC. The pain pattern after LC seems to be different from that after other laparoscopic surgeries [48], and good pain management should be based on an individualized approach. The intensity of preoperative inflammation may sensibilize the peritoneal nociceptors, so a multimodal analgesia could be the best option to control the pain with minimal adverse effects [48]. Several studies found that choosing non-opioid combinations, such as paracetamol and parecoxib 40 mg IV or lornoxicam quick-release 8 mg PO every 12 h, results in the same anti-algic effect as opioids, but limits the risk of pulmonary complications and allows a quick recovery [50,51].
Our study has some limitations: it is a monocentric retrospective study on a limited number of patients. The analyzed values are those from admission, not those from the operative moment. Also, the dynamics of biomarkers regarding the development of postoperative complications were not analyzed. We also could not differentiate between the cases that needed conversion due to sclerosis after multiple previous episodes of mild AC and the impossibility of achieving the critical view of safety due to active inflammation. This might be an explanation for the lower cut-off value found for the NLR when compared to other studies that focus on the gangrenous gallbladder only. However, our study brings valuable information regarding the correlations between the NLR, PLR, and SII and the severity of AC, risk for conversion, and postoperative morbidity.
5. Conclusions
The NLR, PLR, and SII are useful in the preoperative assessment of the AC. The NLR is an early biomarker of inflammation, with higher predictive value when compared to that of PLR, SII, and total leukocytes, and is more versatile than the TG 13/18 scale, being a continuous variable. An NLR value of >4.19 is suggestive of advanced inflammation, while a value of >8.54 is a good predictor for early postoperative sepsis. The PLR and SII correlate significantly with the severity of the inflammation and may be useful in the prediction of the systemic inflammatory response, but they have fair predictive value for advanced AC and risk for conversion in LC.
Author Contributions
Conceptualization, D.S., P.L.S., A.M.D., D.G.B. and C.A.; methodology: P.L.S., B.M.C. and I.M.; software, A.M.D., L.C.T. and G.V.; validation, D.S., C.T, C.S. and D.O.C.; formal analysis, I.M., C.T., M.S.T. and L.C.T.; investigation, D.O.C., C.A., B.M.C. and. D.G.B.; resources, D.S.; data curation, C.A. writing—original draft preparation, D.S., P.L.S., A.M.D., D.G.B., B.M.C., I.M., L.C.T. and C.A.; writing—review and editing, C.S., D.T., G.V., D.O.C. and M.S.T.; visualization, D.S., D.O.C. and C.A.; supervision, D.S.; project administration, P.L.S.; funding acquisition, D.S. Dan Georgian Bratu (D.G.B.), Bogdan Mihai Cristea (B.M.C.) and Catalin Alius (C.A.) contributed equally as the first author at the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sartelli M., Abu-Zidan F.M., Catena F., Griffiths E.A., Di Saverio S., Coimbra R., Ordoñez C.A., Leppaniemi A., Fraga G.P., Coccolini F. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: A prospective multicentre study (WISS Study) World J. Emerg. Surg. 2015;10:61. doi: 10.1186/s13017-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes C., Junior C.S., Di Saverio S., Sartelli M., Kelly M.D., Gomes C.C., Gomes F.C., Corrêa L.D., Alves C.B., Guimarãesm S.F. Acute calculous cholecystitis: Review of current best practices. World J. Gastrointest. Surg. 2017;9:118–126. doi: 10.4240/wjgs.v9.i5.118. Erratum in World J. Gastrointest. Surg. 2017, 9, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tufo A., Pisano M., Ansaloni L., de Reuver P., van Laarhoven K., Davidson B., Gurusamy K.S. Risk Prediction in Acute Calculous Cholecystitis: A Systematic Review and Meta-Analysis of Prognostic Factors and Predictive Models. J. Laparoendosc. Adv. Surg. Technol. A. 2021;31:41–53. doi: 10.1089/lap.2020.0151. [DOI] [PubMed] [Google Scholar]
- 4.Lucocq J., Patil P., Scollay J. Acute cholecystitis: Delayed cholecystectomy has lesser perioperative morbidity compared to emergency cholecystectomy. Surgery. 2022;172:16–22. doi: 10.1016/j.surg.2022.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Bouassida M., Zribi S., Krimi B., Laamiri G., Mroua B., Slama H., Mighri M.M., M’saddak Azzouz M., Hamzaoui L., Touinsi H. C-reactive Protein Is the Best Biomarker to Predict Advanced Acute Cholecystitis and Conversion to Open Surgery. A Prospective Cohort Study of 556 Cases. J. Gastrointest. Surg. 2020;24:2766–2772. doi: 10.1007/s11605-019-04459-8. [DOI] [PubMed] [Google Scholar]
- 6.Turhan V.B., Gök H.F., Ünsal A., Akpınar M., Güler Şimşek G., Buluş H. Pre-operative neutrophil/lymphocyte and platelet/lymphocyte ratios are effective in predicting complicated acute cholecystitis. Ulus. Travma Acil Cerrahi Derg. 2022;28:471–476. doi: 10.14744/tjtes.2021.49956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díez Ares J.Á., Martínez García R., Estellés Vidagany N., Peris Tomás N., Planells Roig M., Valenzuela Gras M., Ripollés González T. Can inflammatory biomarkers help in the diagnosis and prognosis of gangrenous acute cholecystitis? A prospective study. Rev. Esp. Enfermedades Dig. 2021;113:41–44. doi: 10.17235/reed.2020.7282/2020. [DOI] [PubMed] [Google Scholar]
- 8.Yokoe M., Takada T., Strasberg S.M., Solomkin J.S., Mayumi T., Gomi H., Pitt H.A., Garden O.J., Kiriyama S., Hata J., et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos) J. Hepato-Biliary-Pancreat. Sci. 2013;20:35–46. doi: 10.1007/s00534-012-0568-9. [DOI] [PubMed] [Google Scholar]
- 9.Yokoe M., Hata J., Takada T., Strasberg S.M., Asbun H.J., Wakabayashi G., Kozaka K., Endo I., Deziel D.J., Miura F., et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos) J. Hepato-Biliary-Pancreat. Sci. 2018;25:41–54. doi: 10.1002/jhbp.515. [DOI] [PubMed] [Google Scholar]
- 10.Jessica Mok K.W., Goh Y.L., Howell L.E., Date R.S. Is C-reactive protein the single most useful predictor of difficult laparoscopic cholecystectomy or its conversion? A pilot study. J. Minimal Access Surg. 2016;12:26–32. doi: 10.4103/0972-9941.158963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wevers K.P., van Westreenen H.L., Patijn G.A. Laparoscopic cholecystectomy in acute cholecystitis: C-reactive protein level combined with age predicts conversion. Surg. Laparosc. Endosc. Percutan. Technol. 2013;23:163–166. doi: 10.1097/SLE.0b013e31826d7fb0. [DOI] [PubMed] [Google Scholar]
- 12.Díaz-Flores A., Cárdenas-Lailson E., Cuendis-Velázquez A., Rodríguez-Parra A., Trejo-Ávila M.E. C-Reactive Protein as a Predictor of Difficult Laparoscopic Cholecystectomy in Patients with Acute Calculous Cholecystitis: A Multivariate Analysis. J. Laparoendosc. Adv. Surg. Technol. Part A. 2017;27:1263–1268. doi: 10.1089/lap.2017.0139. [DOI] [PubMed] [Google Scholar]
- 13.Vural S., Aydin I., Kesicioglu T. Association of Serum C-Reactive Protein Level and Treatment Duration in Acute Cholecystitis Patients Treated Conservatively. Cureus. 2022;14:e22146. doi: 10.7759/cureus.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çeliktürk E., Salt Ö., Sayhan M.B., Dıbırdık İ. A novel biomarker in acute cholecystitis: YKL-40. Asian J. Surg. 2023;46:1564–1570. doi: 10.1016/j.asjsur.2022.09.073. [DOI] [PubMed] [Google Scholar]
- 15.Park J.W., Kim O.H., Lee S.C., Kim K.H., Hong H.E., Seo H., Choi H.J., Kim S.J. Serum level of visfatin can reflect the severity of inflammation in patients with acute cholecystitis. Ann. Surg. Treat. Res. 2020;99:26–36. doi: 10.4174/astr.2020.99.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaow C.Y.L., Chong R.I.H., Chan K.S., Chia C.T.W., Shelat V.G. Should Procalcitonin Be Included in Acute Cholecystitis Guidelines? A Systematic Review. Medicina. 2023;59:805. doi: 10.3390/medicina59040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D., Yue Z., Weng Y., Zhen G. Diagnostic value of ROC curve evaluation of serum markers in acute cholecystitis with bacterial infection. J. Pak. Med. Assoc. 2022;72:1133–1136. doi: 10.47391/JPMA.3422. [DOI] [PubMed] [Google Scholar]
- 18.Nechita V.I., Hajjar N.A., Drugan C., Cătană C.-S., Moiş E., Nechita M.-A., Graur F. Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study. J. Clin. Med. 2023;12:1641. doi: 10.3390/jcm12041641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modica R., Minotta R., Liccardi A., Cannavale G., Benevento E., Colao A. Evaluation of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Systemic Immune–Inflammation Index (SII) as Potential Biomarkers in Patients with Sporadic Medullary Thyroid Cancer (MTC) J. Pers. Med. 2023;13:953. doi: 10.3390/jpm13060953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia W., Tan Y., Hu S., Li C., Jiang T. Predictive Value of Systemic Immune-Inflammation index and Neutrophil-to-Lymphocyte Ratio in Patients with Severe COVID-19. Clin. Appl. Thromb. Hemost. 2022;28:10760296221111391. doi: 10.1177/10760296221111391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serban D., Papanas N., Dascalu A.M., Kempler P., Raz I., Rizvi A.A., Rizzo M., Tudor C., Silviu Tudosie M., Tanasescu D., et al. Significance of Neutrophil to Lymphocyte Ratio (NLR) and Platelet Lymphocyte Ratio (PLR) in Diabetic Foot Ulcer and Potential New Therapeutic Targets. Int. J. Low Extrem. Wounds. 2021:15347346211057742. doi: 10.1177/15347346211057742. [DOI] [PubMed] [Google Scholar]
- 22.Seyedi S.A., Nabipoorashrafi S.A., Hernandez J., Nguyen A., Lucke-Wold B., Nourigheimasi S., Khanzadeh S. Neutrophil to Lymphocyte Ratio and Spontaneous Bacterial Peritonitis among Cirrhotic Patients: A Systematic Review and Meta-analysis. Can. J. Gastroenterol. Hepatol. 2022;2022:8604060. doi: 10.1155/2022/8604060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajibandeh S., Hajibandeh S., Hobbs N., Mansour M. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: A systematic review and meta-analysis. Am. J. Surg. 2020;219:154–163. doi: 10.1016/j.amjsurg.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Yan Q., Ertao Z., Zhimei Z., Weigang D., Jianjun P., Jianhui C., Chuangqi C. Systemic immune-inflammation index (SII): A More Promising Inflammation-Based Prognostic Marker for Patients with synchronic colorectal peritoneal carcinomatosis. J. Cancer. 2020;11:5264–5272. doi: 10.7150/jca.46446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micić D., Stanković S., Lalić N., Đukić V., Polovina S. Prognostic Value of Preoperative Neutrophil-to-lymphocyte Ratio for Prediction of Severe Cholecystitis. J. Med. Biochem. 2018;37:121–127. doi: 10.1515/jomb-2017-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safari S., Baratloo A., Elfil M., Negida A. Evidence Based Emergency Medicine; Part 5 Receiver Operating Curve and Area under the Curve. Emergency. 2016;4:111–113. [PMC free article] [PubMed] [Google Scholar]
- 27.Er S., Ozden S., Celik C., Yuksel B.C. Can we predict severity of acute cholecystitis at admission? Pak. J. Med. Sci. 2018;34:1293–1296. doi: 10.12669/pjms.345.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz S., Aykota M.R., Ozgen U., Birsen O., Simsek S., Kabay B. Might simple peripheral blood parameters be an early indicator in the prediction of severity and morbidity of cholecystitis? Ann. Surg. Treat. Res. 2023;104:332–338. doi: 10.4174/astr.2023.104.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao C.H., Liu Y.H., Chen W.K., Huang F.W., Hsu T.Y., Cheng H.T., Hsueh P.R., Hsiao C.T., Wu S.Y., Shih H.M. Value of monocyte distribution width for predicting severe cholecystitis: A retrospective cohort study. Clin. Chem. Lab. Med. 2023;61:1850–1857. doi: 10.1515/cclm-2023-0195. [DOI] [PubMed] [Google Scholar]
- 30.Paul S., Khataniar H., Ck A., Rao H.K. Preoperative scoring system validation and analysis of associated risk factors in predicting difficult laparoscopic cholecystectomy in patients with acute calculous cholecystitis: A prospective observational study. Turk. J. Surg. 2022;38:375–381. doi: 10.47717/turkjsurg.2022.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Önder A., Kapan M., Ülger B.V., Oğuz A., Türkoğlu A., Uslukaya Ö. Gangrenous cholecystitis: Mortality and risk factors. Int. Surg. 2015;100:254–260. doi: 10.9738/INTSURG-D-13-00222.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash G., Hasan M. The Accuracy of Neutrophil-to-Lymphocyte Ratio and Abdominal Computed Tomography to Predict the Severity of Acute Cholecystitis. Cureus. 2022;14:e32243. doi: 10.7759/cureus.32243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gojayev A., Karakaya E., Erkent M., Yücebaş S.C., Aydin H.O., Kavasoğlu L., Aydoğan C., Yildirim S. A novel approach to distinguish complicated and non-complicated acute cholecystitis: Decision tree method. Medicine. 2023;102:e33749. doi: 10.1097/MD.0000000000033749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ünal Y., Tuncal S., Küçük B., Barlas A.M., Altıner S., Balık R., Aydın S.M., Senlikci A., Pekcici M.R. An effective and reliable marker in gradıng the severity of acute cholecystitis: Increased immature granulocyte percentage. Akut kolesistitin şiddetini derecelendirmede etkili ve güvenilir bir belirteç: Artmış immatür granülosit yüzdesi. Ulus. Travma Acil Cerrahi Derg. 2022;28:1716–1722. doi: 10.14744/tjtes.2021.86322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato N., Kinoshita A., Imai N., Akasu T., Yokota T., Iwaku A., Koike K., Saruta M. Inflammation-based prognostic scores predict disease severity in patients with acute cholecystitis. Eur. J. Gastroenterol. Hepatol. 2018;30:484–489. doi: 10.1097/MEG.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 36.Cakcak İ.E., Kula O. Predictive evaluation of SIRI, SII, PNI, and GPS in cholecystostomy application in patients with acute cholecystitis. Ulus. Travma Acil Cerrahi Derg. 2022;28:940–946. doi: 10.14744/tjtes.2022.90249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beliaev A.M., Angelo N., Booth M., Bergin C. Evaluation of neutrophil-to-lymphocyte ratio as a potential biomarker for acute cholecystitis. J. Surg. Res. 2017;209:93–101. doi: 10.1016/j.jss.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 38.Kartal M., Kalaycı T. Can neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, prognostic nutrition index, and albumin be used to predict cholecystectomy morbidity in super-elderly patients? Ulus. Travma Acil Cerrahi Derg. 2023;29:890–896. doi: 10.14744/tjtes.2023.31462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manuel-Vázquez A., Latorre-Fragua R., Alcázar C., Requena P.M., de la Plaza R., Blanco Fernández G., Serradilla-Martín M., Ramia J.M., SCORELAP Group Reaching a consensus on the definition of “difficult” cholecystectomy among Spanish experts. A Delphi project. A qualitative study. Int. J. Surg. 2022;102:106649. doi: 10.1016/j.ijsu.2022.106649. [DOI] [PubMed] [Google Scholar]
- 40.Dumitrescu D., Savlovschi C., Borcan R., Pantu H., Serban D., Gradinaru S., Smarandache G., Trotea T., Branescu C., Musat L., et al. Clinical case—voluminous diaphragmatic hernia—surgically acute abdomen: Diagnostic and therapeutical challenges. Chirurgia. 2011;106:657–660. [PubMed] [Google Scholar]
- 41.Savlovschi C., Serban D., Trotea T., Borcan R., Dumitrescu D. Post-surgery morbidity and mortality in colorectal cancer in elderly subjects. Chirurgia. 2013;108:177–179. [PubMed] [Google Scholar]
- 42.Serban D., Badiu D.C., Davitoiu D., Tanasescu C., Tudosie M.S., Sabau A.D., Dascalu A.M., Tudor C., Balasescu S.A., Socea B., et al. Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (Review) Exp. Ther. Med. 2022;23:187. doi: 10.3892/etm.2021.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pisano M., Allievi N., Gurusamy K., Borzellino G., Cimbanassi S., Boerna D., Coccolini F., Tufo A., Di Martino M., Leung J., et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J. Emerg. Surg. 2020;15:61. doi: 10.1186/s13017-020-00336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karamouzos V., Paraskevas T., Mulita F., Karteri S., Oikonomou E., Ntoulias N., Pantzaris N.D., Bourganou V., Velissaris D. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Percentage Ratio as Predictors of In-hospital Mortality in Sepsis. An Observational Cohort Study. Mater. Socio-Medica. 2022;34:33–36. doi: 10.5455/msm.2022.33.33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boicean A., Neamtu B., Birsan S., Batar F., Tanasescu C., Dura H., Roman M.D., Hașegan A., Bratu D., Mihetiu A., et al. Fecal Microbiota Transplantation in Patients Co-Infected with SARS-CoV2 and Clostridioides difficile. Biomedicines. 2023;11:7. doi: 10.3390/biomedicines11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y., Huang X., Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: Interaction effect with disease severity-a retrospective study. BMJ Open. 2019;9:e022896. doi: 10.1136/bmjopen-2018-022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansour N.O., Boraii S., Elnaem M.H., Elrggal M.E., Omar T., Abdelraouf A., Abdelaziz D.H. Evaluation of preoperative duloxetine use for postoperative analgesia following laparoscopic cholecystectomy: A randomized controlled trial. Front. Pharmacol. 2022;13:944392. doi: 10.3389/fphar.2022.944392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chicea R., Bratu D., Chicea A.L., Mihetiu A., Preluca V., Tantar C., Sava M. A comparative Histologic and Immunohistochemistry Evaluation Between Normal Aponeurotic Tissue, Fibrotic Aponeurotic Scars and Polypropylene Embedded Aponeurotic Scars. Mater. Plast. 2017;54:510–512. doi: 10.37358/MP.17.3.4882. [DOI] [Google Scholar]
- 49.Yu T., Zhao L., Zhao H., Fu H., Li J., Yu A. The enhanced recovery after surgery (ERAS) protocol in elderly patients with acute cholecystitis: A retrospective study. Medicine. 2023;102:e32942. doi: 10.1097/MD.0000000000032942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulita F., Karpetas G., Liolis E., Vailas M., Tchabashvili L., Maroulis I. Comparison of analgesic efficacy of acetaminophen monotherapy versus acetaminophen combinations with either pethidine or parecoxib in patients undergoing laparoscopic cholecystectomy: A randomized prospective study. Med. Glas. 2021;18:27–32. doi: 10.17392/1245-21. [DOI] [PubMed] [Google Scholar]
- 51.Kouroukli I., Zompolas V., Tsekoura V., Papazoglou I., Louizos A., Panaretou V. Comparison between lornoxicam quick-release and parecoxib for post-operative analgesia after laparoscopic cholecystectomy: A prospective randomized, placebo-controlled trial. J. Anaesthesiol. Clin. Pharmacol. 2013;29:485–490. doi: 10.4103/0970-9185.119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.