Abstract
Purpose
Androgenetic alopecia (AGA) is the most common type of hair loss in humans, affecting self-esteem and emotional well-being. This study aimed to assess the safety and efficacy of VISPOTM, a standardized saw palmetto oil (2–3% β-sitosterol), in subjects with mild-to-moderate AGA.
Methods
In a double-blind, placebo-controlled, four-arm clinical study, 80 healthy male and female subjects aged 18–50 years were randomly allocated (1:1:1:1) to receive either 400 mg capsules of VISPO or 5 mL of a topical formulation containing 20% VISPO or the respective placebo once daily for 16 weeks. The primary endpoints included hair count (hair comb and hair pull tests) and the self-assessment of perceived efficacy. Objective evaluation was performed using the global photographic assessment score. Hair density, thickness, and anagen/telogen ratio were evaluated using phototrichogram analysis.
Results
At the end of the study, oral and topical formulations of VISPO reduced hair fall by up to 29% (p<0.001) and 22.19% (p<0.01) from the baseline, respectively. Hair density increased by 5.17% and 7.61% in the oral and topical VISPO groups, respectively (p<0.001). In addition, oral ingestion of VISPO resulted in a marked reduction in serum dihydrotestosterone (DHT) levels in the subjects compared to placebo (p<0.001). However, the effect of the VISPO formulations on the anagen/telogen ratio was insignificant. No serious adverse effects were observed during the study.
Conclusion
VISPO formulations reduced hair fall and promoted hair regrowth and scalp appearance in AGA patients.
Keywords: hair loss, androgenetic alopecia, saw palmetto, β-sitosterol, fatty acids
Introduction
Androgenetic alopecia (AGA) is a progressive hair loss condition, with a characteristic pattern in both men and women. In men, AGA, also known as male pattern hair loss (MPHL), is caused by a greater sensitivity of the hair follicles to the androgens;1 however, the cause of female pattern hair loss (FPHL) and its association with androgen remain unclear.2 The incidence of progressive hair loss by AGA has become more prevalent in recent years. AGA is common in nearly 80% of aging men.3 Hair loss negatively impacts the psychological and social well-being of an individual.4 Presently, minoxidil and finasteride are the only FDA (Food and Drug Administration) approved medications for AGA. Minoxidil is used for topical applications and works by stimulating hair follicles via anagen phase induction. Several clinical studies have reported the efficacy of minoxidil against alopecia, including AGA, Alopecia areata, and scarring alopecia.5–7 Minoxidil is associated with non-serious side effects such as scalp irritation, facial hypertrichosis, and allergic contact dermatitis.8 Finasteride, another well-known medication for AGA, functions by inhibiting the conversion of testosterone to dihydrotestosterone (DHT) via regulation of 5α-reductase enzyme activity.9 Despite its efficacy against hair loss, the associated side effects cannot be ignored. Long-term treatment with finasteride has been reported to have sexual side effects such as erectile dysfunction and decreased libido.10
Serenoa repens (Fam.) Arecaceae) dwarf trees, commonly known as saw palmetto (SP), grown largely in parts of North America. Saw palmetto oil extracted from plant berries contains 85–90% fatty acids and other constituents such as β-sitosterol, capric acid, caprylic acid, and caproic acid.11,12 SP competitively inhibits 5α-reductase activity and restricts the conversion of testosterone to DHT.13 Due to its anti-androgenic properties SP has gained potential as a dietary supplement and medication for conditions such as benign prostate hyperplasia and hair loss.14 Previously, SP in food supplements has been clinically evaluated for the prevention of hair loss in AGA subjects.15–17 However, most clinical studies have been conducted on formulations of SP with vitamins and minerals, making it difficult to understand the magnitude of efficacy of SP alone. Hence, it is important to study the hair care potential of SP individually, using randomized clinical trials.
VISPOTM is a proprietary extract containing standardized β-sitosterol and total fatty acid contents. Previously, the efficacy of VISPO was studied in a preclinical model of benign prostate hyperplasia (BPH).18 Further in a comparative clinical study with conventional SP extract, the efficacy and safety of VISPO were confirmed in BPH subjects.19 Here, the protective effect of VISPO as an oral and topical supplement against hair fall was observed in subjects with AGA.
Materials and Methods
VISPOTM
The investigational product VISPOTM used in this study was manufactured by Vidya Herbs Pvt. Ltd. (Bangalore, India) via supercritical fluid extraction. VISPO is a standardized saw palmetto extract containing 2–3% β-sitosterol and ≥ 85% total fatty acids. The extract was formulated for ingestion and topical application. Each 400 mg capsule contained 100 mg of VISPO. The placebo capsules contained maltodextrin and had a similar appearance and color to VISPO capsules. VISPO was formulated into a lotion form for topical application, such that each 100 g lotion contained 20 g VISPO. The composition details of the oral and topical formulations are provided in Supplementary Table 1 and 2.
Study Design
This 16-week double-blind, randomized, placebo-controlled, parallel-group, single-center, four-arm study was conducted on an Indian population at the BGS Global Institute of Medical Sciences, Bengaluru, Karnataka, India. The study Protocol No (Protocol no. LCBS-VH-95) was approved by the Institutional Ethics Committee (IEC) (Reg. No. ECR/1307/Inst/KA/2019), on 11th October 11, 2022. The trial was prospectively registered in the Clinical Trial Registry, India (CTRI/2022/10/046767), on October 25, 2022. This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines.
Sample Size Calculation
A sample size of 80 subjects (20 in each group) was required to establish a power of 80% and 5% level of significance. This sample size was sufficient to demonstrate a clinically meaningful difference between the VISPO and placebo groups in terms of reduction in hair fall from baseline to week 16 (Supplementary Material).
Study Participants
Healthy adult male and female subjects aged 18–50 years with mild-to-moderate AGA were enrolled in this study. Subject inclusion was based on the Norwood Hamilton scale (grade II to grade VA with vertex involvement) in males and the Sinclair scale (Grade II to IV) in females. The exclusion criteria were as follows: (a) conditions such as alopecia areata, alopecia totalis, alopecia universalis, and alopecia diffusa; (b) scarring of the scalp or any other condition or disease of the scalp or hair, including diseases of the hair shaft and inability to discontinue the use of hair weaving; (c) history of chronic health conditions (eg, diabetes, hypertension, chronic renal failure, heart and liver disease), endocrine abnormalities including stable thyroid disease, psychiatric illness, drug abuse, smoking, addiction to alcohol, bariatric surgery/eating disorders such as bulimia or binge eating, cardiovascular surgery, or history of any other major surgery; (d) use of medications such as minoxidil, drugs with anti-androgenic properties (for example, cyproterone acetate, spironolactone, ketoconazole, flutamide, bicalutamide), drugs potentially causing hypertrichosis (eg, cyclosporine, diazoxide, phenytoin psoralens), Anabolic steroids, Lithium and phenothiazines during six months prior to screening; use of systemic steroids for more than 14 days within the past 2 months prior to enrolment in the study; use of isotretinoin, light therapy, radiation to the scalp, or chemotherapy within the past year; (e) subjects allergic to herbal products; (f) subjects with any clinically significant systemic or cutaneous disease, which may interfere with study treatments or procedures. All enrolled subjects signed an IEC-approved informed consent form before participation.
Randomization and Blinding
After baseline measurements, the subjects were randomly assigned to one of the following four groups through block randomization: the VISPO oral intervention group (n=20), placebo oral group (n=20), VISPO topical intervention group (n=20), and placebo topical group (n=20). Each participant received an IP bottle with unique code. The code for each group and patient in the study was generated by a statistician using the R statistical software (version 4.2.1). Randomization numbers and respective IP codes were provided to the investigators. The investigators and study personnel responsible for enrolling the participants and conducting the trial were blinded to the randomization process throughout the study. The test product and placebo were identical in their external form.
Outcomes
This clinical study was performed to evaluate the effectiveness and safety of VISPO against hair falls in male and female patients with AGA. The primary objective was to assess hair fall during the study using dermatological examination (hair comb and hair pull tests) and subject self-assessment. The secondary efficacy objectives included (a) assessment of hair density, thickness, and anagen/telogen ratio as measured using phototrichogram and investigator assessment scoring (photograph scoring) and (b) changes in serum biomarkers 5α-reductase and DHT.
Procedures
In this double-blind parallel-group study, 80 healthy adult subjects meeting all the inclusion criteria and no exclusion criteria were randomly assigned to one of the four treatment arms in a 1:1:1:1 ratio. Active treatments included VISPO 100 mg in 400 mg capsules and 20% in lotion form as oral and topical formulations, respectively. The other two groups were the placebo groups for oral and topical treatments. The subjects in the oral treatment group self-administered 400 mg capsules once daily (after dinner) for 16 weeks. For topical application, approximately 5 mL of hair lotion (one press) was applied once a day on the scalp, followed by a hair wash 30 min after application. Instructions for the product application and hair care to be followed and a subject diary to record the study IP administration, side effects, concomitant medication, changes in scalp condition, and medical condition details during the entire study period were provided.
The dermatological assessment of hair fall reduction included hair comb and hair pull tests, as described elsewhere.20,21 Self-assessment of hair growth and appearance was based on validated questionnaires: the Hair Growth Index (HGI) (3-questioned 7-point scale) and Hair Growth Satisfaction Scale (HGSS) (5-questioned 7-point scale).22
Global photographs of the scalp (vertex and anterior regions) were obtained at baseline, follow-up (8 weeks), and at the end of the study (16 weeks). Photographic assessment was performed by a dermatologist blinded to the treatments using a 7-point rating scale.23 The hair density and thickness were measured using CASLITE Nova (Catseye Systems & Solutions, Mumbai, Maharashtra, India).
Safety Evaluations
The cutaneous tolerability of the topical application was based on a 5-point scale of investigator assessment (erythema, dryness, scaling, allergic reactions, and folliculitis). The self-assessment of the application site included seven parameters: redness, flaking, itching, burning sensation, allergic reaction, eruptions, and boils. Clinical laboratory evaluations included serum biochemical analysis of liver and renal function parameters, and hematological parameters. The subjects were monitored for adverse events (AEs) and serious adverse events (SAEs) throughout the study.
Statistical Analysis
Comparison of baseline demographic and physical characteristics between the study groups and analyses of categorical variables were performed using the chi-square test. All efficacy data were analyzed for normal distribution using the Shapiro–Wilk test. Changes within the group from baseline to weeks 8 and 16 were analyzed using a paired t-test. Comparisons between the groups were performed using an independent t-test. Skewed data were analyzed using the Mann–Whitney test (between the groups) and Wilcoxon signed-rank test (within the group). All data were analyzed using R statistical software (version 4.2.1).
Results
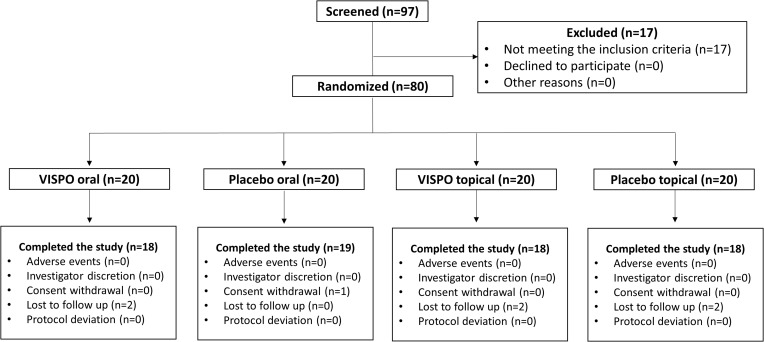
In this clinical trial, 97 human volunteers with mild-to-moderate AGA were screened. Based on the inclusion/exclusion criteria, 80 subjects were enrolled and randomized into 4 treatment arms (n = 20 in each group). During the study, seven subjects withdrew (were lost to follow-up); thus, 73 completed the study (November 2022 to April 2023). The subjects’ disposition during the study is shown in Figure 1. Per-protocol (PP) analysis was used to analyze study outcomes. Subject demographics were similar across the treatment groups, and no significant differences were observed in categorical variables (Table 1).
Figure 1.
Participant flow chart.
Table 1.
Demographic Characteristics of the Subjects
| Group A (VISPO oral) N = 18 | Group B (Placebo oral) N = 19 | Group C (VISPO Topical) N = 18 | Group D (Placebo Topical) N = 18 | p-value (A vs B) | p-value (C vs D) | ||
|---|---|---|---|---|---|---|---|
| Sex | Male, n (%) | 14 (70) | 14 (70) | 16 (80) | 16 (80) | 1.000# | 1.000# |
| Female, n (%) | 6 (30) | 6 (30) | 4 (20) | 4 (20) | |||
| Age (y) | 35.10±6.03 | 36.45±6.01 | 34.05±7.07 | 35.85±6.28 | 0.483‡ | 0.399‡ | |
| Height (cm) | 166.40±10.19 | 165.55±8.12 | 169.05±8.11 | 170.60±8.71 | 0.708‡ | 0.134‡ | |
| Weight (kg) | 67.06±9.90 | 68.23±9.65 | 68.19±10.91 | 73.31±10.22 | 0.772‡ | 0.564‡ | |
| BMI (kg/m2) | 24.20±2.67 | 24.82±2.48 | 23.76±2.74 | 25.13±2.38 | 0.452‡ | 0.101‡ | |
Notes: The values are presented as the mean ± standard deviation. #Chi-square test; ‡Unpaired t-test.
Primary Outcomes
Investigator Assessment of Hair Shedding
In the present study, the protective effect of VISPO on hair shedding was validated using the hair comb and hair pull tests. The hair comb test, also known as 60-second hair count, is a simple and reliable tool for assessing hair shedding.20 (Wasko et al 2008). As shown in Table 2, hair shedding in the VISPO oral treatment group was reduced by 24.74% and 29% (p<0.001) after 8 and 16 weeks of treatment, respectively, compared to baseline. In the topical VISPO group, the hair fall score reduced by 12.08% and 22.19% (p<0.05) from baseline after 8 and 16 weeks of treatment, respectively (Table 3). In contrast, hair fall significantly increased in the respective placebo groups from baseline to the end of the study (p<0.05).
Table 2.
Effect of VISPO Oral Administration on Different Hair Parameters
| VISPO (n=18) | Placebo (n=19) | p-value (VISPO vs Placebo) | |
|---|---|---|---|
| Hair comb test score | |||
| Baseline | 19.56±6.67 | 19.32±7.67 | 0.920‡ |
| Week 8 | 14.72±6.06 | 22.32±7.48 | 0.002‡** |
| Week 16 | 13.89±5.68 | 22.84±6.46 | <0.001‡*** |
| p-value (Baseline vs Week 8) | <0.001#*** | 0.002#** | - |
| p-value (Baseline vs Week 16) | <0.001#*** | 0.030#* | - |
| Hair pull test score | |||
| Baseline | 8.53±0.40 | 8.53±0.31 | 0.990‡ |
| Week 8 | 7.28±0.77 | 8.58±0.38 | <0.001§*** |
| Week 16 | 6.78±0.65 | 8.66±0.37 | <0.001§*** |
| p-value (Baseline vs Week 8) | <0.001†*** | 0.608† | – |
| p-value (Baseline vs Week 16) | <0.001†*** | 0.243† | – |
| Hair density (Hairs/cm2) | |||
| Baseline | 222.56±46.81 | 215.21±29.05 | 0.573‡ |
| Week 8 | 227.56±39.45 | 209.37±30.03 | 0.126‡ |
| Week 16 | 234.06±37.74 | 208.26±29.18 | 0.027‡* |
| p-value (Baseline vs Week 8) | 0.123# | 0.112# | – |
| p-value (Baseline vs Week 16) | 0.009#** | 0.033#* | – |
| Hair thickness (µm) | |||
| Baseline | 16.89±3.46 | 17.16±2.61 | 0.792‡ |
| Week 8 | 17.94±3.90 | 16.89±3.57 | 0.400‡ |
| Week 16 | 19.61±3.26 | 17.53±3.45 | 0.067‡ |
| p-value (Baseline vs Week 8) | 0.249# | 0.759# | - |
| p-value (Baseline vs Week 16) | 0.017#* | 0.636# | - |
| Anagen/Telogen ratio | |||
| Baseline | 3.14±1.01 | 3.19±1.07 | 0.899‡ |
| Week 8 | 3.24±1.32 | 2.94±0.86 | 0.488§ |
| Week 16 | 3.65±1.23 | 2.97±1.20 | 0.112§ |
| p-value (Baseline vs Week 8) | 0.917† | 0.193† | - |
| p-value (Baseline vs Week 16) | 0.139† | 0.533† | - |
Notes: Values are mean±standard deviation (SD). #Paired t-test; ‡Independent t-test; †Wilcoxon signed-rank test; §Mann–Whitney U-test. *p<0.05, **p<0.01 and ***p<0.001.
Table 3.
Effect of VISPO Topical Administration on Different Hair Parameters
| VISPO (n=18) | Placebo (n=19) | p-value (VISPO vs Placebo) | |
|---|---|---|---|
| Hair comb test score | |||
| Baseline | 20.28±7.32 | 18.94±9.93 | 0.650‡ |
| Week 8 | 17.83±5.59 | 21.28±8.32 | 0.007§** |
| Week 16 | 15.78±6.41 | 22.94±8.69 | 0.008‡** |
| p-value (Baseline vs Week 8) | 0.044†* | 0.091† | |
| p-value (Baseline vs Week 16) | 0.029#* | 0.018#* | |
| Hair pull test score | |||
| Baseline | 8.53±0.44 | 8.47±0.40 | 0.693‡ |
| Week 8 | 7.33±0.64 | 8.56±0.34 | <0.001§*** |
| Week 16 | 6.78±0.55 | 8.58±0.35 | <0.001§*** |
| p-value (Baseline vs Week 8) | <0.001†*** | 0.437† | |
| p-value (Baseline vs Week 16) | <0.001†*** | 0.362† | |
| Hair density (Hairs/cm2) | |||
| Baseline | 205.67±34.34 | 230.22±47.15 | 0.084‡ |
| Week 8 | 214.11±35.48 | 222.94±49.96 | 0.545‡ |
| Week 16 | 221.33±37.61 | 221.83±45.90 | <0.001§*** |
| p-value (Baseline vs Week 8) | <0.001#*** | 0.007#** | |
| p-value (Baseline vs Week 16) | 0.0016†** | 0.044†* | |
| Hair thickness (µm) | |||
| Baseline | 16.50±4.40 | 16.61±3.88 | 0.936‡ |
| Week 8 | 17.17±3.38 | 16.17±3.03 | 0.357‡ |
| Week 16 | 17.44±3.67 | 16.56±3.40 | 0.456‡ |
| p-value (Baseline vs Week 8) | 0.378# | 0.606# | |
| p-value (Baseline vs Week 16) | 0.420# | 0.945# | |
| Anagen/Telogen ratio | |||
| Baseline | 3.10±0.95 | 3.25±0.89 | 0.631‡ |
| Week 8 | 2.96±0.81 | 2.97±0.76 | 0.936§ |
| Week 16 | 3.10±0.87 | 2.74±0.95 | 0.193§ |
| p-value (Baseline vs Week 8) | 0.323† | 0.202† | |
| p-value (Baseline vs Week 16) | 0.886† | 0.049†* | |
Notes: Values are mean±standard deviation (SD). #Paired t-test; ‡Independent t-test; †Wilcoxon signed-rank test; §Mann–Whitney U-test. *p<0.05, **p<0.01 and ***p<0.001.
In the hair pull test, the VISPO oral treatment group showed a progressive reduction in the hair fall score from baseline at visits 2 (8 weeks) and 3 (16 weeks). Hair fall was reduced by 14.65% and 20.52% after 8 and 16 weeks of VISPO ingestion, respectively, compared with baseline (p<0.001) (Table 2). A similar trend was observed in the VISPO topical treatment group, wherein hair fall was reduced by 14.06% and 20.52% from the baseline scores at visits 2 and 3, respectively (p<0.001) (Table 3).
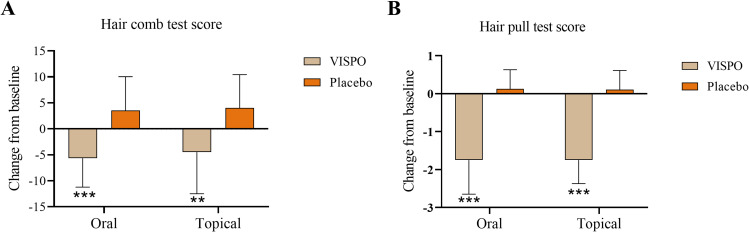
As shown in Figure 2, the reduction in hair fall in the VISPO treatment groups from baseline to the end of the study was significant compared with that in the respective placebo groups (p<0.001).
Figure 2.
Effect of VISPO formulations on the changes in hair fall (count) from baseline to the end of study. (A) Hair comb test and (B) hair pull test. The data were analyzed by independent t-test. **p<0.01 and ***p<0.001.
Subject Self-Assessment Questionnaires
The HGI is a validated, self-administered, 7-point scale hair growth questionnaire (hair thinning, coverage, and overall appearance). In the present study, the percentage of subjects who responded positively to the improvement in hair thickness and shaft appearance was significantly higher in the VISPO oral group than in the placebo (p<0.05). In addition, a considerable proportion of subjects in the VISPO oral group (33.3%) showed improvement in the amount of hair covering the scalp. However, most subjects in the topical treatment group responded with “no change” in the HGI questionnaire (Table 4).
Table 4.
Subject Self-Assessment – Hair Growth Index (HGI) Scoring
| Group A VISPO – Oral N = 18 n (%) | Group B Placebo – Oral N = 19 n (%) | Group C VISPO – Topical N = 18 n (%) | Group D Placebo – Topical N = 18 n (%) | p-value (A vs B) | p-value (C vs D) | |
|---|---|---|---|---|---|---|
| The area of thinning hair | ||||||
| Decreased | 0 (0) | 1 (5.26) | 0 (0) | 1 (5.56) | 0.170‡ | 0.597‡ |
| Increased | 6 (33.33) | 2 (10.53) | 2 (11.11) | 2 (11.11) | ||
| No change | 12 (66.67) | 16 (84.21) | 16 (88.89) | 15 (83.33) | ||
| The amount of hair covering the scalp | ||||||
| Decreased | 0 (0) | 0 (0) | 1 (5.56) | 1 (5.56) | 0.092‡ | 0.833‡ |
| Increased | 6 (33.33) | 2 (10.53) | 1 (5.56) | 2 (11.11) | ||
| No change | 12 (66.67) | 17 (89.47) | 16 (88.89) | 15 (83.33) | ||
| The quality of existing hair in terms of thickness and hair shaft appearance | ||||||
| Decreased | 0 (0) | 1 (5.26) | 1 (5.56) | 1 (5.56) | 0.022‡* | 0.833‡ |
| Increased | 9 (50) | 2 (10.53) | 1 (5.56) | 2 (11.11) | ||
| No change | 9 (50) | 16 (84.21) | 16 (88.89) | 15 (83.33) | ||
Note: ‡Chi square test; *p<0.05.
Hair growth satisfaction scale (HGSS) is a validated, self-administered 7-point scale that assesses satisfaction with the appearance of scalp hair (overall, scalp appearance, coverage, amount of hair in the thinning areas, and hair growth in the thinning areas). A high proportion of subjects in all treatment groups responded with “no change” to the HGSS questionnaire at the end of the study (Table 5). However, 33.33% of the VISPO oral group subjects provided positive feedback on “the overall appearance”, ‘the amount of hair covering the scalp”, and “new hair growth”.
Table 5.
Subject Self-Assessment – Hair Growth Satisfaction Scale (HGSS)
| Group A VISPO – Oral N = 18 n (%) | Group B Placebo – Oral N = 19 n (%) | Group C VISPO – Topical N = 18 n (%) | Group D Placebo – Topical N = 18 n (%) | p-value (A vs B) | p-value (C vs D) | |
|---|---|---|---|---|---|---|
| Overall appearance | ||||||
| Decreased | 0 (0) | 0 (0) | 0 (0) | 2 (11.11) | 0.092‡ | 0.344‡ |
| Increased | 6 (33.33) | 2 (10.53) | 2 (11.11) | 2 (11.11) | ||
| No change | 12 (66.67) | 17 (89.47) | 16 (88.89) | 14 (77.78) | ||
| The appearance of the thinning scalp | ||||||
| Decreased | 0 (0) | 1 (5.26) | 0 (0) | 1 (5.56) | 0.170‡ | 0.597‡ |
| Increased | 6 (33.33) | 2 (10.53) | 2 (11.11) | 2 (11.11) | ||
| No change | 12 (66.67) | 16 (84.21) | 16 (88.89) | 15 (83.33) | ||
| The area of thinning scalp | ||||||
| Decreased | 0 (0) | 1 (5.26) | 0 (0) | 1 (5.56) | 0.170‡ | 0.597‡ |
| Increased | 6 (33.33) | 2 (10.53) | 2 (11.11) | 2 (11.11) | ||
| No change | 12 (66.67) | 16 (84.21) | 16 (88.89) | 15 (83.33) | ||
| The amount of hair covering the scalp | ||||||
| Decreased | 0 (0) | 0 (0) | 1 (5.55) | 1 (5.56) | 0.092‡ | 0.833‡ |
| Increased | 6 (33.33) | 2 (10.53) | 1 (5.55) | 2 (11.11) | ||
| No change | 12 (66.67) | 17 (0.89) | 16 (88.89) | 15 (83.33) | ||
| New hair growth | ||||||
| Decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.092‡ | 1.000‡ |
| Increased | 6 (33.33) | 2 (10.53) | 2 (11.11) | 2 (11.11) | ||
| No change | 12 (66.67) | 17 (0.89) | 16 (88.89) | 16 (88.89) | ||
Note: ‡Chi square test.
Secondary Outcomes
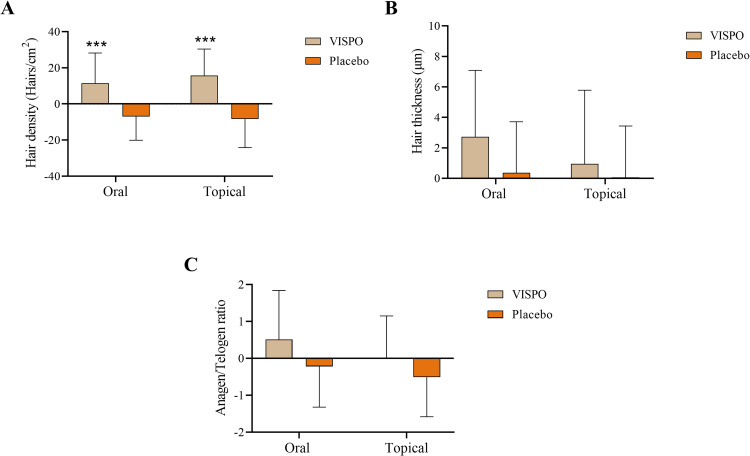
In the present study, phototrichogram analysis was used to measure secondary parameters such as hair density, thickness, and anagen/telogen ratio. At the end of the study, hair density in the VISPO oral group increased by 5.17% (p<0.01), while in the respective placebo group, it was reduced by 3.23% relative to the baseline (p<0.05). Furthermore, there was a significant increase in the mean hair thickness in the VISPO oral group compared with the baseline measurement (p<0.05). The anagen/telogen ratio increased in the VISPO oral group at the end of the study. However, these differences were not statistically significant (Table 2). The VISPO topical group showed a significant increase in hair density after 8 (4.1%, p<0.001) and 16 weeks (7.61%, p<0.001) of treatment compared to the baseline. However, in comparison with the baseline data, there was an insignificant change in hair thickness and anagen/telogen ratio in the VISPO topical group (Table 3). The change in hair density from baseline to the end of the study was significant in the VISPO oral and topical treatment groups compared with the respective placebo groups (p<0.001), whereas the changes with respect to hair thickness and anagen/telogen ratio were not statistically significant (Figure 3).
Figure 3.
Effect of VISPO formulations on changes in hair growth parameters – Phototrichogram analysis. (A) Hair density, (B) hair thickness and (C) anagen/telogen ratio. The data were analyzed by independent t-test. ***p<0.001.
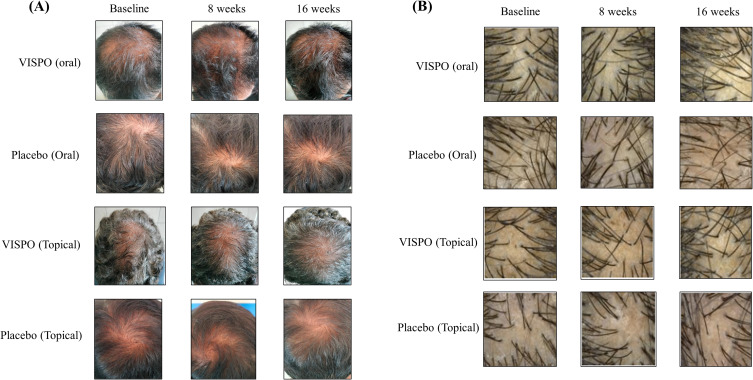
The global photographic assessment of scalp hair is presented in Table 6. The proportion of subjects showing improvement in both vertex and anterior scalp views at the end of the study was higher in the VISPO oral group than in the placebo group. In contrast, most participants in the VISPO topical treatment group showed no change in the photograph score. However, the proportion of subjects with a minimal decrease in the assessment score was lower in the topical VISPO group than in the placebo group. Figure 4 shows representative images of the photographic assessment and phototrichogram analysis.
Table 6.
Investigator Assessment Scoring of Photographs
| Group A VISPO – Oral N = 18 n (%) | Group B Placebo – Oral N = 19 n (%) | Group C VISPO – Topical N = 18 n (%) | Group D Placebo – Topical N = 18 n (%) | |
|---|---|---|---|---|
| Vertex | ||||
| Greatly decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderately decreased | 0 (0) | 0 (0) | 1 (5.56) | 1 (5.56) |
| Minimally decreased | 2 (11.11) | 6 (31.58) | 0 (0) | 4 (22.22) |
| Unchanged | 7 (38.89) | 11 (57.89) | 17 (94.44) | 11 (61.11) |
| Minimally increased | 8 (44.44) | 2 (10.53) | 0 (0) | 2 (11.11) |
| Moderately increased | 1 (5.56) | 0 (0) | 0 (0) | 0 (0) |
| Greatly increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anterior | ||||
| Greatly decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Moderately decreased | 0 (0) | 0 (0) | 1 (5.56) | 3 (16.67) |
| Minimally decreased | 4 (22.22) | 6 (31.58) | 2 (11.11) | 4 (22.22) |
| Unchanged | 8 (44.44) | 11 (57.89) | 15 (83.33) | 9 (50.00) |
| Minimally increased | 6 (33.33) | 2 (10.53) | 0 (0) | 2 (11.11) |
| Moderately increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Greatly increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Figure 4.
Representative images showing the changes in scalp hair following 16-week treatment with oral/topical VISPO formulations. (A) Representative photographs showing the vertex region of the scalp. (B) Phototrichogram analysis performed at baseline and at 8 and 16 weeks.
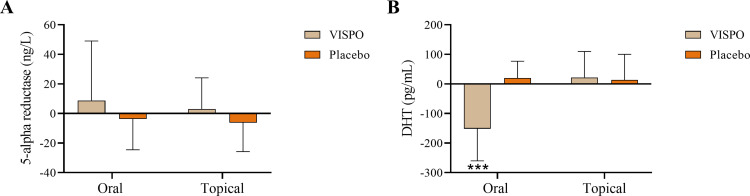
In the present study, serum samples from the subjects at baseline and at the end of the study were quantified for 5α-reductase levels using ELISA. As shown in Figure 5A, the VISPO oral and topical groups showed no significant change in the enzyme level from baseline to the end of the study compared to the respective placebo groups. Interestingly, the serum DHT level was significantly reduced in the VISPO oral treatment group by 1.29-fold at the end of the study compared to that at baseline (p<0.001). This change in the DHT level was significant compared to that in the placebo (p<0.001). However, the VISPO topical treatment group showed no significant change in DHT from baseline to the end of the study compared to the placebo group (Figure 5B). These data clearly suggest that oral administration of VISPO could markedly inhibit 5α-reductase activity, and thus, the accumulation of DHT in the subjects.
Figure 5.
Effect of VISPO formulations on the androgenic markers in serum. (A) 5α-reductase expression and (B) dihydrotestosterone (DHT) level. The data were analyzed by independent t-test. ***p<0.001.
Safety Evaluation
The topical formulations used in this study showed cutaneous tolerability based on investigator and subject self-assessments.
Serum biochemical analysis showed no significant changes in liver function parameters (aspartate aminotransferase AST and alanine aminotransferase (ALT) from baseline to the end of the study. Serum creatinine levels were significantly reduced from baseline to the end of the study in all treatment groups (p<0.05). No significant changes were observed in BUN levels of the treatment groups during the study (Table 7). The hematological parameters were within the normal range, except for some marginal variations (Table 8). Vital signs were within normal levels in all subjects in all groups throughout the study (data not shown).
Table 7.
Safety Evaluation – Analysis of Clinical Chemistry Parameters
| Parameter | Visit | Group A (N=18) VISPO-Oral | Group B (N=19) Placebo-Oral | Group C (N=18) VISPO-Topical | Group A (N=18) Placebo-Topical | ||||
|---|---|---|---|---|---|---|---|---|---|
| AST (U/L) | Baseline | 26.44±15.33 | 0.311# | 24.95±10.16 | 0.091 | 22.22±7.18 | 0.836# | 24.28±9.58 | 0.065 |
| Week 16 | 27.61±15.26 | 21.53±4.13 | 21.94±10.65 | 29.06±15.59 | |||||
| Change | 1.17±4.74 | −3.42±8.36 | −0.28±5.60 | 4.78±10.28 | |||||
| ALT (U/L) | Baseline | 29.17±15.43 | 0.731# | 24.05±12.88 | 0.149 | 22.61±12.98 | 0.332# | 27.00±14.00 | 0.684 |
| Week 16 | 28.44±15.45 | 21.11±8.36 | 21.22±10.90 | 26.50±12.03 | |||||
| Change | −0.72±8.75 | −2.95±8.53 | −1.39±5.90 | −0.50±5.12 | |||||
| Serum creatinine (mg/dL) | Baseline | 0.91±0.24 | <0.001#*** | 0.90±0.14 | <0.001#*** | 0.85±0.16 | 0.012#* | 0.88±0.15 | <0.001#*** |
| Week 16 | 0.84±0.22 | 0.81±0.14 | 0.78±0.19 | 0.81±0.16 | |||||
| Change | −0.07±0.07 | −0.09±0.09 | −0.06±0.10 | −0.07±0.06 | |||||
| BUN (mg/dL) | Baseline | 10.07±3.02 | 0.295# | 9.44±2.06 | 0.535 | 10.15±3.26 | 0.549 | 8.62±2.58 | 0.311 |
| Week 16 | 9.37±2.54 | 9.05±2.36 | 9.71±2.94 | 9.27±2.31 | |||||
| Change | −0.70±2.75 | −0.40±2.73 | −0.44±3.06 | 0.65±2.64 | |||||
Notes: Values are mean±SD. #Paired t-test; *p<0.05 and ***p<0.001.
Abbreviations: N, number of subjects; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BUN, Blood urea nitrogen.
Table 8.
Safety Evaluation – Analysis of Hematological Parameters
| Parameter | Visit | Group A (N=18) VISPO-Oral | Group B (N=19) Placebo-Oral | Group C (N=18) VISPO-Topical | Group A (N=18) Placebo-Topical | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/dL) | Baseline | 13.89±1.94 | 0.579# | 13.55±2.12 | 0.722# | 13.18±1.39 | 0.820# | 13.46±1.64 | 0.660# |
| EOS | 13.96±2.10 | 13.60±1.96 | 13.15±1.30 | 13.55±2.06 | |||||
| Change | 0.07±0.50 | 0.05±0.63 | −0.03±0.61 | 0.09±0.89 | |||||
| WBC (cells/mm3) | Baseline | 7426.67±2074.32 | 0.820# | 7093.10±1857.26 | 0.811# | 7861.67±1689.33 | 0.142# | 6938.33±1308.80 | 0.837# |
| EOS | 7362.78±1992.54 | 7026.32±1992.96 | 7453.89±1658.60 | 6869.44±1020.93 | |||||
| Change | −63.89±1171.13 | −66.84±1199.46 | 407.78±1122.87 | −68.89±1395.90 | |||||
| Neutrophils (%) | Baseline | 58.18±7.74 | 0.213# | 58.35±9.18 | 0.960# | 59.34±6.37 | 0.858# | 56.21±7.03 | 0.269# |
| EOS | 56.22±8.55 | 58.26±6.89 | 59.04±9.16 | 59.08±9.32 | |||||
| Change | −1.96±6.43 | −0.09±7.75 | −0.30±6.98 | 2.87±10.67 | |||||
| Lymphocytes (%) | Baseline | 31.14±6.19 | 0.073# | 30.86±8.82 | 0.954# | 29.11±5.73 | 0.328# | 32.37±8.13 | 0.433# |
| EOS | 33.97±7.16 | 30.96±5.84 | 30.38±7.83 | 30.95±8.39 | |||||
| Change | 2.82±6.26 | 0.10±7.46 | 1.27±5.36 | −1.42±7.52 | |||||
| Eosinophils (%) | Baseline | 3.99±2.57 | 0.285# | 3.97±2.13 | 0.798# | 5.18±4.37 | 0.079# | 3.11±1.79 | 0.618# |
| EOS | 3.65±2.34 | 3.86±2.50 | 4.45±4.08 | 2.94±1.74 | |||||
| Change | −0.34±1.30 | −0.11±1.86 | 0.73±1.66 | −0.17±1.44 | |||||
| Monocytes (%) | Baseline | 6.19±1.35 | 0.099# | 6.11±1.93 | 0.682# | 5.92±1.60 | 0.436# | 6.01±1.50 | 0.110# |
| EOS | 5.78±1.43 | 6.28±1.64 | 5.59±1.63 | 6.70±1.59 | |||||
| Change | −0.41±1.00 | 0.17±1.82 | −0.33±1.77 | 0.69±1.75 | |||||
| Basophils (%) | Baseline | 0.50±0.27 | 0.176# | 0.72±0.27 | 0.092# | 0.44±0.20 | 0.054# | 0.52±0.24 | 0.004#** |
| EOS | 0.39±0.23 | 0.56±0.28 | 0.54±0.21 | 0.33±0.20 | |||||
| Change | −0.11±0.33 | −0.15±0.37 | 0.10±0.21 | −0.19±0.24 | |||||
| PCV (%) | Baseline | 43.17±5.70 | 0.166# | 42.65±5.69 | 0.423# | 41.21±3.29 | 0.413# | 42.36±4.41 | 0.249# |
| EOS | 42.54±5.77 | 42.25±5.11 | 40.91±3.90 | 41.62±5.49 | |||||
| Change | −0.62±1.82 | −0.41±2.15 | −0.30±1.52 | −0.73±2.61 | |||||
| MCV (fL) | Baseline | 84.93±9.06 | 0.371# | 83.85±8.81 | 0.565# | 79.05±9.00 | 0.215# | 83.04±7.43 | 0.332# |
| EOS | 85.37±9.73 | 83.53±9.29 | 79.48±8.72 | 133.80±215.00 | |||||
| Change | 0.21±0.98 | −0.15±1.13 | 0.32±1.06 | 49.91±211.86 | |||||
| MCH (pg) | Baseline | 27.43±3.36 | 0.005#** | 26.61±3.69 | 0.110# | 25.55±3.25 | 0.807# | 26.65±2.82 | 0.004#** |
| EOS | 28.02±3.56 | 26.94±3.78 | 25.59±3.14 | 27.27±2.87 | |||||
| Change | 0.59±0.77 | 0.33±0.86 | 0.04±0.76 | 0.62±0.77 | |||||
| MCHC (g/dL) | Baseline | 32.17±0.78 | 0.028#* | 31.69±1.20 | 0.105# | 32.29±1.81 | 0.735# | 31.74±0.74 | 0.012#* |
| EOS | 32.78±1.33 | 32.16±1.63 | 32.15±1.12 | 32.52±1.38 | |||||
| Change | 0.61±1.08 | 0.46±1.18 | −0.14±1.78 | 0.77±1.16 | |||||
| Platelet count (Lakhs/cm2) | Baseline | 2.86±0.60 | 0.610# | 3.27±0.98 | 0.333# | 2.95±0.65 | 0.953# | 2.67±0.76 | 0.682# |
| EOS | 2.80±0.59 | 3.17±1.01 | 2.95±0.67 | 2.73±0.70 | |||||
| Change | −0.06±0.45 | 0.10±0.43 | 0.00±0.27 | 0.05±0.55 | |||||
Notes: Values are mean±standard deviation (SD). #Paired t-test; *p<0.05 and **p<0.01.
Abbreviations: N, number of subjects; Hb, Hemoglobin; WBC, White blood cell; PCV, Packed cell volume; MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; cmm, cubic millimeter; g/dl, gram/deciliter; fL, femtolitres; pg, picograms.
Ten subjects experienced mild AEs, including a common cold and headaches. The investigator’s diagnosis revealed that the reported AEs were unrelated to investigational products. None of the participants reported SAEs during the study.
Discussion
In the present study, oral and topical formulations of standardized SP oil (VISPO) were investigated for hair loss arrest in male and female patients with AGA. In this study, we compared the formulations with their respective placebo groups. A 16-week treatment with either formulation of VISPO significantly arrested hair fall compared with placebo. In addition, phototrichogram analysis revealed that the VISPO formulations considerably increased hair density from baseline to the end of the study. These results are consistent with those of previous studies. In a randomized clinical trial, Narda et al reported that a food supplement containing 100 mg of SP showed significant improvement in the hair pull test compared to placebo.15 In another prospective cohort study, a 24-week application of SP-containing hair lotion increased the total hair count in male AGA subjects by 4.9%.17
In the self-assessment of perceived treatment efficacy, the VISPO oral group participants responded positively to the HGI and HGSS scores. In addition, the VISPO group showed a higher proportion of respondents with an increase in vertex and anterior hair growth in the investigator’s photographic assessment score. These findings are in line with those of previous studies, where the oral ingestion of SP showed better patient satisfaction scores in the perceived subjective assessments than the placebo.24,25 However, most subjects in the VISPO topical group responded with “no change” in the subjective assessment.
One of the limitations of the present study is that the duration of treatment was restricted to 16 weeks. Our results clearly demonstrated that VISPO formulations were effective in reducing hair loss at the end of treatment. However, the formulations did not significantly change the anagen/telogen ratio from the baseline to the end of the study. Previously, SP extracts were used for longer durations to observe a significant change with respect to perceived efficacy and anagen induction.15,26 Overall, these observations clearly suggest that prolonged treatment with VISPO formulations could stimulate hair growth in subjects. Although no adverse effects were recorded during the study, it should be noted that the study duration was short and the sample size was small. Another limitation of this study was that we did not use any standard medications for efficacy comparisons.
Experimental studies of SP extracts have demonstrated their anti-androgenic effects as a function of 5α-reductase inhibition. SP extracts are rich in saturated fatty acids, such as lauric acid, myristic acid, linoleic acid, oleic acid, and sterols (β-sitosterol), which contribute significantly to 5α-reductase inhibition.27,28 These attributes could be responsible for the observed reduction in DHT levels in subjects after oral intake of VISPO. Interestingly, in the topical VISPO group, there was no significant reduction in the serum DHT level from baseline compared to the placebo group. One possible explanation for this observation is that the topical application of the actives generally results in higher DHT reduction in the scalp than in systemic circulation.29 In a recent double-blind study by Piraccini et al, the topical administration of finasteride showed similar efficacy to that of oral treatment in improving the hair count of subjects, but with less impact on serum DHT levels.30 Given that there is a clear DHT inhibition by VISPO oral treatment, it would seem there may also be sexual side effects, menstrual effects and mood changes in women, as in case of finasteride.31,32 However, none of the subjects reported such side effects during the study. The adverse effects reported with oral SP are mostly limited to mild gastrointestinal issues like nausea, diarrhea and constipation.33
In addition to its anti-androgenic action, SP extracts have been demonstrated to mediate hair regrowth in murine models via transforming growth factor-β (TGF-β) signaling.34 Further, SP formulations have been reported to exert anti-inflammatory effects in human keratinocytes.35 There are reports that β-sitosterol, the key constituent of SP, can instigate vascularization through its angiogenic ability.36 Based on these reports, it can be presumed that VISPO potentiates hair loss arrest by its anti-androgenic effect and further supports hair regrowth via stimulation of growth factors and neovascularization in the scalp.
Most clinical studies have been conducted with SP extract as an ingredient in formulations containing other active ingredients, such as vitamins and minerals.15,16 In such a scenario, it is difficult to ascertain the contribution of SP extract to hair care. In contrast, in the present study, the observed efficacy of the product (VISPO) could be attributed to SP extract alone. VISPO supports hair growth by reducing hair fall and increasing the hair density. This study provides further insights into the functionality of SP as key ingredient in hair care.
Conclusion
A 16-week treatment of AGA patients with oral and topical formulations of VISPO was effective for hair fall arrest. Furthermore, oral ingestion of VISPO significantly inhibited 5α-reductase activity and hence reduced DHT accumulation in the subjects. Though the data from this study provide limited evidence on the topical application of VISPO, long-term treatment with these formulations may be required to establish the complete efficacy of VISPO for hair regrowth in patients with AGA. Overall, VISPO can be effectively used as an active ingredient in functional and cosmetic formulations.
Acknowledgments
The authors acknowledge Mr. Sathish Mukashi, Vin Super Foods, Bangalore, India, for assistance with the formulation development.
Data Sharing Statement
The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Disclosure
The investigational product VISPOTM is a proprietary extract manufactured by Vidya Herbs Pvt., Ltd. Heggar Venkataramana Sudeep, Richards Aleksander, Kuluvar Gouthamchandra, and Kodimule Shyamprasad are employed by Vidya Herbs Pvt. Ltd., and Sriram Rashmi s employed by the BGS Global Institute of Medical Sciences, Bangalore, India. Thomas V. Jestin s employed by Leads Clinical Research and Bio Services Private Ltd. (Bangalore, India). The authors declare no conflicts of interest.
References
- 1.English RS. A hypothetical pathogenesis model for androgenic alopecia: clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med Hypotheses. 2018;111:73–81. doi: 10.1016/j.mehy.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 2.Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57:9–17. doi: 10.1007/s12020-017-1280-y [DOI] [PubMed] [Google Scholar]
- 3.York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21(5):603–612. doi: 10.1080/14656566.2020.1721463 [DOI] [PubMed] [Google Scholar]
- 4.Kranz D. Young men’s coping with androgenetic alopecia: acceptance counts when hair gets thinner. Body Image. 2011;8(4):343–348. doi: 10.1016/j.bodyim.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141.e135. doi: 10.1016/j.jaad.2017.02.054 [DOI] [PubMed] [Google Scholar]
- 6.Triyangkulsri K, Suchonwanit P. Role of Janus kinase inhibitors in the treatment of alopecia areata. Drug Des Devel Ther. 2018;12:2323–2335. doi: 10.2147/DDDT.S172638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fertig R, Tosti A. Frontal fibrosing alopecia treatment options. Intractable Rare Dis Res. 2016;5(4):314–315. doi: 10.5582/irdr.2016.01065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777–2786. doi: 10.2147/DDDT.S214907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zuuren EJ, Fedorowicz Z. Interventions for female pattern hair loss. JAMA Dermatol. 2017;153(3):329–330. doi: 10.1001/jamadermatol.2016.5790 [DOI] [PubMed] [Google Scholar]
- 10.Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5-alpha reductase inhibitors (Finasteride, Dutasteride): a systematic review. J Clin Aesthet Dermatol. 2016;9(7):56–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Abe M, Ito Y, Suzuki A, Onoue S, Noguchi H, Yamada S. Isolation and pharmacological characterization of fatty acids from saw palmetto extract. Anal Sci. 2009;25:553‐557. doi: 10.2116/analsci.25.553 [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Sharma V, Chauhan N, et al. Hair growth: focus on herbal therapeutic agent. Curr Drug Discov Technol. 2015;12:21‐42. doi: 10.2174/1570163812666150610115055 [DOI] [PubMed] [Google Scholar]
- 13.Pais P, Villar A, Rull S. Determination of the potency of a novel saw palmetto supercritical CO2 extract (SPSE) for 5α-reductase isoform II inhibition using a cell-free in vitro test system. Res Rep Urol. 2016;8:41–49. doi: 10.2147/RRU.S96576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon Y. Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci Biotechnol. 2019;28(6):1599–1606. doi: 10.1007/s10068-019-00605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narda M, Aladren S, Cestone E, Nobile V. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3:127. doi: 10.4172/2471-9323.1000127 [DOI] [Google Scholar]
- 16.Gómez Grau E, Lladós Sevilla M, Mira J, Vivancos F. Efficacy of a food supplement with Serenoa serrulata and tocotrienol-tocopherol against androgenetic alopecia and female telogen effluvium: about a pilot study. Rev. Argent Dermatol. 2015;96(1):43–55. [Google Scholar]
- 17.Wessagowit V, Tangjaturonrusamee C, Kootiratrakarn T, et al. Treatment of male androgenetic alopecia with topical products containing Serenoa repens extract. Australas J Dermatol. 2016;57(3):e76–82. doi: 10.1111/ajd.12352 [DOI] [PubMed] [Google Scholar]
- 18.Sudeep HV, Venkatakrishna K, Amrutharaj B, Shyamprasad K, Shyamprasad K. A phytosterol-enriched saw palmetto supercritical CO2 extract ameliorates testosterone-induced benign prostatic hyperplasia by regulating the inflammatory and apoptotic proteins in a rat model. BMC Complement Altern Med. 2019;19(1):270. doi: 10.1186/s12906-019-2697-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudeep HV, Thomas JV, Shyamprasad K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020;20(1):86. doi: 10.1186/s12894-020-00648-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasko CA, Mackley CL, Sperling LC, Mauger D, Miller JJ. Standardizing the 60-second hair count. Arch Dermatol. 2008;144:759–762. doi: 10.1001/archderm.144.6.759 [DOI] [PubMed] [Google Scholar]
- 21.Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1(2):108–119. doi: 10.4103/0974-7753.58553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubelin Harcha W, Barboza Martínez J, Tsai TF, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014;70(3):489–498.e3. doi: 10.1016/j.jaad.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 23.Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J Am Acad Dermatol. 1998;39(4 Pt 1):578–589. doi: 10.1016/S0190-9622(98)70007-6 [DOI] [PubMed] [Google Scholar]
- 24.Zanzottera F, Bizzaro G, Michelotti A, Nobile V. Efficacy of a nutritional supplement, standardized in fatty acids and phytosterols, on hair loss and hair health in both women and men. J Cosmo Trichol. 2017;3(121):2. doi: 10.4172/2471-9323.1000121 [DOI] [Google Scholar]
- 25.Farris PK, Rogers N, McMichael A, Kogan S. A novel multi-targeting approach to treating hair loss, using standardized nutraceuticals. J Drugs Dermatol. 2017;16(11):S141–8. [PubMed] [Google Scholar]
- 26.Pezza M, Carlomagno V, Casucci G. Telogen effluvium treated with Serenoa repens supplement. J Senses Sci. 2014;1:1. [Google Scholar]
- 27.Abe M, Ito Y, Oyunzul L, Oki-Fujino T, Yamada S. Pharmacologically relevant receptor binding characteristics and 5 alpha-reductase inhibitory activity of free fatty acids contained in saw palmetto extract. Biol Pharm Bull. 2009;32(4):646–650. doi: 10.1248/bpb.32.646 [DOI] [PubMed] [Google Scholar]
- 28.Cabeza M, Bratoeff E, Heuze I, Ramirez E, Sanchez M, Flores E. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc. West Pharmacol Soc. 2003;46:153–155. [PubMed] [Google Scholar]
- 29.Caserini M, Radicioni M, Leuratti C, Terragni E, Iorizzo M, Palmieri R. Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. Int. J Clin Pharmacol Ther. 2016;54(1):19–27. [DOI] [PubMed] [Google Scholar]
- 30.Piraccini BM, Blume-Peytavi U, Scarci F, et al. Topical finasteride study group. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a Phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol. 2022;36(2):286–294. doi: 10.1111/jdv.17738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8:1747–1753. doi: 10.1111/j.1743-6109.2011.02255.x [DOI] [PubMed] [Google Scholar]
- 32.Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichology. 2018;10(1):48–50. doi: 10.4103/ijt.ijt_73_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agbabiaka TB, Pittler MH, Wider B, Ernst E. Serenoa repens (saw palmetto): a systematic review of adverse events. Drug Saf. 2009;32(8):637–647. doi: 10.2165/00002018-200932080-00003 [DOI] [PubMed] [Google Scholar]
- 34.Zhu HL, Gao YH, Yang JQ, Li JB, Gao J. Serenoa repens extracts promote hair regeneration and repair of hair loss mouse models by activating TGF-β and mitochondrial signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):4000–4008. [DOI] [PubMed] [Google Scholar]
- 35.Chittur S, Parr B, Marcovici G. Inhibition of inflammatory gene expression in keratinocytes using a composition containing carnitine, thioctic acid and saw palmetto extract. Evid Based Complement Alternat Med. 2011;2011(985345):1–7. doi: 10.1093/ecam/nep102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi S, Kim KW, Choi JS, et al. Angiogenic activity of beta-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med. 2002;68(4):330–335. doi: 10.1055/s-2002-26750 [DOI] [PubMed] [Google Scholar]