Abstract
Shiga toxin (Stx)-positive Escherichia coli O157:H7 readily colonize and persist in specific-pathogen-free (SPF) chicks, and we have shown that an Stx-negative E. coli O157:H7 isolate (NCTC12900) readily colonizes SPF chicks for up to 169 days after oral inoculation at 1 day of age. However, the role of intimin in the persistent colonization of poultry remains unclear. Thus, to investigate the role of intimin and flagella, which is a known factor in the persistence of non-O157 E. coli in poultry, isogenic single- and double-intimin and aflagellar mutants were constructed in E. coli O157:H7 isolate NCTC12900. These mutants were used to inoculate (105 CFU) 1-day-old SPF chicks. In general, significant attenuation of the aflagellate and intimin-aflagellate mutants, but not the intimin mutant, was noted at similar time points between 22 and 92 days after inoculation. The intimin-deficient mutant was still being shed at the end of the experiment, which was 211 days after inoculation, 84 days more than the wild type. Shedding of the aflagellar and intimin-aflagellar mutants ceased 99 and 113 days after inoculation, respectively. Histological analysis of gastrointestinal tissues from inoculated birds gave no evidence for true microcolony formation by NCTC12900 or intimin and aflagellar mutants to epithelial cells. However, NCTC12900 mutant derivatives associated with the mucosa were observed as individual cells and/or as large aggregates. Association with luminal contents was also noted. These data suggest that O157 organisms do not require intimin for the persistent colonization of chickens, whereas flagella do play a role in this process.
Shiga toxin (Stx)-positive Escherichia coli O157:H7 was first associated with a major food-borne outbreak involving contaminated hamburgers, and subsequent analysis has shown that the associated disease in humans includes hemorrhagic colitis, hemolytic uremic syndrome, and thrombocytopenic purpura (7, 36, 56). Entry into the food chain occurs via food or water contaminated with animal feces harboring E. coli O157:H7 (4, 26, 31, 47). Cattle are recognized as the main animal reservoir (28, 29, 73). However, E. coli O157:H7 has been isolated from other animals, including sheep, goats, deer, wild rabbits, and rats, and from birds, including seagulls (11, 23, 38, 53, 54, 55, 68).
The mechanisms of colonization and persistent carriage of E. coli O157:H7 in ruminants have been studied by bacteriological and histological analyses after deliberate oral inoculations (8, 12, 14, 16, 17, 25, 37, 43, 48, 67, 70). Intimin-dependent intimate attachment to colonic epithelial cells was demonstrated in newborn piglets (21, 65) and newborn colostrum-deprived calves (18). A recent study has demonstrated that in experimental cattle and sheep models of E. coli O157:H7 infection, the wild-type isolate was shed in significantly greater numbers than an isogenic intimin mutant (15). However, although the wild type was shed in sheep for a longer period of time than the intimin mutant, in cattle, albeit in only one animal, the intimin mutant was still detected in bovine feces at the end of the study (day 60). Another ruminant study has confirmed that an Stx-negative E. coli O157:H7 isolate (NCTC12900) persists in conventionally weaned sheep and that the duration of shedding is intimin dependent (69). The general conclusion from these studies is that there is a primary role for intimin for colonization in ruminants.
Other E. coli O157 bacterial components, such as lipopolysaccharide, long polar fimbriae (LPF), and the outer membrane protein OmpA, may also play a role in colonization (35, 51, 64). However, a role for E. coli O157:H7 flagella, a known pathogenic determinant of other E. coli pathogens (41), is equivocal. Purified H7 antigen did not inhibit adherence of E. coli O157:H7 to HEp-2 cells (58), whereas purified H6 flagella of E. coli have been implicated with in vitro adherence (24). Also, E. coli O157:H− strains have been associated with zoonotic disease (6, 43).
Isolation of E. coli O157:H7 from poultry is infrequently reported, although isolation from retail chicken and turkey has been done (22). However, although no direct link with the consumption of poultry and O157 disease in humans has been reported, an O157 food-borne outbreak was reported where poultry was on the menu (9). Experimental infections of chicks demonstrated that Stx-positive E. coli O157:H7 can readily colonize poultry, although the exact mechanisms of colonization remain to be confirmed (3, 58, 61, 63). A recent study from our laboratory demonstrated that Stx-negative E. coli O157:H7 isolate NCTC12900 readily colonizes and persists in poultry for at least 24 weeks after infection (5). These data suggest that Shiga toxins have little or no involvement in the persistent colonization of this host and that poultry could be a major risk to human health should this sector of the farming industry become infected.
In order to understand the basis of persistent colonization of poultry by E. coli O157:H7, young chicks were inoculated with intimin- and flagellum-deficient mutants of E. coli O157:H7 (NCTC12900). The choice of mutants was made because intimin is regarded as a primary colonization factor for mammalian species, whereas flagella are regarded as primary virulence determinants for avian pathogenic E. coli (41). Here, we report our findings.
MATERIALS AND METHODS
Bacteria, culture, preparation of inocula, and media.
Stx-negative E. coli O157:H7 strain NCTC12900 is a naturally occurring Stx1- and Stx2-negative isolate that was isolated from a public health laboratory in Austria during 1992 and was described previously (5, 19).
Bacterial isolates were stored at −80°C in heart infusion broth supplemented with glycerol (30%, wt/vol). Working cultures were maintained at 4°C on sheep blood agar (5%). Luria-Bertani (LB) agar (Oxoid), supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Promega) at 25 mg/ml and ampicillin (Penbrithin; GlaxoSmith Kline) at 100 μg/ml, was used for all cloning procedures. Minimal medium was M9 salts medium supplemented with 0.2% (wt/vol) glucose, 10 mM magnesium sulfate, and the appropriate antibiotic (chloramphenicol at 10 μg/ml, kanamycin at 25 μg/ml, streptomycin at 25 μg/ml, or tetracycline at 20 μg/ml). Bacteria for visualization by electron microscopy and motility tests were prepared from overnight LB broth cultures grown with agitation at 37°C.
Inocula for HEp-2 tissue culture adherence and invasion assays were prepared from overnight LB broth cultures grown with agitation at 37°C as described previously (19, 35). Briefly, bacterial cultures were resuspended in 0.1 M phosphate-buffered solution (PBS; pH 7.2) to give an optical density reading of 1.2 absorbance (ABS) at 540 nm. These were then resuspended in minimal Eagles modified medium supplemented with 1% (wt/vol) nonessential amino acids (Sigma) and 1% (vol/vol) l-glutamine (Sigma) to give approximately 107 CFU/ml immediately prior to inoculation.
Inocula for chick experiments were prepared as described previously (5). Briefly, overnight cultures were diluted in 0.1 M PBS (pH 7.2) to contain approximately 106 CFU/ml. The number of E. coli O157 bacteria given in each inoculum (100 μl) was determined by plating 10-fold serial dilutions on sorbitol-MacConkey agar supplemented with cefixime and tellurite (CT-SMAC; Oxoid) for the wild-type isolate NCTC12900 or the appropriate antibiotic (chloramphenicol at 10 μg/ml, streptomycin at 25 μg/ml) for each isogenic mutant.
PCR.
All primer sequences listed run 5′ to 3′. Amplification of NCTC12900 fliC and eae genes was performed using primers FliCF (CTCTCGCTGATCACTCAA) with FliCR (CGACATGTTGGACACTTC) and EaeF (CTTTACCGGCGGAACTGGA) with EaeR (GGACCCGGCACAAGCATAAG), respectively. FliCF and FliCR primers amplified a 1,641-bp DNA product from the fliC encoding region (GenBank accession no. AF228488; position [bp] 28 through 45 and 1668 through 1651) and primers EaeF and EaeR amplified a 2,172-bp DNA product from the eae encoding region (GenBank accession no. AF061251; position [bp] 609 through 627 and 2780 through 2761). Amplification of the fliC gene for complementation of NCTC12900 nonmotile isogenic mutants was performed using previously reported primers (38) that were modified by our laboratory: FliC1 (ACCGGGGTTATCGGCCTG) and FliC2 (GGATGCGGCGTAAACGCC). These primers amplified a 2,126-bp DNA product, where FliC1 anneals 211-bp upstream of the fliC start codon and FliC2 anneals 121-bp downstream of the fliC stop codon (GenBank accession no. AF228488).
PCRs consisted of thermophilic DNA polymerase 10× magnesium-free buffer (5 μl), 1.5 mM MgCl2, 2.5 U of Taq (Promega), 200 μM (each) deoxynucleoside triphosphates (dNTPs; Amersham Biosciences), 10 pmol of each primer (Oswell), and 1 ng of complete genomic DNA and were made to a final volume of 50 μl by using sterile distilled H2O. PCR amplifications were performed for 1 cycle at 94°C for 2 min; 30 cycles of 94°C for 45 s, 56°C for 45 s, and 72°C for 2 min; and 1 cycle at 72°C for 10 min. PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining and were purified for cloning by using the Sephaglas DNA purification kit (Amersham Biosciences). PCR products required for complementation studies consisted of the above-mentioned reagents. However, Taq polymerase was replaced with Pfu polymerase (Promega) and the annealing temperature was changed to 58°C.
Construction of E. coli O157:H7 NCTC12900 fliC and eae mutants.
Insertional inactivation of eae in NCTC12900 followed previously described methods (13). Briefly, a 1,558-bp EcoRI-SalI fragment from the conserved N-terminal region of eae was excised from an eae clone of an E. coli O157:H7 strain A84 cosmid library made in E. coli XL1-Blue MR. This EcoRI-SalI fragment was cloned into EcoRI-SalI cut pUC18 to give pALC6. The identity of the insert was confirmed by restriction enzyme mapping, and pALC6 was linearized with EcoRV and dephosphorylated with shrimp alkaline phosphatase (Amersham Biosciences). A 1.1-kb chloramphenicol cassette excised from pBCSK (Stratagene) was ligated with the plasmid pALC6 to give pALC8. The eae::Camr fragment of pALC8 was cloned into the SrfI site of the plasmid suicide vector pEFORMT to give pALC10. The host for pALC10 was E. coli K-12 S17 λ pir, permissive for the replication of the suicide vector. In the study reported here, conjugations were performed between E. coli K-12 S17 λ pir harboring pALC10 and the recipient strain NCTC12900 with selection made on minimal medium plates supplemented with chloramphenicol. Well-isolated colonies were picked and streaked onto minimal medium plates supplemented separately with chloramphenicol and tetracycline. Recombinants that grew only on chloramphenicol were subcultured onto the same medium twice to purify from any E. coli K-12 S17 λ pir carryover and then tested genotypically and phenotypically to confirm inactivation of eae.
Insertional inactivation of fliC in NCTC12900 followed previously described methods (1, 40). Briefly, primers FliCF and FliCR were used to amplify the 1,641-bp DNA product from NCTC12900. The DNA product was purified and ligated into the multiple cloning site of the cloning vector pCR2.1 to give pGUS1. The insert was mapped by restriction enzyme digestion. A blunt-ended 1.2-kb PvuII fragment that carried a streptomycin resistance gene was excised from p723 (Stratagene) and cloned into the centrally located Eco47III site of the 1,641-bp DNA product within pGUS1 to give pGUS2. EcoRI and SacI were used to cleave the fliC::Strr construct from pGUS2, which was cloned into the SrfI site of pERFORMK to give pGUS3. The host for pGUS3 was E. coli K-12 S17 λ pir. Conjugations were performed between E. coli K-12 S17 λ pir harboring pGUS3 and the recipient strain NCTC12900 with selection made on minimal medium plates supplemented with streptomycin. Well-isolated colonies were picked and streaked onto minimal medium plates supplemented separately with streptomycin and kanamycin. Recombinants that grew only on streptomycin were subcultured onto the same medium to prevent any E. coli K-12 S17 λ pir carryover and then tested genotypically and phenotypically to confirm inactivation of fliC.
A second round of allelic exchange was done to construct an isogenic double-mutant defective for both eae and fliC. This was done by performing a conjugation between S17 λ pir carrying the pGUS3 pERFORMK fliC::Strr suicide vector construct and the isogenic intimin-deficient mutant. The isogenic intimin and aflagellar mutants and the isogenic intimin-aflagellar double mutant were designated DM3, DM4, and DM5, respectively.
Southern hybridizations were done for all mutants following the procedures described previously (57) using purified probes created by using the primers listed above for the generation of fliC and eae DNA products. Suicide vectors without inserts and the antibiotic resistance gene cassettes were used also as probes.
Complementaion of E. coli O157:H7 NCTC12900 nonmotile mutants.
For complementation studies of both aflagellar mutants, DM4 and DM5, primers FliC1 and FliC2 were used to amplify a 2,126-bp DNA product that included the promoter region and the start and stop codons for the fliC gene. This DNA product was cloned into pCR2.1 to give pJAMIE1. After EcoRI digestion, the fliC amplicon was cloned into the unique EcoRI site in the chloramphenicol resistance cassette of pACYC184 to give pJAMIE2, which was subsequently electroporated into NCTC12900 aflagellar mutants, DM4 and DM5.
Motility and elaboration of flagella.
Isolates were cultured on 5% sheep blood agar plates for 16 h at 37°C aerobically, and single colonies were picked with an inoculation needle and stabbed into the center of 0.35% semisolid “sloppy” LB agar contained within a glass universal. These were incubated at 25, 37, and 42°C for 24 h. Motility was observed as diffuse growth out from the inoculation stab.
For the elaboration of any surface appendages of interest, isolates were cultured in an appropriate medium. Bacterial cultures (1 ml) were centrifuged at 4,147 × g, and the supernatant was removed. Bacterial pellets were resuspended in 0.5 ml of 0.1 M PBS (pH 7.2), and 50-μl drops were placed on sterile dental wax. Formvar carbon-coated grids were placed (silver side down) on top of each drop for 15 min. The grid was lifted by forceps, excess liquid was removed on blotting paper, and the grid (silver side down) was placed onto a 50-μl drop of potassium phosphate, tungsten (KPT)-negative stain for no longer than 15 s. Grids were carefully blotted dry and viewed using a Philips CM 10 transmission electron microscope.
E. coli O157 adherence and invasion assays.
Adherence and invasion assays of NCTC12900 and isogenic derivatives with HEp-2 cells were performed as described previously (19, 42). Briefly, E. coli O157 inoculum resuspended in minimal essential Eagle medium (MEM) (Sigma) was added to give a concentration of 107 CFU/ml in each well. The monolayers were then incubated for 3 h at 37°C in the presence of CO2. The inoculum was removed, and each monolayer was washed three times with Hanks' balanced salt solution (Sigma) to remove nonadherent bacteria. The monolayer was then disrupted for 10 min by using a solution of 1% Triton X-100 (Sigma) and a 12-mm-long magnetic stirrer. After disruption, serial 10-fold dilutions were plated onto LB agar and incubated overnight to determine the number of CFU/ml. For invasion assays, bacteria were allowed to adhere as described above for 3 h, at which point the monolayers were washed three times with Hanks' balanced salt solution before adding MEM containing gentamicin (100 μg/ml). Plates were incubated as described above for a further 2 h. The numbers of internalized bacteria were determined as described above. All adherence and invasion assays were performed in duplicate on five separate occasions. The numbers of internalized E. coli O157 were determined by bacterial enumeration and subtracted from the numbers of associated bacteria to give a true value for adhesion. For statistical analyses, counts were transformed to log 10 values and analyses of variance were performed, followed by Student's t tests.
For fluorescent actin staining (FAS) and the visualization of adherence by scanning electron microscopy (SEM), E. coli O157 cell adherence assays (6 h) were prepared and stained using methods described previously (19, 41). For all assays after incubation for 3 h, the medium was removed and replaced with fresh medium and incubated for a further 3 h. Preparation of cells for visualization by FAS and SEM was performed as described previously (42).
SPF chick model.
Colonization and invasion experiments were performed essentially as described previously (5, 41) under home office license 70/4987. A total of 120-day-old specific-pathogen-free (SPF) White Leghorn (SPAFAS) chicks were assorted randomly into four groups, designated A through D, each comprised of 30 birds. Each group was housed on sterile wood shaving flooring in separate large heated isolators with feed (Dodgeson & Horrell) and water supplied ad libitum. At 24 h of age, all birds in groups A were dosed with 105 CFU of E. coli O157:H7 NCTC12900, all birds in group B were dosed with 105 CFU of DM3, all birds in group C were dosed with 105 CFU of DM4, and all birds in group D were dosed with 105 CFU of DM5. All doses were given by oral gavage. Isolators were partially cleaned every 5 to 6 weeks, where some E. coli O157-contaminated bedding was left in the isolator after each clean.
For all experiments, on days 1, 2, and 5 after inoculation, six birds were selected at random and culled by cervical dislocation for postmortem examination. Immediately, liver, spleen, duodenum, jejunum, ileum, colon, ceca, cecal tonsils, and crop were removed aseptically from each bird. Tissues from five birds were enumerated for E. coli O157 bacteria, and tissues from one bird were enumerated for histological studies. On days 57 and 92 after inoculation, two birds selected at random from the remaining birds and on day 211 after inoculation, the remaining three birds (all females) were killed and the bacteria in tissues were enumerated. Additionally, two, one, and two birds were removed at day 43, 50, and 92 after inoculation from each isolator, respectively, to prevent overcrowding. This was in accordance with United Kingdom Home Office regulations.
To determine the extent and duration of fecal shedding of the inoculated strain, cloacal swabbing was performed on day 1 after inoculation and on 10 birds or the number remaining after selective removal from each group weekly thereafter until day 211 after inoculation. Swabs were streaked onto CT-SMAC and/or SMAC supplemented with the appropriate antibiotic to select the mutant. Negative E. coli O157 swabs were placed in buffered peptone water (BPW) for 6 h at 37°C and then plated. Plates were incubated aerobically overnight at 37°C and scored as follows: high was confluent, medium was >200 colonies, low was <200 colonies, positive was after enrichment only, clear had no colonies.
The latex agglutination test (Oxoid), consisting of latex particles coated with specific antibody reactive with O157 somatic antigen, was used to test 10% of sorbitol nonfermenting single colonies retrieved on CT-SMAC/SMAC-antibiotic plates.
Statistical analysis.
For analysis of bacterial counts for in vivo colonization and invasion studies, the Wilcoxon-Mann-Whitney nonparametric test was used to compare the wild type with each mutant. For the distribution of bacterial shedding, the scores for wild type and each mutant were converted as follows: 0 for clear, 1 for positive after enrichment, 2 for low (<200 colonies), 3 for medium (>200 colonies), and 4 for high (confluent growth). These data were also compared using the Wilcoxon-Mann-Whitney test. Exact P values were calculated using StatXact software.
Analysis of eggs.
Egg examination followed previously described methods (5, 71). Briefly, 50 eggs were collected per group and transferred to a class I cabinet in which all further manipulations were performed. The egg shell, white, and yolk were separated and placed into a separate volume of 100 ml of BPW. Sterile gloves were worn that were changed between preparation of each egg. Individual samples were incubated at 37°C for 6 h. Immunomagnetic separation (10) was performed on 1 ml of each egg sample. Each bead sample was subcultured onto CT-SMAC/SMAC-antibiotic plates.
Examination of tissues by immunohistochemistry.
All tissues were examined by light microscopy after staining with hematoxylin and eosin stain. Tissues that showed evidence of adherent bacteria were stained using specific E. coli O157 antibodies by using the methods described previously (5).
RESULTS
Construction and characterization of mutants.
Single isogenic intimin (DM3) and aflagellar (DM4) mutants and an isogenic intimin-aflagellar (DM5) double mutant were constructed in NCTC12900 as described in Materials and Methods. PCR confirmed that target genes had been disrupted in each mutant because the original-sized PCR product was not amplified, whereas a product equal in size to the wild-type product plus the antibiotic-resistant cassette was (data not shown).
Southern hybridization analysis of each isogenic mutant probed with the appropriate target gene confirmed an increase in size of the target gene after the insertion of an antibiotic-resistant cassette compared with the wild-type genes (Fig. 1). Also, Southern blots using the suicide vector DNA alone and the antibiotic resistance gene cassettes alone as probes showed that the mutants did not harbor any remnant of the suicide vector plasmid and that the resistance genes were located to fragments the same size as the altered target genes (data not shown).
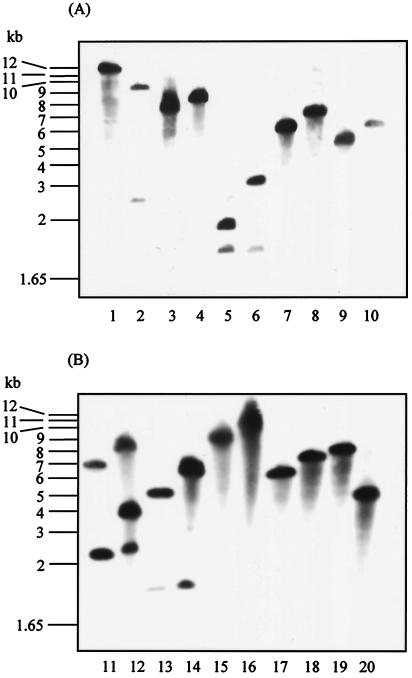
FIG. 1.
Southern blot hybridization of E. coli O157:H7 (NCTC12900) and isogenic intimin- and flagellum-deficient mutants to demonstrate insertional inactivation of target genes. (A) Lanes: 1, 3, 5, 7, and 9, NCTC12900; 2, 4, 6, 8, and 10, DM3 (eae::Camr) digested with PvuII (lanes 1 and 2), EcoRI (lanes 3 and 4), SalI (lanes 5 and 6), EcoRV (lanes 7 and 8), and AvaI (lanes 9 and 10). (B) Lanes: 11, 13, 15, 17, and 19, NCTC12900; lanes 12, 14, 16, 18, and 20, DM4 (fliC::Strr) digested with ClaI (lanes 11 and 12), MfeI (lanes 13 and 14), EcoRI (lanes 15 and 16), EcoRV (lanes 17 and 18), and BglI (lanes 19 and 20). DM5 (eae::Camr, fliC::Strr) gave a pattern that was a combination of both DM3 and DM4. Purified probes were created using the primers listed for the amplification of fliC and eae genes in the construction of mutants section of Materials and Methods.
Stab motility tests demonstrated that DM4 and DM5 were nonmotile, whereas the parental strain and DM3 were motile. DM4 and DM5 did not elaborate flagella as demonstrated by SEM, whereas the parental strain and DM3 did (data not shown). Complementation of both aflagellar mutants, DM4 and DM5, did restore the elaboration of flagella and the motility phenotype.
Intimin-deficient mutant of E. coli O157 adheres to HEp-2 cells less efficiently.
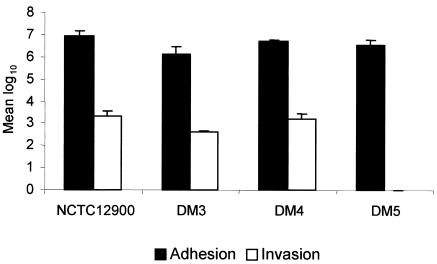
The results of adherence and invasion assays are shown in Fig. 2. The numbers of DM3 and DM5 bacteria adhering to HEp-2 cells were significantly lower than for NCTC12900 (P < 0.05). The number of DM4 bacteria adhering was lower than that of NCTC12900, although the difference was not significant (P = 0.053). The number of internalized DM3 was significantly lower than NCTC12900 (P < 0.001), whereas the number of internalized DM4 was similar to that of NCTC12900 and significantly higher than that of DM3 (P < 0.001). No DM5 bacteria were internalized.
FIG. 2.
Adhesion and invasion of HEp-2 cells by Stx-negative E. coli O157:H7 NCTC12900 wild-type, intimin, and aflagellar mutants. DM3, isogenic intimin-deficient mutant; DM4, isogenic flagellum-deficient mutant; DM5, isogenic intimin-flagellum-deficient mutant. Mean log 10 = CFU/ml.
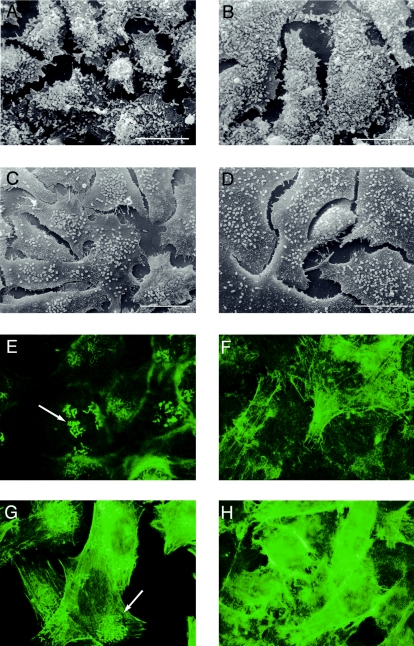
Bacteria adhering to HEp-2 cells were observed by SEM. Like the parent strain NCTC12900, DM4 generated the localized adherence pattern and characteristic microcolonies (Fig. 3). However, diffuse adherence patterns were observed for DM3 and DM5 on HEp-2 cell monolayers.
FIG. 3.
Observation of E. coli O157 adherence patterns for intimin- and flagellum-deficient mutants as demonstrated by SEM (A through D) and FAS (E through H). Wild-type NCTC12900 (A and E), isogenic intimin-deficient mutant DM3 (C and F), isogenic flagellum-deficient mutant DM4 (B and G), and isogenic intimin-flagellum-deficient mutant DM5 (D and H) are shown. Bar = 20 μm. FAS magnification, ×1000.
Actin rearrangements within HEp-2 cells were observed by FAS with both NCTC12900 and DM4. However, no FAS reaction was observed for either DM3 or DM5 (Fig. 3).
SPF chicks were readily colonized by E. coli O157:H7 NCTC12900 and isogenic mutants defective for intimin and flagella.
There was no morbidity or mortality in any experimental group, and the birds remained healthy, growing at the anticipated rate.
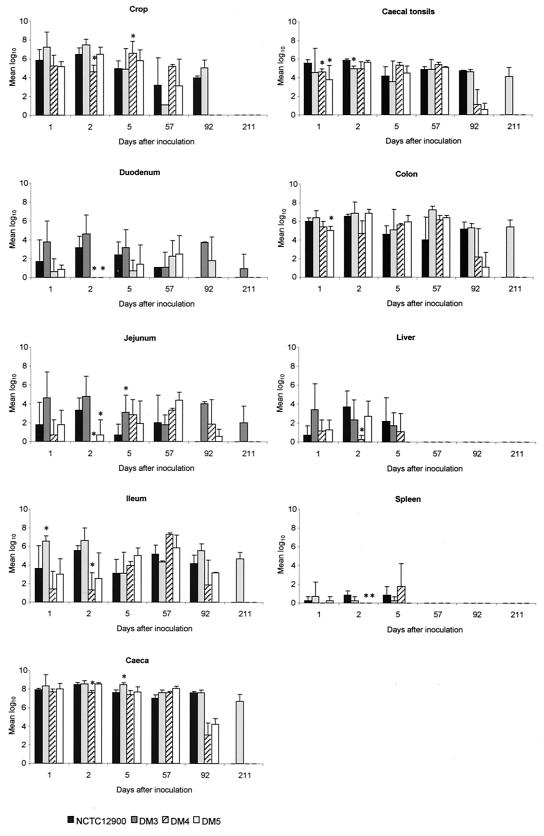
From each experimental group, birds were removed periodically and killed and the tissues were collected for bacteriological analysis. These data are presented in Fig. 4. Each of the inoculated strains colonized all of the regions of the gastrointestinal tract examined. The distal region of the tract was well colonized by all strains within 24 h of inoculation, and the highest numbers, in the region of 108 CFU/gram of tissue, were recovered from the cecum. The small intestine was generally less well colonized, whereas the crop was also well colonized. High numbers of each of the four strains were recovered from the cecum, colon, and crop for up to 92 days after inoculation. However, by the close of the experiment 211 days after inoculation, only the intimin-deficient mutant (DM3) was recovered from the duodenum, jejunum, ileum, cecum, colon, and cecal tonsils. By this time point, wild-type NCTC12900 and both DM4 and DM5 had been cleared from all tissues from all remaining birds. Significant differences between NCTC12900 and each mutant are indicated (Fig. 4). There were relatively few significant differences except that the aflagellar mutants DM4 and DM5 were recovered in lower numbers from the small intestine early after inoculation. A trend was observed that the intimin-deficient mutant (DM3) was recovered from the ceca in higher numbers than all other strains, including NCTC12900, but only one data point showed a statistically significantly higher number of DM3 than NCTC12900 and that was on day 5 after inoculation.
FIG. 4.
Colonization and invasion of SPF chicks by Stx-negative E. coli O157:H7 NCTC12900 wild-type and intimin- and flagellum-deficient mutants. Post mortem examinations were performed on five birds from each group on days 1, 2, and 5; on two birds from each group on days 57 and 92; and on three birds from each group on day 211. DM3, isogenic intimin-deficient mutant; DM4, isogenic flagellum-deficient mutant; DM5, isogenic intimin-flagellum-deficient mutant. *, P < 0.05. Statistical comparisons were not applicable when the number of birds in each group was less than five.
All four strains were recovered from the liver and/or spleen during the first 5 days after inoculation. The aflagellar mutant DM4 was recovered in lower numbers than in wild-type NCTC12900, except from the spleen on day 5 after inoculation. The intimin-deficient mutant DM3 was recovered in the highest numbers (day 1) from the liver and spleen. However, from these tissues on days 2 and 5 after inoculation, the numbers of recovered DM3 were lower than those of recovered NCTC12900. The double mutant DM5 was not detected in the spleen on day 4 after inoculation and the liver and spleen on day 5 after inoculation. However, the majority of the aforementioned differences were not significant.
Flagella are required for long-term persistence.
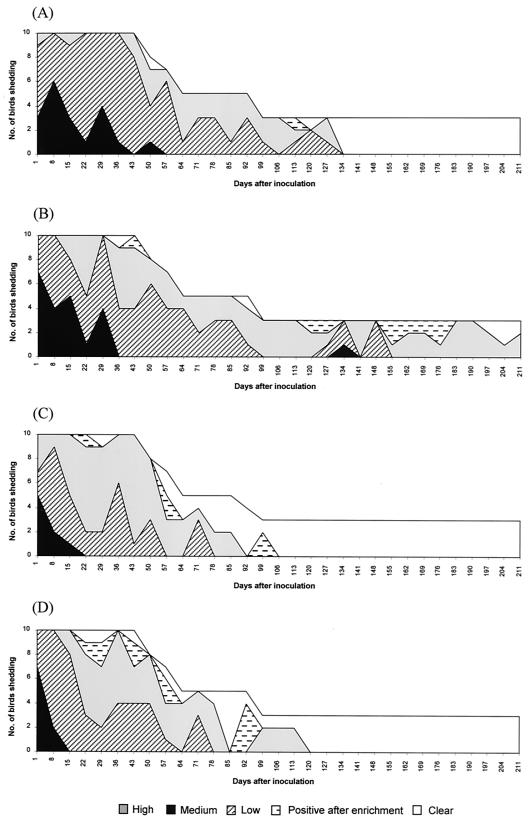
Cloacal swabs were taken from each experimental group and plated onto the appropriate media. A semiquantitative measure of shedding of these E. coli O157 strains was made by assessing the density of growth on the plates as described in Materials and Methods, and the data are presented in Fig. 5. The shedding profile was assessed for the remaining birds in each group over 211 days.
FIG. 5.
The distribution of bacterial shedding over time by SPF chicks of wild-type NCTC12900 (A), DM3 (isogenic intimin-deficient mutant) (B), DM4 (isogenic flagellum-deficient mutant) (C), and DM5 (intimin-flagellum-deficient mutant) (D). Recovery of E. coli O157 strains was scored as follows: high, confluent growth; medium, >200 colonies; low, <200 colonies; positive after enrichment; and clear, no colonies.
Wild-type NCTC12900 was cleared by day 134 after inoculation, whereas the intimin-deficient mutant DM3 was still being shed at day 211 after inoculation. Heavy shedding by SPF chicks was observed immediately after inoculation for NCTC12900 and mutant DM3 but declined gradually for over 57 and 36 days, respectively. However, an increase in shedding was observed for both isolates, especially DM3, for some of the birds prior to coming into lay.
Aflagellar mutants DM4 and DM5 were cleared by days 106 and 120 after inoculation, respectively. Generally, the number of birds shedding DM4 and DM5 was less than for the wild-type NCTC12900 and at certain time points was significant. For DM4, significantly fewer bacteria were recovered on days 22 (P < 0.001), 29 (P < 0.001), 36 (P = 0.05), 43 (P = 0.006), 57 (P = 0.002), 78 (P = 0.048), and 92 (P = 0.008) after inoculation. For DM5, significantly fewer bacteria were recovered on days 22 (P = 0.002), 29 (P < 0.001), 36 (P = 0.008), 57 (P = 0.017), 85 (P = 0.008), and 92 (P = 0.008) after inoculation. Although not significant, far less DM5 were recovered on day 43 (P = 0.073) after inoculation.
Eggshell contamination by DM3.
Birds inoculated with the intimin-deficient mutant DM3 (group B) came into lay around day 145 after inoculation, and 50 eggs were collected for bacteriological examination. Seven eggshells, but no egg contents, were culture positive. Culture-positive eggs were detected between days 145 and 182 after inoculation.
Birds inoculated with wild-type NCTC12900 (group A) came into lay on day 123 after inoculation, but none of the eggs collected were culture positive. Similarly, eggs laid by birds inoculated with DM4 and DM5 were culture negative for shells and contents. Both groups (C and D) laid their first egg on day 130 after inoculation.
Adherence in vivo of E. coli O157:H7 NCTC12900 was not associated with AE lesions.
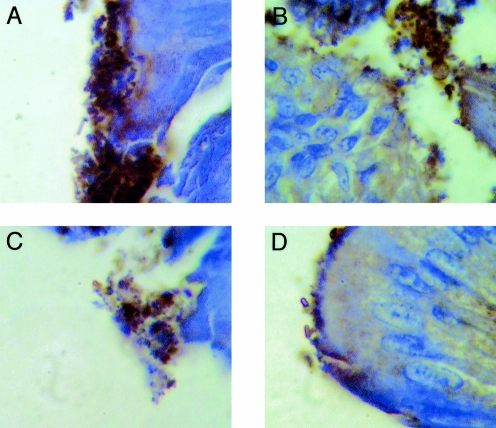
Tissue samples from all groups of birds were subjected to hematoxylin and eosin staining, and samples adjacent to tissues showing bacteria associated with the gastrointestinal epithelium were examined after staining with anti-O157 specific sera. E. coli O157 organisms were identified in various gastrointestinal tissue samples from each of the four experimental groups and at all time points examined. The bacteria were associated mostly with debris located near rather than intimately associated with the epithelium (Fig. 6). Discrete individual bacteria were observed occasionally in association with the mucosa; however, the association was not intimate (Fig. 6). No attaching and effacing (AE) lesions or true microcolonies were observed in any gastrointestinal tissue sample taken from birds inoculated with either wild-type NCTC12900 or aflagellar mutant DM4. Overall, most association of E. coli O157 with the epithelium occurred in the ceca.
FIG. 6.
Association of bacteria specifically stained with somatic anti-E. coli O157 sera during the first 5 days of experimental infection. Magnification, ×1,000. Cecal tissue from SPF chicks dosed orally by gavage with wild-type NCTC12900 (A), isogenic intimin-deficient mutant DM3 (B), isogenic flagellum-deficient DM4 (C), and isogenic intimin-flagellum-deficient mutant DM5 (D) are shown.
DISCUSSION
Unequivocal evidence was gained that intimin- and aflagellar-deficient mutants of Stx-negative E. coli O157:H7 strain NCTC12900 were constructed by allelic exchange. These mutants possessed no residual plasmid suicide vector anywhere in the genome but had an antibiotic resistance cassette inserted in the targeted genes. As allelic exchange is a well-established method, it is likely that no changes other than those anticipated had arisen and that the differences in the phenotypes of the mutants compared with the parental strain NCTC12900 were associated with the specific mutations. Furthermore, the flagellum phenotype was restored after complementation of the aflagellar and intimin-aflagellar mutants, DM4 and DM5, with the fliC gene on a plasmid.
In the in vitro tissue culture adherence and invasion assays, the intimin and intimin-aflagellar mutants DM3 and DM5 showed significantly decreased adhesion and invasion and were unable to form microcolonies or AE lesions. It is possible that reduced invasion by the intimin mutants may be attributed to the lower levels of bacteria adhering. The aflagellar mutants DM4 and DM5 were nonmotile and did not produce flagella. There were no statistically significant differences in the adherence and invasion by DM4 of tissue culture compared with the parental strain NCTC12900. These data suggest that flagella play little or no role in the adherence process of O157, at least to the tissue types tested here, and support previously reported findings (59). However, in our study, invasion of HEp-2 cells by E. coli O157:H7 strain NCTC12900 was demonstrated, which is at variance with the findings of this report. However, low-level invasion of HCT-8 cells by E. coli O157:H7 has been noted (60) and the authors reported that cell invasion was strongly dependent on eukaryotic microfilaments. In another study, low-level E. coli O157:H7 invasion of this cell type was also noted (46) but these authors concluded that E. coli O157:H7 do not invade HCT-8 cells to any significant extent and that HCT-8 cells take up low numbers of E. coli O157:H7 nonspecifically. Nevertheless, E. coli O157:H7 invasion of HEp-2 cells has recently been reported (66).
Experimental infection of young chicks with Stx-positive E. coli O157:H7 demonstrated that this host may be readily colonized and that this pathogen can persist for many months (3, 58). Stx-negative E. coli O157:H7 NCTC12900 colonized SPF chicks and persisted for many months, indicating that Stx may play little or no role in colonization of the gastrointestinal tract of the chicken (5). The findings of the present study indicate that the in vivo behavior of the intimin-deficient mutant was not significantly different from that of the wild type. Indeed, the mutant persisted longer. Histological analysis of gastrointestinal tissues taken from dosed chicks from each experiment showed that NCTC12900 wild-type and intimin- and flagellum-deficient mutants interacted with the epithelium. The epithelial surfaces of the ceca appeared to have the most associated E. coli O157 bacteria. However, no true microcolonies were observed for any test strain. Furthermore, it is possible that any AE lesions induced by the eae-proficient strains were present but too sparse to observe. Collectively, these findings suggest that while intimin appears to be required for microcolony formation, attaching and lesion formation and persistence in mammalian models (15, 21, 67, 69), intimin plays no or possibly only a very minor role in the colonization and persistence of E. coli O157:H7 in SPF chicks. Studies have demonstrated, in vitro, that E. coli O157:H7 intimin is able to its bind to its own encoded receptor, Tir (19, 33), which is injected into the host cell surface via a type III secretion system (34). A recent study has demonstrated that E. coli O157H7 intimin γ (gamma) does bind to, albeit in vitro, the eukaryotic receptor nucleolin (60), known to be expressed on the cell surface of mammalian cells in association with the actin cytoskeleton (32). It has been suggested that intimin γ requires both nucleolin and Tir to induce pedestal formation (60). Nucleolin protein is expressed in chickens, but whether it is expressed on the epithelial surface or has similar homology to mammalian nucleolin, or that E. coli O157:H7 intimin would bind to avian nucleolin expressed on the cell surface, remains unclear (45). However, from the data presented in this study, it seems possible that no E. coli O157:H7 intimin receptor, whether bacterial or eukaryotic, was required for persistent colonization of an avian host. Another interpretation of the data is that there are fundamental differences in either the in vivo expression of the locus of enterocyte effacement (LEE) or the competence of the LEE-encoded effector molecules to induce intimate attachment in the avian host. Indeed, possession of intimin may be disadvantageous in this host. These are areas for future research.
Other E. coli O157:H7 factors are likely to be involved in adherence and persistence in the avian host. In this study, we addressed the possible role of flagella and clear evidence was gained that the aflagellar mutants were attenuated for long-term persistence. This was not unexpected because in previous studies, we demonstrated that the flagella of E. coli O78:K80, an avian pathogen, and Salmonella enterica serotype Enteritidis were shown to play a role in colonization and persistence in the SPF chick model (2, 41). However, compared to the wild type, the defective intimin, aflagellar, and intimin-aflagellar mutants were associated in similar numbers with most tissues taken from SPF chicks during the first 5 days of E. coli O157:H7 infection. Potentially this observation may be a result of recycling between the chicks and the isolator environment, as uninoculated SPF chicks are infected by NCTC12900 (unpublished data). Nevertheless, lower numbers and more rapid clearance of the aflagellar mutants from the liver and spleen were noted, suggesting that flagella may be required for low-level invasion. Although controversial, low-level E. coli O157:H7 invasion in the SPF chick model was reported by our laboratory previously (5). However, and to the best of our knowledge, E. coli O157:H7 invasion of host tissues has not been reported in mammalian in vivo models of infection, although non-O157 EHEC in vivo enterocyte invasion has been reported (62). This may suggest that low-level E. coli O157:H7 invasion occurs only in nonmammalian hosts. Nevertheless, as very few data points showed any significant differences between any of the four strains in the first 5 days after inoculation, flagella may still play little or no role in the early events of colonization. Other age-related factors that may have been attributed to this observation are the development of intestinal mucus and of the immune system. Flagella are important for the invasion of cell mucus by avian pathogenic E. coli (40). With regard to the immune status of the birds, chicks hatch with immature T lymphocytes (44). It should also be borne in mind that the competitive effects of a developing native flora would not be observed early in the 1-day-old model but would be seen as the bird aged. As the aflagellar mutants were cleared earlier than the wild type and the intimin-deficient mutant, this suggests a possible role for flagella in competition with the native flora. Thus, while flagella may play a role in long-term persistence, other factors may influence colonization early after oral dosing. Genome sequencing (30, 53) has revealed five novel fimbrial operons, including two for long polar fimbriae (LPF1 and LPF2). Furthermore, a recent study has reported that both LPF1 and LPF2 contribute to the long-term colonization of E. coli O157:H7 in sheep and pigs (35). Also, a low-molecular-weight outer membrane protein expressed by an Stx-positive E. coli O157:H7 isolate was associated with adherence to chicken ceca (72). These and other factors, such as lipopolysaccharide, may also play a role in the colonization of poultry.
The intimin mutant was still being shed by birds 30 weeks after inoculation, 84 days more than NCTC12900. It is possible that defective intimin or the specific mutation in DM3 may confer an advantage to E. coli O157:H7 in this host and this testable hypothesis is the subject of further analyses.
Stx-positive and Stx-negative E. coli O157:H7 isolates can contaminate the shells of eggs (5, 58) but not the egg contents as described for Salmonella (27). The obvious route of eggshell contamination is fecal contamination during lay. Also, it was noted that the extent of shedding of the intimin mutant increased prior to the onset of lay. It is possible that shedding and clearance were influenced by the physiological changes within birds at the onset of laying. In this experiment, the wild-type isolate was found not to contaminate the eggshell, whereas previous contamination of eggshells (6%) was reported (5).
The studies conducted here are the first to analyze the role of E. coli O157:H7 intimin and flagella in a poultry model. The lack of a role for intimin in persistent colonization is at variance with mammalian studies in which the formation of AE lesions is considered a primary mechanism for colonization and persistent infection. The role of flagella in persistence is in common with the role of this organelle in the persistence of other avian adapted bacteria. Collectively, these data support the notion that E. coli O157:H7 may readily colonize avian species by non-LEE-associated mechanisms. That avian species are susceptible to colonization by E. coli O157:H7 indicates that continued vigilance is required to ensure the poultry industry does not become infected with this agent.
Acknowledgments
We acknowledge funding for the study from the Department for the Environment, Food and Rural Affairs (United Kingdom) (project grant OZ0706) and the BBSRC.
We also acknowledge the support of Bill Cooley for Electron Microscopy, the animal service unit, and the cell and tissue culture and histopathology departments at the VLA.
Editor: J. B. Bliska
REFERENCES
- 1.Allen-Vercoe, E., M. P. Dibb-Fuller, C. J. Thorns, and M. J. Woodward. 1997. SEF17 fimbriae are essential for the convoluted colonial morphology of Salmonella enteritidis. FEMS Microbiol. Lett. 153:33-42. [DOI] [PubMed] [Google Scholar]
- 2.Allen-Vercoe, E., and M. J. Woodward. 1999. Colonisation of the chicken caecum by afimbriate and aflagellate derivatives of Salmonella enterica serotype Enteritidis. Vet. Microbiol. 69:265-275. [DOI] [PubMed] [Google Scholar]
- 3.Beery, J. T., M. P. Doyle, and J. L. Schoeni. 1985. Colonization of chicken cecae by Escherichia coli associated with hemorrhagic colitis. Appl. Environ. Microbiol. 49:310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 5.Best, A., R. M. La Ragione, W. A. Cooley, C. D. O'Connor, P. Velge, and M. J. Woodward. 2003. Interaction with avian cells and colonisation of specific pathogen free chicks by Shiga-toxin negative Escherichia coli O157:H7 (NCTC 12900). Vet. Microbiol. 93:207-222. [DOI] [PubMed] [Google Scholar]
- 6.Bielaszewska, M., H. Schmidt, A. Liesegang, R. Prager, W. Rabsch, H. Tschäpe, A. Cízek, J. Janda, K. Bláhová, and H. Karch. 2000. Cattle can be a reservoir of sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H− strains and a source of human diseases. J. Clin. Microbiol. 38:3470-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt, J. R., L. S. Fouser, S. L. Watkins, I. Zelikovic, P. I. Tarr, V. Nazer-Stewart, and E. D. Avner. 1984. Escherichia coli O157:H7-associated hemolytic-uremic syndrome after ingestion of contaminated hamburgers. J. Pediatr. 25:519-526. [DOI] [PubMed] [Google Scholar]
- 8.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter, A. O., A. A. Borczyk, J. A. Carlson, B. Harvey, J. C. Hockin, M. A. Karmali, C. Krishnan, D. A. Korn, and H. Lior. 1987. A severe outbreak of Escherichia coli O157:H7-associated hemorrhagic colitis in a nursing home. N. Engl. J. Med. 317:1496-1500. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, P. A., D. J. Wright, and C. A. Siddons. 1994. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from bovine faeces. J. Med. Microbiol. 40:424-427. [DOI] [PubMed] [Google Scholar]
- 11.Cizek, A., P. Alexa, I. Literak, J. Hamrik, P. Novak, and J. Smola. 1999. Shiga toxin-producing Escherichia coli O157 in feedlot cattle and Norwegian rats from a large-scale farm. Lett. Appl. Microbiol. 28:435-439. [DOI] [PubMed] [Google Scholar]
- 12.Cookson, A. L., A. D. Wales, J. M. Roe, C. M. Hayes, G. R. Pearson, and M. J. Woodward. 2002. Variation in the persistence of Escherichia coli O157:H7 in experimentally inoculated 6-week-old conventional lambs. J. Med. Microbiol. 51:1032-1040. [DOI] [PubMed] [Google Scholar]
- 13.Cookson, A. L., and M. J. Woodward. 2003. The role of intimin in the adherence of enterohaemorrhagic Escherichia coli (EHEC) O157:H7 to HEp-2 tissue culture cells and to bovine gut explant tissues. Int. J. Med. Microbiol. 292:547-553. [DOI] [PubMed] [Google Scholar]
- 14.Cornick, N. A., S. L. Booher, T. A. Casey, and H. W. Moon. 2000. Persisent colonization of sheep by Escherichia coli O157:H7 and other E. coli pathotypes. Appl. Environ. Microbiol. 66:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dibb-Fuller, M. P., A. Best, D. A. Stagg, W. A. Cooley, and M. J. Woodward. 2001. An in-vitro model for studying the interaction of Escherichia coli O157:H7 and other enteropathogens in bovine primary cell cultures. J. Med. Microbiol. 50:759-769. [DOI] [PubMed] [Google Scholar]
- 21.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle, M. P., and J. L. Schoeni. 1987. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl. Environ. Microbiol. 53:2394-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer, J. R., T. Zhao, M. P. Doyle, M. R. Goldberg, C. A. Brown, C. T. Sewell, D. M. Kavanaugh, and C. D. Bauman. 2001. Experimental and field studies of Escherichia coli O157:H7 in white-tailed deer. Appl. Environ. Microbiol. 67:1218-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 25.Grauke, L. J., I. T. Kudva, J. Won Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 27.Guard-Petter, J. 2001. The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 3:421-430. [DOI] [PubMed] [Google Scholar]
- 28.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington State. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancock, D. D., T. E. Besser, D. H. Rice, D. E. Herriott, and P. I. Tarr. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epideminol. Infect. 118:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genome comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 31.Hilborn, E. D., J. H. Mermin, P. A. Mshar, J. L. Hadler, A. Voetsch, C. Wojtkunski, M. Swartz, R. Mshar, M. A. Lambert-Fair, J. A. Farrar, M. K. Glynn, and L. Slutsker. 1999. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Int. Med. 159:1758-1764. [DOI] [PubMed] [Google Scholar]
- 32.Hovanessian, A. G., F. Puvion-Dutilleul, S. Nisole, J. Svab, E. Perret, J. S. Deng, and B. Krust. 2000. The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp. Cell Res. 261:312-328. [DOI] [PubMed] [Google Scholar]
- 33.Ismaili, A., D. J. Philpott, M. T. Dytoc, and P. M. Sherman. 1995. Signal transduction responses following adhesion of verotoxin-producing Escherichia coli. Infect. Immun. 63:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarvis, K. G., and J. B. Kaper. 1996. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect. Immun. 64:4826-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan, D. M., N. Cornick, A. G. Torres, E. A. Dean-Nystrom, J. B. Kaper, and H. W. Moon. 2004. Long polar fimbriae contribute to colonization by Escherichia coli O157:H7 in vivo. Infect. Immun. 72:6168-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, and B. T. Steele. 1983. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorraghic colitis. Lancet ii:1299-1300. [DOI] [PubMed] [Google Scholar]
- 37.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1997. Characterization of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli serotypes isolated from sheep. J. Clin. Microbiol. 35:892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landini, P., and A. J. B. Zehnder. 2002. The global regulatory hns gene negatively affects adhesion to solid surfaces by anaerobically grown Escherichia coli by modulating expression of flagellar genes and lipopolysaccharide production. J. Bacteriol. 184:1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Ragione, R. M., W. A. Cooley, and M. J. Woodward. 2000. The role of fimbriae and flagella in the adherence of avian strains of Escherichia coli O78:K80 to tissue culture cells and tracheal and gut explants. J. Med. Microbiol. 49:327-338. [DOI] [PubMed] [Google Scholar]
- 41.La Ragione, R. M., A. R. Sayers, and M. J. Woodward. 2000. The role of fimbriae and flagella in the colonization, invasion and persistence of Escherichia coli O78:K80 in the day-old-chick model. Epidemiol. Infect. 124:351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Ragione, R. M., I. M. McLaren, G. Foster, W. A. Cooley, and M. J. Woodward. 2002. Phenotypic and genotypic characterization of avian Escherichia coli O86:K61 isolates possessing a gamma-like intimin. Appl. Environ. Microbiol. 68:4932-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeJeune, J. T., T. E. Besser, and D. D. Hancock. 2001. Cattle water troughs as reservoirs of Escherichia coli O157. Appl. Environ. Microbiol. 67:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowenthal, J. W., T. E. Connick, P. G. McWaters, and J. J. York. 1994. Development of T cell immune responsiveness in the chicken. Immunol. Cell Biol. 72:115-122. [DOI] [PubMed] [Google Scholar]
- 45.Maridor, G., W. Krek, and E. A. Nigg. 1990. Structure and developmental expression of chicken nucleolin and NO38: coordinate expression of two abundant non-ribosomal nucleolar proteins. Biochim. Biophys. Acta 1049:126-133. [DOI] [PubMed] [Google Scholar]
- 46.McKee, M. L., and A. D. O'Brien. 1995. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potentials reveals a new attachment pattern shared by intestinal E. coli. Infect. Immun. 63:2070-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mead, P. S., and P. M. Griffin. 1998. Escherichia coli O157:H7. Lancet 352:1207-1212. [DOI] [PubMed] [Google Scholar]
- 48.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. E. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonisation of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien, S. J., P. S. Murdoch, A. H. Riley, I. King, M. Barr, S. Murdoch, A. Greig, R. Main, W. J. Reilly, and F. M. Thomson-Carter. 2001. A foodborne outbreak of Vero cytotoxin-producing Escherichia coli O157:H-phage type 8 in hospital. J. Hosp. Infect. 49:167-172. [DOI] [PubMed] [Google Scholar]
- 50.Oelschlaeger, T. A., T. J., Barrett, and D. J. Kopecko. 1994. Some structures and processes of human epithelial cells involved in uptake of enterohemorrhagic Escherichia coli O157:H7 strains. Infect. Immun. 62:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paton, A. W., E. Voss, P. A. Manning, and J. C. Paton. 1998. Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia coli to human intestinal epithelial (Henle 407) cells. Microb. Pathog. 24:57-63. [DOI] [PubMed] [Google Scholar]
- 52.Perna, N. T., G. Plunket III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Pósfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 53.Pritchard, G. C., S. Williamson, T. Carson, J. R. Bailey, L. Warner, G. Willshaw, and T. Cheasty. 2001. Wild rabbits—a novel vector for verocytotoxigenic Escherichia coli O157. Vet. Rec. 149:567. [PubMed] [Google Scholar]
- 54.Pritchard, G. C., G. A. Willshaw, J. R. Bailey, T. Carson, and T. Cheasty. 2000. Verocytotoxin-producing Escherichia coli O157 on a farm open to the public: outbreak investigation and longitudinal bacteriological study. Vet. Rec. 147:259-264. [DOI] [PubMed] [Google Scholar]
- 55.Rice, D. H., D. D. Hancock, and T. E. Besser. 1995. Verotoxigenic E. coli O157 colonisation of wild deer and range cattle. Vet. Rec. 137:524. [DOI] [PubMed] [Google Scholar]
- 56.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorraghic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Schoeni, J. L., and M. P. Doyle. 1994. Variable colonization of chickens perorally inoculated with Escherichia coli O157:H7 and subsequent contamination of eggs. Appl. Environ. Microbiol. 60:2958-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherman, P. M., and R. Soni. 1988. Adherence of Vero cytotoxin-producing Escherichia coli of serotype O157:H7 to human epithelial cells in tissue culture: role of outer membranes as bacterial adhesins. J. Med. Microbiol. 26:11-17. [DOI] [PubMed] [Google Scholar]
- 60.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesion intimin-γ of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 277:2876-2885. [DOI] [PubMed] [Google Scholar]
- 61.Stavric, S., B. Buchanan, and T. M. Gleeson. 1993. Intestinal colonization of young chicks with Escherichia coli O157:H7 and other verotoxin-producing serotypes. J. Appl. Microbiol. 74:557-563. [PubMed] [Google Scholar]
- 62.Stordeur, P., B. China, G. Charlier, S. Roels, and J. Mainil. 2000. Clinical signs, reproduction of attaching/effacing lesions, and enterocyte invasion after oral inoculation of an O118 enterohaemorrhagic Escherichia coli in neonatal calves. Microbes Infect. 2:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sueyoshi, M., and M. Nakazawa. 1994. Experimental infection of young chicks with attaching and effacing Escherichia coli. Infect. Immun. 62:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres, A. G., and J. B. Kaper. 2003. Multiple elements controlling adherence of enterohemorraghic Escherichia coli O157:H7 to HeLa cells. Infect. Immun. 71:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immun. 63:3621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2002. Variations in the csgD promoter of Escherichia coli O157:H7 associated with increased virulence in mice and increased invasion of HEp-2 cells. Appl. Environ. Microbiol. 70:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wales, A. D., G. R. Pearson, A. M. Skuse, J. M. Roe, C. M. Hayes, A. L. Cookson, and M. J. Woodward. 2001. Attaching and effacing lesions caused by Escherichia coli O157:H7 in experimentally inoculated neonatal lambs. J. Med. Microbiol. 50:752-758. [DOI] [PubMed] [Google Scholar]
- 68.Wallace, J. S., T. Cheasty, and K. Jones. 1997. Isolation of Vero cytotoxin-producing Escherichia coli O157 from wild birds. J. Appl. Microbiol. 82:399-404. [DOI] [PubMed] [Google Scholar]
- 69.Woodward, M. J., A. Best, K. A. Sprigings, G. A. Pearson, A. M. Skuse, A. Wales, C. M. Hayes, J. M. Roe, J. C. Low, and R. M. La Ragione. 2003. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 293:299-308. [DOI] [PubMed] [Google Scholar]
- 70.Woodward, M. J., D. Gavier-Widen, I. M. McLaren, C. Wray, M. Sozmen, and G. R. Pearson. 1999. Infection of gnotobiotic calves with Escherichia coli O157:H7 strain A84. Vet. Rec. 144:466-470. [DOI] [PubMed] [Google Scholar]
- 71.Woodward, M. J., G. C. Gettinby, M. F. Breslin, J. Corkish, and S. Houghton. 2002. The efficacy of Salenvac, a Salmonella enterica subsp. Enterica serotype Enteritidis iron-restricted bacterin vaccine, in laying chickens. Avian Pathol. 31:383-392. [DOI] [PubMed] [Google Scholar]
- 72.Zhao, S., J. Meng, M. P. Doyle, R. Meinersman, G. Wang, and P. Zhao. 1996. A low molecular weight outer-membrane protein of Escherichia coli O157:H7 associated with adherence to INT407 cells and chicken caeca. J. Med. Microbiol. 45:90-96. [DOI] [PubMed] [Google Scholar]
- 73.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]