ABSTRACT
Physiological changes during pregnancy may alter the pharmacokinetics (PK) of antituberculosis drugs. The International Maternal Pediatric Adolescent AIDS Clinical Trials Network P1026s was a multicenter, phase IV, observational, prospective PK and safety study of antiretroviral and antituberculosis drugs administered as part of clinical care in pregnant persons living with and without HIV. We assessed the effects of pregnancy on rifampin, isoniazid, ethambutol, and pyrazinamide PK in pregnant and postpartum (PP) persons without HIV treated for drug-susceptible tuberculosis disease. Daily antituberculosis treatment was prescribed following World Health Organization-recommended weight-band dosing guidelines. Steady-state 12-hour PK profiles of rifampin, isoniazid, ethambutol, and pyrazinamide were performed during second trimester (2T), third trimester (3T), and 2–8 of weeks PP. PK parameters were characterized using noncompartmental analysis, and comparisons were made using geometric mean ratios (GMRs) with 90% confidence intervals (CI). Twenty-seven participants were included: 11 African, 9 Asian, 3 Hispanic, and 4 mixed descent. PK data were available for 17, 21, and 14 participants in 2T, 3T, and PP, respectively. Rifampin and pyrazinamide AUC0–24 and C max in pregnancy were comparable to PP with the GMR between 0.80 and 1.25. Compared to PP, isoniazid AUC0–24 was 25% lower and C max was 23% lower in 3T. Ethambutol AUC0–24 was 39% lower in 3T but limited by a low PP sample size. In summary, isoniazid and ethambutol concentrations were lower during pregnancy compared to PP concentrations, while rifampin and pyrazinamide concentrations were similar. However, the median AUC0–24 for rifampin, isoniazid, and pyrazinamide met the therapeutic targets. The clinical impact of lower isoniazid and ethambutol exposure during pregnancy needs to be determined.
KEYWORDS: rifampin, isoniazid, ethambutol, pyrazinamide, pregnancy, drug-susceptible tuberculosis, pharmacokinetics
INTRODUCTION
Tuberculosis (TB) disease, caused by Mycobacterium tuberculosis, remains a global health emergency and is one of the world’s most deadly infections, despite being a preventable and curable disease. In 2021, an estimated 10.6 million people developed TB disease, of which 3.4 million were women (1). Many of these women are of reproductive age and maternal TB disease is associated with a threefold increased maternal mortality rate, particularly in the setting of concomitant HIV (2 – 8).
Pregnant and postpartum (PP) persons are at risk for developing TB and for progression from latent to active TB disease (9, 10). TB disease during pregnancy increases the risk of preterm birth, intrauterine fetal growth restriction, low birth weight, and small for gestational age. Fortunately, treatment of TB disease during pregnancy decreases these adverse pregnancy outcomes (7, 11).
The World Health Organization (WHO) recommends rifampin (RIF), isoniazid (INH), ethambutol (EMB), and pyrazinamide (PZA) as first-line antituberculosis therapy for drug-susceptible TB (DS-TB) disease (12). RIF, INH, EMB, and PZA are used in pregnant persons with DS-TB disease despite a lack of pregnancy-specific pharmacokinetic (PK) and safety data for these drugs. Data from a 2022 systematic review and meta-analysis of 2,563 pregnant and postpartum persons showed that preventive therapy with variable combinations of antituberculosis drugs did not increase the risk of serious maternal adverse events but did not include pregnant persons treated for DS-TB disease (13).
Pregnancy induces important changes in plasma volume, cardiac output, and renal filtration as well as gastrointestinal function and hepatic drug-metabolizing enzymes that are most prominent in the third trimester (14). These unique physiological changes may result in clinically significant changes in drug PK and pharmacodynamics across the trimesters of pregnancy and postpartum. Currently, PK data of first-line TB drugs when used for treatment of DS-TB disease during pregnancy are limited, and trimester-specific PK differences have not been described. Optimal exposure to TB treatment is essential to achieve a cure as achieving minimum plasma concentration targets is predictive of favorable treatment outcomes (15). To remedy the uncertainty regarding TB drug exposure in pregnancy using standard WHO weight-banded dosing, we explored the PK of RIF, INH, EMB, and PZA in pregnant and postpartum persons without HIV treated for DS-TB disease. These PK parameters were compared to therapeutic targets described in the literature for non-pregnant adult DS-TB patients. The minimum target AUC0-24 for RIF, INH, and PZA selected were 35.4, 10.52, and 363 µg·h/mL, respectively (15 – 17) and the minimum target C max concentrations selected for RIF, INH, EMB, and PZA were 8, 3, 2, and 20 µg/mL, respectively (18 – 20).
RESULTS
Participant characteristics
Between November 2013 and October 2019, 27 pregnant women without HIV taking first-line TB drugs were enrolled in the study and had evaluable PK data. Maternal and infant clinical characteristics are summarized in Table 1. Eleven (41%) women were Black African, 9 (33%) Asian, 3 Hispanic (11%), and 4 (15%) of mixed descent. The median age at delivery was 26.1 (range 16.2–39.4) years and the median weight in third trimester (3T) was 57.0 (range 46.1–92.3) kg. The median gestational age at birth was 38.6 (range 28.1–41.6) weeks of pregnancy and the median birth weight was 3,100 (range 1,195–3,960) g. Thirteen (54%) of the women were classified as slow INH metabolizers, 7 (29%) as intermediate, and 4 (17%) as fast. Eighteen (67%) women were taking fixed-dose combination (FDC) tablets and 9 (33%) used separate drug tablets throughout the study.
TABLE 1.
Maternal and infant clinical characteristics
| n (%) or median (range) | |
|---|---|
| Maternal demographics (n = 27) | |
| Age at delivery (years) | 26.1 (16.2–39.4) |
| Weight at 3T (kg) (n = 21) | 57.0 (46.1–92.3) |
| PK sampling age (gestational or postpartum weeks) | |
| 2T (n = 17) | 24.9 (20.0–28.9) |
| 3T (n = 21) | 32.9 (30.1–36.9) |
| PP (n = 14) | 4.9 (2.3–7.1) |
| Race/ethnicity | |
| Black African | 11 (41) |
| Asian | 9 (33) |
| Mixed descent | 4 (15) |
| Hispanic | 3 (11) |
| Country | |
| South Africa | 10 (37) |
| Thailand | 9 (33) |
| Tanzania | 3 (11) |
| Botswana | 2 (7) |
| Brazil | 2 (7) |
| USA | 1 (4) |
| NAT2 metabolizer type (n = 24) | |
| Slow | 13 (54) |
| Intermediate | 7 (29) |
| Fast | 4 (17) |
| Infant demographics (n = 27) | |
| Gestational age (weeks) | 38.6 (28.1–41.6) |
| Birth weight (g) | 3,100 (1,195–3,960) |
Paired pregnancy and PP data were available for 6 of 16 women who had second-trimester (2T) visits and 12 of 22 women who had third-trimester (3T) visits. Twenty-one women were sampled on more than one occasion (2T, 3T, or PP) and 11 women had both 2T and 3T data. Participants receiving EMB and PZA in the PP period were limited (n = 4 and n = 2, respectively) due to the short 2-month duration of intensive phase TB treatment. The median drug concentrations versus time curves per sampling time point and distributions of AUC0-24 are shown in Fig. 1 to 3 for each drug and PK parameters presented in Tables 2 to 5. The tables present only the results of the univariate mixed-effects model comparisons between trimesters and PP for consistency, as the smaller sample size within-participant comparisons that could be done (Tables S1 to S3) agreed with the results from the larger sample size univariate mixed-effects model comparisons. Dosing ranges for all the drugs were comparable between sample time points, as well as between participants who did or did not meet the minimum C max target.
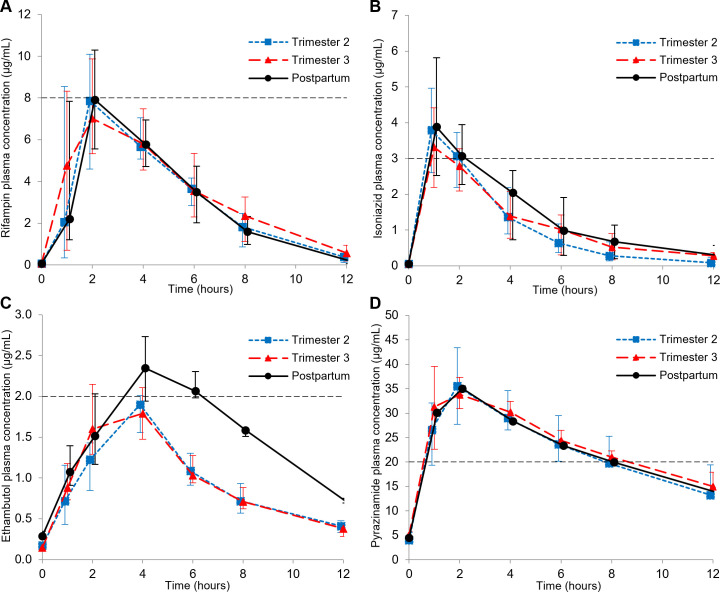
Fig 1.
Median plasma concentration-time profiles of (A) rifampin, (B) isoniazid, (C) ethambutol, and (D) pyrazinamide during the second and third trimesters and postpartum (error bars indicate the IQR). The minimum target C max of each drug is represented by the horizontal dashed lines.
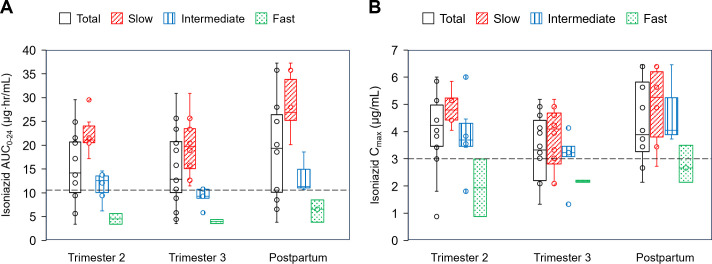
Fig 3.
Box and whisker plots showing plasma isoniazid (A) AUC0-24 and (B) C max for the total group and per metabolizer type during the second and third trimesters and postpartum (median, IQR, and range). The dots represent additional individual values. The minimum target AUC0-24 and C max are represented by the dashed lines.
TABLE 2.
Maternal rifampin dose, pharmacokinetic parameters, and target attainment a
| Median value for group (IQR) | 2T/PP comparison b | 3T/PP comparison b | |||||
|---|---|---|---|---|---|---|---|
| Second trimester n = 16 |
Third trimester n = 20 |
Postpartum n = 13 |
GMR (90% CI) | P-value | GMR (90% CI) | P-value | |
| Dose (mg/kg) | 9.19 (8.25–9.81) | 8.59 (8.02–10.14) | 9.30 (8.84–10.00) | – | – | – | – |
| AUC0–24 (µg·h/mL) | 38.89 (31.88–53.19) | 44.53 (31.01–56.96) | 38.38 (30.22–57.89) | 0.91 (0.74–1.11) | 0.420 | 1.06 (0.89–1.26) | 0.586 |
| CL/F (L/h) | 14.44 (10.66–17.11) | 13.26 (9.59–16.39) | 11.72 (9.82–19.35) | 1.18 (0.98–1.42) | 0.150 | 1.00 (0.85–1.18) | 0.983 |
| T max (h) | 2 (1–6) | 2 (1–6) | 2 (1–4) | – | – | – | – |
| T ½ (h) | 1.69 (1.51–2.14) | 2.18 (1.83–2.73) | 1.73 (1.62–2.07) | 0.99 (0.83–1.17) | 0.888 | 1.28 (1.09–1.50) | 0.014 |
| C max (µg/mL) | 8.66 (5.44–10.23) | 7.69 (5.99–10.32) | 7.92 (5.77–10.30) | 0.90 (0.74–1.09) | 0.348 | 0.92 (0.78–1.09) | 0.409 |
| C 0 (µg/mL) | BQL (BQL–BQL) | BQL (BQL–BQL) | BQL (BQL–BQL) | – | – | – | – |
| C 12 (µg/mL) | 0.35 (0.12–0.63) | 0.56 (0.18–0.94) | 0.27 (BQL −0.51) | 1.22 (0.69–2.14) | 0.556 | 2.11 (1.28–3.48) | 0.018 |
| n (%) C max <8 µg/mL target | 8 (50) | 12 (60) | 7 (54) | – | – | – | – |
| n (%) AUC0–24 <35.4 µg·h/mL target | 8 (50) | 8 (40) | 7 (54) | – | – | – | – |
Summary statistics for second/third trimester and postpartum presented as median (IQR), except T max, which is presented as median (range). AUC0-24: area under the concentration versus time curve over the dosing interval; CL/F: apparent oral clearance; T max: time to maximum plasma concentration; t 1/2: half-life; C max: maximum plasma concentration; C 0: pre-dose concentration; C 12: 12 hours post-dose concentration; BQL: below quantification limit.
Comparisons between pregnancy and postpartum presented as univariate mixed-effect model GMRs with a 90% confidence interval and P-value. Values in italic type are significant P-values (<0.1).
TABLE 3.
Maternal isoniazid dose, pharmacokinetic parameters, and target attainment a
| Median value for group (IQR) | 2T/PP comparison b | 3T/PP comparison b | |||||
|---|---|---|---|---|---|---|---|
| Second trimester n = 14 |
Third trimester n = 19 |
Postpartum n = 12 |
GMR (90% CI) | P-value | GMR (90% CI) | P-value | |
| Dose (mg/kg) | 4.82 (4.16–5.45) | 4.88 (4.07–5.45) | 4.70 (4.44–5.36) | – | – | – | – |
| AUC0–24 (µg·h/mL) | 14.18 (9.41–20.70) | 12.87 (9.29–21.05) | 19.36 (9.58–26.90) | 0.82 (0.69–0.97) | 0.055 | 0.75 (0.65–0.86) | 0.002 |
| CL/F (L/h) | 21.81 (12.08–31.90) | 19.03 (12.63–27.84) | 15.52 (10.00–31.31) | 1.25 (1.08–1.46) | 0.019 | 1.39 (1.22–1.57) | <0.001 |
| T max (h) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | – | – | – | – |
| T ½ (h) | 2.19 (1.49–2.75) | 2.93 (1.61–3.31) | 2.92 (1.51–3.34) | 0.83 (0.69–0.98) | 0.074 | 0.96 (0.83–1.12) | 0.668 |
| C max (µg/mL) | 4.23 (3.42–5.14) | 3.32 (2.18–4.60) | 3.89 (3.09–6.01) | 0.91 (0.72–1.16) | 0.519 | 0.77 (0.63–0.95) | 0.044 |
| C 0 (µg/mL) | BQL (BQL–BQL) | BQL (BQL–BQL) | BQL (BQL–BQL) | – | – | – | – |
| C 12 (µg/mL) | 0.09 (BQL–0.26) | 0.28 (BQL −0.38) | 0.30 (BQL −0.62) | – | – | – | – |
| n (%) C max <3 µg/mL target | 3 (21) | 6 (32) | 3 (25) | – | – | – | – |
| n (%) AUC0–24 <10.52 µg·h/mL target | 4 (29) | 5 (26) | 3 (25) | – | – | – | – |
Summary statistics for second/third trimester and postpartum presented as median (IQR), except T max, which is presented as median (range). AUC0-24: area under the concentration versus time curve over the dosing interval; CL/F: apparent oral clearance; T max: time to maximum plasma concentration; t 1/2: half-life; C max: maximum plasma concentration; C 0: pre-dose concentration; C 12: 12 hours post-dose concentration; BQL: below quantification limit.
Comparisons between pregnancy and postpartum presented as univariate mixed-effect model GMR with a 90% confidence interval and P-value. Values in italic type are significant P-values (<0.1).
TABLE 4.
Maternal ethambutol dose, pharmacokinetic parameters, and target attainment a
| Median value for group (IQR) | 2T/PP comparison b | 3T/PP comparison b | |||||
|---|---|---|---|---|---|---|---|
| Second trimester n = 8 |
Third trimester n = 11 |
Postpartum n = 4 |
GMR (90% CI) | P-value | GMR (90% CI) | P-value | |
| Dose (mg/kg) | 16.85 (15.40–17.67) | 16.37 (14.92–18.86) | 16.49 (15.10–17.03) | – | – | – | – |
| AUC0–24 (µg·h/mL) | 13.77 (12.86–17.08) | 14.53 (12.57–16.87) | 23.55 (22.35–24.68) | 0.59 (0.41–0.85) | 0.032 | 0.61 (0.44–0.86) | 0.032 |
| CL/F (L/h) | 72.72 (61.61–85.56) | 73.75 (65.65–75.75) | 45.43 (42.59–49.15) | 1.57 (1.36–1.80) | 0.001 | 1.51 (1.32–1.74) | 0.002 |
| T max (h) | 4 (2–4) | 4 (2–4) | 4 (2–6) | – | – | – | – |
| T ½ (h) | 3.90 (3.51–4.70) | 4.04 (3.16–4.31) | 4.08 (3.76–4.67) | 0.96 (0.74–1.24) | 0.752 | 0.93 (0.73–1.19) | 0.576 |
| C max (µg/mL) | 1.91 (1.60–2.64) | 2.10 (1.67–2.93) | 2.77 (2.65–2.87) | 0.75 (0.58–0.98) | 0.083 | 0.82 (0.63–1.06) | 0.180 |
| C 0 (µg/mL) | 0.17 (0.13–0.20) | 0.15 (0.13–0.17) | 0.29 (0.26–0.38) | 0.50 (0.37–0.69) | 0.007 | 0.51 (0.38–0.69) | 0.007 |
| C 12 (µg/mL) | 0.41 (0.33–0.48) | 0.38 (0.27–0.46) | 0.71 (0.69–0.77) | 0.64 (0.55–0.75) | 0.002 | 0.60 (0.52–0.70) | 0.001 |
| n (%) C max <2 µg/mL target | 4 (50) | 3 (27) | 0 (0) | – | – | – | – |
Summary statistics for second/third trimester and postpartum presented as median (IQR), except T max, which is presented as median (range). AUC0-24: area under the concentration versus time curve over the dosing interval; CL/F: apparent oral clearance; T max: time to maximum plasma concentration; t 1/2: half-life; C max: maximum plasma concentration; C 0: pre-dose concentration; C 12: 12 hours post-dose concentration.
Comparisons between pregnancy and postpartum presented as univariate mixed-effect model GMRs with a 90% confidence interval and P-value. Values in italic type are significant P-values (<0.1).
TABLE 5.
Maternal pyrazinamide dose, pharmacokinetic parameters, and target attainment a
| Median value for group (IQR) | 2T/PP comparison b | 3T/PP comparison b | |||||
|---|---|---|---|---|---|---|---|
| Second trimester n = 6 |
Third trimester n = 9 |
Postpartum n = 2 |
GMR (90% CI) | P-value | GMR (90% CI) | P-value | |
| Dose (mg/kg) | 22.75 (21.58–26.18) | 25.00 (21.70–26.03) | 22.29 (20.83–23.74) | – | – | – | – |
| AUC0–24 (µg·h/mL) | 373.73 (340.25–560.14) | 404.69 (389.42–432.82) | 388.30 (370.85–405.74) | 1.03 (0.51–2.07) | 0.928 | 1.12 (0.61–2.04) | 0.650 |
| CL/F (L/h) | 3.55 (3.25–3.98) | 3.55 (2.77–3.79) | 4.01 (3.70–4.31) | 0.90 (0.63–1.27) | 0.453 | 0.84 (0.58–1.20) | 0.282 |
| T max (h) | 2 (1–4) | 2 (1–4) | 2 (2) | – | – | – | – |
| T ½ (h) | 8.40 (7.08–9.34) | 8.32 (7.60–9.49) | 7.85 (7.77–7.94) | 1.07 (0.76–1.50) | 0.630 | 1.13 (0.78–1.65) | 0.440 |
| C max (µg/mL) | 35.55 (28.80–46.70) | 33.80 (32.10–39.60) | 35.05 (33.20–36.90) | 1.04 (0.64–1.70) | 0.831 | 1.10 (0.62–1.95) | 0.669 |
| C 0 (µg/mL) | 4.01 (3.57–6.06) | 5.08 (3.98–5.72) | 4.53 (4.51–4.55) | 1.03 (0.35–3.04) | 0.935 | 1.10 (0.40–3.06) | 0.812 |
| C 12 (µg/mL) | 13.25 (12.40–21.40) | 15.00 (14.00–17.90) | 13.90 (13.20–14.60) | 1.02 (0.45–2.34) | 0.941 | 1.14 (0.55–2.37) | 0.647 |
| n (%) C max <20 µg/mL target | 0 (0) | 0 (0) | 0 (0) | – | – | – | – |
| n (%) AUC0–24 <363 µg·h/mL target | 2 (33) | 2 (22) | 0 (0) | – | – | – | – |
Summary statistics for second/third trimester and postpartum presented as median (IQR), except T max, which is presented as median (range). AUC0-24: area under the concentration versus time curve over the dosing interval; CL/F: apparent oral clearance; T max: time to maximum plasma concentration; t 1/2: half-life; C max: maximum plasma concentration; C 0: pre dose concentration; C 12: 12h post-dose concentration.
Comparisons between pregnancy and postpartum presented as univariate mixed-effect model GMRs with a 90% confidence interval and P-value.
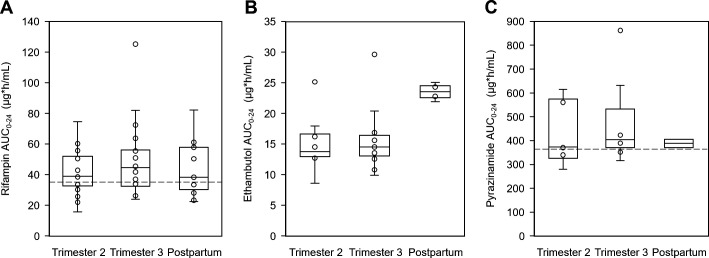
Fig 2.
Box and whisker plots showing plasma AUC0-24 of (A) rifampin, (B) ethambutol, and (C) pyrazinamide during the second and third trimesters and postpartum (median, IQR, and range). The dots represent additional individual values. The minimum target AUC0-24 of rifampin and pyrazinamide is represented by the dashed lines.
Rifampin pharmacokinetics
A total of 16, 20, and 13 women completed 2T, 3T, and PP RIF PK sampling, respectively, and parameters are presented in Table 2. Median RIF AUC0-24 was above target in 2T, 3T, and PP, and median RIF C max was above target in 2T and approximating target in 3T and PP. When comparing 2T and 3T to PP, AUC0-24 and C max were similar as the 90% CI for the geometric mean ratio (GMR) approximated the 0.80–1.25 range for both trimesters in both the paired comparisons (Table S1) and the univariate mixed-effect models. However, compared to PP, 3T RIF C 12 and T ½ were significantly higher by 111% [GMR 2.11 (90% CI 1.28–3.48); P = 0.018] and 28% [GMR 1.28 (90% CI 1.09–1.50); P = 0.014], respectively. During 3T, 60% of participants did not reach target C max, and 40% did not reach target AUC0-24.
Isoniazid pharmacokinetics
A total of 14, 19, and 12 women completed 2T, 3T, and PP INH PK sampling, respectively, and parameters are presented in Table 3. Median INH AUC0-24 and C max were above target in 2T, 3T, and PP. Compared to PP, 2T INH CL/F was higher by 25% [GMR 1.25 (90% CI 1.08–1.46); P = 0.019]. Compared to PP, 3T INH AUC0-24 and C max were lower by 25% [GMR 0.75 (90% CI 0.65–0.86); P = 0.002] and 23% [GMR 0.77 (90% CI 0.63–0.95); P = 0.044], respectively, with correspondingly higher INH CL/F by 39% [GMR 1.39 (90% CI 1.22–1.57), P < 0.001]. Within-participant comparison (Table S2) showed similar 27% lower INH AUC0-24 [GMR 0.73 (90% CI 0.66–0.81); P = 0.002, n = 10] and 25% lower INH C max [GMR 0.75 (90% CI 0.60–0.94); P = 0.002, n = 9] in 3T, with corresponding 41% higher CL/F compared to PP. In 3T, 32% and 26% of participants did not reach target C max and AUC0-24, respectively. INH AUC0-24 depended on the N-acetyltransferase 2 (NAT2) metabolizer type (Fig. 3) with significant differences between the metabolizer groups in each sampling time point (P-value for Kruskal-Wallis = 0.004, 0.001, and 0.009 for 2T, 3T, and PP, respectively). C max was similar between the metabolizer groups (Fig. 3, P-value for Kruskal-Wallis = 0.06, 0.24, and 0.10 for 2T, 3T, and PP, respectively). Slow metabolizers had the highest median AUC0-24 and C max at all timepoints. The four fast metabolizers did not reach the AUC0-24 target at any timepoint and only one reached the C max target concentration at postpartum.
Ethambutol pharmacokinetics
A total of 8, 11, and 4 women completed 2T, 3T, and PP EMB PK sampling, respectively, and parameters are presented in Table 4. While median C max was marginally below and above target in 2T and 3T, 50% and 27% of participants did not reach target C max, respectively.
A within-participant comparison could be done in three participants (Table S3), and results corresponded with the univariate mixed-effect model. Compared to postpartum, EMB clearance was higher by more than 50% in pregnancy, and 3T C 0 and C 12 were lower by 49% and 40%, respectively. Both 2T and 3T EMB AUC0-24 were lower by 41% [GMR 0.59 (90%CI 0.41–0.85); P = 0.032] and 39% [GMR 0.61 (90% CI 0.44–0.86); P = 0.032], respectively. Correspondingly, EMB C max was 25% lower in 2T [GMR 0.75 (90%CI 0.58–0.98); P = 0.083] and showed 18% lower trend in 3T.
Pyrazinamide pharmacokinetics
Six, nine, and two women completed 2T, 3T, and PP PZA PK sampling, respectively, and parameters are presented in Table 5. Univariate mixed-effect models did not identify any differences in PZA PK parameters between pregnancy and postpartum timepoints. None of the participants had a PZA C max below the minimum treatment target in any of the sampling timepoints and only 22% did not reach target AUC in 3T.
Delivery samples
One pair of maternal and cord blood samples were collected at delivery and had a RIF cord/maternal concentration ratio of 0.59, but INH was below quantification limit (BQL). No statistical analysis could be performed.
Maternal and infant safety outcomes
Maternal and infant safety events are summarized in Table 6. Twelve (44%) women experienced one or more adverse events (AEs) grade 3 or greater after study entry, including five (19%) cases of drug-induced liver injury (DILI); four were slow NAT2 metabolizers and one was intermediate. Two (7%) grade 2 TB drug-related skin eruptions occurred before study entry. All DS-TB treatment-related events resolved after permanent treatment discontinuation (n = 1) or treatment completion (n = 2) or had successful treatment rechallenge after temporary interruption (n = 2). TB drug concentrations were not statistically different between women who developed DILI and those who did not, although INH AUC0-24 were higher in women who developed DILI [median INH AUC0-24 (IQR) 21.1 (13.8–23.8) n = 5 versus 11.5 (8.8–19.0) n = 15, P-value 0.06]. Seven mothers (26%) had grade 2 anemia (Hb 8.5 to <9.5 g/dL) of which three (11%) had grade 3 anemia (Hb 6.5 to <8.5 g/dL) at entry. TB treatment outcomes in the mothers were not collected. Adverse pregnancy outcomes included preterm delivery in seven (26%) women, two of whom were severe (<32 weeks gestation). All preterm deliveries were classified as not treatment-related. Other adverse pregnancy outcomes included one case of preeclampsia with placental ischemia, one severe perineal tear hemorrhage requiring surgical treatment, and one pregnancy-induced hypertension.
TABLE 6.
Maternal and infant safety outcomes and TB treatment-related adverse events d
| Maternal safety events (n = 27) | n (%) | Infant safety events (n = 27) | n (%) |
|---|---|---|---|
| Mothers with one or more ≥ grade 3 adverse event | 12 (44) | Infants with one or more ≥ grade 3 adverse event | 8 (30) |
| TB treatment-related events ≥ grade 3 | TB treatment-related events ≥ grade 3 | 2 (7) | |
| Drug-induced liver injury | 5 (19) | Neutropenia at birth | 1 (4) |
| Slow metabolizer | 4 (15) | Hyperbilirubinemia in infant on INH prophylaxis | 1 (4) |
| Intermediate metabolizer | 1 (4) | ||
| Neutropenia a | 1 (4) | ||
| Other events ≥ grade 3 | Other events ≥ grade 3 | ||
| Anemia | 3 (11) | Severe birth asphyxia | 1 (4) |
| Respiratory distress of prematurity | 2 (7) | ||
| Neonatal sepsis | 1 (4) | ||
| Congenital abnormalities | 3 (11) | ||
| Jeune syndrome with intestinal malrotation | 1 (4) | ||
| Umbilical hernia c | 1 (4) | ||
| Inguinal hernia c | 1 (4) | ||
| Infant death (<3 months after birth) | 2 (7) | ||
| Acute watery diarrhea | 1 (4) | ||
| Probable sudden infant death syndrome | 1 (4) | ||
| TB treatment-related events < grade 3 | TB treatment-related events < grade 3 | ||
| Drug-induced skin rash b | 2 (7) | Elevated liver enzymes at birth | 1 (4) |
| Adverse pregnancy outcomes | Adverse birth weight outcomes | ||
| Preterm delivery (<37 weeks) | 7 (26) | Low birth weight (<2,500g) | 10 (37) |
| Moderate to late preterm (32–36+6 weeks) | 5 (19) | Very low birth weight (<1,500g) | 2 (7) |
| Severe preterm (<32 weeks) | 2 (7) | SGA | 3 (11) |
| Preeclampsia with placental ischemia | 1 (4) | IUGR | 2 (7) |
| Perineal tear hemorrhage | 1 (4) | ||
| Pregnancy-induced hypertension | 1 (4) |
Reported as possibly treatment-related, but more likely TB disease-related.
Both grade 2 and resolved before entry on TB drug discontinuation.
Grade 1 but grouped under congenital abnormalities.
SGA: small for gestational age; IUGR: intrauterine growth retardation.
Eight (30%) infants experienced adverse events grade ≥3 of which two (7%) were classified as DS-TB treatment-related, namely neutropenia accompanied by grade 1 raised liver enzymes at birth due to intrauterine TB drug exposure and hyperbilirubinemia at 7 weeks of age in 1 out of 10 infants that received INH prophylaxis. No cases of congenital TB were reported. Three (11%) congenital abnormalities were reported, including Jeune syndrome, an umbilical hernia, and an inguinal hernia, all classified as not treatment-related. Two infant (<3 months) deaths were reported, both classified as not treatment-related; one case of acute watery diarrhea with dehydration at 6 weeks of age and one case of probable sudden infant death syndrome at 11 weeks of age in an ex-premature infant, born at 28-week gestation, who was well at the time of hospital discharge.
Ten (37%) infants had low birth weight (birth weight <2,500 g) of which two (7%) had a very low birth weight (<1,500 g). Three (11%) infants were small for gestational age (birth weight < tenth percentile) and two (7%) infants were diagnosed with intrauterine growth retardation (birth weight < third percentile), all classified as not treatment-related.
DISCUSSION
This is the first opportunistic study that made use of intensive PK sampling methods in both second and third trimesters of pregnancy and postpartum in participants with DS-TB disease without HIV using standard WHO-recommended weight-banded dosing of available FDC tablets. We described the PK parameters of RIF, INH, EMB, and PZA. We found that the AUC0-24 and C max of RIF and PZA were similar during pregnancy and postpartum, but that the AUC0-24 of INH and EMB were lower during pregnancy compared to postpartum. We observed wide between-patient PK variability for RIF and INH, consistent with findings from previous studies in non-pregnant participants with DS-TB disease (21 – 27).
Pregnancy is associated with an increase in volume of distribution due to water retention, changes in percentage fat distribution and quantity, and decreased plasma protein concentrations, which may alter drug PK. RIF is a lipophilic drug and pregnancy-associated increases in fat distribution may lead to lower plasma concentrations. Decreased plasma protein concentrations can potentially decrease the pharmacologically active free fraction of RIF at equilibrium (28). These physiologic changes may result in increased maintenance dosage requirements to prevent sub-therapeutic drug exposure of RIF during pregnancy. Additionally, RIF causes the pregnane X receptor (PXR) to activate its own metabolism, a process known as clearance autoinduction, which can potentially reduce RIF exposure at a steady state (29).
However, we found that RIF AUC0-24 and C max are similar in 2T and 3T compared to PP with similar or higher exposures compared to previously published non-pregnant cohorts (19, 24, 30 – 32) and consistent with previously reported pregnancy population PK model predicted values (33). Our finding of 28% higher T ½ and 111% higher C 12 in 3T did not manifest in differences in AUC0-24, which agrees with the model predicted 14% lower clearance in pregnancy not translating into clinically relevant differences in exposure. The physiological determinants of lower RIF clearance are not fully understood.
One possible explanation of the modest increases in plasma concentrations of RIF can be explained by its disposition in the liver and bile. Following intestinal absorption, RIF is transported into the hepatocytes where it is deacetylated before being eliminated in the bile and urine (34). Hepatobiliary elimination of RIF is mediated by canalicular membrane transporters, mainly multidrug resistance-associated protein 2 (MRP2) (35). RIF is a potent inhibitor of MRP2 (35, 36), a process that can potentially lead to delayed elimination (increased T 1/2). In addition, the high concentrations of estrogens during pregnancy may further inhibit MRP2 (37) and increase the T 1/2 and plasma concentrations of RIF.
Preclinical and in vitro research suggests that prolonged exposure to estrogen and progesterone during pregnancy can alter drug pharmacokinetics, which may also include TB drugs. Estrogen and progesterone have concentration-dependent biological effects. In human hepatocytes, estrogen upregulates the expression of some CYP enzymes (CYP2A6, CYP2B6, and CYP3A4) through the activation of the estrogen receptor, while progesterone upregulates the expression of other enzymes via the PXR, which raises the possibility that changes in both estrogen and progesterone plasma concentrations can alter RIF metabolism during pregnancy (38).
It is also theoretically plausible that pregnancy-induced cholestasis (decrease in hepatobiliary bilious flow due to estrogen-induced intrahepatic inhibition of MRP2, resulting in a decrease in bile transport) primarily occurring in 3T could be a contributing factor, but this has not been previously described in the literature and needs further investigation.
Data from preclinical studies and physiologically based pharmacokinetic models have demonstrated that the activities of P-glycoprotein (P-gp), a transmembrane efflux pump with excretory function found on the apical membranes of many epithelial cells with excretory function, can be altered by induction and inhibitory effects of RIF (38). However, RIF is also a substrate of P-gp (39), and it is unknown if pregnancy induces P-gp expression and potentially alters RIF disposition.
Similar high proportions of participants not reaching target RIF AUC0-24 and C max have been previously described in non-pregnant cohorts (26, 40, 41), and as low exposures have been associated with worse treatment outcomes (15, 24), it is reassuring that pregnancy itself does not seem to lower RIF exposure further.
INH exposure in this study was 18%–25% lower in pregnancy compared to postpartum. As INH is predominantly excreted in the urine, the increased glomerular filtration rate occurring in pregnancy could explain our findings (42). This finding has not been previously described in a non-HIV pregnancy cohort on treatment for DS-TB disease. A 26% increase in INH clearance during pregnancy was described by Gausi et al. in a TB prevention treatment cohort of pregnant persons living with HIV (PPHIV) mainly on efavirenz-based ART, where model-predicted postpartum AUC0-24 was 1.4-fold greater compared to antepartum (43). In contrast, Mathad et al. reported no differences in INH plasma concentrations in pregnancy versus postpartum in a cohort of pregnant persons on TB preventative therapy containing INH and rifapentine (44). Different rates of INH and its metabolite N-acetyl-isoniazid (ACL) clearance can result from genetic variations in the phenotype of the phase II conjugating liver enzyme NAT2. As our sample consisted of 54% slow NAT2 metabolizers, which is higher than most previously reported cohorts (30, 43, 45), it is important to perform within-participant comparisons to adjust for this pharmacogenomic influence. The results of both the smaller sample size within-participant comparison and larger sample size univariate mixed-effect model comparison were comparable. The difference is thus likely attributable to pregnancy-mediated factors. A modest reduction in NAT2 activity during pregnancy was described by Tsutsumi et al. (46), but we could not determine the effect of pregnancy on NAT2 activity on INH and ACL elimination rates. The impact of 25% lower INH exposure in pregnancy could particularly be of clinical importance in pregnant and postpartum persons who are fast NAT2 metabolizers and thus, subsequently at risk of subtherapeutic dosing at standard weight-banding guidelines already in non-pregnant state (21).
No EMB AUC targets have been described in the literature. EMB AUC0-24 was lower by 40% in pregnancy. EMB is predominantly eliminated unchanged through urine excretion, and pregnancy-induced hyperfiltration could be explanatory. In contrast, a previously published two-compartment first-order population PK model with data from a sub-study of the Tshepiso trial by Abdelwahab et al. (47) involving 15 PPHIV with DS-TB disease on a standard daily dosing of EMB (15–25 mg/dL) showed no significant differences in EMB’s clearance and bioavailability during pregnancy compared to postpartum (n = 3, one paired sample). Abdelwahab et al. reported comparable median C max values of 1.82 and 2.1 µg/mL, and AUC0–24 values of 16.5 and 19.0 µg·h/mL for antepartum and postpartum persons, respectively. Our results show higher EMB C max but lower AUC0-24 during pregnancy, with significantly higher AUC0–24 PP. These findings are comparable to results from other published non-pregnant cohorts (18, 45) or higher (32, 48). A larger sample of PP persons on EMB to enable within-participant analysis is urgently needed to confirm these findings.
PZA PK parameters were similar in pregnancy compared to PP, and concentrations were comparable to previously described non-pregnant cohorts (24, 49). Pregnancy did not appear to affect the dosage or disposition of PZA according to the findings from a two-compartment first-order population PK model that used data from a sub-study of the Tshepiso trial involving 15 PPHIV with DS-TB disease who were given a standard daily dose of PZA (20–30 mg/kg of body weight). PZA’s clearance and bioavailability were not significantly different during pregnancy compared to PP (n = 3, one paired sample) (47). PZA is mainly eliminated in metabolized form through various pathways, and it is unlikely that pregnancy has a significant influence on exposure. All participants reached target C max, and the majority reached target AUC0-24, which is reassuring, as PZA concentrations are reported to be the most important predictor of both sputum conversion and sterilizing activity when used as part of combination therapy (15, 24).
AEs reported during the study were primarily DS-TB disease related. Seven (26%) participants experienced treatment-related events, of which five (19%) were DILI. These findings are consistent with previous reports (11, 50, 51). This underlines the need for close monitoring of liver functions in pregnancy whilst on TB treatment, as pregnancy itself increases the risk for DILI significantly (52, 53). As previously reported (54, 55), slow metabolizers are more at risk of adverse drug reactions via higher plasma and tissue exposure, and this seemed evident in our sample where four out of five drug-induced liver injuries occurred in slow metabolizers. Although no statistically significant differences in drug concentrations were found between participants who developed DILI and those who did not, higher INH AUC0-24 was observed in participants with DILI. However, causality between higher INH exposure and DILI could not be established as INH was co-administered with other commonly associated DILI culprit drugs RIF, EMB, and PZA in four out of five participants who developed DILI.
High rates of preterm delivery and low birth weight were expected to occur in association with TB disease during pregnancy (5, 7, 56, 57), demonstrating the need for early diagnosis and treatment to prevent these adverse pregnancy and neonatal outcomes (6). Although our sample size was limited, no congenital abnormalities were attributable to DS-TB treatment, and treatment-related adverse events in infants were sparse and mild and support the fetal safety of DS-TB drugs in pregnancy as summarized by Zhou et al. (13) and Shiu et al. (58).
Our study has several strengths. First, this opportunistic PK study used intensive sampling methods in both second and third trimesters of pregnancy and postpartum including the use of FDC tablets according to WHO weight-banded dosing guidelines where available, as applicable to typical treatment settings worldwide, providing findings that would be representative of typical use. Second, within-participant comparison using the PP period as control, adjusting for possible pharmacogenetic and other participant-specific influences, was achieved for RIF and INH in more than 10 participants per group, confirming the reliability of the univariate mixed-effect model-derived GMRs for these drugs. Third, the study participants were followed up longitudinally until 16–24 weeks postpartum, allowing us to record AEs related to DS-TB disease as well as DS-TB treatment exposure at regular intervals in both mother and infant.
This study had several limitations. First, there were few participants who continued EMB and PZA through the postpartum period, limiting within-participant comparisons. Second, participants were only sampled until 12 hours post-dose, necessitating extrapolation of terminal concentrations at 24 hours to report AUC0-24 for the longer half-life drugs EMB and PZA. Third, TB treatment outcomes were not collected, which limited the interpretation of the clinical consequences of our PK findings. Fourth, although C max and AUC0-24 targets are the most widely used PK parameters to evaluate optimal dose ranges, treatment efficacy targets combined with minimum inhibitory concentration data could have strengthened our findings but could not be defined for our participants. Fifth, while the median dosing ranges were comparable between the sampling times, the pregnancy weight gain and post-delivery weight loss that pregnant and postpartum participants experienced might have led to dosing alterations during the study when the weight band cut-offs are crossed, allowing for within-participant dosing variability. Sixth, as fixed-dose combination TB treatment formulations and meal recommendations were not standardized, heterogeneity in absorption related to formulation, dietary intake at dosing time as well as pregnancy-mediated decreased gastric motility may have influenced PK profiles. Seventh, selection bias might have occurred in cases where serious AEs occurred, which required regimen adjustments, as such participants receiving alternative regimens would not have been eligible for inclusion in this study. Safety reporting began only at the time of participant enrollment, and participants were required to have been on TB treatment for at least 2 weeks prior to enrollment, which could have caused favorable outcomes to be overstated and treatment-related AEs to be understated. Besides treatment exposure, variety in duration and severity of TB disease antepartum can influence the occurrence of treatment-related AEs.
This study was intended to determine whether pregnant and postpartum persons who received antituberculosis therapy in accordance with the most recent DS-TB treatment recommendations achieved serum concentrations that met the therapeutic targets and were comparable to those in non-pregnant adults. It is important to emphasize that this PK study was not powered for efficacy or safety outcomes and recruited a heterogeneous population of pregnant and postpartum persons >2 weeks stable on TB treatment, with a diverse spectrum of DS-TB disease at entry.
Achieving adequate TB treatment drug concentrations during pregnancy is important to cure TB disease and minimize adverse pregnancy and TB outcomes. In pregnant persons without HIV receiving treatment for DS-TB disease, RIF and PZA AUC0–24 and C max in pregnancy were similar but INH AUC0–24 and C max and EMB AUC0–24 were lower compared to non-pregnant concentrations. However, the median AUC0–24 for RIF, INH, and PZA met the therapeutic targets in all sampling timepoints. The clinical relevance of low INH and EMB exposure when treating pregnant persons with DS-TB disease needs to be determined. More PK data for the evaluation of pregnancy effects on EMB and PZA exposure are needed.
MATERIALS AND METHODS
Study population and design
The International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1026s is a non-blinded, phase IV, prospective opportunistic study of antiretroviral and antituberculosis PK and safety in pregnant women living with and without HIV and registered in ClinicalTrials.gov with identifier NCT00042289. Recruitment sites are situated in North and South America, Asia, and Africa. The study included an arm for pregnant women living without HIV receiving DS-TB treatment with at least two of the following first-line TB drugs: RIF, INH, EMB, and PZA. Participant eligibility further included being pregnant ≥20 weeks gestation and ≥18 years of age at screening and treated for DS-TB disease (intensive or continuation phase) as part of local clinical care for ≥2 weeks before the day of the first PK evaluation. Participants continued to take their prescribed TB medications throughout pregnancy and postpartum until treatment completion. Participants were excluded if they had concurrent use of medication known to interact with the TB treatment, a clinical condition or laboratory abnormality that might require a change in the TB regimen during the study period or multiple gestations. The use of local generic formulations was evaluated by the protocol pharmacologist prior to enrollment to confirm adequate bioequivalence. All infants were required to be enrolled in utero immediately after maternal enrollment. Local institutional review boards approved the protocol at all participating sites and signed informed consent was obtained from all participants prior to participation. The specific regimen and duration of antituberculosis treatment were decided by the participant’s local healthcare provider, who remained responsible for clinical management and prescription throughout the study. Maternal and infant safety follow-up continued until 24 weeks PP.
Clinical and laboratory monitoring
Demographics, medical history, physical examination, and hematology and serum biochemistry safety were assessed at each study visit. All infants received physical examinations after birth and laboratory evaluations were performed if clinically indicated. Where birth weight classification data were lacking, 2013 Fenton growth chart standards were used to classify (59). AEs from entry onwards were reported at each study visit per protocol guidelines and clinical management was determined by each participant’s clinician. Causality assessment was performed by the individual site clinicians and reviewed by the protocol team. The National Institute of Allergy and Infectious Diseases, Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0, dated November 2014 (60), was used to grade AE severity. All toxicities were followed until resolution.
Sample collection
Intensive steady-state 12-hour PK profiles of RIF, INH, ACL, EMB, and PZA were performed during the second trimester (2T; 20+0–26+6 weeks gestation), third trimester (3T; 30+0–36+6 weeks gestation), and 2–8 weeks PP. Daily antituberculosis treatment using fixed-dose combination or separate tablets was given according to WHO-recommended weight-banded dosing guidelines with prespecified dosing ranges (Table S4) and dose ingestion was directly observed on the day of PK sampling. Blood samples were collected pre-dose and at 1-, 2-, 4-, 6-, 8-, and 12-hour post-dose. At delivery, a single maternal plasma sample and an umbilical cord sample after the cord was clamped were collected if logistically feasible. The blood samples were centrifuged, aliquoted, and stored at −70°C within 1 hours of sampling until analysis. In consenting participants, a maternal dried blood spot sample was collected for DNA extraction to determine the NAT2 metabolizer genotype.
Assays
Plasma PK samples were analyzed at the Division of Clinical Pharmacology, University of Cape Town, using previously described methods (61). The laboratory adheres to ISO15189 and performs standardized inter-laboratory testing through the AIDS Clinical Trial Group clinical pharmacology quality assurance and quality control program. RIF, INH, ACL, EMB, and PZA plasma concentrations were measured using liquid chromatography-tandem mass spectrometry; lower limits of quantification being 0.117, 0.105, 0.053, 0.084, and 0.203 µg/mL, respectively. DNA extraction was performed at Bio-Analytical Research Corporation SA laboratories, Johannesburg, using the Maxwell Promega instrument and the single-nucleotide polymorphism (SNP) analysis was done using the ABI ViiA7 Real-Time instrument to analyze seven NAT2 SNPs: rs1801279 (191G > A), rs1041983 (282C > T), rs1801280 (341T > C), rs1799929 (481C > T), rs1799930 (590G > A), rs1208 (803A > G), and rs1799931 (857G > A). The alleles were defined following the SNPedia classification (62).
Pharmacokinetic analyses
For all the four TB drugs, the pre-dose (C 0), maximum (C max), minimum (C min), and last plasma concentration (C last) along with corresponding time points (T max, T min) were reported. Stata version 16.1 (Stata Corporation, College Station, TX, USA) was used to characterize the PK parameters using non-compartmental analyses and reviewed by the protocol pharmacologists. The steady-state area under the plasma concentration-time curve from time 0 to 12-hour post-dose (AUC0-12) was calculated using the linear trapezoidal rule. All samples prior to terminal concentrations that were BQL were regarded as 0 to estimate the total sum of trapezoids for AUC most conservatively. AUC was reported over 24 hours in order to provide exposure data in line with the daily dosing regimen and enable comparison with PK data from recently published non-pregnant cohorts. AUC0-24 was estimated using extrapolated concentrations for time point 24 h (C 24) using Excel 2016 (Microsoft Corporation, Redmond, WA, USA). If terminal concentrations were BQL before or at time point 12 hours, AUC0-∞ was extrapolated using a linear extension of log concentration and regarded as AUC0-24. Apparent clearance (CL/F) from plasma was calculated as dose divided by AUC0–24. Half-life (t 1/2) was calculated as ln (2)/k e ; k e is the elimination rate constant derived from the terminal slope of the log concentration versus the time curve using all measurable concentrations after the peak. To be able to perform GMR analysis between pregnant and postpartum time points, BQL concentrations were set at half the lower limit of quantification to avoid noughts in the statistical summary calculations. Maternal metabolizer status was classified as slow, intermediate, or rapid, based on the number of NAT2 acetylator gene polymorphisms after sequencing NAT2 SNPs (63). To classify the metabolizer status for four participants lacking pharmacogenomic data, we made use of the 3-hour ACL/INH concentration ratio as published elsewhere (64). To do so, we calculated a timepoint 3-hour post-dose for ACL and INH concentrations by using the 2 and 4-hour timepoints.
Statistical methods
Analysis of data was done using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were calculated for PK parameters of interest during each study period. As pre-specified in the protocol, PK parameters during the 2T versus PP and during 3T versus PP were compared at the within-participant level using the Wilcoxon signed rank test, with a two-sided P-value <0.10 considered statistically significant. Within-participant GMRs and 90% confidence intervals (CI) for PK parameters in the 2T and 3T versus PP conditions were calculated for all four TB drugs to assess whether there was a clinically important difference in exposure. A 90% CI between 0.8 and 1.25 was considered to indicate that the PK parameter was comparable during pregnancy versus postpartum. Univariate mixed-effects models were also fitted to allow the inclusion of data from pregnant and postpartum women who have missing data for some time points, as intensive phase treatment including EMB and PZA could have been completed before delivery. In each mixed effects model, the PK evaluation time points were the independent variables, and the log-transformed TB PK parameter was the dependent variable. AUC0-24 and C max differences between NAT2 metabolizer groups were analyzed using the non-parametric Kruskal-Wallis test in Stata version 16.1 (Stata Corporation, College Station, TX, USA). The percentages of participants with AUC0-24 and C max below the minimum targets were determined during pregnancy and postpartum.
ACKNOWLEDGMENTS
The IMPAACT P1026s protocol team acknowledges the valued contribution of the women who volunteered to participate in the protocol with their infants. We gratefully acknowledge the contributions of the clinical research site investigators and staff who conducted P1026s: 8950 Family Centre for Research with Ubuntu, South Africa (Lynne Cornelissen and Jeanne De Jager); 5115 Siriraj Hospital Mahidol University, Thailand (Chokephaibulkit Kulkanya, Peerawong Werarak, and Amphan Chalermchockcharoenkit); 5116 Chiang Rai Regional Hospital, Thailand (Jullapong Achalapong, Pra-ornsuda Sukrakanchana, and Nusara Krapunpongsakul); 5118 Kilimanjaro Christian Medical Centre, Tanzania (James Samwel Ngocho, Blandina T. Mmbaga, and Boniface Njau); 5071 Institute of Pediatrics, Federal University of Rio de Janeiro, Brazil (Cristina Brarroso Hofer, Elizabeth S. Machado, Ricardo Hugo S. Oliveira, and Thalita F. Abreu); 5040 SUNY Stony Brook, USA (Sharon Nachman, Cecilia Avila, Jennifer Griffin, and Barsha Chakraborty); 8051 Wits RHI Shandukani Research Centre, South Africa (Lee Fairlie, Faeezah Patel, Hedwig Kowo, and Tammy Meyers); 12701 Gaborone and 12702 Molepolole Prevention/Treatment Trials, Botswana (Gaerolwe Masheto, Ponego L. Ponatshego, Mpho S. Raesi, and Unoda Chakalisa). In addition to the authors, members of the IMPAACT P1026s protocol team include Alexander Benns, Sandra Burchett, Nantasak Chotivanich, Anthony Garcia-Prats, Amita Gupta, Anneke Hesseling, Jennifer Hughes, Gonzague Jourdain, Regis Kreitchmann, Pooja Mehta, Diane Costello, Lisa M. Frenkel, Emily Barr, and Christina Reding.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Marije Van Schalkwyk, Email: marije@sun.ac.za.
James E. Leggett, Providence Portland Medical Center, Portland, Oregon, USA
DATA AVAILABILITY
The data cannot be made publicly available due to the ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (email address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00737-23.
Within-participant paired comparison tables per drug and WHO FDC dosing target table.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. World Health Organization . 2022. Global tuberculosis report 2022. Available from: https://www.who.int/publications/i/item/9789240061729. Retrieved 24 Aug 2023.
- 2. Gupta A, Nayak U, Ram M, Bhosale R, Patil S, Basavraj A, Kakrani A, Philip S, Desai D, Sastry J, Bollinger RC, Byramjee Jeejeebhoy Medical College-Johns Hopkins University Study Group . 2007. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002-2005. Clin Infect Dis 45:241–249. doi: 10.1086/518974 [DOI] [PubMed] [Google Scholar]
- 3. Zumla A, Bates M, Mwaba P. 2014. The neglected global burden of tuberculosis in pregnancy. Lancet Glob Health 2:e675–6. doi: 10.1016/S2214-109X(14)70338-9 [DOI] [PubMed] [Google Scholar]
- 4. Sugarman J, Colvin C, Moran AC, Oxlade O. 2014. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health 2:e710–6. doi: 10.1016/S2214-109X(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 5. Figueroa-Damián R, Arredondo-García JL. 2001. Neonatal outcome of children born to women with tuberculosis. Arch Med Res 32:66–69. doi: 10.1016/s0188-4409(00)00266-6 [DOI] [PubMed] [Google Scholar]
- 6. Tripathy SN, Tripathy SN. 2003. Tuberculosis and pregnancy. Int J Gynaecol Obstet 80:247–253. doi: 10.1016/s0020-7292(02)00393-4 [DOI] [PubMed] [Google Scholar]
- 7. Sobhy S, Babiker Z, Zamora J, Khan KS, Kunst H. 2017. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG 124:727–733. doi: 10.1111/1471-0528.14408 [DOI] [PubMed] [Google Scholar]
- 8. Dennis EM, Hao Y, Tamambang M, Roshan TN, Gatlin KJ, Bghigh H, Ogunyemi OT, Diallo F, Spooner KK, Salemi JL, Olaleye OA, Khan KZ, Aliyu MH, Salihu HM. 2018. Tuberculosis during pregnancy in the United States: racial/ethnic disparities in pregnancy complications and in-hospital death. PLoS One 13:e0194836. doi: 10.1371/journal.pone.0194836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zenner D, Kruijshaar ME, Andrews N, Abubakar I. 2012. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 185:779–784. doi: 10.1164/rccm.201106-1083OC [DOI] [PubMed] [Google Scholar]
- 10. Mathad JS, Yadav S, Vaidyanathan A, Gupta A, LaCourse SM. 2022. Tuberculosis infection in pregnant people: current practices and research priorities. Pathogens 11:1481. doi: 10.3390/pathogens11121481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asuquo B, Vellore AD, Walters G, Manney S, Mignini L, Kunst H. 2012. A case-control study of the risk of adverse perinatal outcomes due to tuberculosis during pregnancy. J Obstet Gynaecol 32:635–638. doi: 10.3109/01443615.2012.704436 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . 2022. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment. Available from: https://apps.who.int/iris/handle/10665/353829 [PubMed]
- 13. Zhou X, Fang G, Xie Y, Wei A, Huang F. 2022. Safety evaluation of antituberculosis drugs during pregnancy: a systematic review and meta-analysis. Front Surg 9:871321. doi: 10.3389/fsurg.2022.871321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frederiksen MC. 2001. Physiologic changes in pregnancy and their effect on drug disposition. Semin Perinatol 25:120–123. doi: 10.1053/sper.2001.24565 [DOI] [PubMed] [Google Scholar]
- 15. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, Gumbo T, McIlleron H. 2015. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 59:38–45. doi: 10.1128/AAC.03931-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donald PR, Parkin DP, Seifart HI, Schaaf HS, van Helden PD, Werely CJ, Sirgel FA, Venter A, Maritz JS. 2007. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur J Clin Pharmacol 63:633–639. doi: 10.1007/s00228-007-0305-5 [DOI] [PubMed] [Google Scholar]
- 18. Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, Lockman S, Oyewo A, Talbot EA, Kenyon TA, Moeti TL, Moffat HJ, Peloquin CA. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 41:461–469. doi: 10.1086/431984 [DOI] [PubMed] [Google Scholar]
- 20. Kwara A, Cao L, Yang H, Poethke P, Kurpewski J, Tashima KT, Mahjoub BD, Court MH, Peloquin CA. 2014. Factors associated with variability in rifampin plasma pharmacokinetics and the relationship between rifampin concentrations and induction of efavirenz clearance. Pharmacotherapy 34:265–271. doi: 10.1002/phar.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilkins JJ, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson USH. 2011. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br J Clin Pharmacol 72:51–62. doi: 10.1111/j.1365-2125.2011.03940.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Oosterhout JJ, Dzinjalamala FK, Dimba A, Waterhouse D, Davies G, Zijlstra EE, Molyneux ME, Molyneux EM, Ward S. 2015. Pharmacokinetics of antituberculosis drugs in HIV-positive and HIV-negative adults in Malawi. Antimicrob Agents Chemother 59:6175–6180. doi: 10.1128/AAC.01193-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abulfathi AA, Decloedt EH, Svensson EM, Diacon AH, Donald P, Reuter H. 2019. Clinical pharmacokinetics and pharmacodynamics of rifampicin in human tuberculosis. Clin Pharmacokinet 58:1103–1129. doi: 10.1007/s40262-019-00764-2 [DOI] [PubMed] [Google Scholar]
- 24. Burhan E, Ruesen C, Ruslami R, Ginanjar A, Mangunnegoro H, Ascobat P, Donders R, van Crevel R, Aarnoutse R. 2013. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother 57:3614–3619. doi: 10.1128/AAC.02468-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIlleron H, Rustomjee R, Vahedi M, Mthiyane T, Denti P, Connolly C, Rida W, Pym A, Smith PJ, Onyebujoh PC. 2012. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother 56:3232–3238. doi: 10.1128/AAC.05526-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daskapan A, Idrus LR, Postma MJ, Wilffert B, Kosterink JGW, Stienstra Y, Touw DJ, Andersen AB, Bekker A, Denti P, Hemanth Kumar AK, Jeremiah K, Kwara A, McIlleron H, Meintjes G, van Oosterhout JJ, Ramachandran G, Rockwood N, Wilkinson RJ, van der Werf TS, Alffenaar J-WC. 2019. A systematic review on the effect of HIV infection on the pharmacokinetics of first-line tuberculosis drugs. Clin Pharmacokinet 58:747–766. doi: 10.1007/s40262-018-0716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devaleenal Daniel B, Ramachandran G, Swaminathan S. 2017. The challenges of pharmacokinetic variability of first-line anti-TB drugs. Expert Rev Clin Pharmacol 10:47–58. doi: 10.1080/17512433.2017.1246179 [DOI] [PubMed] [Google Scholar]
- 28. Eke AC. 2022. An update on the physiologic changes during pregnancy and their impact on drug pharmacokinetics and pharmacogenomics. J Basic Clin Physiol Pharmacol 33:581–598. doi: 10.1515/jbcpp-2021-0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J, Raymond K. 2006. Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob 5:3. doi: 10.1186/1476-0711-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aarnoutse RE, Kibiki GS, Reither K, Semvua HH, Haraka F, Mtabho CM, Mpagama SG, van den Boogaard J, Sumari-de Boer IM, Magis-Escurra C, Wattenberg M, Logger JGM, Te Brake LHM, Hoelscher M, Gillespie SH, Colbers A, Phillips PPJ, Plemper van Balen G, Boeree MJ, PanACEA Consortium . 2017. Pharmacokinetics, tolerability, and bacteriological response of rifampiadministered at 600, 900, and 1,200 milligrams daily in patients with pulmonary tuberculosis. Antimicrob Agents Chemother 61:e01054-17. doi: 10.1128/AAC.01054-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M, PanACEA consortium . 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, Msandiwa R, Castel S, Wiesner L, Chaisson RE, McIlleron H, Dooley KE. 2015. Population pharmacokinetics of rifampin in pregnant women with tuberculosis and HIV coinfection in Soweto, South Africa. Antimicrob Agents Chemother 60:1234–1241. doi: 10.1128/AAC.02051-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Acocella G. 1978. Clinical pharmacokinetics of rifampicin. Clin Pharmacokinet 3:108–127. doi: 10.2165/00003088-197803020-00002 [DOI] [PubMed] [Google Scholar]
- 35. Chen J, Wu H, Tang X, Chen L. 2022. 4-Phenylbutyrate protects against rifampin-induced liver injury via regulating MRP2 ubiquitination through inhibiting endoplasmic reticulum stress. Bioengineered 13:2866–2877. doi: 10.1080/21655979.2021.2024970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Te Brake LHM, Russel FGM, van den Heuvel J, de Knegt GJ, de Steenwinkel JE, Burger DM, Aarnoutse RE, Koenderink JB. 2016. Inhibitory potential of tuberculosis drugs on ATP-binding cassette drug transporters. Tuberculosis (Edinb) 96:150–157. doi: 10.1016/j.tube.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 37. Zu Y, Yang J, Zhang C, Liu D. 2021. The pathological mechanisms of estrogen-induced cholestasis: current perspectives. Front Pharmacol 12:761255. doi: 10.3389/fphar.2021.761255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asaumi R, Nunoya KI, Yamaura Y, Taskar KS, Sugiyama Y. 2022. Robust physiologically based pharmacokinetic model of rifampicin for predicting drug-drug interactions via P-glycoprotein induction and inhibition in the intestine, liver, and kidney. CPT Pharmacometrics Syst Pharmacol 11:919–933. doi: 10.1002/psp4.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuetz EG, Schinkel AH, Relling MV, Schuetz JD. 1996. P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome P4503A in mice and humans. Proc Natl Acad Sci U S A 93:4001–4005. doi: 10.1073/pnas.93.9.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tostmann A, Mtabho CM, Semvua HH, van den Boogaard J, Kibiki GS, Boeree MJ, Aarnoutse RE. 2013. Pharmacokinetics of first-line tuberculosis drugs in Tanzanian patients. Antimicrob Agents Chemother 57:3208–3213. doi: 10.1128/AAC.02599-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perumal R, Naidoo K, Naidoo A, Ramachandran G, Requena-Mendez A, Sekaggya-Wiltshire C, Mpagama SG, Matteelli A, Fehr J, Heysell SK, Padayatchi N. 2020. A systematic review and meta-analysis of first-line tuberculosis drug concentrations and treatment outcomes. Int J Tuberc Lung Dis 24:48–64. doi: 10.5588/ijtld.19.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopes van Balen VA, van Gansewinkel TAG, de Haas S, Spaan JJ, Ghossein-Doha C, van Kuijk SMJ, van Drongelen J, Cornelis T, Spaanderman MEA. 2019. Maternal kidney function during pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 54:297–307. doi: 10.1002/uog.20137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gausi K, Wiesner L, Norman J, Wallis CL, Onyango-Makumbi C, Chipato T, Haas DW, Browning R, Chakhtoura N, Montepiedra G, Aaron L, McCarthy K, Bradford S, Vhembo T, Stranix-Chibanda L, Masheto GR, Violari A, Mmbaga BT, Aurpibul L, Bhosale R, Nevrekhar N, Rouzier V, Kabugho E, Mutambanengwe M, Chanaiwa V, Nyati M, Mhembere T, Tongprasert F, Hesseling A, Shin K, Zimmer B, Costello D, Jean-Philippe P, Sterling TR, Theron G, Weinberg A, Gupta A, Denti P, IMPAACT P1078 (TB APPRISE) Study Group Team . 2021. Pharmacokinetics and drug-drug interactions of isoniazid and efavirenz in pregnant women living with HIV in high TB incidence settings: importance of genotyping. Clin Pharmacol Ther 109:1034–1044. doi: 10.1002/cpt.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathad JS, Savic R, Britto P, Jayachandran P, Wiesner L, Montepiedra G, Norman J, Zhang N, Townley E, Chakhtoura N, Bradford S, Patil S, Popson S, Chipato T, Rouzier V, Langat D, Chalermchockcharoentkit A, Kamthunzi P, Gupta A, Dooley KE. 2022. Pharmacokinetics and safety of 3 months of weekly rifapentine and isoniazid for tuberculosis prevention in pregnant women. Clin Infect Dis 74:1604–1613. doi: 10.1093/cid/ciab665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Denti P, Jeremiah K, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, Castel S, Wiesner L, Hagen CM, Christiansen M, Changalucha J, McIlleron H, Friis H, Andersen AB. 2015. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One 10:e0141002. doi: 10.1371/journal.pone.0141002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsutsumi K, Kotegawa T, Matsuki S, Tanaka Y, Ishii Y, Kodama Y, Kuranari M, Miyakawa I, Nakano S. 2001. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activities in humans. Clin Pharmacol Ther 70:121–125. doi: 10.1067/mcp.2001.116495 [DOI] [PubMed] [Google Scholar]
- 47. Abdelwahab MT, Leisegang R, Dooley KE, Mathad JS, Wiesner L, McIlleron H, Martinson N, Waja Z, Letutu M, Chaisson RE, Denti P. 2020. Population pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in pregnant South African women with tuberculosis and HIV. Antimicrob Agents Chemother 64:e01978-19. doi: 10.1128/AAC.01978-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruslami R, Nijland HMJ, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. 2007. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother 51:2546–2551. doi: 10.1128/AAC.01550-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Donald PR, Maritz JS, Diacon AH. 2012. Pyrazinamide pharmacokinetics and efficacy in adults and children. Tuberculosis (Edinb) 92:1–8. doi: 10.1016/j.tube.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 50. Thompson NP, Caplin ME, Hamilton MI, Gillespie SH, Clarke SW, Burroughs AK, McIntyre N. 1995. Anti-tuberculosis medication and the liver: dangers and recommendations in management. Eur Respir J 8:1384–1388. doi: 10.1183/09031936.95.08081384 [DOI] [PubMed] [Google Scholar]
- 51. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WCM, van der Ven AJAM, Dekhuijzen R. 2008. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x [DOI] [PubMed] [Google Scholar]
- 52. Beck-Friis J, Studahl M, Yilmaz A, Andersson R, Lönnermark E. 2020. Increased risk of hepatotoxicity and temporary drug withdrawal during treatment of active tuberculosis in pregnant women. Int J Infect Dis 98:138–143. doi: 10.1016/j.ijid.2020.06.069 [DOI] [PubMed] [Google Scholar]
- 53. Kamath P, Kamath A, Ullal SD. 2021. Liver injury associated with drug intake during pregnancy. World J Hepatol 13:747–762. doi: 10.4254/wjh.v13.i7.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang D, Hao J, Hou R, Yu Y, Hu B, Wei L. 2020. The role of NAT2 polymorphism and methylation in anti-tuberculosis drug-induced liver injury in Mongolian tuberculosis patients. J Clin Pharm Ther 45:561–569. doi: 10.1111/jcpt.13097 [DOI] [PubMed] [Google Scholar]
- 55. Richardson M, Kirkham J, Dwan K, Sloan DJ, Davies G, Jorgensen AL. 2019. NAT2 variants and toxicity related to anti-tuberculosis agents: a systematic review and meta-analysis. Int J Tuberc Lung Dis 23:293–305. doi: 10.5588/ijtld.18.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bekker A, Schaaf HS, Draper HR, Kriel M, Hesseling AC. 2016. Tuberculosis disease during pregnancy and treatment outcomes in HIV-infected and uninfected women at a referral hospital in Cape town. PLoS One 11:e0164249. doi: 10.1371/journal.pone.0164249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lewis PF, Budhewar AS, Bavdekar NB. 2021. Fetomaternal outcome of pregnant women infected with tuberculosis: an analytical study. J South Asian Fed Obstet Gynecol 13:197–201. doi: 10.5005/jp-journals-10006-1948 [DOI] [Google Scholar]
- 58. Shiu JR, Min A, Kiang TKL. 2021. Clinical pharmacokinetics and pharmacodynamics of anti-tubercular drugs in pregnancy. Eur J Drug Metab Pharmacokinet 46:1–24. doi: 10.1007/s13318-020-00657-x [DOI] [PubMed] [Google Scholar]
- 59. Fenton TR, Kim JH. 2013. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59. doi: 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Division of AIDS . 2014. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Available from: https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf
- 61. Chirehwa MT, McIlleron H, Wiesner L, Affolabi D, Bah-Sow O, Merle C, Denti P, team R. 2019. Effect of efavirenz-based antiretroviral therapy and high-dose rifampicin on the pharmacokinetics of isoniazid and acetyl-isoniazid. J Antimicrob Chemother 74:139–148. doi: 10.1093/jac/dky378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. NAT2 genotype - Specia. 2023. Available from: https://www.snpedia.com/index.php/NAT2
- 63. Isoniazid . 2023. Drug bank online. Available from: https://go.drugbank.com/drugs/DB00951
- 64. Seifart HI, Parkin DP, Botha FJ, Donald PR, Van Der Walt BJ. 2001. Population screening for isoniazid acetylator phenotype. Pharmacoepidemiol Drug Saf 10:127–134. doi: 10.1002/pds.570 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Within-participant paired comparison tables per drug and WHO FDC dosing target table.
Data Availability Statement
The data cannot be made publicly available due to the ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (email address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.