Abstract
Previously, our laboratory showed that Holstein cattle experimentally infected with Neospora caninum develop parasite-specific CD4+ cytotoxic T lymphocytes (CTL) that lyse infected, autologous target cells through a perforin-granzyme pathway. To identify specific parasite antigens inducing bovine CTL and helper T-lymphocyte responses for vaccine development against bovine neosporosis, the tachyzoite major surface proteins NcSAG1 and NcSRS2 were targeted. In whole tachyzoite antigen-expanded bovine T-lymphocyte lines, recombinant NcSRS2 induced potent memory CD4+- and CD8+-T-lymphocyte activation, as indicated by proliferation and gamma interferon (IFN-γ) secretion, while recombinant NcSAG1 induced a minimal memory response. Subsequently, T-lymphocyte epitope-bearing peptides of NcSRS2 were mapped by using overlapping peptides covering the entire NcSRS2 sequence. Four experimentally infected cattle with six different major histocompatibility complex (MHC) class II haplotypes were the source of immune cells used to identify NcSRS2 peptides presented by Holstein MHC haplotypes. NcSRS2 peptides were mapped by using IFN-γ secretion by rNcSRS2-stimulated, short-term T-lymphocyte cell lines, IFN-γ enzyme-linked immunospot (ELISPOT) assay with peripheral blood mononuclear cells, and 51Cr release cytotoxicity assay of rNcSRS2-stimulated effector cells. Four N. caninum-infected Holstein cattle developed NcSRS2 peptide-specific T lymphocytes detected ex vivo in peripheral blood by IFN-γ ELISPOT and in vitro by measuring T-lymphocyte IFN-γ production and cytotoxicity. An immunodominant region of NcSRS2 spanning amino acids 133 to 155 was recognized by CD4+ T lymphocytes from the four cattle. These findings support investigation of subunit N. caninum vaccines incorporating NcSRS2 gene sequences or peptides for induction of NcSRS2 peptide-specific CTL and IFN-γ-secreting T lymphocytes in cattle with varied MHC genotypes.
Neospora caninum is an intracellular apicomplexan protozoan parasite that causes abortion and congenital infection in cattle (21). Transplacental parasite transmission is the major mode of infection (18, 45) which persists in the herd over successive generations, causing significant economic losses due to abortion, culling, and decreased milk production (13, 20, 25, 52, 53). Recrudescence of chronic, congenitally acquired infection due to downregulation of Th1 immune responses during pregnancy may be largely responsible for transplacental N. caninum transmission (10, 22, 27, 42). A vaccine that could control tachyzoite parasitemia and decrease congenital infection or abortion rates is needed.
Studies of Toxoplasma gondii, a protozoan parasite closely related to N. caninum in biology, phylogeny, and immunogenicity, unequivocally show the importance of T lymphocytes in resistance to infection by adoptive transfer, gene knockout, and depletion studies with mouse models (19). The presence of parasite-specific CD4+ and CD8+ cytotoxic T lymphocytes (CTL) is also associated with immunity to natural T. gondii infection in humans (14, 41). In murine models of neosporosis, a T-lymphocyte-mediated immune response, characterized by increased levels of gamma interferon (IFN-γ) and interleukin 12 (IL-12), correlates with disease resistance (5, 30, 33), and IFN-γ alone inhibits tachyzoite growth and cellular invasion (24, 38, 51, 55). In pregnant mice, protection against vertical transmission correlates with induction of parasite-specific T-lymphocyte-mediated immunity (39). Cattle infected with N. caninum produce parasite-specific, major histocompatibility complex (MHC)-restricted, CD4+ CTL (50) that could reduce N. caninum infection indirectly by IFN-γ activation of mononuclear phagocytes and directly by killing N. caninum-infected cells.
To identify vaccine candidate parasite antigens for induction of CTL and IFN-γ secretion in cattle against neosporosis abortion, this study focused on major tachyzoite glycosylphosphatidylinositol-anchored surface proteins, N. caninum surface antigen 1 (NcSAG1) and N. caninum SAG1-related sequence 2 (NcSRS2) (26, 28). The T. gondii surface protein TgSAG1, an orthologue of NcSAG1 (28), protected against congenital T. gondii infection in BALB/c mice (31). In murine models of neosporosis, NcSRS2 vaccination induced protection against lethal challenge or vertical transmission (11, 37, 39). These data provided the rationale to test the hypothesis that NcSAG1 and NcSRS2 induce CTL and IFN-γ secretion in T lymphocytes in cattle, the natural outbred host for N. caninum infection.
Immunogens containing killed N. caninum tachyzoites do not protect against fetal infection in challenged cattle (1), and a whole-antigen immunogen exacerbated encephalitis in a mouse model of neosporosis (6), highlighting a need to identify specific protection-inducing antigens. Advantages to the epitope approach of vaccine development can include induction of more-potent responses, increased qualitative control of the immune response, targeting of dominant and subdominant epitopes, and overcoming of safety concerns regarding attenuated whole-organism preparations (46). A formidable obstacle to developing broadly efficacious epitope-based vaccines stimulating effective T-lymphocyte responses in cattle hinges on the polymorphism of the MHC molecules. It has been shown in humans that some MHC class I and class II molecules share common peptide binding motifs, so called supermotifs, and bind many of the same epitopes (34, 46-48). Thus, instead of identifying many single epitopes for each MHC class I or MHC class II molecule, identification of supertype epitopes (peptide epitopes presented by different MHC molecules) or epitope clusters (contiguous epitopes on a given antigen) capable of binding multiple MHC molecules is desirable for induction of immune responses in multiple individuals within outbred populations. Identification of epitope clusters or supertype epitopes in N. caninum proteins for inclusion in subunit immunogens may be advantageous for specific manipulation of immune responses in outbred cattle with known MHC haplotypes.
Herein, we showed that T-lymphocyte cell lines of cattle infected with N. caninum readily proliferated and secreted IFN-γ when stimulated with the recombinant tachyzoite surface protein rNcSRS2 but not rNcSAG1. Therefore, NcSRS2 was targeted for further study, and overlapping peptides of NcSRS2 were tested to identify candidate peptides for inclusion in vaccines against bovine neosporosis abortion. NcSRS2 peptide-specific CD4+ CTL and IFN-γ-secreting T lymphocytes were present in four infected Holstein cattle with six MHC class II haplotypes, suggesting that identified NcSRS2 peptides could stimulate T-lymphocyte-mediated immune responses in outbred Holstein populations.
MATERIALS AND METHODS
Cattle.
Four MHC-heterozygous female, nonpregnant, 1- to 7-year-old Friesian-Holstein cattle (Bos taurus) representing six MHC class I haplotypes and six class II haplotypes were selected for MHC haplotype disparity (MHC mismatched) and similarity (MHC half-matched). Each cow's bovine lymphocyte antigen (BoLA)-A class I alleles and DRB3 and DQA alleles were characterized by microarray typing (described below). The BoLA haplotypes of the cows were inferred from BoLA class I, DRB3, and DQA typing on the basis of previously defined haplotypes of Holstein cattle (15, 17, 32).
Cattle were infected with an initial dose of 107 N. caninum strain Nc-1 tachyzoites via intramuscular inoculation. They were reinfected intramuscularly at monthly intervals with 5 × 106 Nc-1 tachyzoites for 10 or more months and monitored periodically for persistent N. caninum infection, using a competitive inhibition enzyme-linked immunosorbent assay (ELISA) specific for N. caninum antibodies (3) (VMRD, Inc., Pullman, Wash.). Animals were housed and cared for in accordance with Animal Care and Use Regulations of Washington State University, Pullman, Wash.
N. caninum tachyzoites.
The Nc-1 strain of N. caninum tachyzoites was maintained by regular serial passage in Vero cells as described previously (5). Tachyzoites were used for infection of experimental cattle and for infection of stimulator and target cells in 51Cr release cytotoxicity assays. Sonicated tachyzoite antigen (NSo) from washed tachyzoites and sonicated Vero cell antigen were prepared as described previously (4) for positive and negative controls, respectively, in 51Cr release, enzyme-linked immunospot (ELISPOT), and ELISA assays.
Synthesis of NcSRS2 peptides.
Eighty-one 15-mer peptides overlapping by 11 amino acids were synthesized commercially (Genemed, San Francisco, Calif.) based upon the protein sequence of NcSRS2 (GenBank accession no. AAD45521) (36). Custom peptides were >80% pure as analyzed by high-performance liquid chromatography. Peptides were dissolved in 100% dimethyl sulfoxide to 20 mg per ml and stored at −20°C. Peptides were further diluted to 100 or 200 μg/ml in RPMI prior to use in 51Cr release, ELISPOT, and ELISA assays.
Preparation of recombinant NcSRS2 and NcSAG1.
Recombinant proteins NcSRS2 and NcSAG1 were prepared and purified as histidine-tagged thioredoxin fusion proteins, using the pBAD/thioTOPO system (Invitrogen) per the manufacturer's instructions. The NcSRS2 open reading frame was amplified from genomic N. caninum DNA with the following primer set: NcSRS2-F (5′-ATGGCGACGCATGCTTGTGTGGTG-3′) and NcSRS2-R (5′-GTACGCAAAGATTGCCGTTGC-3′). The NcSAG1 open reading frame was amplified from genomic N. caninum DNA using the following primer set: NcSAG1-F (5′-ATGTTTCCTCGGGCAGTGAGAC-3′) and NcSAG1-R (5′-CGCGACGCCAGCCGCTATCGAGC-3′). The amplicons containing the open reading frames of NcSRS2 and NcSAG1 were cloned into pBAD/thioTOPO to yield plasmids pNcSRS2-pBAD and pNcSAG1-pBAD. The correct NcSRS2 and NcSAG1 sequences and orientations of the inserts in plasmids NcSRS2-pBAD and pNcSAG1-pBAD were confirmed by nucleotide sequencing. Recombinant NcSRS2 and NcSAG1 were produced in Escherichia coli-transformed cultures after induction with 0.1% arabinose at 37°C for 3 h and purified on Ni-agarose columns after elution with Imidazol, as described by the manufacturer (Invitrogen). The purified fusion proteins were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie blue staining and by Western blotting, using immune mouse serum that detected N. caninum native and recombinant proteins of the expected molecular weights for NcSAG1 and NcSRS2, respectively.
MHC typing.
MHC typing was performed by hybridization of biotinylated, genomic PCR products from the MHC class I, DRB3, and DQA genes to short, 15- to 22-bp oligonucleotide microarrays as described previously (40). The only significant modification from the published procedure was that instead of using heminested PCR for the class I and DRB3 genes, all of the biotinylated PCR products were generated by single-round amplification.
Although class I typing was performed by microarray hybridization, the serological names have been used to denote the haplotypes (16). Class II haplotypes are indicated by using the D-region haplotype (DH) nomenclature (15, 32, 40, 43). The official allele names assigned by the Bovine Leukocyte Antigen Nomenclature Committee of the International Society for Animal Genetics are used for the class II alleles present in each haplotype (17, 44).
Immunofluorescence flow cytometry.
Peripheral blood mononuclear cells (PBMC) and stimulated T lymphocytes were analyzed for cell phenotype by two-color flow cytometry (50), using bovine monoclonal antibodies (MAb) against CD4 (ILA11A), CD8 (CACT 80C or 7C2B), γδ (GB21A), CD3 (MM1A) and CD2 (16-1E10) (Washington State University Monoclonal Antibody Center, Pullman, Wash). The fluorescence-labeled cells were counted and analyzed on a Becton Dickinson FACSScan instrument by using CellQuest analysis software (Becton, Dickinson and Company, Franklin Lakes, N.J.).
Tachyzoite-specific short-term T-lymphocyte cell lines for proliferation and IFN-γ assay.
Antigen-specific, short-term T-lymphocyte cell lines were expanded from PBMC of cows numbered 495 and 562 experimentally infected with N. caninum tachyzoites. Short-term N. caninum antigen-specific CD4+- or CD8+-T-lymphocyte lines from PBMC were developed by stimulation with NSo (10 μg/ml) (sonicated whole tachyzoite antigen) (4) for 7 days followed by expansion with 2 ng of recombinant human IL-2 per ml for 7 days in RPMI 1640 supplemented with 10% fetal bovine serum (Sigma Chemical Co.), 2 mM glutamine, 10 mM HEPES buffer, 10 μg of gentamicin per ml, and 5 × 10−5 M 2-mercaptoethanol (complete RPMI). To enrich CD4+ and CD8+ T lymphocytes in effector populations, aliquots of effector T lymphocytes were partially depleted of CD4+ or CD8+ T lymphocytes using immunomagnetic beads as described previously (50). T lymphocytes were analyzed for cell phenotype by flow cytometry as described above.
NcSRS2-specific short-term T-lymphocyte cell lines for IFN-γ ELISA and 51Cr release cytotoxicity assays.
For expansion of NcSRS2-specific effector cells from PBMC of infected cattle, approximately 108 γδ-T-lymphocyte-depleted PBMC were cultured with 107 tachyzoite-infected (multiplicity of infection, 2:1), irradiated (3,000 rads), peripheral blood adherent cells (PBAC) in 75-cm3 polystyrene culture flasks for 5 to 7 days in complete RPMI 1640 at 37°C, 5% CO2, and 95% humidity. Following the first round of stimulation, effectors were cultured with 10 μg of rNcSRS2 per ml and autologous, irradiated (3,000 rads) PBMC at an antigen-presenting cell-to-effector ratio of 7:1 for an additional 5 to 7 days. γδ T lymphocytes were depleted from PBMC prior to stimulation by using complement-mediated lysis as described previously (50). The phenotypes of effector T lymphocytes were determined by immunofluorescence flow cytometry prior to use, and if γδ T lymphocytes and CD8+ T lymphocytes comprised more than 2% (each) of the effector populations, they were depleted to less than 2% from effector cultures by immunomagnetic bead separation (Dynal), following methods previously described (50).
Lymphocyte proliferation assay using rNcSRS2 and rNcSAG1.
Tachyzoite-specific T-lymphocyte lines (stimulated with NSo as described above) were plated in round-bottom, 96-well plates, 2 × 106 per ml. The cell lines were stimulated with 10 μg of rNcSAG1 or rNcSRS2 per ml, medium only (antigen negative control), 5 μg of concanavalin A (positive control) per ml, 10 μg of NSo (positive control) per ml, 10 μg of recombinant green fluorescent protein per ml expressed in the same vector system used for N. caninum surface proteins (irrelevant recombinant antigen negative control), or 10 μg of uninfected Vero cell lysate per ml. Lymphocytes were then cultured for 5 days, and aliquots of culture supernatants were collected for IFN-γ assay, described in the following section. [3H]thymidine (0.5 μCi per well) was added and incubated for 18 h, and supernatants were harvested on day 6 to measure counts per minute, determined with a beta scintillation counter. Results are presented as stimulation index, calculated by dividing mean counts per minute for treated wells by mean counts per minute for wells containing uninfected Vero cell lysates. Proliferation was considered significant if the stimulation index was >3.0 and the mean number of counts per minute was >1,000 (7).
Detection of peptide-specific T lymphocytes by IFN-γ ELISA.
The Bovigam (Biocor Animal Health, Inc., Omaha, Neb.) ELISA kit measuring bovine IFN-γ was used to identify activated T lymphocytes of N. caninum-infected cattle specific for whole rNcSRS2 and rNcSAG1 antigen and for NcSRS2 epitope-containing peptides. For the IFN-γ ELISA assay with cell lines developed with NSo and recombinant human IL-2 and then stimulated with rNcSRS2 and rNcSAG1, supernatants from stimulated wells were removed after 5 days of stimulation with the recombinant peptides as described for the lymphocyte proliferation assay above. For the IFN-γ assay with 15-mer NcSRS2 peptides, the short-term NcSRS2-specific T-lymphocyte cell lines were stimulated for an additional 3 days with irradiated, autologous PBMC and individual 15-mer NcSRS2 peptides. T-lymphocyte effectors (3 × 104) plus 2 × 105 irradiated, autologous PBMC per well were stimulated with one of 81 overlapping NcSRS2 15-mer peptides at 10 μg/ml. Positive controls were concanavalin A (5 μg per ml) and NSo (10 μg per ml). The negative controls were medium alone and Vero cell lysate (10 μg per ml). All experiments were performed in 96-well, round-bottom culture plates, three replicates per treatment, in complete RPMI, 100 μl per well, 37°C, 5% CO2, 95% humidity. Supernatants were diluted eightfold, and IFN-γ was measured by following kit directions. Absorbance was read on a Titertek Multiskan MCC/340 ELISA plate reader at 540 nm, and the IFN-γ concentration was determined by using a standard curve prepared by serial dilutions of a supernatant from a Mycobacterium bovis purified protein derivative-specific T-helper lymphocyte clone that contained 440 U of IFN-γ per ml as determined by the neutralization of vesicular stomatitis virus (9). In this assay, 0.6 U corresponds to 1 ng of IFN-γ. The results are presented as units per milliliter of IFN-γ (8).
The response of T-lymphocyte short-term cell lines to NcSRS2 peptides was considered positive if two criteria were met: (i) IFN-γ amounts after peptide stimulation were significantly different from those with medium as analyzed by one-way analysis of variance with control and Bonferroni correction, alpha = 0.05; and (ii) the IFN-γ amounts were greater than 2.5 times background levels. The background IFN-γ value was calculated as mean IFN-γ from triplicate wells containing lymphocytes and medium alone.
Enumeration of NcSRS2-specific PBMC by IFN-γ ELISPOT.
To detect NcSRS2-specific T lymphocytes from PBMC without in vitro expansion, IFN-γ-expressing cells in PBMC were quantified by using an ELISPOT specific for bovine IFN-γ. The ELISPOT assays were conducted as described previously (56) with the following modifications. NcSRS2 15-mer peptides overlapping by 11 amino acids were added to freshly isolated PBMC (106 cells) in 100 μl of complete RPMI at final concentrations of 10 μg per ml. Positive controls included concanavalin A (5 μg per ml), rNcSRS2 (10 μg per ml), and NSo (10 μg per ml). Negative control wells contained PBMC in complete RPMI alone and with 10 μg of Vero cell lysate per ml. The ELISPOT assays were conducted with triplicate wells of MultiScreen-Immobilon-P plates (Millipore) coated with mouse anti-bovine IFN-γ MAb CC330 (8 μg/ml), incubated for 72 h at 37°C with 5% CO2, and detected with biotinylated mouse anti-bovine IFN-γ MAb CC302 (5 μg/ml). Both anti-bovine IFN-γ MAbs were the kind gift of Christopher Howard (Compton, United Kingdom).
For each animal, the mean number of spots in medium negative control wells was subtracted from the mean number of spots in test wells to determine the number of NcSRS2 peptide-specific spot-forming cells (SFC). Results were presented as the number NcSRS2 peptide-specific IFN-γ secreting SFC per 106 PBMC.
PBMC response to NcSRS2 peptides was considered positive if two criteria were met: (i) number of spot-forming cells per million PBMC after peptide stimulation was significantly different from that with medium as analyzed by one-way analysis of variance with control and Bonferroni correction (alpha = 0.05); and (ii) the number of IFN-γ spot-forming cells per million was greater than 2.5 times the background level. The background IFN-γ values for lymphocytes stimulated with individual NcSRS2 peptides was calculated as mean number of IFN-γ-secreting SFC per million from triplicate wells containing medium alone for each 96-well plate.
51Chromium release cytotoxicity assay.
51Cr release cytotoxicity assays were performed by following methods previously described (50) with the following modifications. Effectors consisted of short-term T-lymphocyte cell lines stimulated with NcSRS2 as described above for use in ELISA and 51Cr release cytotoxicity assays. In several cases, CD8+ and γδ T lymphocytes exceeded 2% of the effector populations, and immunomagnetic beads were used as previously described (50) to further deplete CD8+ and γδ T lymphocytes to <2% each prior to CTL assays. For peptide-pulsed targets, the PBAC were pulsed with 10 μg of NcSRS2 15-mer peptides, rNcSRS2, NSo, or Vero cell lysate/ml for 16 to 18 h. Test groups consisted of (i) effectors plus N. caninum-infected, autologous, PBAC targets (positive control), (ii) effectors plus uninfected, autologous PBAC targets (negative control), (iii) effectors plus NcSRS2 peptide-pulsed autologous targets, (iv) effectors plus rNcSRS2-pulsed autologous targets, (v) effectors plus NSo-pulsed, autologous targets (positive control), and (vi) effectors plus Vero antigen-pulsed autologous targets. Test groups were plated in replicates of three at various effector-to-target-cell ratios of 3:1 to 30:1. Counts were determined with a MicroBeta TriLux beta emission counter (PerkinElmer Wallac, Inc., Gaithersburg, Md.) windowed for 51Cr and transformed into percent specific lysis by using the following equation:
 |
Percent spontaneous lysis (mean counts per minute spontaneous/mean counts per minute maximal) was <20% in every experiment. A significant difference in percent specific lysis between the experimental groups was defined as >2.5 times the standard error of the mean (SEM). SEM was calculated by using a formula that takes into account individual variances within maximal, spontaneous, and experimental release wells (49).
RESULTS
rNcSRS2 stimulates memory T lymphocytes.
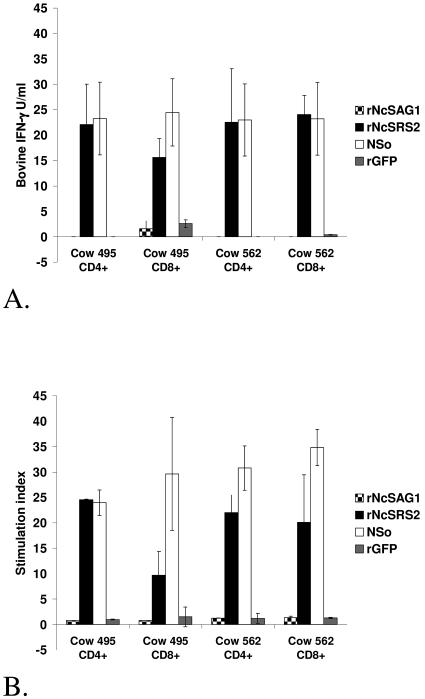
rNcSRS2 activated CD4+ and CD8+ enriched T-lymphocyte cell lines from cows 495 and 562 as indicated by high IFN-γ secretion and stimulation indices (Fig. 1A and B), whereas minimal stimulation was seen in response to rNcSAG1. For both cows, the CD4+ and CD8+ phenotypes comprised 60% or more of the total T lymphocytes in respective, enriched effector cultures. Increased proliferation and IFN-γ secretion after rNcSRS2 stimulation provided the rationale for mapping NcSRS2 CD4+-T-lymphocyte epitopes for vaccine development.
FIG. 1.
In vitro stimulation of memory bovine T-lymphocytes with rNcSAG1 and rNcSRS2. Both CD4+ and CD8+ enriched T lymphocytes responded to NcSRS2 but not NcSAG1 as indicated by secretion of IFN-γ (A) and cell proliferation stimulation index (B). In both panels A and B, short-term T-lymphocyte lines were stimulated with NSo and interleukin-2, and the CD4+ and CD8+ T lymphocytes were purified for assay. Cell proliferation was measured by standard [3H]thymidine incorporation assay. Stimulation index was calculated by dividing mean counts per minute for treated wells by mean counts per minute for wells containing uninfected Vero cell lysates. rNcSAG1, recombinant NcSAG1; rNcSRS2, recombinant NcSRS2; NSo, whole, sonicated N. caninum tachyzoites; rGFP, recombinant green fluorescent protein. Error bar = ± standard deviation.
Mapping NcSRS2 T-lymphocyte epitopes by IFN-γ ELISA.
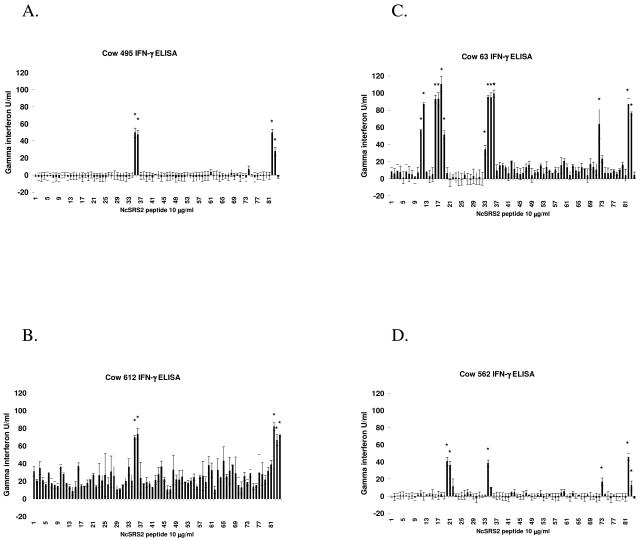
Multiple NcSRS2 peptides induced IFN-γ secretion from short-term T-lymphocyte cell lines of four N. caninum-infected Holstein cattle with six different MHC haplotypes (Table 1). Measurement of IFN-γ in the cell supernatants by ELISA in units/milliliter after peptide stimulation is shown in Fig. 2.
TABLE 1.
MHC haplotypes of experimental Holstein cattle
| Cow no. | BoLA-A serotype | Class II haplotypea | DRB3 PCR-RFLPc | DRB3 allele | DQA allele | DQB allele |
|---|---|---|---|---|---|---|
| 63 | A11 | DH24A | 24 | 0101b | 0101b | 0101b |
| A14 | DH27A | 27 | 14011b | 1401b | 1401b | |
| 562 | A11 | DH24A | 24 | 0101b | 0101b | 0101b |
| A12(A30) | DH16A | 16 | 1501b | 10011b | 0102 | |
| 22021b | 1101 | |||||
| 612 | A13 | DH23A | 23 | 2703b | 0101b | 0103b |
| 22031b | 1803b | |||||
| A15(A8) | DH22H | 22 | 1101b | 10011b | 1002b | |
| 2206b | 1402b | |||||
| 495 | A15(A8) | DH22H | 22 | 1101b | 10011b | 1002b |
| 2206b | 1402b | |||||
| A20 | DH08A | 8 | 1201b | 12011b | 1005 | |
| 2201b | 1201 |
FIG. 2.
IFN-γ secretion from short-term T-lymphocyte effector cell lines stimulated with overlapping peptides of NcSRS2. T lymphocytes were obtained from PBMC of N. caninum-infected cows 495 (A), 612 (B), 63(C), and 562 (D) and cultured with antigen-presenting cells stimulated sequentially with live tachyzoites, rNcSRS2, and synthetic peptide as described in the text. Culture supernatants were tested for bovine IFN-γ using commercial Bovigam kit, and units were calculated from a standard curve. The far-right three bars show results for control cultures stimulated with ConA, NSo (whole N. caninum tachyzoite lysate) (positive control), or rNcSRS2 (positive control), respectively. A response was considered positive (*) as described in the text.Error bars indicate ± standard deviations.
NcSRS2 peptides recognized by short-term T-lymphocyte lines from cow 562 (DH16A/DH24A) were peptides 20, 21, 34, and 73. Short-term T-lymphocyte lines from cow 63 (DH24A/DH27A) recognized peptides 11, 12, 16, 17, 18, 19, 34, 35, 36, and 72. Peptides recognized by cow 495 (DH08A/DH22H) were 35, 36, and 74. Peptides recognized by cow 612 (DH22H/DH23A) were 35 and 36.
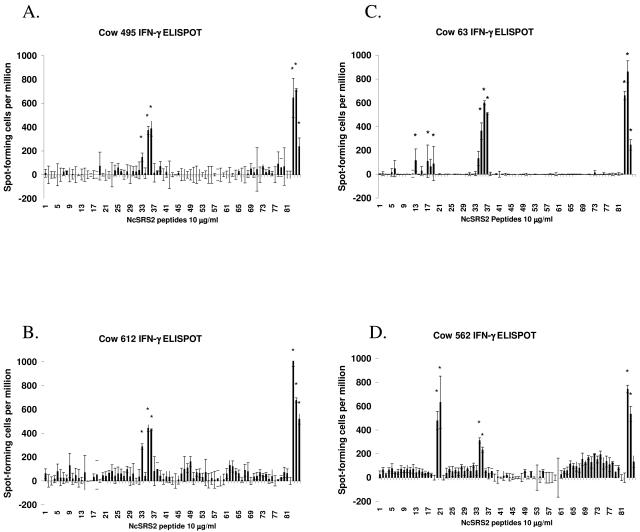
Mapping NcSRS2 T-lymphocyte epitopes by using IFN-γ ELISPOT with PBMC.
The results of one ELISPOT assay per cow are presented in Fig. 3, and results of both ELISPOT assays are summarized in Table 2. Peptides recognized by PBMC from the cattle that met the selection criteria described in Materials and Methods in one or more ELISPOT assays were the following: (i) cow 562 (DH16A/DH24A), peptides 20, 21, 34, 72, and 74; (ii) cow 63 (DH24A/DH27A), peptides 13, 16, 18, 34, 35, and 36; (iii) cow 495 (DH08A/DH22H), peptides 33, 35, and 36; and (iv) cow 612 (DH22H/DH23A), peptides 33, 35, and 36.
FIG. 3.
IFN-γ secretion from PBMC stimulated with overlapping peptides of NcSRS2. PBMC were obtained from N. caninum-infected cows 495 (A), 612 (B), 63 (C), and 562 (D) and cultured with antigen-presenting cells and NcSRS2 peptides for 72 h as described in the text. Bovine IFN-γ spot-forming cells per million were measured by ELISPOT assay. The far-right three bars show results for control cultures stimulated with ConA (positive control), NSo (whole N. caninum tachyzoite lysate) (positive control), or rNcSRS2, respectively. A response was considered positive (*) as described in the text. Error bars indicate ± standard deviations.
TABLE 2.
Summary of NcSRS2-specific CD4+ CTL and IFN-γ-secreting T lymphocytes from four Holstein cattle
| NcSRS2 peptide (position) | Amino acid sequence | Result for cow no.d:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63
|
562
|
612
|
495
|
||||||||||
| IFN-γ
|
CTLc | IFN-γ
|
CTLc | IFN-γ
|
CTLc | IFN-γ
|
CTLc | ||||||
| Ba | Eb | Ba | Eb | Ba | Eb | Ba | Eb | ||||||
| 11 (41-55) | LLSEDDGLIVCNESD | * | |||||||||||
| 12 (45-59) | DDGLIVCNESDGEDE | * | |||||||||||
| 13 (49-63) | IVCNESDGEDECEKN | * | |||||||||||
| 16 (61-75) | EKNAAPLSTFLPGAK | * | * | ||||||||||
| 17 (65-79) | APLSTFLPGAKKEWV | * | |||||||||||
| 18 (69-83) | TFLPGAKKEWVTGTL | * | * | ||||||||||
| 19 (73-87) | GAKKEWVTGTLQQGI | * | |||||||||||
| 20 (77-91) | EWVTGTLQQGIKITI | * | ** | * | |||||||||
| 21 (81-95) | GTLQQGIKITIPDEH | * | ** | * | |||||||||
| 33 (129-143) | GQVAHCAYSSNVRLR | * | * | * | |||||||||
| 34 (133-147) | HCAYSSNVRLRPITV | * | ** | * | ** | * | |||||||
| 35 (137-151) | SSNVRLRPITVNPEN | * | ** | * | * | ** | * | * | ** | * | |||
| 36 (141-155) | RLRPITVNPENNGVT | * | * | * | * | * | * | * | ** | * | |||
| 61 (241-255) | PVNIEEVAKPAGAGS | ||||||||||||
| 72 (285-299) | SGGATTGKQNASQNA | * | * | ||||||||||
| 73 (289-303) | TTGKQNASQNAKDKG | * | * | * | |||||||||
| 74 (293-307) | QNASQNAKDKGETGG | * | * | ||||||||||
IFN-γ secretion in peptide-specific short-term T-lymphocyte cell lines from N. caninum-infected cattle, detected by ELISA.
IFN-γ secretion in peptide-specific PBMC, detected by IFN-γ ELISPOT.
Peptide-specific CD4+ CTL detected by 51Cr release cytotoxicity assays.
*, NcSRS2 peptides that induced a positive response; **, NcSRS2 peptides that induced a positive response in two of two ELISPOT assays.
NcSRS2 peptide-specific CD4+ CTL in short-term cell lines from N. caninum-infected cattle.
NcSRS2 peptides were chosen for testing in 51Cr release cytotoxicity assays if epitope-containing peptides were recognized in one ELISA and one or more ELISPOT assays or if epitope clusters were suspected (peptides 33, 34, 35, and 36 and peptides 72, 73, and 74). Stimulated effector cells were comprised of more than 95% CD4+ T lymphocytes and less than 2% CD8+ T lymphocytes, γδ T lymphocytes, or CD2+/CD3− (NK-like) cells (23) when analyzed by flow cytometry (data not shown). NcSRS2-specific CTL identified in at least two 51Cr release cytotoxicity assays for cows 495, 612, and 562 are shown in Table 2. For cow 63, NcSRS2-specific CTL identified in a single 51Cr release cytotoxicity assay are shown, since the cow developed unrelated disease and was subsequently excluded from further study. Results were considered significant when percent specific lysis of the experimental group was >2.5 SEM for autologous, unpulsed targets and Vero antigen-pulsed targets.
NcSRS2 peptides recognized by CD4+ CTL from short-term cell lines of cow 562 (DH16A/DH24A) were peptides 20, 21, 34, and 73. CD4+ CTL from short-term cell lines of cow 63 (DH24A/DH27A) killed autologous PBAC targets pulsed with peptides 35 and 36. NcSRS2 peptides recognized by CD4+ CTL of cow 495 (DH08A/DH22H) were peptides 35, 36, and 73. Autologous targets pulsed with peptides 35 and 36 were killed by CTL of cow 612 (DH22H/DH23A).
DISCUSSION
T-lymphocyte cell lines of Holstein cattle infected with N. caninum showed robust proliferative and IFN-γ responses to tachyzoite surface protein NcSRS2 but responded poorly to NcSAG1. The minimal response of bovine T-lymphocyte cell lines to NcSAG1 was unexpected, since vaccination studies using TgSAG1 protein and DNA constructs have demonstrated protection against acute toxoplasmosis models in rodent systems (2, 12, 29, 54). This highlighted the need to evaluate immune responses in the target species, and emphasis was placed on mapping NcSRS2 epitope-containing peptides recognized by CD4+ CTL and IFN-γ-secreting T lymphocytes from infected cattle with known MHC haplotypes.
Not all genetically different MHC molecules are functionally different, and for humans, the functional binding specificities of predominant MHC class I molecules fall into nine supertypes (46). Human HLA-DR functional binding specificities have recently been clustered into nine supertype classes (34) based on binding pocket specificity; however, supertypes in cattle have not yet been characterized. For this study, four N. caninum-infected MHC class II heterozygous Holstein cattle with half-matched and mismatched haplotype combinations were chosen (Table 1). The four cows had CD4+ CTL directed against peptides within NcSRS2 amino acids (aa) 133 to 155. Peptides within this region also induced IFN-γ secretion in T lymphocytes from these four cattle as measured by ELISPOT and ELISA. Overlapping peptides 35 and 36, spanning aa 137 to 155, were recognized by CD4+ CTL of cows 63 (DH24A/DH27A), 612 (DH22H/DH23A), and 495 (DH08A/DH22H). Cows 612 and 495 shared the class II haplotype DH22H but did not share an MHC class II haplotype with cow 63. The NcSRS2 region aa 137 to 155 could contain an epitope broadly recognized by a Holstein “supertype.” Peptide 34 (aa 133 to 147) was recognized by CD4+ CTL and IFN-γ-secreting T lymphocytes of cow 562 (DH16A/DH24A). Thus, overlapping peptides 34, 35, and 36 spanning NcSRS2 aa 133 to 155 were recognized by the four cows and may represent an epitope cluster. This region is considered a candidate antigen for inclusion in a multiepitope protein or DNA immunization construct to induce CTL and IFN-γ-secreting T lymphocytes against N. caninum infection in Holstein cattle. Microarray MHC typing was used to determine the genotypic frequencies of MHC class II haplotypes carried by 101 Holstein cattle at the Washington State University and University of Idaho (Moscow, Idaho) dairy farms. Bernstein's square root formula was used to calculate the combined phenotypic frequency for five MHC class II haplotypes (DH08A, DH16A, DH22A, DH24A, and DH27A) shown to present NcSRS2 peptides. Approximately 79% of the Holstein cattle at these dairies carried one or two MHC class II haplotypes shown to present NcSRS2 peptides. Thus, a minimum of 79% of Holstein cattle in the Pacific Northwest of the United States could be expected to respond to immunization with the identified NcSRS2 epitopes.
Cows 63 (DH24A/DH27A) and 562 (DH16A/DH24A) had half-matched MHC class II haplotypes, and peptide 34 was recognized by T-lymphocyte cell lines and PBMC of both cows. Therefore, peptide 34 (aa 133 to 147) may be presented by MHC class II molecules in the shared haplotype DH24A. Peptides 11 and 12 (aa 41 to 59) and peptides 16, 17, 18, and 19 (aa 61 to 87) were uniquely recognized by short-term T-lymphocyte cell lines of cow 63 and not by cell lines of cow 562, suggesting that these peptides were presented by MHC class II molecules unique to cow 63 within the unshared haplotype DH27A. T-lymphocyte cell lines of cow 562 uniquely recognized peptides 20 and 21, and these peptides were likely presented by allelic products within the unshared haplotype DH16A.
T-lymphocyte lines and PBMC of MHC half-matched cow 495 (DH08A/DH22H) and cow 612 (DH22H/DH23A) secreted IFN-γ in response to stimulation with NcSRS2 peptides 35 and 36. Epitopes within NcSRS2 amino acid region 137 to 155 were likely presented by MHC class II molecules within the shared haplotype DH22H.
In some cases, a positive response to NcSRS2 peptides detected by ELISA in short-term cell lines was not detected by ELISPOT in PBMC. This may reflect preferential expansion of NcSRS2-specific T lymphocytes to subdominant epitopes in culture that were below the level of detection by ELISPOT assay from the peripheral blood pool. However, measuring the IFN-γ from PBMC may more closely reflect the in vivo response to infection than measuring IFN-γ from cell lines, and the ELISPOT should be valuable for measuring the response to subunit immunogens ex vivo in naive cattle.
To find CTL epitope-containing peptides, NcSRS2 peptides that induced IFN-γ secretion were used to pulse autologous PBAC targets in 51Cr release cytotoxicity assays. This strategy was effective at identifying NcSRS2 peptides that elicited both IFN-γ production and CTL responses by CD4+ T lymphocytes from infected cattle.
Short-term T-lymphocyte cell lines of MHC-half-matched cows 495 and 612 each killed autologous PBAC targets pulsed with peptides 35 and 36, which is consistent with the possibility that one or more CTL epitopes within amino acid sequence 137 to 155 were presented by the MHC class II molecules in shared haplotype DH22H. Interestingly, CTL of cow 63 also killed autologous targets pulsed with peptides 35 and 36, and cow 63 shared no class II haplotype with cows 495 and 612. Therefore, the NcSRS2 region spanning aa 137 to 155 may contain one or more CTL supertype epitopes broadly recognized by Holstein cattle and/or represent a CTL epitope cluster.
Some NcSRS2 peptides that induced IFN-γ secretion in short-term T-lymphocyte cell lines expanded from γδ-depleted PBMC were not recognized by CD4+ CTL from similarly expanded cell cultures. This suggested that the NcSRS2 peptide-specific IFN-γ secreting effectors could be T-helper lymphocytes. The NcSRS2 peptides that induced IFN-γ secretion as detected by ELISA and ELISPOT that were not recognized by CTL were the following: for cow 63 (DH24A/DH27A), 16, 17, 18, 19, 34, and 72; for cow 562 (DH16A/DH24A), 72 and 74; for cow 612 (DH22H/DH23A), 33; and for cow 495 (DH08A/DH22H), 33, 73, 74, and 80.
NcSRS2 peptides 72, 73, and 74 (aa 285 to 307) induced IFN-γ secretion in three of four cattle with shared and unshared MHC class II haplotypes. Thus, NcSRS2 aa 285 to 307, a possible epitope cluster, is also considered a vaccine candidate antigen for induction of IFN-γ secretion and requires further testing in naive cattle of known MHC haplotype.
The primary method of N. caninum transmission is transplacental and is likely related to recrudescence of chronic infection during pregnancy and the altered maternal immunity which complicates parasite control. The immunologic balance that favors fetal survival also may facilitate N. caninum replication and dissemination. A type 2 cytokine bias has been associated with successful implantation and pregnancy maintenance in mammals (22), and downregulation of proinflammatory cytokines, such as IFN-γ, IL-2, and tumor necrosis factor alpha, is thought to limit potentially damaging effects of inflammation on the placenta and fetus (35). Manipulation of the immune response with subunit immunogens designed for specific gestational times (pregnant or nonpregnant), maternal infection status (naive or chronically infected), and specific Holstein MHC haplotypes may be required. Results from this study showed that some NcSRS2 peptides induced IFN-γ only, whereas response to other peptides was associated with both CD4+ CTL killing and IFN-γ secretion. As more information about protective immune responses against neosporosis abortion becomes available, the selection of specific peptides for immunization may be one method of inducing the desired immune responses.
The overall contribution of CD4+ CTL to immune protection against N. caninum-induced abortion in cattle is not known. CD4+ CTL may protect through IFN-γ secretion, CTL killing of infected monocytes, and the effects of granulysin on tachyzoites. The NcSRS2 peptides recognized by T lymphocytes of infected cattle identified in this study will facilitate testing whether protective NcSRS2-specific CD4+ CTL and IFN-γ secreting T lymphocytes can be induced in naive cattle with known MHC haplotypes.
Additionally, determining what bovine MHC molecules present protective N. caninum CTL epitopes could help select Holstein MHC haplotypes that are more resistant to N. caninum abortion. Discovery of CTL supertype epitopes, the sequences of their MHC molecules, and anchor residues required in different MHC molecules in cattle is of significant practical importance not only to vaccine development against neosporosis but also generally to future investigation of protective CTL-based immunity to other infectious agents in outbred cattle populations.
Acknowledgments
This research was supported by National Institutes of Health, Department of Health and Human Services, Public Health Service grant 1K08 AI49182-03 and by research grant no. US 2913-97 from BARD, the United States-Israel Binational Agricultural Research Development Fund.
We acknowledge the Institute for Animal Health, Compton, Newbury, Berkshire, United Kingdom, for the generous provision of MAb for the IFN-γ ELISPOT.
With gratitude, we acknowledge the technical expertise and support of Jennifer Eldridge, Monica Florin-Christensen, and Emma Karel.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Andrianarivo, A. G., J. D. Rowe, B. C. Barr, M. L. Anderson, A. E. Packham, K. W. Sverlow, L. Choromanski, C. Loui, A. Grace, and P. A. Conrad. 2000. A POLYGEN-adjuvanted killed Neospora caninum tachyzoite preparation failed to prevent foetal infection in pregnant cattle following i.v./i.m. experimental tachyzoite challenge. Int. J. Parasitol. 30:985-990. [DOI] [PubMed] [Google Scholar]
- 2.Angus, C. W., D. Klivington-Evans, J. P. Dubey, and J. A. Kovacs. 2000. Immunization with a DNA plasmid encoding the SAG1 (P30) protein of Toxoplasma gondii is immunogenic and protective in rodents. J. Infect. Dis. 181:317-324. [DOI] [PubMed] [Google Scholar]
- 3.Baszler, T. V., S. Adams, J. Vander-Schalie, B. A. Mathison, and M. Kostovic. 2001. Validation of a commercially available monoclonal antibody-based competitive-inhibition enzyme-linked immunosorbent assay for detection of serum antibodies to Neospora caninum in cattle. J. Clin. Microbiol. 39:3851-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baszler, T. V., D. P. Knowles, J. P. Dubey, J. M. Gay, B. A. Mathison, and T. F. McElwain. 1996. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 34:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baszler, T. V., M. T. Long, T. F. McElwain, and B. A. Mathison. 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol. 29:1635-1646. [DOI] [PubMed] [Google Scholar]
- 6.Baszler, T. V., T. F. McElwain, and B. A. Mathison. 2000. Immunization of BALB/c mice with killed Neospora caninum tachyzoite antigen induces a type 2 immune response and exacerbates encephalitis and neurological disease. Clin. Diagn. Lab. Immunol. 7:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett, S., and E. M. Riley. 1992. The statistical analysis of data from immunoepidemiological studies. J. Immunol. Methods 146:229-239. [DOI] [PubMed] [Google Scholar]
- 8.Beyer, J. C., R. W. Stich, D. S. Hoover, W. C. Brown, and W. P. Cheevers. 1998. Cloning and expression of caprine interferon-gamma. Gene 210:103-108. [DOI] [PubMed] [Google Scholar]
- 9.Brown, W. C., S. Zhao, A. C. Rice-Ficht, K. S. Logan, and V. M. Woods. 1992. Bovine helper T cell clones recognize five distinct epitopes on Babesia bovis merozoite antigens. Infect. Immun. 60:4364-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton, D., M. M. McAllister, and J. P. Dubey. 2002. The comparative pathogenesis of neosporosis. Trends Parasitol. 18:546-552. [DOI] [PubMed] [Google Scholar]
- 11.Cannas, A., A. Naguleswaran, N. Muller, B. Gottstein, and A. Hemphill. 2003. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J. Parasitol. 89:44-50. [DOI] [PubMed] [Google Scholar]
- 12.Chen, G., H. Chen, H. Guo, and H. Zheng. 2002. Protective effect of DNA-mediated immunization with a combination of SAG1 and IL-2 gene adjuvant against infection of Toxoplasma gondii in mice. Chin. Med. J. (Engl. Ed.). 115:1448-1452. [PubMed] [Google Scholar]
- 13.Cramer, G., D. Kelton, T. F. Duffield, J. C. Hobson, K. Lissemore, S. K. Hietala, and A. S. Peregrine. 2002. Neospora caninum serostatus and culling of Holstein cattle. J. Am. Vet. Med. Assoc. 221:1165-1168. [DOI] [PubMed] [Google Scholar]
- 14.Curiel, T. J., E. C. Krug, M. B. Purner, P. Poignard, and R. L. Berens. 1993. Cloned human CD4+ cytotoxic T lymphocytes specific for Toxoplasma gondii lyse tachyzoite-infected target cells. J. Immunol. 151:2024-2031. [PubMed] [Google Scholar]
- 15.Davies, C. J., I. Joosten, L. Andersson, M. A. Arriens, D. Bernoco, B. Bissumbhar, G. Byrns, M. J. van Eijk, B. Kristensen, H. A. Lewin, et al. 1994. Polymorphism of bovine MHC class II genes. Joint report of the Fifth International Bovine Lymphocyte Antigen (BoLA) Workshop, Interlaken, Switzerland, 1 August 1992. Eur. J. Immunogenet. 21:259-289. [DOI] [PubMed] [Google Scholar]
- 16.Davies, C. J., I. Joosten, D. Bernoco, M. A. Arriens, J. Bester, G. Ceriotti, S. Ellis, E. J. Hensen, H. C. Hines, P. Horin, et al. 1994. Polymorphism of bovine MHC class I genes. Joint report of the Fifth International Bovine Lymphocyte Antigen (BoLA) Workshop, Interlaken, Switzerland, 1 August 1992. Eur. J. Immunogenet. 21:239-258. [DOI] [PubMed] [Google Scholar]
- 17.Davies, C. J., L. Anderson, S. A. Ellis, E. J. Hensen, H. A. Lewin, S. Mikko, N. E. Muggli-Cockett, J. J. van der Poel, and G. C. Russell. 1997. Nomenclature for factors of the BoLA system, 1996. Report of the ISAG BoLA Nomenclature Committee. Anim. Genet. 28:159-168. [Google Scholar]
- 18.Davison, H. C., A. Otter, and A. J. Trees. 1999. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int. J. Parasitol. 29:1683-1689. [DOI] [PubMed] [Google Scholar]
- 19.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey, J. P. 1999. Recent advances in Neospora and neosporosis. Vet. Parasitol. 84:349-367. [DOI] [PubMed] [Google Scholar]
- 21.Dubey, J. P. 2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Entrican, G. 2002. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J. Comp. Pathol. 126:79-94. [DOI] [PubMed] [Google Scholar]
- 23.Goff, W. L., W. C. Johnson, R. H. Horn, G. M. Barrington, and D. P. Knowles. 2003. The innate immune response in calves to Boophilus microplus tick transmitted Babesia bovis involves type-1 cytokine induction and NK-like cells in the spleen. Parasite Immunol. 25:185-188. [DOI] [PubMed] [Google Scholar]
- 24.Gomez Marin, J. E., A. Bonhomme, M. Guenounou, and J. M. Pinon. 1996. Role of interferon-gamma against invasion by Toxoplasma gondii in a human monocytic cell line (THP1): involvement of the parasite's secretory phospholipase A2. Cell Immunol. 169:218-225. [DOI] [PubMed] [Google Scholar]
- 25.Hobson, J. C., T. F. Duffield, D. Kelton, K. Lissemore, S. K. Hietala, K. E. Leslie, B. McEwen, G. Cramer, and A. S. Peregrine. 2002. Neospora caninum serostatus and milk production of Holstein cattle. J. Am. Vet. Med. Assoc. 221:1160-1164. [DOI] [PubMed] [Google Scholar]
- 26.Howe, D. K., A. C. Crawford, D. Lindsay, and L. D. Sibley. 1998. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 66:5322-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innes, E. A., S. E. Wright, S. Maley, A. Rae, A. Schock, E. Kirvar, P. Bartley, C. Hamilton, I. M. Carey, and D. Buxton. 2001. Protection against vertical transmission in bovine neosporosis. Int. J. Parasitol. 31:1523-1534. [DOI] [PubMed] [Google Scholar]
- 28.Jung, C., C. Y. Lee, and M. E. Grigg. 2004. The SRS superfamily of Toxoplasma surface proteins. Int. J. Parasitol. 34:285-296. [DOI] [PubMed] [Google Scholar]
- 29.Khan, I. A., K. H. Ely, and L. H. Kasper. 1991. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J. Immunol. 147:3501-3506. [PubMed] [Google Scholar]
- 30.Khan, I. A., J. D. Schwartzman, S. Fonseka, and L. H. Kasper. 1997. Neospora caninum: role for immune cytokines in host immunity. Exp. Parasitol. 85:24-34. [DOI] [PubMed] [Google Scholar]
- 31.Letscher-Bru, V., A. W. Pfaff, A. Abou-Bacar, D. Filisetti, E. Antoni, O. Villard, J. P. Klein, and E. Candolfi. 2003. Vaccination with Toxoplasma gondii SAG-1 protein is protective against congenital toxoplasmosis in BALB/c mice but not in CBA/J mice. Infect. Immun. 71:6615-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewin, H. A., G. C. Russell, and E. J. Glass. 1999. Comparative organization and function of the major histocompatibility complex of domesticated cattle. Immunol. Rev. 167:145-158. [DOI] [PubMed] [Google Scholar]
- 33.Long, M. T., T. V. Baszler, and B. A. Mathison. 1998. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum. J. Parasitol. 84:316-320. [PubMed] [Google Scholar]
- 34.Lund, O., M. Nielsen, C. Kesmir, A. G. Petersen, C. Lundegaard, P. Worning, C. Sylvester-Hvid, K. Lamberth, G. Roder, S. Justesen, S. Buus, and S. Brunak. 2004. Definition of supertypes for HLA molecules using clustering of specificity matrices. Immunogenetics 55:797-810. [DOI] [PubMed] [Google Scholar]
- 35.Maley, S. W., D. Buxton, A. G. Rae, S. E. Wright, A. Schock, P. M. Bartley, I. Esteban-Redondo, C. Swales, C. M. Hamilton, J. Sales, and E. A. Innes. 2003. The pathogenesis of neosporosis in pregnant cattle: inoculation at mid-gestation. J. Comp. Pathol. 129:186-195. [DOI] [PubMed] [Google Scholar]
- 36.Marsh, A. E., D. K. Howe, G. Wang, B. C. Barr, N. Cannon, and P. A. Conrad. 1999. Differentiation of Neospora hughesi from Neospora caninum based on their immunodominant surface antigen, SAG1 and SRS2. Int. J. Parasitol. 29:1575-1582. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa, Y., N. Inoue, X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Protective efficacy of vaccination by recombinant vaccinia virus against Neospora caninum infection. Vaccine 19:1381-1390. [DOI] [PubMed] [Google Scholar]
- 38.Nishikawa, Y., A. Iwata, H. Nagasawa, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Comparison of the growth inhibitory effects of canine IFN-alpha, -beta and -gamma on canine cells infected with Neospora caninum tachyzoites. J. Vet. Med. Sci. 63:445-448. [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 19:1710-1716. [DOI] [PubMed] [Google Scholar]
- 40.Park, Y. H., Y. S. Joo, J. Y. Park, J. S. Moon, S. H. Kim, N. H. Kwon, J. S. Ahn, W. C. Davis, and C. J. Davies. 2004. Characterization of lymphocyte subpopulations and major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J. Vet. Sci. 5:29-39. [PubMed] [Google Scholar]
- 41.Purner, M. B., R. L. Berens, P. B. Nash, A. van Linden, E. Ross, C. Kruse, E. C. Krug, and T. J. Curiel. 1996. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 64:4330-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn, H. E., J. T. Ellis, and N. C. Smith. 2002. Neospora caninum: a cause of immune-mediated failure of pregnancy? Trends Parasitol. 18:391-394. [DOI] [PubMed] [Google Scholar]
- 43.Russell, G. C. 2000. Sequence duplication at the 3′ end of BoLA-DQB genes suggests multiple allelic lineages. Immunogenetics 52:101-106. [DOI] [PubMed] [Google Scholar]
- 44.Russell, G. C., C. J. Davies, L. Anderson, S. A. Ellis, E. J. Hensen, H. A. Lewin, S. Mikko, N. E. Muggli-Cockett, and J. J. van der Poel. 1997. BoLA class II nucleotide sequences, 1996. Report of the ISAG BoLA Nomenclature Committee. Anim. Genet. 28:169-180. [Google Scholar]
- 45.Schares, G., M. Peters, R. Wurm, A. Barwald, and F. J. Conraths. 1998. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet. Parasitol. 80:87-98. [DOI] [PubMed] [Google Scholar]
- 46.Sette, A., and J. Sidney. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50:201-212. [DOI] [PubMed] [Google Scholar]
- 47.Sidney, J., H. M. Grey, S. Southwood, E. Celis, P. A. Wentworth, M. F. del Guercio, R. T. Kubo, R. W. Chesnut, and A. Sette. 1996. Definition of an HLA-A3-like supermotif demonstrates the overlapping peptide-binding repertoires of common HLA molecules. Hum. Immunol. 45:79-93. [DOI] [PubMed] [Google Scholar]
- 48.Sidney, J., S. Southwood, M. F. del Guercio, H. M. Grey, R. W. Chesnut, R. T. Kubo, and A. Sette. 1996. Specificity and degeneracy in peptide binding to HLA-B7-like class I molecules. J. Immunol. 157:3480-3490. [PubMed] [Google Scholar]
- 49.Siliciano, R. F., A. D. Keegan, R. Z. Dintzis, H. M. Dintzis, and H. S. Shin. 1985. The interaction of nominal antigen with T cell antigen receptors. I. Specific binding of multivalent nominal antigen to cytolytic T cell clones. J. Immunol. 135:906-914. [PubMed] [Google Scholar]
- 50.Staska, L. M., T. C. McGuire, C. J. Davies, H. A. Lewin, and T. V. Baszler. 2003. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 71:3272-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka, T., H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. Growth-inhibitory effects of interferon-gamma on Neospora caninum in murine macrophages by a nitric oxide mechanism. Parasitol. Res. 86:768-771. [DOI] [PubMed] [Google Scholar]
- 52.Thurmond, M. C., and S. K. Hietala. 1996. Culling associated with Neospora caninum infection in dairy cows. Am. J. Vet. Res. 57:1559-1562. [PubMed] [Google Scholar]
- 53.Thurmond, M. C., and S. K. Hietala. 1997. Effect of Neospora caninum infection on milk production in first-lactation dairy cows. J. Am. Vet. Med. Assoc. 210:672-674. [PubMed] [Google Scholar]
- 54.Velge-Roussel, F., P. Marcelo, A. C. Lepage, D. Buzoni-Gatel, and D. T. Bout. 2000. Intranasal immunization with Toxoplasma gondii SAG1 induces protective cells into both NALT and GALT compartments. Infect. Immun. 68:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamane, I., H. Kitani, T. Kokuho, T. Shibahara, M. Haritani, T. Hamaoka, S. Shimizu, M. Koiwai, K. Shimura, and Y. Yokomizo. 2000. The inhibitory effect of interferon gamma and tumor necrosis factor alpha on intracellular multiplication of Neospora caninum in primary bovine brain cells. J. Vet. Med. Sci. 62:347-351. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, Y., G. H. Palmer, J. R. Abbott, C. J. Howard, J. C. Hope, and W. C. Brown. 2003. CpG ODN 2006 and IL-12 are comparable for priming Th1 lymphocyte and IgG responses in cattle immunized with a rickettsial outer membrane protein in alum. Vaccine 21:3307-3318. [DOI] [PubMed] [Google Scholar]