ABSTRACT
Type II toxin-antitoxin systems are highly prevalent in bacterial genomes and play crucial roles in the general stress response. Previously, we demonstrated that the type II antitoxin PfMqsA regulates biofilm formation through the global regulator AgtR in Pseudomonas fluorescens. Here, we found that both the C-terminal DNA-binding domain of PfMqsA and AgtR are involved in bacterial antibiotic susceptibility. Electrophoretic mobility shift assay (EMSA) analyses revealed that AgtR, rather than PfMqsA, binds to the intergenic region of emhABC-emhR, in which emhABC encodes an resistance-nodulation-cell division efflux pump and emhR encodes a repressor. Through quantitative real-time reverse-transcription PCR and EMSA analysis, we showed that AgtR directly activates the expression of the emhR by binding to the DNA motif [5´-CTAAGAAATATACTTAC-3´], leading to repression of the emhABC. Furthermore, we demonstrated that PfMqsA modulates the expression of EmhABC and EmhR. These findings enhance our understanding of the mechanism by which antitoxin PfMqsA contributes to antibiotic susceptibility.
KEYWORDS: type II antitoxin, antibiotic susceptibility, transcription factor, Pseudomonas fluorescens
INTRODUCTION
The tripartite resistance-nodulation-cell division (RND) efflux pumps, which are widely distributed in Gram-negative bacteria, are well known for their roles in antibiotic resistance (1). Spanning across the two membranes of Gram-negative bacteria, the RND efflux pumps typically consist of an inner membrane transporter, an outer membrane porin, and a periplasmic membrane fusion protein that connects the inner and outer membrane components (2). Driven by the proton motive force, the RND efflux pumps actively expel diverse antibiotics from the bacteria interior, and overexpression of these pumps can reduce the susceptibility of bacterial pathogens to antibiotics (3). More than 12 RND-type operons have been identified and characterized in Pseudomonas aeruginosa, an opportunistic pathogen notorious for its high-level resistance to clinically used antibiotics (4). Among these RND efflux pumps, MexAB-OprM- (5), MexXY- (6), MexCD-OprJ- (7), and MexEF-OprN- (8) overproducing mutants have been found in clinical strains, while the clinical relevance of MexJK-OprM, which is involved in resistance or virulence, remains unclear (9). Several RND efflux systems, including MexMN-OprM (10), MexPQ-OpmE (10), MexVW-OprM (11), MuxABC-OpmB (12, 13), and TriABC-OpmH (14, 15), are capable of conferring resistance to P. aeruginosa or Escherichia coli host deficient in major RND pumps (16). Besides their role in antibiotic susceptibility, RND efflux pumps also play pivotal roles in bacterial physiology, such as quorum sensing (QS) (17), virulence (18), bacterial stress response, secondary metabolism, and bacterial pathogenicity (19).
Pseudomonas fluorescens is an opportunistic human pathogen that is responsible for nosocomial infection, particularly in immunocompromised patients (20). As emhABC genes of P. fluorescens, which are orthologous to MexAB-OprM of P. aeruginosa and TtgABC of Pseudomonas putida (21), play a role in antibiotic susceptibility, it has been of considerable interest in understanding the functions and regulation mechanisms of EmhABC. The EmhABC pump in P. fluorescens exhibits the ability to extrude polycyclic aromatic hydrocarbons (21), antibiotics (e.g., chloramphenicol, nalidixic acid, ampicillin, and tetracycline), and dyes (21 – 23). Of note, unlike MexAB-OprM and TtgABC, EmhABC does not display broad substrate specificity to antibiotics (21). Currently, two regulators, EmhR and two-component system (TCS) RstAB, directly participate in the regulation of emhABC. EmhR negatively regulates (23) while RstAB positively regulates emhABC expression under non-inducing conditions (24). Interestingly, exposure to indole induces a conformational change in the EmhR dimer, which attenuates the EmhR-DNA interaction and relieves the inhibitory effect on emhABC (23). Considering that most RND operons are tightly controlled by various transcription regulators, a deeper understanding of the regulation mechanisms governing the EmhABC efflux pump is necessary.
Toxin-antitoxin (TA) systems are genetic modules consisting of two or three closely linked genes that encode a stable toxin protein and its cognate antitoxin (25). To date, TA systems are categorized into eight types (26, 27), with type II TA systems being the most extensively studied (28). Under normal conditions, the type II antitoxin neutralizes the toxicity of the toxin. However, under stressed growth conditions, the antitoxin protein is degraded by endogenous proteases such as Lon and/or Clp family, resulting in the release of toxin, which then alters metabolism or inhibits growth (29). Type II antitoxin typically contains a C-terminal DNA-binding domain and serves as a transcription factor (30). One notable example is the antitoxin MqsA from E. coli, which binds to the promoter regions of csgD (31) and rpoS (32) to regulate biofilm formation and stress response. Similar antitoxins, for example, MqsA in P. putida KT2440 (33) and HigA in P. aeruginosa have been identified (34, 35). A comprehensive investigation into the regulatory mechanism of type II antitoxins could greatly enhance our understanding of the biological functions of TA systems.
The primary roles of TA systems have been demonstrated to include maintaining genetic stabilization of chromosomes (36, 37), inhibiting phage propagation (38), and promoting antibiotic tolerance (39). Previously, we characterized a type II TA pair PfMqsRA in P. fluorescens and revealed that antitoxin PfMqsA acts as a repressor of the GntR-type transcriptional factor AgtR to influence biofilm formation (40). In this study, we demonstrate that AgtR enhances antibiotic susceptibility in P. fluorescens by directly regulating EmhR, a repressor that controls the expression of the RND efflux pump EmhABC. Given that PfMqsA represses AgtR, our findings propose a novel role for PfMqsA in antibiotic susceptibility.
RESULTS
The C-terminal DNA-binding domain of PfMqsA and AgtR are involved in antibiotic susceptibility
Although antitoxin PfMqsA was demonstrated to regulate transcription factor AgtR, which regulates up to 252 genes in P. fluorescens (40), it is not clear if these two genes were involved in antibiotic susceptibility. To address this, we conducted susceptibility assessments on several strains: the wild-type strain, the ΔPfmqsA-C mutant lacking the C-terminal DNA-binding domain (residue 80 to residue 160) of PfMqsA, and the ΔagtR mutant strain, using a range of antibiotics. As shown in Table 1, deletion of agtR gene resulted in the increase of ampicillin (AMP), chloramphenicol (CHL), gentamicin (GEN), piperacillin (PIP), lomefloxacin (LOM) and tetracycline (TET) minimal inhibitory concentrations (MICs), compared to the wild-type strains. However, the PfmqsA-C deletion mutant exhibited higher susceptibility to these antibiotics. No significant difference was observed with kanamycin (KAN). In addition, the susceptibility of two deletion mutants (ΔagtR and ΔPfmqsA-C) was fully restored by the addition of plasmid carrying the agtR or PfmqsA-C allele complementation (Table 1). Together, these data show that PfMqsA-C and AgtR are responsible for the changed susceptibility of P. fluorescens to these antimicrobial agents.
TABLE 1.
MICs of P. fluorescens and its derivatives to antibiotics a
| Strains | Antibiotics (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| AMP | CHL | KAN | TET | GEN | LOM | PIP | CB | |
| Wild type | 256 | 128 | 2 | 4 | 4 | 0.5 | 4 | >512 |
| ΔPfmqsA-C | 128 | 64 | 2 | 2 | 0.5 | <0.25 | 2 | >512 |
| ΔagtR | >512 | 256 | 2 | 8 | 8 | 2 | 8 | >512 |
| ΔemhR | >512 | 256 | 2 | 16 | 16 | 2 | 8 | >512 |
| ΔemhABC | 16 | 32 | 2 | 1 | 0.5 | <0.25 | 2 | 8 |
| ΔemhRΔagtR | >512 | 256 | 2 | 16 | 16 | 2 | 8 | >512 |
| ΔemhR::agtR | >512 | 256 | 8 | ND | 16 | 2 | 8 | >512 |
| ΔagtR::emhR | 64 | 64 | 2 | ND | <0.25 | <0.25 | 2 | 256 |
| ΔemhR::emhR | 256 | 128 | 2 | ND | 4 | 0.5 | 4 | >512 |
| ΔemhRΔagtR::agtR | >512 | 256 | 8 | ND | 16 | 2 | 8 | >512 |
| ΔPfmqsA-C::PfmqsA-C | 256 | 128 | 2 | ND | 4 | 0.5 | 4 | >512 |
| ΔagtR::agtR | 256 | 128 | 8 | ND | 4 | 0.5 | 4 | >512 |
AMP, ampicillin; CHL, chloramphenicol; KAN, kanamycin; TET, tetracycline; GEN, gentamicin; LOM, lomefloxacin; PIP, piperacillin; CB, carbenicillin. ND represents not determined, due to TET resistance gene encoded on the plasmid pRK415.
AgtR binds to the intergenic region between emhABC and emhR
Previous study has suggested that the EmhABC efflux pump, which is negatively controlled by EmhR, is a key determinant to the resistance to antimicrobial agents including AMP, CHL, TET, LOM, ethidium bromide, and crystal violet (22, 23). Therefore, we speculated that the changes in MICs observed for the ΔagtR and ΔPfmqsA-C mutants were likely due to the transcription factors PfMqsA and AgtR controlling the expression of emhABC or emhR. We first confirmed the involvement of EmhR and EmhABC in bacterial susceptibility to AMP, CHL, PIP, LOM, GEN, and TET. MIC analysis revealed that deletion of emhR gene led to decreased susceptibility to these antibiotics, while the ΔemhABC mutant displayed increased antibiotic susceptibility compared to the wild-type strain (Table 1). Regarding carbenicillin (CB), P. fluorescens exhibited high resistance (MIC >512 µg/mL), and deletion of emhABC resulted in moderate resistance (MIC = 8 µg/mL). In contrast, P. aeruginosa only displayed moderate resistance (MIC = 16 µg/mL) (41). These findings suggest that other factors, besides emhABC, contribute to the intrinsic CB resistance observed in P. fluorescens.
Next, we aimed to determine if these two proteins physically interact with the promoter region of the emhR operon or emhABC operon. Genomic context analysis of emhABC reveals that emhR is adjacent to the emhABC operon and transcribed in the opposite direction (Fig. 3A) (22). The emhABC-emhR intergenic region (designated F0, 331 bp) was amplified, and electrophoretic mobility shift assays (EMSAs) were employed to detect the binding of PfMqsA or AgtR to the DNA fragment. As depicted in Fig. 1A, AgtR bound and shifted the F0 probe in a dose-dependent manner. In contrast, the PfMqsA protein did not shift the F0 probe (Fig. 1B), demonstrating that AgtR, rather than PfMqsA, is likely directly involved in controlling the expression of EmhR or EmhABC.
Fig 1.
Transcription factor AgtR binds to the intergenic region of emhABC-emhR. (A) EMSAs were employed to determine the interaction between AgtR and F0. F0 (0.05 µM) was incubated with different amounts of AgtR (0.1–4 μM) in total reaction mixtures of 20 µL each. (B) EMSAs were used to determine the interaction between PfMqsA and F0. F0 (0.04 µM) was incubated with different amounts of PfMqsA (1–6 μM) in total reaction mixtures of 20 µL each. (C) The transcription levels of emhR, emhA, emhB, and emhC were measured between the wild-type strain and the ΔagtR mutant. Cells were grown into an OD600 (optical density of a sample measured at a wavelength of 600 nm) of 0.5 for quantitative real-time reverse-transcription PCR assays. The expression level of the 16S rRNA gene was used to normalize data. At least four replicates were performed. P < 0.01 was displayed as **, P < 0.001 was displayed as ***. (D) The β-gal activities of pRG970-PemhABC (left) and pRG970-PemhR (right) were measured between the wild-type strain and the ΔagtR mutant. β-gal activities were measured until the OD600 reached approximately 0.5. At least four replicates were performed. P < 0.01 was displayed as **.
To systematically understand the impact of AgtR on the emhR and emhABC operons, we used quantitative real-time reverse-transcription PCR (qRT-PCR) assay to compare the transcription levels of emhR, emhA, emhB, and emhC between the wild-type strain and the ΔagtR mutant. As illustrated in Fig. 1C and Table S3, emhR mRNA level in the ΔagtR mutant was significantly repressed by ninefold compared to wild-type strain. Conversely, the relative mRNA levels of the genes from the emhABC operon were up-regulated, ranging from twofold to fourfold. The mRNA levels of these four genes in the ΔagtR mutant were restored to wild-type levels by complementation with a plasmid containing the agtR gene (Fig. 1C; Table S3). To further support these findings, we performed the lacZ promoter assays to test the effects of AgtR on emhABC and emhR at the translational level. Compared to the wild-type strain, emhABC promoter activity was increased by twofold in the ΔagtR mutant, while emhR promoter activity was repressed by nearly 1.8-fold (Fig. 1D). Collectively, these results indicate that AgtR positively regulates the expression of emhR but negatively regulates emhABC.
AgtR directly controls the expression of emhR
Given that emhABC is negatively regulated by EmhR in P. fluorescens (22), we hypothesized that AgtR may decrease antibiotic susceptibility by controlling the emhABC transcription or regulating the expression of EmhR. To elucidate the molecular mechanism of AgtR-mediated antibiotic susceptibility, an emhR and agtR double-gene knockout mutant (ΔemhRΔagtR) and several complementary strains were constructed. We then determined their susceptibilities to antibiotics. As expected, the ΔemhRΔagtR double mutant and the ΔemhR::agtR complementary strain exhibited no significant changes in susceptibility to AMP, CHL, TET, LOM, GEN, and PIP compared to the ΔemhR mutant (Table 1). In contrast, ΔagtR::emhR complementary strain displayed increased sensitivity to antibiotics compared to the ΔagtR mutant and the wild-type strain.
Furthermore, expression levels of genes from emhABC operon among different strains were also determined. As shown in Fig. 2 and Table S3, the mRNA levels of emhABC operon in the ΔemhRΔagtR mutant and the ΔemhR::agtR complementary strain were similar to those in the ΔemhR mutant. When compared to the ΔagtR mutant, the expression level of genes from the emhABC operon was significantly reduced in the ΔagtR::emhR complementary strain. Taken together, these results indicate that AgtR directly activates the expression of emhR and thus represses emhABC, which in turn increases the antibiotic susceptibility of P. fluorescens.
Fig 2.
Comparison of transcription levels of emhA (A), emhB (B), and emhC (C) among different bacterial cells. Cells were grown into an OD600 of 0.5 for qRT-PCR assays. At least four replicates were performed. P < 0.001 was displayed as ***, ns stands for non-significant.
Identification of the AgtR-binding site in the promoter region of the emhR operon
To characterize the AgtR-binding motif in the promoter region of the emhR operon (F0), the F0 was divided into three fragments (174, 120, and 157 bp), with 60 bp overlapping one by one, for EMSAs (Fig. 3). The strongly shifted bands were observed for both the 174 bp fragment and 120 bp fragment (Fig. 3B), implying the presence of binding sites within these regions. Next, the 174 bp fragment was further divided into a 114 bp fragment and a 60 bp fragment, and the 114 bp fragment was used in subsequent EMSAs. The affinity of the F0-114 bp for AgtR was completely abolished (Fig. 3C), demonstrating a binding site overlapping or within the 60 bp fragment. We recently showed that 17 bp fragment is the minimal for AgtR-binding sequence (S.-W. Quan, unpublished data). Interestingly, we identified three palindromic sequences in the 60 bp fragment referred to as H1, H2, and H3 (Table S2) using the UGENE software, and it was shown that AgtR bound to H3 rather than H1 and H2 (Fig. 3E). By using the BPROM program, the −35 region (TTGTAA) and −10 region (GCGTAATAT) were predicted to be present in the promoter of emhR. In particular, the AgtR-binding site was found adjacent to the −35 site of the emhR promoter, further confirming that AgtR positively regulates emhR expression by facilitating binding of polymerase RNA polymerase (RNAP) (Fig. 3D). This mechanism is similar to the recently described NtcA transcription factor, where its binding site in the nirA promoter (PnirA) overlaps with the −35 element, leading to DNA bending toward RNAP and facilitating RNAP accessibility to NtcA (42). Taken together, our data suggest that the binding motif of AgtR in the emhR promoter is (5´-CTAAGAAATATACTTAC-3´).
Fig 3.
Identification of the AgtR-binding sites in the promoter region of the emhR operon. (A) The promoter region of the emhR operon (F0) was divided into three fragments (174, 120, and 157 bp), and the 174 bp fragment of F0 was further divided into two fragments (F0-114-bp fragment and a 60 bp fragment). (B) EMSAs were used to determine the interaction between AgtR and 174, 120, and 157 bp fragments. DNA fragments (0.04 µM) were incubated with different amounts of AgtR (0.5–2 μM) in total reaction mixtures of 20 µL each. (C) EMSAs were employed to determine the interaction between AgtR and F0-114. F0-114 (0.05 µM) was incubated with different amounts of AgtR (0.5–2 μM) in total reaction mixtures of 20 µL each. (D) The emhR promoter region. The black lines indicate the −35 and −10 promoter region of emhR. The binding motif of AgtR is highlighted in red letters. (E) EMSAs of AgtR binding to H1, H2, and H3. DNA fragments (0.05 µM) were incubated with different amounts of AgtR (0.5–2 μM) in total reaction mixtures of 20 µL each.
PfMqsA regulates the expression of EmhR and EmhABC
Considering that the C-terminal DNA-binding domain of PfMqsA represses agtR (40), it is plausible that deletion of the PfmqsA-C-terminal domain could lead to changes in the expression of emhR and emhABC. To investigate this, we compared the mRNA levels of the emhR operon and emhABC operon in the wild-type strain and the ΔPfmqsA-C mutant using qRT-PCR assays. Indeed, in comparison to the wild-type strain or the ΔPfmqsA-C mutant with complementation, the emhR transcript in the ΔPfmqsA-C mutant was increased by threefold, while the transcription of emhA, emhB, and emhC was reduced by threefold to sevenfold (Fig. 4A; Table S3). To confirm these results, lacZ-reporter activities of emhABC and emhR in the ΔPfmqsA-C mutant were tested. Consistent with the qRT-PCR results, the ΔPfmqsA-C mutant exhibited higher emhR promoter activity and a fold lower emhABC promoter activity compared to the wild-type strain (Fig. 4B). These results demonstrate that PfMqsA modulates antibiotic susceptibility in bacteria by controlling emhR and emhABC.
Fig 4.
PfMqsA regulates the expression of emhR and emhABC operons. (A) The transcription levels of emhR, emhA, emhB, and emhC were measured between the wild-type strain and the ΔPfmqsA-C mutant. Cells were grown into an OD600 of 0.5 for qRT-PCR assays. The expression level of the 16S rRNA gene was used to normalize data. At least four replicates were performed. P < 0.001 was displayed as ***. (B) The β-gal activities of pRG970-PemhABC (left) and pRG970-PemhR (right) were measured between the wild-type strain and the ΔPfmqsA-C mutant. β-gal activities were measured until the OD600 reached approximately 0.5. At least four replicates were performed. P < 0.01 was displayed as **, P < 0.05 was displayed as *.
DISCUSSION
RND efflux pumps have been extensively reported in various bacterial pathogens, including E. coli, P. aeruginosa, Klebsiella pneumoniae, and Salmonella typhimurium (43). The expression of RND efflux pumps is usually linked with increased energy consumption and nonspecific leakage of metabolites required for growth, thus causing unwanted signaling molecules and imposing a metabolic burden on bacterial cells (44). Therefore, it is not surprising that a series of local and global transcriptional regulators are involved in tightly controlling the expression of RND efflux pumps (19). A local repressor is typically located proximal to the operon coding for the efflux pump, ensuring its expression remains at a basal level (45). Inactivation of local repressors, for example, AcrR in E. coli (46), MexR in P. aeruginosa (47), can lead to high-level expression of the operons encoding the corresponding RND efflux pumps. Global response regulators such as MarA, RamA, and SoxS can activate the housekeeping efflux pump AcrAB expression in E. coli (48). Additionally, it has been demonstrated that TCSs are involved in the expression of RND efflux pumps to sense various environmental stimuli. For instance, the BaeSR system in E. coli activates the expression of acrD and mdtABC in response to effector molecule indole (49). It was demonstrated that P. fluorescens is intrinsically resistant to certain antibiotics and toxic compounds (21, 22); however, the mechanisms underlying antibiotic susceptibility regulation in P. fluorescens remain largely unknown. In this study, we found that AgtR plays a crucial role in antibiotic susceptibility by positively regulating the expression of the repressor EmhR through its interaction with the DNA motif (5´-CTAAGAAATATACTTAC-3´), which blocks the transcript of emhABC efflux pump. On the other hand, as EmhABC may play other important roles in QS, virulence, and secondary metabolism, understanding the mechanism and regulation of the EmhABC helps us gain insights into its broader roles in bacterial physiology as well as responding to the presence of antibiotics in the environment.
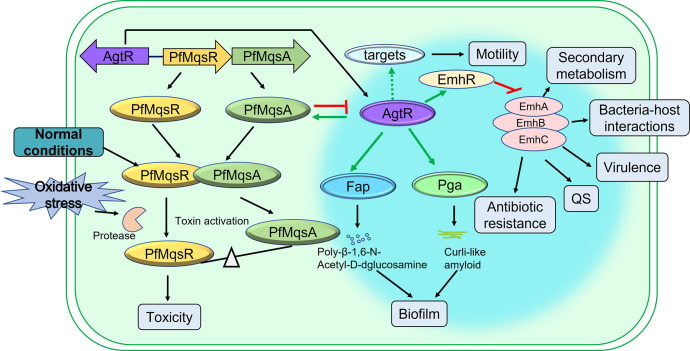
Previously, it has been reported that antitoxin PfMqsA in P. fluorescens controls the biofilm formation through the AgtR/Fap-AgtR/Pga pathway. Moreover, quantitative proteomics analysis revealed the broad regulatory roles of agtR, which controls up to 252 genes in P. fluorescens (40). In this study, we establish, for the first time, a link between the TA system and bacterial antibiotic susceptibility by showing that the PfMqsA represses agtR, leading to suppression of emhR, which in turn activates emhABC and indirectly increases the bacterial antibiotic susceptibility. A schematic diagram illustrating our current understanding of the involvement of the antitoxin PfMqsA in mediating cellular processes is shown in Fig. 5. Of note, AgtR acts as a global transcriptional factor and influences diverse biological processes in bacteria. Therefore, Fig. 5 briefly summarizes the identified roles of PfMqsA and AgtR. Our findings reveal that under oxidative stress, PfMqsA is degraded by endogenous proteases such as Lon and/or Clp family, resulting in an increased mRNA level of AgtR. This activation of AgtR promotes the expression of genes involved in poly-β-1,6-N-acetyl-D-glucosamine (PGA) biosynthesis, curli-like amyloid production, cell motility, and bacterial sensitivity to AMP, CHL, PIP, GEN, LOM, and TET. In addition, AgtR positively regulates the PfmqsRA operon, and the PfmqsA-agtR regulatory circuit manner can well tune the expression level of these two proteins under the ordinary laboratory growth conditions.
Fig 5.
Model for the regulation of PfMqsRA system in P. fluorescens. Under normal conditions, the antitoxin PfMqsA forms a tight protein complex with the toxin PfMqsR resulting in its inactivation. PfMqsA is a negative regulator of AgtR. Many genes such as fap, pga, and emhR are activated by AgtR through direct binding. Under unfavorable conditions (e.g., oxidative stresses), PfMqsA is degraded by protease Lon and/or Clp family, and the expression levels of AgtR is increased to activate genes encoding PGA biosynthesis, curli-like amyloid production, and cell motility. As EmhR represses the gene encoding RND efflux pump emhABC, PfMqsA could mediate the expression of EmhABC via the AgtR/EmhR pathway. In addition, the PfmqsRA operon is also positively regulated by AgtR, and the manner of the PfmqsA-agtR regulatory circuit can finely tune the expression level of these two proteins during balanced growth. Speculative interaction is indicated by stippled lines.
The GntR family of transcription regulators is highly abundant in bacterial genome (50), and controls a large variety of cellular processes, including virulence, pathogenesis, and glucose metabolism (51). Members of the GntR family consist of two functional domains: N-terminal DNA-binding domain containing a conserved winged helix-turn-helix motif and a larger variable C-terminal effector-binding domain (52). Increasing evidence indicates that GntR regulators are important for the expression of genes associated with antibiotic susceptibility. For instance, the novel GntR family transcriptional regulator in Mycobacterium tuberculosis Rv1152 was reported to inhibit four vancomycin-responsive genes to regulate susceptibility to vancomycin (53). The GntR transcriptional regulator Ms0535 in Mycobacterium smegmatis was found to positively regulate susceptibility to isoniazid by controlling the expression of the Ms0534 (54). In this work, we discovered that the GntR-type regulator AgtR positively regulates the expression of emhR operon by directly binding to its upstream region, leading to the inhibition of the EmhABC efflux pump and modulation of bacterial antibiotic susceptibility. This highlights the important roles of certain members of the GntR family in the regulation of bacterial antibiotic susceptibility. It has been demonstrated that EmhR is an indole-responsive regulator (23); therefore, we speculate that indole may be involved in mediating the AgtR/EmhR pathway and this molecular mechanism warrants further investigation. Additionally, it is important to mention that complementation expression of agtR resulted in a fourfold increase in MIC for Kan, while deletion of agtR and emhR did not cause this phenotype. One possible reason is that increasing agtR gene expression beyond the normal physiological level may introduce artifacts that enhance the bacterial susceptibility to Kan.
It has been observed that cells in biofilm exhibit decreased susceptibility to a wide range of antimicrobial agents and host immune defenses compared to planktonic cells (55). This mechanism is linked to metabolic alterations, exopolymer matrices, and changes in gene/protein expression (56). In fact, cells in a biofilm community can display a 10-fold to 1,000-fold increase in antibiotic resistance (57, 58). This phenomenon is observed across various bacterial species, including Staphylococcus epidermidis (58), P. aeruginosa (59), and E. coli (60). Biofilm-related genes include those encoding QS molecules (61) and TA systems (62), and a growing body of evidence suggests that TA systems are tightly linked to bacterial biofilm formation. For instance, MqsRA from E. coli is the first TA system found to be involved in biofilm formation by regulating rpoS (32). Similarly, deletion of antitoxin HigA leads to enhanced biofilm formation in P. aeruginosa PA14 (63). These lines of evidence indicate that TA systems such as RelBE (64), MqsRA (32, 33, 40), HigBA (34, 35), MazEF (65), and GraTA (66) can contribute to antibiotic resistance by influencing biofilm formation. Our previous work found that compared to wild-type strain, the ΔPfmqsA-C strain showed a similar level of biofilm formation (40). This promotes us to speculate whether the PfMqsRA system directly or indirectly regulates bacterial sensitivity to antibiotics. Interestingly, our data provide evidence of an interaction between PfMqsA and the RND efflux pump: antitoxin PfMqsA represses agtR, leads to induction of emhR, thereby suppressing EmhABC, and increases antibiotic susceptibility. Of note, results showed that changes in antibiotic susceptibility of the ΔPfmqsA-C mutant and ΔemhABC mutant to AMP, CHL, PIP, and TET are inconsistent, suggesting that besides regulating emhABC expression, PfMqsA may mediate bacterial susceptibility through additional mechanisms.
Our previous whole-proteomics analysis revealed that deletion of agtR led to an increase in the expression of outer membrane porins (i.e., tolB, oprD, oprI, and oprA) (40). Among these, TolB is known to contribute to bacterial resistance against multiple antimicrobials, such as GEN, TET, streptomycin, colistin, polymyxin B, ciprofloxacin, imipenem, and ceftazidime (67). In P. aeruginosa, OprD (68) and OprI (69) play critical roles in carbapenem resistance and cationic-helical antimicrobial peptides resistance, respectively. OprA is responsible for aminoglycoside and macrolide resistance (70). Therefore, it is plausible that PfMqsA is involved in bacterial sensitivity to antibiotics by positively regulating these genes. In addition, considering the regulation of efflux pumps is highly complex and interconnected (19); it is possible that other RND efflux pumps might synergistically interact with EmhABC to influence antibiotic sensitivity.
Over the past few decades, numerous type II TA systems have been proposed to play roles in maintaining genetic stabilization, inhibiting phage propagation and improving antibiotic tolerance. For example, the CcdAB system of plasmid F in E. coli has been shown to prevent plasmid loss via a mechanism known as post-segregational killing (36, 37). Another TA system RnlAB in E. coli can impede bacteriophage T4 infections (71). Furthermore, HipA kinases in various bacteria inhibit cell growth by phosphorylating aminoacyl-tRNA synthetases (72 – 75). Here, we revealed a novel role for the PfMqsRA system in antibiotic susceptibility and may have important implications for the study of molecular mechanisms of resistance development in environments and clinical settings. Additionally, our results strongly support the notion that exploring the regulatory mechanism of the global transcription factor AgtR greatly expands our understanding of the biological functions of PfMqsRA systems. Therefore, additional investigations are needed to explore the regulatory mechanism of AgtR in diverse processes, such as oxidative phosphorylation and carbon/nitrogen metabolism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions
The plasmids and bacterial strains used in this study are listed in Table S1, primers used for PCR amplification and qRT-PCR analysis are listed in Table S2. Luria-Bertani (LB) medium with constant shaking at 200 rpm or LB agar plate at 37°C was used to culture E. coli and its derivatives. King’s B (KB) broth with constant shaking at 200 rpm or KB agar plate at 28°C was used to culture wild -type strain of P. fluorescens and its derivatives. When required, the growth broth was added with ampicillin (50 µg/mL), chloramphenicol (34 µg/mL), kanamycin (50 µg/mL), tetracycline (20 µg/mL), isopropyl β-D-thiogalactoside (IPTG, 0.2 mM), and X-Gal (40 µg/mL).
Construction, expression, and purification of PfMqsA and AgtR
The genes encoding PfmqsA and agtR were amplified, digested with NdeI and NotI restriction enzymes and individually ligated into the pET-28b-derived vector to give plasmid pET28-PfmqsA, pET28-agtR. The recombination plasmids were transformed into E. coli BL21 (DE3) cells and grown to an optical density (OD600) of 0.6 at 200 rpm at 37°C. The target proteins were induced with a final concentration of 0.2 mM IPTG and cells were continued cultured for another 20 h at 16°C before harvesting. Then, bacterial cells were collected by centrifugation for 5 min at 12,000 × g and resuspended in the lysis buffer containing 20 mM Tris-HCl pH 7.5, 300 mM NaCl, and 10 mM imidazole. After 30 min of sonication and centrifugation at 12,000 × g, 4°C for 30 min, the supernatant was loaded onto a Ni-NTA affinity column. Proteins were finally eluted with 20 mM Tris-HCl pH 7.5, 250 mM imidazole, and 300 mM NaCl. All purified proteins were then pooled, loaded to a HiLoad 26/60 Superdex 75 pg column, and equilibrated with 300 mM NaCl and 20 mM Tris-HCl pH 7.5.
EMSA
EMSAs were performed as described in the previous work (76). 6-Carboxyfluorescein-F0 was obtained from PCR amplification. The DNA probe was incubated with different amounts of purified proteins in a 20 µL final volume of binding buffer [50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 50 mM KCl, 0.5 mM dithiothreitol (DTT), 3 µM human serum albumin (HSA), 10% (vol/vol) glycerol] for 30 min on ice. The samples were then mixed with 1× sucrose solution (5%, vol/vol) and electrophoresed on a 5% Native-PAGE at 80 V in 1× Tris-borate-EDTA buffer for 90 min. The gels were visualized by a gel imaging system (BIO-LAB, PharosFX) at 488 nm.
Construction and complementation of deletion mutants
We have already reported the construction of ΔPfmqsA-C mutant and ΔagtR mutant (40), and the ΔemhR mutant strain was constructed using a two-step homologous recombination according to a modification protocol of Wang et al. (77). Briefly, two flanking regions of ~0.8 kb of the target gene were amplified, digested, and ligated into the suicide vector pK18mobsacB-km to generate the replacement plasmid. The plasmid was transformed into E. coli S17-1 and conjugated into wild -type strain of P. fluorescens by biparental mating. After two recombination exchanges, deleted mutant was selected on the KB agar plate supplied with 10% (vol/vol) sucrose and ampicillin. To obtain the emhR and agtR double-gene knockout mutant (ΔemhRΔagtR), we used the ΔemhR mutant. All deletion mutants were confirmed by PCR amplification. Complementary plasmids for deletion mutants were constructed using the broad-host-range vector pRK415.
Construction of reporter strains and β-galactosidase assay
Plasmids for lacZ reporter were constructed using pRG970Km as previously described (76). Briefly, DNA fragments containing 331 bp of the upstream regions of the emhABC operon and emhR were amplified, digested, and cloned ahead of lacZ gene in pRG970Km, respectively. The resulting plasmids were conjugated to mutant and wild-type strain of P. fluorescens by biparental mating, as described above. To monitor β-galactosidase activity, the strains were grown in 100 mL flasks containing 50 mL KB broth at 200 rpm at 28°C. β-Galactosidase activities were quantified according to Miller method (78).
RNA isolation and qRT-PCR
Total RNA was isolated from wild-type strains of P. fluorescens and mutants with Trizol (Lablead, Beijing, China) and chloroform. qRT-PCR assays were performed as described previously (76). Briefly, a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) was used to synthesize single-strand cDNAs; 100 ng of total RNA and specific primers were used for real-time PCR analysis, according to the manufacturer’s instructions [SYBR Premix Ex Taq II (Tli RNaseH Plus) dkit, TaKaRa, Dalian, China]. The qRT-PCR conditions were 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s using a CFX real-time PCR system (Bio-Rad, Hercules, CA). The 16S rRNA was used as an internal control to normalize all data and all measurements were performed in four biological replicates. The fold change of each gene was calculated using the 2 -ΔΔCt formula method (79).
MIC determination
MIC analysis was performed according to the procedures described previously (80). Briefly, the single colonies from wild-type strain of P. fluorescens and mutants were inoculated in 2 mL fresh KB broth and grown to early exponential phase at 28°C with shaking. The cultures were then diluted with KB broth (final inoculum density of 1 × 106 cells/mL) and incubated with 96-well microtiter plates at 28°C for 20 h without shaking. Control experiment was KB broth containing no antibiotics. The cell viability was monitored by a multi-mode microplate reader (Bio-Tek, USA at 570 nm. All antibiotics were purchased from Lab Lead Co. (Beijing, China) and assays were performed in four biological replicates.
ACKNOWLEDGMENTS
The authors thank the Core Facility of School of Life Sciences of Lanzhou University for proteomic analysis. This work was supported by the grants from the National Natural Science Foundation of China (grant numbers 31971422 and 31972319), the Gansu Research Program (22CX8NA001), the Gansu Association for Science and Technology (GSHZSF2023-06), and the Research Funds for the Central Universities from Lanzhou University (grant no. lzujbky-2023-eyt02)
Contributor Information
Yong Wang, Email: wangyongdd@lzu.edu.cn.
Yong-Xing He, Email: heyx@lzu.edu.cn.
Laurent Poirel, University of Fribourg, Fribourg, Switzerland .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00812-23.
supplemental table.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Nikaido H, Takatsuka Y. 2009. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta 1794:769–781. doi: 10.1016/j.bbapap.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nikaido H. 2009. Multidrug resistance in bacteria. Annu Rev Biochem 78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eswaran J, Koronakis E, Higgins MK, Hughes C, Koronakis V. 2004. Three's company: component structures bring a closer view of tripartite drug efflux pumps. Curr Opin Struct Biol 14:741–747. doi: 10.1016/j.sbi.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 4. de Bentzmann S, Plésiat P. 2011. The pseudomonas aeruginosa opportunistic pathogen and human infections. Environ Microbiol 13:1655–1665. doi: 10.1111/j.1462-2920.2011.02469.x [DOI] [PubMed] [Google Scholar]
- 5. Li XZ, Nikaido H, Poole K. 1995. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother 39:1948–1953. doi: 10.1128/AAC.39.9.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and Mexxy-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother 44:3322–3327. doi: 10.1128/AAC.44.12.3322-3327.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraud S, Campigotto AJ, Chen Z, Poole K. 2008. MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa: involvement in chlorhexidine resistance and induction by membrane-damaging agents dependent upon the algu stress response sigma factor. Antimicrob Agents Chemother 52:4478–4482. doi: 10.1128/AAC.01072-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fetar H, Gilmour C, Klinoski R, Daigle DM, Dean CR, Poole K. 2011. mexEF-oprN multidrug efflux operon of Pseudomonas aeruginosa: regulation by the mext activator in response to nitrosative stress and chloramphenicol. Antimicrob Agents Chemother 55:508–514. doi: 10.1128/AAC.00830-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li XZ, Nikaido H. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623. doi: 10.2165/11317030-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mima T, Sekiya H, Mizushima T, Kuroda T, Tsuchiya T. 2005. Gene cloning and properties of the RND-type multidrug efflux pumps MexPQ-OpmE and MexMN-OprM from Pseudomonas aeruginosa. Microbiol Immunol 49:999–1002. doi: 10.1111/j.1348-0421.2005.tb03696.x [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Mima T, Komori Y, Morita Y, Kuroda T, Mizushima T, Tsuchiya T. 2003. A new member of the tripartite multidrug efflux pumps, MexVW-OprM, in Pseudomonas aeruginosa. J Antimicrob Chemother 52:572–575. doi: 10.1093/jac/dkg390 [DOI] [PubMed] [Google Scholar]
- 12. Mima T, Kohira N, Li Y, Sekiya H, Ogawa W, Kuroda T, Tsuchiya T. 2009. Gene cloning and characteristics of the RND-type multidrug efflux pump MuxABC-OpmB possessing two RND components in Pseudomonas aeruginosa. Microbiology (Reading) 155:3509–3517. doi: 10.1099/mic.0.031260-0 [DOI] [PubMed] [Google Scholar]
- 13. Yang L, Chen L, Shen L, Surette M, Duan K. 2011. Inactivation of MuxABC-OpmB transporter system in Pseudomonas aeruginosa leads to increased ampicillin and carbenicillin resistance and decreased virulence. J Microbiol 49:107–114. doi: 10.1007/s12275-011-0186-2 [DOI] [PubMed] [Google Scholar]
- 14. Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. 2007. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol 189:7600–7609. doi: 10.1128/JB.00850-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perron K, Caille O, Rossier C, Van Delden C, Dumas JL, Köhler T. 2004. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J Biol Chem 279:8761–8768. doi: 10.1074/jbc.M312080200 [DOI] [PubMed] [Google Scholar]
- 16. Li XZ, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alcalde-Rico M, Olivares-Pacheco J, Alvarez-Ortega C, Cámara M, Martínez JL. 2018. Role of the multidrug resistance efflux pump MexCD-OprJ in the Pseudomonas aeruginosa Quorum sensing response. Front Microbiol 9:2752. doi: 10.3389/fmicb.2018.02752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeannot K, Elsen S, Köhler T, Attree I, van Delden C, Plésiat P. 2008. Resistance and virulence of Pseudomonas aeruginosa clinical strains overproducing the MexCD-OprJ efflux pump. Antimicrob Agents Chemother 52:2455–2462. doi: 10.1128/AAC.01107-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du D, Wang-Kan X, Neuberger A, van Veen HW, Pos KM, Piddock LJV, Luisi BF. 2018. Multidrug efflux pumps: structure, function and regulation. Nat Rev Microbiol 16:523–539. doi: 10.1038/s41579-018-0060-x [DOI] [PubMed] [Google Scholar]
- 20. Singh P, Montano A, Bostick A. 2021. Rapid severe sepsis from Pseudomonas fluorescens/ putida bacteremia due to skin and soft tissue infection – a case report. Ann Med Surg (Lond) 70:102845. doi: 10.1016/j.amsu.2021.102845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hearn EM, Dennis JJ, Gray MR, Foght JM. 2003. Identification and characterization of the emhABC efflux system for polycyclic aromatic hydrocarbons in Pseudomonas fluorescens Clp6A. J Bacteriol 185:6233–6240. doi: 10.1128/JB.185.21.6233-6240.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian T, Wu XG, Duan HM, Zhang LQ. 2010. The resistance-nodulation-division efflux pump emhabc influences the production of 2,4-diacetylphloroglucinol in Pseudomonas fluorescens 2P24. Microbiology 156:39–48. doi: 10.1099/mic.0.031161-0 [DOI] [PubMed] [Google Scholar]
- 23. Han JT, Li DY, Zhang MY, Yu XQ, Jia XX, Xu H, Yan X, Jia WJ, Niu S, Kempher ML, Tao X, He YX. 2021. Emhr is an indole-sensing transcriptional regulator responsible for the indole-induced antibiotic tolerance in Pseudomonas fluorescens. Environ Microbiol 23:2054–2069. doi: 10.1111/1462-2920.15354 [DOI] [PubMed] [Google Scholar]
- 24. Li DY, Han JT, Zhang MY, Yan X, Zhang N, Ge H, Wang Z, He YX, Bulman Z. 2021. The two-component system RstA/RstB regulates expression of multiple efflux pumps and influences anaerobic nitrate respiration in Pseudomonas fluorescens. mSystems 6:e0091121. doi: 10.1128/mSystems.00911-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33:966–976. doi: 10.1093/nar/gki201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Yao J, Sun Y-C, Wood TK. 2021. Type VII toxin/antitoxin classification system for antitoxins that enzymatically neutralize toxins. Trends Microbiol 29:388–393. doi: 10.1016/j.tim.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 27. Jurėnas D, Fraikin N, Goormaghtigh F, Van Melderen L. 2022. Biology and evolution of bacterial toxin–antitoxin systems. Nat Rev Microbiol 20:335–350. doi: 10.1038/s41579-021-00661-1 [DOI] [PubMed] [Google Scholar]
- 28. Harms A, Brodersen DE, Mitarai N, Gerdes K. 2018. Toxins, targets, and triggers: an overview of toxin-antitoxin biology. Mol Cell 70:768–784. doi: 10.1016/j.molcel.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 29. Chan WT, Espinosa M, Yeo CC. 2016. Keeping the wolves at bay: antitoxins of prokaryotic type II toxin-antitoxin systems. Front Mol Biosci 3:9. doi: 10.3389/fmolb.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayes F, Van Melderen L. 2011. Toxins-antitoxins: diversity, evolution and function. Crit Rev Biochem Mol Biol 46:386–408. doi: 10.3109/10409238.2011.600437 [DOI] [PubMed] [Google Scholar]
- 31. Soo VW, Wood TK. 2013. Antitoxin MqsA represses curli formation through the master biofilm regulator CsgD. Sci Rep 3:3186. doi: 10.1038/srep03186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Kim Y, Hong SH, Ma Q, Brown BL, Pu M, Tarone AM, Benedik MJ, Peti W, Page R, Wood TK. 2011. Antitoxin MqsA helps mediate the bacterial general stress response. Nat Chem Biol 7:359–366. doi: 10.1038/nchembio.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun C, Guo Y, Tang K, Wen Z, Li B, Zeng Z, Wang X. 2017. MqsR/MqsA toxin/antitoxin system regulates persistence and biofilm formation in Pseudomonas putida Kt2440. Front Microbiol 8:840. doi: 10.3389/fmicb.2017.00840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo Y, Sun C, Li Y, Tang K, Ni S, Wang X. 2019. Antitoxin Higa inhibits virulence gene mvfR expression in Pseudomonas aeruginosa. Environ Microbiol 21:2707–2723. doi: 10.1111/1462-2920.14595 [DOI] [PubMed] [Google Scholar]
- 35. Song Y, Zhang S, Luo G, Shen Y, Li C, Zhu Y, Huang Q, Mou X, Tang X, Liu T, Wu S, Tong A, He Y, Bao R. 2021. Type II antitoxin higa is a key virulence regulator in Pseudomonas aeruginosa. ACS Infect Dis 7:2930–2940. doi: 10.1021/acsinfecdis.1c00401 [DOI] [PubMed] [Google Scholar]
- 36. Dao-Thi M-H, Van Melderen L, De Genst E, Afif H, Buts L, Wyns L, Loris R. 2005. Molecular basis of gyrase poisoning by the addiction toxin Ccdb. Journal of Molecular Biology 348:1091–1102. doi: 10.1016/j.jmb.2005.03.049 [DOI] [PubMed] [Google Scholar]
- 37. Madl T, Van Melderen L, Mine N, Respondek M, Oberer M, Keller W, Khatai L, Zangger K. 2006. Structural basis for nucleic acid and toxin recognition of the bacterial Antitoxin Ccda. J Mol Biol 364:170–185. doi: 10.1016/j.jmb.2006.08.082 [DOI] [PubMed] [Google Scholar]
- 38. Dy RL, Richter C, Salmond GPC, Fineran PC. 2014. Remarkable mechanisms in microbes to resist phage infections. Annu Rev Virol 1:307–331. doi: 10.1146/annurev-virology-031413-085500 [DOI] [PubMed] [Google Scholar]
- 39. Moyed HS, Bertrand KP. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of Murein synthesis. J Bacteriol 155:768–775. doi: 10.1128/jb.155.2.768-775.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Zhang SP, Zhang MY, Kempher ML, Guo DD, Han JT, Tao X, Wu Y, Zhang LQ, He YX. 2019. The antitoxin MqsA homologue in Pseudomonas fluorescens 2P24 has a rewired regulatory circuit through evolution. Environ Microbiol 21:1740–1756. doi: 10.1111/1462-2920.14538 [DOI] [PubMed] [Google Scholar]
- 41. Mesaros N, Glupczynski Y, Avrain L, Caceres NE, Tulkens PM, Van Bambeke F. 2007. A combined phenotypic and genotypic method for the detection of Mex efflux pumps in Pseudomonas aeruginosa. J Antimicrob Chemother 59:378–386. doi: 10.1093/jac/dkl504 [DOI] [PubMed] [Google Scholar]
- 42. Han SJ, Jiang YL, You LL, Shen LQ, Yang F, Cui N, Kong WW, Sun H, Zhou K, Meng HC, Chen ZP, Chen Y, Zhang Y, Zhou CZ. 2022. DNA Looping mediates cooperative transcription activation. bioRxiv. doi: 10.1101/2022.10.10.511513 [DOI] [PubMed]
- 43. Trastoy R, Manso T, Fernández-García L, Blasco L, Ambroa A, Pérez Del Molino ML, Bou G, García-Contreras R, Wood TK, Tomás M. 2018. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin Microbiol Rev 31:e00023-18. doi: 10.1128/CMR.00023-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olivares Pacheco J, Alvarez-Ortega C, Alcalde Rico M, Martínez JL, Davies JE. 2017. Metabolic compensation of fitness costs is a general outcome for antibiotic-resistant Pseudomonas aeruginosa mutants overexpressing efflux pumps. mBio 8. doi: 10.1128/mBio.00500-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grkovic S, Brown MH, Skurray RA. 2002. Regulation of bacterial drug export systems. Microbiol Mol Biol Rev 66:671–701, doi: 10.1128/MMBR.66.4.671-701.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma D, Alberti M, Lynch C, Nikaido H, Hearst JE. 1996. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol 19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x [DOI] [PubMed] [Google Scholar]
- 47. Evans K, Adewoye L, Poole K. 2001. MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: identification of MexR binding sites in the mexA-mexR Intergenic region. J Bacteriol 183:807–812. doi: 10.1128/JB.183.3.807-812.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holden ER, Webber MA. 2020. MarA, RamA, and SoxS as mediators of the stress response: survival at a cost. Front Microbiol 11:828. doi: 10.3389/fmicb.2020.00828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nishino K, Nikaido E, Yamaguchi A. 2007. Regulation of multidrug efflux systems involved in multidrug and metal resistance of Salmonella enterica serovar typhimurium. J Bacteriol 189:9066–9075. doi: 10.1128/JB.01045-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rigali S, Derouaux A, Giannotta F, Dusart J. 2002. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J Biol Chem 277:12507–12515. doi: 10.1074/jbc.M110968200 [DOI] [PubMed] [Google Scholar]
- 51. Liu GH. 2021. Progress on the GntR Family Transcription Regulators in Bacteria. Available from: 10.16288/j.yczz.20-245. https://doi.org/10.16288/j.yczz.20-245 [DOI] [PubMed]
- 52. Jain D. 2015. Allosteric control of transcription in GntR family of transcription regulators: a structural overview. IUBMB Life 67:556–563. doi: 10.1002/iub.1401 [DOI] [PubMed] [Google Scholar]
- 53. Zeng J, Deng W, Yang W, Luo H, Duan X, Xie L, Li P, Wang R, Fu T, Abdalla AE, Xie J. 2016. Mycobacterium tuberculosis Rv1152 is a novel GntR family transcriptional regulator involved in intrinsic vancomycin resistance and is a potential vancomycin adjuvant target. Sci Rep 6:28002. doi: 10.1038/srep28002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu J, Zhao L, Yang M. 2015. A GntR family transcription factor positively regulates mycobacterial isoniazid resistance by controlling the expression of a putative permease. BMC Microbiol 15:214. doi: 10.1186/s12866-015-0556-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mah TF, O’Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39. doi: 10.1016/s0966-842x(00)01913-2 [DOI] [PubMed] [Google Scholar]
- 56. Muranaka LS, Takita MA, Olivato JC, Kishi LT, de Souza AA. 2012. Global expression profile of biofilm resistance to antimicrobial compounds in the plant-pathogenic bacterium Xylella fastidiosa reveals evidence of persister cells. J Bacteriol 194:4561–4569. doi: 10.1128/JB.00436-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gristina AG, Hobgood CD, Webb LX, Myrvik QN. 1987. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 8:423–426. doi: 10.1016/0142-9612(87)90077-9 [DOI] [PubMed] [Google Scholar]
- 58. Evans RC, Holmes CJ. 1987. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob Agents Chemother 31:889–894. doi: 10.1128/AAC.31.6.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nickel JC, Ruseska I, Wright JB, Costerton JW. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27:619–624. doi: 10.1128/AAC.27.4.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. 2004. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol 64:515–524. doi: 10.1007/s00253-003-1517-y [DOI] [PubMed] [Google Scholar]
- 61. Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762–764. doi: 10.1038/35037627 [DOI] [PubMed] [Google Scholar]
- 62. Hemati S, Azizi-Jalilian F, Pakzad I, Taherikalani M, Maleki A, Karimi S, Monjezei A, Mahdavi Z, Fadavi MR, Sayehmiri K, Sadeghifard N. 2014. The correlation between the presence of quorum sensing, toxin-antitoxin system genes and MIC values with ability of biofilm formation in clinical isolates of Pseudomonas aeruginosa. Iran J Microbiol 6:133–139. [PMC free article] [PubMed] [Google Scholar]
- 63. Wood TL, Wood TK. 2016. The Higb/Higa toxin/antitoxin system of Pseudomonas aeruginosa influences the virulence factors pyochelin, pyocyanin, and biofilm formation. Microbiologyopen 5:499–511. doi: 10.1002/mbo3.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Y, Wang H, Hay AJ, Zhong Z, Zhu J, Kan B, Vadivelu J. 2015. Functional relbe-family toxin-antitoxin pairs affect biofilm maturation and intestine colonization in Vibrio cholerae. PLoS ONE 10:e0135696. doi: 10.1371/journal.pone.0135696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tripathi A, Dewan PC, Siddique SA, Varadarajan R. 2014. Mazf-induced growth inhibition and persister generation in Escherichia coli. J Biol Chem 289:4191–4205. doi: 10.1074/jbc.M113.510511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tamman H, Ainelo A, Ainsaar K, Hõrak R. 2014. A moderate toxin, Grat, modulates growth rate and stress tolerance of Pseudomonas putida. J Bacteriol 196:157–169. doi: 10.1128/JB.00851-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lo Sciuto A, Fernández-Piñar R, Bertuccini L, Iosi F, Superti F, Imperi F, Bengoechea JA. 2014. The periplasmic protein tolb as a potential drug target in Pseudomonas aeruginosa. PLoS ONE 9:e103784. doi: 10.1371/journal.pone.0103784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li H, Luo YF, Williams BJ, Blackwell TS, Xie CM. 2012. Structure and function of Oprd protein in Pseudomonas aeruginosa: from antibiotic resistance to novel therapies. Int J Med Microbiol 302:63–68. doi: 10.1016/j.ijmm.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chang T-W, Wang C-F, Huang H-J, Wang I, Hsu S-TD, Liao Y-D. 2015. Key residues of outer membrane protein OprI involved in hexamer formation and bacterial susceptibility to cationic antimicrobial peptides. Antimicrob Agents Chemother 59:6210–6222. doi: 10.1128/AAC.01406-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moore RA, DeShazer D, Reckseidler S, Weissman A, Woods DE. 1999. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother 43:465–470. doi: 10.1128/AAC.43.3.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koga M, Otsuka Y, Lemire S, Yonesaki T. 2011. Escherichia coli rnlA and rnlB compose a novel toxin-antitoxin system. Genetics 187:123–130. doi: 10.1534/genetics.110.121798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kaspy I, Rotem E, Weiss N, Ronin I, Balaban NQ, Glaser G. 2013. Hipa-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat Commun 4:3001. doi: 10.1038/ncomms4001 [DOI] [PubMed] [Google Scholar]
- 73. Germain E, Castro-Roa D, Zenkin N, Gerdes K. 2013. Molecular mechanism of bacterial persistence by hipa. Molecular Cell 52:248–254. doi: 10.1016/j.molcel.2013.08.045 [DOI] [PubMed] [Google Scholar]
- 74. Vang Nielsen S, Turnbull KJ, Roghanian M, Bærentsen R, Semanjski M, Brodersen DE, Macek B, Gerdes K, Laub MT, Helaine S, Arraiano C. 2019. Serine-threonine kinases encoded by split hipA homologs inhibit tryptophanyl-tRNA synthetase. mBio 10. doi: 10.1128/mBio.01138-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou X, Eckart MR, Shapiro L, Blaser MJ. 2021. A bacterial toxin perturbs intracellular amino acid balance to induce persistence. mBio 12. doi: 10.1128/mBio.03020-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yan X, Yang R, Zhao R-X, Han J-T, Jia W-J, Li D-Y, Wang Y, Zhang N, Wu Y, Zhang L-Q, He Y-X, Zhou N-Y. 2017. Transcriptional regulator PhlH modulates 2,4-Diacetylphloroglucinol biosynthesis in response to the biosynthetic intermediate and end product. Appl Environ Microbiol 83. doi: 10.1128/AEM.01419-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang P, Yu Z, Li B, Cai X, Zeng Z, Chen X, Wang X. 2015. Development of an efficient conjugation-based genetic manipulation system for pseudoalteromonas. Microb Cell Fact 14:11. doi: 10.1186/s12934-015-0194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Miller J. 1972. Experiments in molecular genetics. Q Rev Biol 49. doi: 10.1086/408025 [DOI] [Google Scholar]
- 79. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔδCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 80. Schwalbe R, Steele-Moore L, Goodwin AC. 2007. Antimicrobial susceptibility testing protocols. Antimicrobial susceptibility testing protocol. CRC Press. doi: 10.1201/9781420014495 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplemental table.