Abstract
Vibrio cholerae O1 strain TSI-4 (El Tor, Ogawa) can shift to a rugose colony morphology from its normal translucent colony morphology in response to nutrient starvation. We have investigated differences between the rugose and translucent forms of V. cholerae O1 strain TSI-4. Electron microscopic examination of the rugose form of TSI-4 (TSI-4/R) revealed thick, electron-dense exopolysaccharide materials surrounding polycationic ferritin-stained cells, while the ferritin-stained material was absent around the translucent form of TSI-4 (TSI-4/T). The exopolysaccharide produced by V. cholerae TSI-4/R was found to have a composition of N-acetyl-d-glucosamine, d-mannose, 6-deoxy-d-galactose, and d-galactose (7.4:10.2:2.4:3.0). The expression of an amorphous exopolysaccharide promotes biofilm development under static culture conditions. Biofilm formation by the rugose strain was determined by scanning electron microscopy, and most of the surface of the film was colonized by actively dividing rod cells. The corresponding rugose and translucent strains were compared for stress resistance. By having exopolysaccharide materials, the rugose strains acquired resistance to osmotic and oxidative stress. Our data indicated that an exopolysaccharide material on the surface of the rugose strain promoted biofilm formation and resistance to the effects of two stressing agents.
Cholera is a serious epidemic disease that has killed millions of people and continues to be a major health problem worldwide. Vibrio cholerae, the bacterium that causes cholera, is a motile, gram-negative, curved rod with a single polar flagellum. The hypothesis that V. cholerae occupies an ecological niche in the estuarine environment requires that this organism be able to survive the dynamics of various physiochemical changes, including variations in nutrient concentrations. As a response to nutrient depletion, copiotrophic (31, 42), heterotrophic bacteria may undergo considerable morphological, physiological, and chemical changes (13, 22, 23, 26–28). In fact, to survive energy- and nutrient-deprived conditions, non-spore-forming, heterotrophic bacteria are known to undergo an active adaptation program (28). Brown and Williams have provided detailed experimental evidence that the molecular composition of the bacterial cell walls is essentially plastic and is remarkably responsive to the cell’s growth environment (5). Rice et al. (33) discovered that V. cholerae O1 from the Peru epidemic was able to shift to a phenotype having a wrinkled or rugose colony morphology. They also suggested that the V. cholerae rugose phenotype represents a fully virulent survival form of the organism that can persist in the presence of free chlorine. Morris et al. (29) reported that V. cholerae can shift to a rugose colony morphology associated with the expression of an amorphous exopolysaccharide (EPS) that promotes cell aggregation, and they also confirmed that rugose strains displayed resistance to killing by chlorine and complement-mediated serum bactericidal activity. They also indicated that these rugose strains cause human disease. However, the phenotypic characteristics associated with rugose morphology, relationships between these characteristics, and their relative importance in pathogenicity still remained to be identified.
A large variety of EPSs are synthesized by gram-negative bacteria. While some have been implicated in the pathogenicity of plant and mammalian hosts, others have not been assigned a function, but many serve a structural role, benefiting the bacterium by enabling attachment to surfaces, improving nutrient acquisition, or providing protection from environmental stresses and host defenses (36). The EPSs cover the surfaces of many gram-negative and gram-positive bacteria. They may form a capsule composed of a high-molecular-weight polysaccharide attached to the cell surface, or they may produce slime either loosely attached to the cell surface or released to the culture fluid. Bacterial cells initiate the process of irreversible adhesion by binding to the surface by using EPS glycocalyx polymers and the development of microcolonies. The eventual production of a continuous biofilm on the colonized surface is a function of cell division within microcolonies and recruitment of bacteria from the planktonic phase. The biofilm concept has drawn attention to the bacterium’s ecological and biotechnological importance (8–11). We must now accept the unequivocal evidence that bacteria respond to changes in their environment by profound phenotypic variations in enzymatic activity, cell wall composition (34), and surface structure (2).
In this study, we have isolated the rugose variants of V. cholerae O1 strain TSI-4 from starvation medium and determined EPS expression on the cell surface of the rugose strain by polycationic ferritin-labeled thin-section electron microscopy. While examining the morphological characteristics of these rugose strains, we found that they produced a continuous biofilm on the colonized surface and culture tube walls. Directly sampled, intact biofilms were subjected to electron microscopic analysis. We have also studied the role of the slime polysaccharide of V. cholerae TSI-4 in the bacterium’s resistance to osmotic and oxidative stress.
MATERIALS AND METHODS
Organism and microcosm conditions.
V. cholerae O1 strain TSI-4 (El Tor Ogawa) was used in this study. Frozen stocks were maintained at −80°C in L broth (25) containing 50% glycerol. The original isolate of strain TSI-4 had a translucent colony morphology. Cells of TSI-4 were routinely grown at 37°C on a rotary shaker in L broth. The culture was incubated to mid-log phase, which corresponded to an A600 of 0.4. The cells were then harvested by centrifugation (13,000 × g for 10 min), washed three times with cold M9 salts (37), resuspended in starvation medium (M9 salts) to give a final concentration of approximately 5 × 107 cells/ml, and incubated at 16°C without shaking. Strain TSI-4 exhibits a shift of colony morphology to the rugose form under starvation conditions at 2 months after inoculation. The rate of phase variation from the rugose form to the translucent form was assessed by inoculating an isolated rugose colony into L broth and incubating it overnight with shaking at 37°C and then plating serial dilutions of the bacteria onto L agar incubated overnight at 37°C.
Polycationic ferritin labeling and electron microscopy.
Bacteria were grown on L agar overnight at 37°C, harvested, and washed twice with cacodylate buffer (0.1 M, pH 7.0). Bacterial cells were fixed for 2 h at 20°C in cacodylate buffer containing 5% glutaraldehyde. Fixed bacteria were washed and resuspended in cacodylate buffer and allowed to react with polycationic ferritin (Sigma Chemical Co., St. Louis, Mo.) (final concentration, 1.0 mg/ml) for 30 min at 20°C (12, 15, 19). The samples were diluted 1:10 with the same buffer, and the bacterial cells were centrifuged and washed three times in cacodylate buffer. Bacterial cells were then immobilized in 4% agar, postfixed for 2 h with 2% osmium tetroxide, and then washed three times. The samples were dehydrated in a graded series of acetone washes, washed twice in propylene oxide, and then embedded in Epon by a rapid embedding technique (30). Thin sections were poststained with uranyl acetate and lead citrate and examined with a JEM 2000EX electron microscope (JEOL, Ltd., Tokyo, Japan) at 100 kV.
Antiserum.
An anti-EPS antibody was prepared by the specific-adsorption method from the serum of a rabbit immunized with rugose variant strain TSI-4/R as follows. A rabbit was immunized with a bacterial suspension (108 CFU/ml) of strain TSI-4/R by three injections at 5-day intervals, and 7 days after the last injection, blood was collected from the ear vein. Antibodies other than the anti-EPS antibody were removed from the serum by the adsorption technique. About 2 ml of the serum and about 109 CFU of heat-inactivated strain TSI-4/T (translucent variant) per ml were mixed, and the mixture was stirred during overnight incubation at 4°C. The bacteria were then removed by centrifugation at 5,000 × g for 10 min, and the supernatant was again mixed with the same strain. This adsorption process was repeated three times, and the final supernatant was used as anti-EPS serum. The final titer of the antiserum was measured by tube agglutination. Strain TSI-4/R was used as the antigen to determine the titer of the anti-EPS antibody. The agglutination titer of anti-EPS serum against TSI-4/R was 1:256, and it did not agglutinate strain TSI-4/T.
Immunoelectron microscopy.
For immunoelectron microscopy, a colloidal gold probe (Wako Pure Chemical Industries Ltd., Osaka, Japan) was used to label the specific reaction sites of anti-EPS serum and an anti-O1 monoclonal antibody (mouse immunoglobulin G anti-V. cholerae monoclonal antibody reacting with lipopolysaccharide [LPS] epitopes present in the bacterial cell wall). The anti-V. cholerae monoclonal antibody was purchased from Cosmo Bio Co., Ltd. (6). To label the specimens, 1 ml of a bacterial suspension of about 108 CFU/ml was treated with antiserum appropriately diluted with phosphate-buffered saline (PBS) for 30 min at 37°C. The bacteria then were separated from the serum by centrifugation at 12,000 × g for 10 min. After being washed three times with PBS, the bacteria were mixed with a suspension of the colloidal gold probe, and the mixture was kept at room temperature for 30 min. After being washed with PBS to remove nonreacted gold particles, the bacteria were processed for thin-section electron microscopy (1).
EPS isolation and purification.
Cells were harvested from a 24-h culture on an L agar plate and resuspended in physiological saline (0.85% sodium chloride). Samples were centrifuged at 1,600 × g for 20 min, and supernatants were dialyzed with multiple changes of distilled water. The specimens were then ultracentrifuged at 154,000 × g for 15 h at 20°C, and the supernatants were removed and subjected to enzymatic digestion with RNase (100 μg/ml), DNase I (50 μg/ml plus 1 mM MgCl2), and pronase (250 μg/ml), followed by sequential phenol-chloroform extraction (32). Purity was assessed by lack of detectable protein on silver-stained sodium dodecyl sulfate-polyacrylamide gel and by wavelength scanning spectrophotometric analysis (Milton Roy Spectronic Genesys 5 spectrophotometer; Milton Co. Ltd., New York, N.Y.). The total neutral sugar content was determined by the phenol-sulfuric acid method (14). Qualitative and quantitative sugar analyses were carried out after pyridylamination by using a Palstation pyridylamination reagent kit (Takara Biomedicals, Kyoto, Japan) (35).
Outer membrane and LPS preparation.
The outer membrane was prepared from a broth culture of either strain TSI-4/T or strain TSI-4/R by the method of Filip et al. (16). LPS was prepared from 1 ml of an overnight culture (18). LPS and outer membrane samples were electrophoresed and detected by silver staining as previously described (24).
Preparation of DNA.
The high-molecular-weight chromosomal DNAs of V. cholerae TSI-4/T and TSI-4/R were prepared essentially by the method of Berns and Thomas (4). Plasmid DNA was isolated by the alkaline method (25). HindIII, BglII, and PstI were used for chromosomal DNA and plasmid fingerprinting (40).
Scanning electron microscopy.
V. cholerae TSI-4 was cultured in L broth at 37°C without shaking for 3 to 5 days. The biofilms growing on the upper surface of the L broth and on the wall of a culture tube were sampled for scanning electron microscopy. The specimens were fixed with 2% glutaraldehyde in PBS for 1 h followed by 1% osmium tetroxide overnight at 4°C, dehydrated with a series of acetone concentrations ranging from 50 to 100%, dried by the critical-point drying method, coated with gold-palladium for surface conductivity, and examined with a scanning image observing device (ASID) equipped with a JEOL JEM 2000EX electron microscope.
Stress resistance assay.
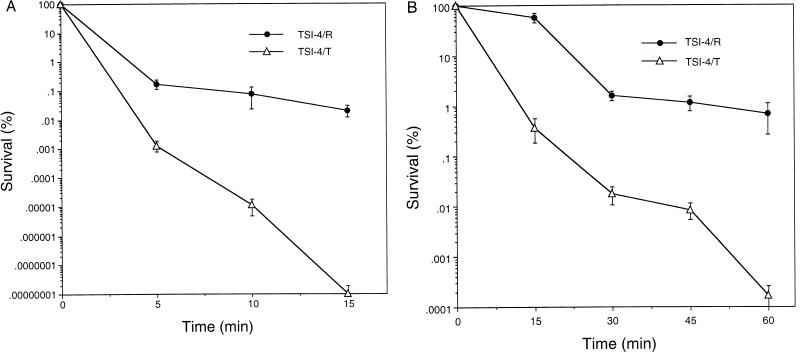
Stress resistance was assessed by using a 1:1,000 dilution of an overnight culture of either TSI-4/T or TSI-4/R in 3.0 ml of minimal glucose medium. The diluted cultures were grown to approximately 2 × 109 cells per ml, which corresponded to an A600 of 0.4, and cells were harvested by centrifugation and resuspended in minimal glucose medium (pH 7.0) which contained either 20 mM H2O2 (oxidative stress) or 2.5 M NaCl (osmotic stress). Survival was measured at various times, depending upon the type of stress applied. Percent survival was calculated by dividing the number of CFUs at a given time by the number of CFUs at time zero and multiplying the result by 100. One hundred percent viability was taken to be approximately 2 × 109 cells per ml and represents the viable counts obtained immediately before challenge. Percent survival of TSI-4/T and that of TSI-4/R were compared (see Fig. 6).
FIG. 6.
Increased resistance of strain TSI-4/R to oxidative stress (A) and osmotic stress (B). Strains TSI-4/R and TSI-4/T were collected at mid-exponential phase and tested as described in Materials and Methods. The time points for oxidative challenge (H2O2) were 0, 5, 10, and 15 min, and the time points for osmotic challenge (NaCl) were 0, 15, 30, 45, and 60 min. Survival is expressed as the percentage of the initial cell input that survived the treatment. Bars show standard errors (each point is the average of three separate experiments). The P values (t test) for the 10-min time point in the oxidative stress resistance test and the 30-min time point in the osmotic stress resistance test are <0.001 and <0.01, respectively.
Statistical analysis.
Data are expressed as means ± standard errors. Differences between experimental groups were analyzed by Student’s t test (unpaired), and a P of <0.05 was accepted as statistically significant.
RESULTS
Isolation of the rugose strain and phase variation.
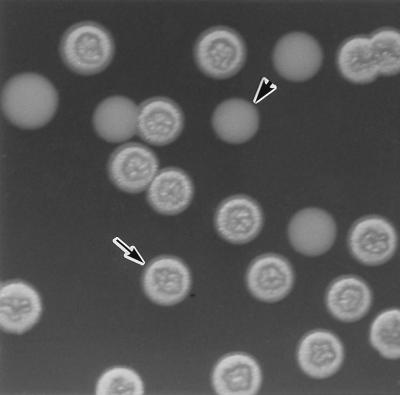
We have found that V. cholerae O1 strain TSI-4 is able to shift to a phenotype having a rugose colony morphology under starvation conditions. To isolate spontaneous rugose variants, bacteria from smooth colonies were starved in M9 salts for 2 months at 16°C and then plated on L agar at 37°C. TSI-4 underwent phase variation, converting from translucent to rugose in M9 salts and back again at a low rate in L broth. Rugose TSI-4/R colonies inoculated into L broth and subcultured on L agar produced translucent TSI-4/T colonies at a frequency of 1.5 × 10−5. The two distinct colony morphologies are shown in Fig. 1.
FIG. 1.
Photomicrograph of V. cholerae O1 rugose TSI-4/R (arrow) and translucent TSI-4/T (arrowhead) colonies.
Thin-section electron microscopy.
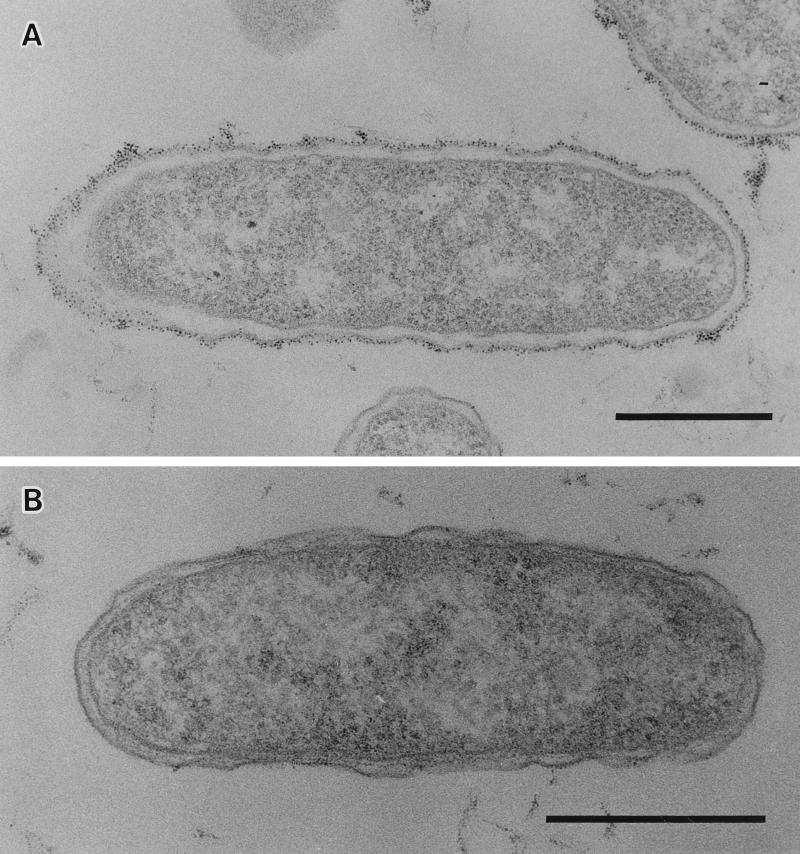
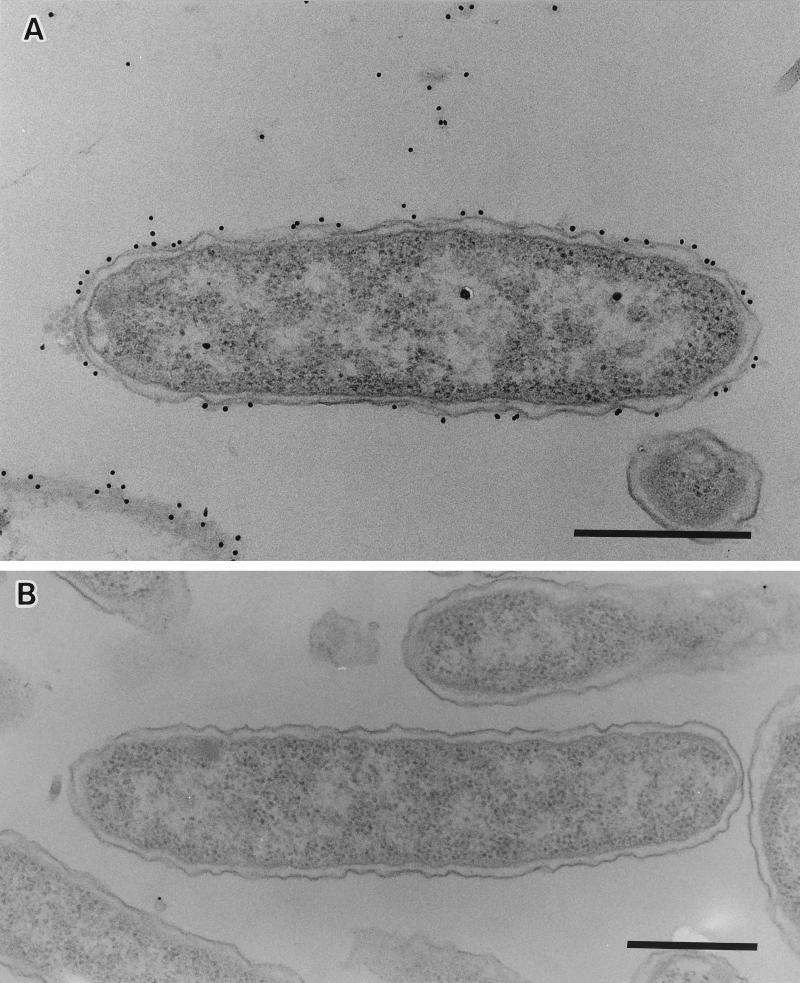
To determine the nature of the colony morphology differences, bacterial pellets were stained with polycationic ferritin and thin sections were observed by electron microscopy. Representative profiles are shown in Fig. 2. EPS materials of TSI-4/R were recognized as a heavy, fibrous, electron-dense, ferritin-stained layer completely surrounding the cell (Fig. 2A), but TSI-4/T did not appear to have this external layer surrounding its cells (Fig. 2B). The staining of the extracellular fibrous layer by polycationic ferritin strongly suggests the presence of acidic polysaccharide, and it shows the electrostatic binding of a derivative of ferritin to anionic sites on the cell surface (12, 20).
FIG. 2.
Thin sections of V. cholerae stained with polycationic ferritin showing a thick, electron-dense EPS layer completely surrounding TSI-4/R cells (A) and the absence of this layer surrounding a cell of TSI-4/T (B). Bars, 0.5 μm.
Immunoelectron microscopy.
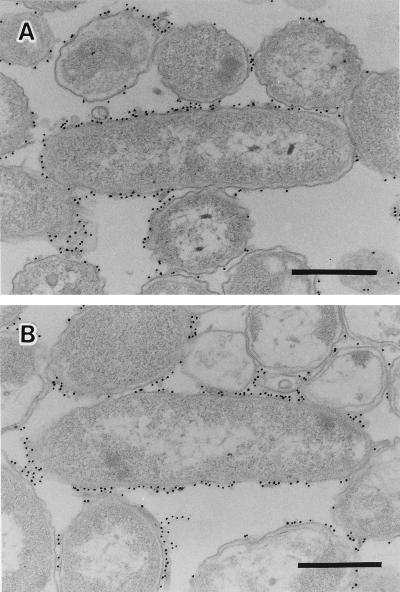
The reactivity of the anti-V. cholerae O1 monoclonal antibody with the cell surfaces was tested with both strains TSI-4/R and TSI-4/T. The reaction of anti-O1 serum with both strains is shown in Fig. 3. The gold particles were specifically found on the outer membrane surfaces and were believed to be the antibody reacting with the surface O antigens of both strains. The anti-EPS serum prepared by specific adsorption of anti-TSI-4/R serum with strain TSI-4/T was reactive only with TSI-4/R and not with TSI-4/T (Fig. 4A and B). The gold particles were specifically bound to the EPS material on the surface of strain TSI-4/R and at the intercellular spaces (Fig. 4A).
FIG. 3.
Immunoelectron micrographs of the surface labeling of strains TSI-4/R (A) and TSI-4/S (B) with anti-V. cholerae O1 monoclonal antibody. Bars, 0.5 μm.
FIG. 4.
Strains TSI-4/R (A) and TSI-4/T (B) treated with anti-EPS serum and labeled with a gold probe. Bars, 0.5 μm.
LPS and outer membrane profiles.
Additional testing of the two morphotypes was undertaken to identify differences which might contribute to different colony morphologies, outer membrane proteins and LPS were prepared from both cell types of TSI-4, electrophoresed, and detected. No outer membrane protein or LPS differences between cell types were detected (data not shown). To further ensure that the rugose strain was a phenotypic variant of V. cholerae O1 strain TSI-4, we also performed plasmid and DNA fingerprint analyses. The plasmid and DNA patterns of the two strains were the same (data not shown).
EPS extraction.
The purified EPS from TSI-4/R was quantified by the phenol-sulfuric acid method of Dubois et al. (14). The reaction of extracted EPS material with phenol and sulfuric acid confirms the presence of sugars and suggests that these sugars are hexoses and methylhexoses because of a characteristic absorbance peak at 490 nm. Spectrophotometric scanning of the purified EPS also showed a lack of absorbance at 260 or 280 nm, indicating an absence of contaminating protein or nucleic acids. Qualitative and quantitative sugar analyses showed that the extracellular polysaccharide of V. cholerae TSI-4/R contains N-acetyl-d-glucosamine, d-mannose, 6-deoxy-d-galactose, and d-galactose at a molar ratio of 7.4:10.2:2.4:3.0.
Biofilm growth of V. cholerae TSI-4/R and scanning electron microscopy.
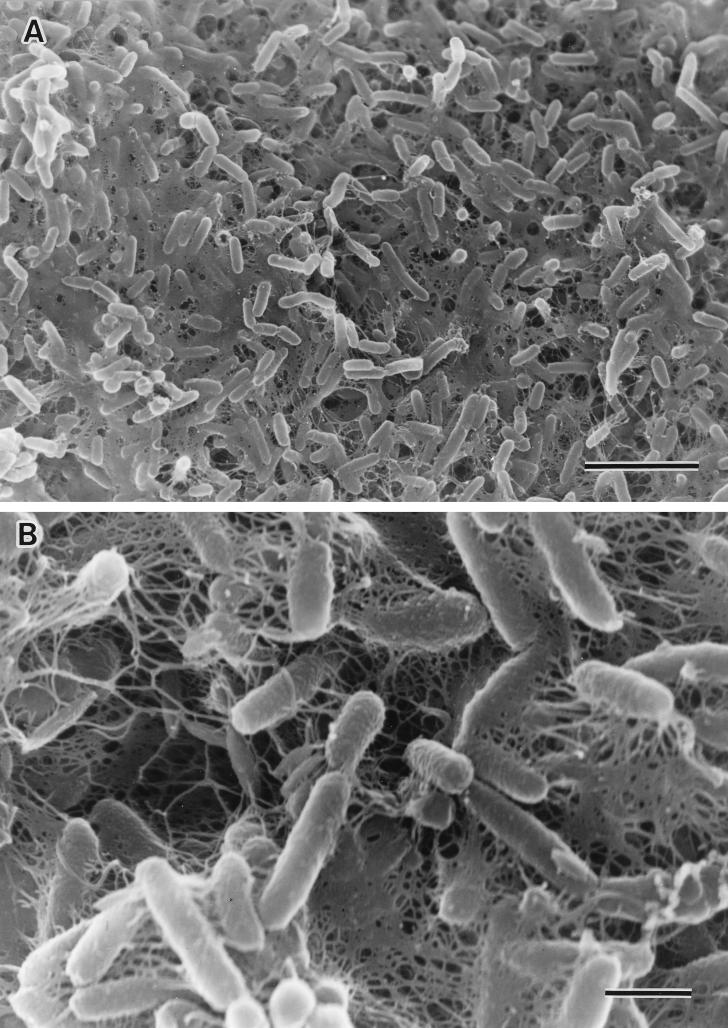
The biofilms of V. cholerae TSI-4/R were clearly visible on the upper surface of L broth and culture tube walls after 5 days of static incubation at 37°C, whereas TSI-4/T did not have the biofilm-forming property and produced a smooth suspension of bacteria. Figure 5 shows a biofilm under scanning electron microscopy; the surface of the film was completely covered with a layer of contiguous bacterial cells embedded within a polymeric matrix. Throughout the biofilm, cells were interconnected by a finger-like glycocalyx matrix that extended from the substratum to the outer boundaries of the biofilm.
FIG. 5.
Scanning electron micrographs of biofilm formation by V. cholerae O1 strain TSI-4/R. (A) Most of the surface has been colonized with actively dividing rod cells, and finger-like projections of extracellular polymeric material are present. Bar, 5 μm. (B) High magnification indicates the presence of extracellular polymeric materials on the surfaces of bacterial cells. Bar, 1 μm.
Rugose TSI-4 exhibits elevated resistance to osmotic and oxidative stress.
Cells of both TSI-4/T and TSI-4/R were collected at mid-exponential phase and tested as described in Materials and Methods. V. cholerae O1 strain TSI-4/R was much more resistant to oxidative and osmotic stress than was strain TSI-4/T, showing viability more than 10 times greater than that of strain TSI-4/T (Fig. 6).
DISCUSSION
When bacteria are transported from one environment to another, the environmental changes with which they are confronted include changes in temperature, nutrient concentration, salinity, osmotic pressure, pH, and many other factors. However, bacterial cells dynamically adapt to shifts in environmental parameters by employing a variety of genotypic and phenotypic mechanisms (7). Starvation-induced changes in bacterial surfaces have been reported for several strains of marine bacteria by Kjelleberg and Hermansson (21). The cells of V. cholerae demonstrate a predilection for association with chitinaceous surfaces and the mucilaginous sheath of algae that can be interpreted as an ecological advantage (3).
In this paper, we report that the growth of V. cholerae O1 strain TSI-4 (El Tor, Ogawa) in starvation medium resulted in a change to a wrinkled or rugose colony morphology from the normal translucent colony morphology and, at the same time, significant production of EPSs, some of which are loosely attached to the cell surface and some of which are released into the intercellular spaces. Analogies to rugosity can be found in a number of other bacterial species, including the expression of alginate by mucoid strains of Pseudomonas aeruginosa and the expression of an adhesive EPS by members of the marine genus Hyphomonas. Wrangstadh et al. (41) demonstrated that energy and nutrient starvation of marine Pseudomonas sp. strain S9 induced the production and release of an EPS with resulting pronounced effects on the adhesion and aggregation of the bacterial cells. Our data indicate that cell surface EPS materials confer a rugose colony morphology, biofilm formation ability, and resistance to osmotic and oxidative stress. We suggest that the spontaneous and reversible variation in cell-associated and cell-free EPS production represents an optimal adaptive mechanism that facilitates survival in stressful environments.
The rugose form of V. cholerae was first described in 1938 by Bruce White, who recognized that it might serve as a survival form of the organism (39). Rice et al. (33) suggested that the V. cholerae rugose phenotype represents a fully virulent survival form of the organism that can persist in the presence of free chlorine and that this phenotype may limit the usefulness of chlorination in blocking the endemic and epidemic spread of cholera. Morris et al. (29) have supported and confirmed his finding that rugose strains appear to produce an EPS that promotes cell aggregation and causes human disease. This aggregation may shield individual cells from killing by disinfectants, such as chlorine, or lysis by complement. He also suggested that the EPS produced by V. cholerae plays a role in marine biofilm formation; this, in turn, may contribute to attachment of bacteria to marine organisms, such as plankton. Our findings confirm and support their suggestions that EPS-producing rugose vibrios promote cell aggregation in a shaking culture at stationary phase and that this adhesive EPS and rugose vibrio can form a biofilm in a static culture. We identified the biofilm of TSI-4/R macroscopically and electron microscopically. EPS materials were also identified by ferritin-labeled thin-section electron microscopy. Polycationic ferritin has previously been used to visualize the capsular material of many organisms, such as Klebsiella sp. (38), Neisseria gonorrhoeae (17), and non-O1 V. cholerae (20).
Routine practices in clinical laboratories and researchers working with V. cholerae have routinely selected smooth colonies from culture plates for investigation. A rugose colony is easily confused with a contaminated colony because of its unusual morphology and is likely to be dismissed as an avirulent rough variant or a contaminant. This rugose form can survive in the presence of the first-line disinfectant chlorine and remain fully virulent (29, 33). Our studies extend previous findings by indicating that the rugose strain acquired high resistance to stress conditions and biofilm formation ability. EPS materials of this rugose variant are considered to be important for shielding of the organism from adverse environmental conditions. The rugose form might serve as a survival form of the organism. The ability of V. cholerae O1 to assume this phenotype and survive environmental stress is very important in the ecology of this organism.
ACKNOWLEDGMENTS
We thank H. Nakayama for the scientific discussion.
This work was supported by a grant from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Amako K, Meno Y, Takade A. Fine structures of the capsules of Klebsiella pneumoniae and Escherichia coli K1. J Bacteriol. 1988;170:4960–4962. doi: 10.1128/jb.170.10.4960-4962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anwar H, Shand G H, Ward K H, Brown M R W, Alpar K E, Gowar J. Antibody response to acute Pseudomonas aeruginosa infection in a burn wound. FEMS Microbiol Lett. 1985;29:225–230. [Google Scholar]
- 3.Baker R M, Singleton F L, Hood M A. Effect of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983;46:930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berns K I, Thomas C A., Jr Isolation of high molecular weight DNA from Haemophilus influenzae. J Mol Biol. 1965;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown M R W, Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections and in nature. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- 6.Castillo L, Castillo D, Silva W, Zapata L, Reoid M, Ulloa M T, Maldonado A, Valenzuela M E, Buatos R, Tamplin M L, Martinez M A, De Ioannes A E, Jamett A, Yudelevich A, Becker M I. Development of highly specific monoclonal antibodies for the diagnosis of Vibrio cholerae O1. Hybridoma. 1995;14(3):271–278. doi: 10.1089/hyb.1995.14.271. [DOI] [PubMed] [Google Scholar]
- 7.Colwell R R, Huq A. Vibrios in the environment: viable but nonculturable Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 117–133. [Google Scholar]
- 8.Costerton J W, Cheng K J, Geesey G G, Ladd T I, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 9.Costerton J W, Irwin R T, Cheng K J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- 10.Costerton J W, Marrie T J. The role of the bacterial glycocalyx in resistance to antimicrobial agents. In: Easmon C S F, Jelijaszewicz J, Brown M R W, Lambert P A, editors. Role of the envelope in the survival of bacteria in infection. New York, N.Y: Academic Press, Inc.; 1983. pp. 63–85. [Google Scholar]
- 11.Costerton J W, Marrie T J, Cheng K J. Phenomena of bacterial adhesion. In: Savage D C, Fletcher M, editors. Bacterial adhesion. New York, N.Y: Plenum Publishing Corp.; 1985. pp. 3–43. [Google Scholar]
- 12.Danon D L, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972;38:500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- 13.Dawson M P, Humphrey B, Marshall K C. Adhesion: a tactic in the survival strategy of a marine Vibrio during starvation. Curr Microbiol. 1981;6:195–201. [Google Scholar]
- 14.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smiths F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;3:350–356. [Google Scholar]
- 15.Erdos G W. Localization of carbohydrate-containing molecules. In: Aldrich H C, Todd W J, editors. Ultrastructure techniques for microorganisms. New York, N.Y: Plenum Publishing Corp.; 1986. pp. 399–420. [Google Scholar]
- 16.Filip C, Fletcher G, Wulff J L, Earhart C J. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckel J E, Blackett B, Everson J S, Ward M E. The influence of surface charge on the attachment of Neisseria gonorrhoeae to human cells. J Gen Microbiol. 1976;96:359–364. doi: 10.1099/00221287-96-2-359. [DOI] [PubMed] [Google Scholar]
- 18.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacques M, Foiry B. Electron microscopic visualization of capsular material of Pasteurella multocida types A and D labeled with polycationic ferritin. J Bacteriol. 1987;169:3470–3472. doi: 10.1128/jb.169.8.3470-3472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson J A, Panigrahi P, Morris J G., Jr Non-O1 Vibrio cholerae NRT36S produces a polysaccharide capsule that determines colony morphology, serum resistance, and virulence in mice. Infect Immun. 1992;60:864–869. doi: 10.1128/iai.60.3.864-869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjelleberg S, Hermansson M. Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol. 1984;48:497–503. doi: 10.1128/aem.48.3.497-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjelleberg S, Hermansson M, Mården P, Jones G W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- 23.Kjelleberg S, Humphrey B A, Marshall K C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982;43:1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macpherson D F, Morona R, Beger D W, Cheah K C, Manning P A. Genetic analysis of the rfb region of Shigella flexneri encoding the Y serotype O-antigen specificity. Mol Microbiol. 1991;5:1491–1499. doi: 10.1111/j.1365-2958.1991.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 25.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 26.Mården P, Nyström T, Kjelleberg S. Uptake of leucine by a marine gram-negative heterotrophic bacterium during exposure to starvation conditions. FEMS Microbiol Ecol. 1987;45:233–241. [Google Scholar]
- 27.Mården P, Tunlid A, Malmcrona-Friberg K, Odham G, Kjelleberg S. Physiological and morphological changes during short term starvation of marine bacteria isolate. Arch Microbiol. 1985;149:326–332. [Google Scholar]
- 28.Matin A, Auger E A, Blum P H, Schultz J E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;42:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- 29.Morris J G, Jr, Sztein M B, Rice E W, Nataro J P, Losonsky G A, Panigrahi P, Tacket C O, Johnson J A. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J Infect Dis. 1996;174:1364–1368. doi: 10.1093/infdis/174.6.1364. [DOI] [PubMed] [Google Scholar]
- 30.Panigrahi P, Tall B D, Russell R G, Detolla L J, Morris J G., Jr Development of an in vitro model for study of non-O1 Vibrio cholerae virulence using Caco-2 cells. Infect Immun. 1990;58:3415–3424. doi: 10.1128/iai.58.10.3415-3424.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poindexter J S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981;45:123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy G P, Hayat U, Abeygunawardana C, Fox C, Wright A C, Maneval D R, Jr, Bush C A, Morris J G., Jr Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus M06-24. J Bacteriol. 1992;174:2620–2630. doi: 10.1128/jb.174.8.2620-2630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice E W, Johnson C J, Clark R M, Fox K R, Reasoner D J, Dunnigan M E, Panigrahi P, Johnson J A, Morris J G., Jr Chlorine and survival of “rugose” Vibrio cholerae. Lancet. 1992;340:740. doi: 10.1016/0140-6736(92)92289-r. [DOI] [PubMed] [Google Scholar]
- 34.Shand G H, Anwar H, Kadurugamuwa J, Brown M R W, Silverman S H, Melling J. In vivo evidence that bacteria in urinary tract infections grow under iron-restricted conditions. Infect Immun. 1985;48:35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takemoto H, Hase S, Ikenaka T. Microquantitative analysis of neutral and amino sugars as fluorescent pyridylamino derivatives by high-performance liquid chromatography. Anal Biochem. 1985;145:245–250. doi: 10.1016/0003-2697(85)90357-4. [DOI] [PubMed] [Google Scholar]
- 36.Torres-Cabassa A, Gottesman S, Frederick R D, Dolph P J, Coplin D L. Control of extracellular polysaccharide synthesis in Erwinia stewartii and Escherichia coli K-12: a common regulatory function. J Bacteriol. 1987;169:4525–4531. doi: 10.1128/jb.169.10.4525-4531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wai S N, Moriya T, Kondo K, Misumi H, Amako K. Resuscitation of Vibrio cholerae O1 strain TSI-4 from a viable but nonculturable state by heat shock. FEMS Microbiol Lett. 1996;136:187–191. doi: 10.1111/j.1574-6968.1996.tb08047.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiss R, Schiefer H-G, Kruss H. Ultrastructural visualization of Klebsiella capsules by polycationic ferritin. FEMS Microbiol Lett. 1979;6:435–437. [Google Scholar]
- 39.White P B. The rugose variant of Vibrios. J Pathol Bacteriol. 1938;46:1–6. [Google Scholar]
- 40.Wolcott M J. Advances in nucleic acid-based detection methods. Clin Microbiol Rev. 1992;5:370–386. doi: 10.1128/cmr.5.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrangstadh M, Conway P L, Kjelleberg S. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch Microbiol. 1986;145:220–227. doi: 10.1007/BF00443649. [DOI] [PubMed] [Google Scholar]
- 42.Wrangstadh M, Szewzyk U, Östling J, Kjelleberg S. Starvation-specific formation of a peripheral exopolysaccharide by a marine Pseudomonas sp., strain S9. Appl Environ Microbiol. 1990;56:2065–2072. doi: 10.1128/aem.56.7.2065-2072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]