Abstract
Organ failure in Plasmodium falciparum malaria is associated with neutrophil activation and endothelial damage. This study investigates whether neutrophil-induced endothelial damage involves apoptosis and whether it can be prevented by neutralization of neutrophil secretory products. Endothelial cells from human umbilical veins were coincubated with neutrophils from healthy donors and with sera from eight patients with P. falciparum malaria, three patients with P. vivax malaria, and three healthy controls. Endothelial apoptosis was demonstrated by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) and annexin V staining. The rate of apoptosis of cells was markedly increased after incubation with patient serum compared to that with control serum. Apoptosis was most pronounced after incubation with sera from two patients with fatal cases of P. falciparum malaria, followed by sera of survivors with severe P. falciparum malaria and, finally, by sera of patients with mild P. falciparum and P. vivax malaria. Ascorbic acid, tocopherol, and ulinastatin reduced the apoptosis rate, but gabexate mesilate and pentoxifylline did not. Furthermore, in fatal P. falciparum malaria, apoptotic endothelial cells were identified in renal and pulmonary tissue by TUNEL staining. These findings show that apoptosis caused by neutrophil secretory products plays a major role in endothelial cell damage in malaria. The antioxidants ascorbic acid and tocopherol and the protease inhibitor ulinastatin can reduce malaria-associated endothelial apoptosis in vitro.
Severe Plasmodium falciparum malaria is associated with activation of neutrophils and monocytes, elevated cytokine levels, and endothelial damage. In vitro studies have shown that neutrophils can be activated by products of malaria parasites (32) and by host cytokines (43, 44, 55), which are increased in the sera of patients suffering from malaria (18, 30, 34).
Activated neutrophils and their secretory products may generate not only antiparasitic activity (16) but also endothelial damage (55), which can lead to organ failure in severe malaria. We have previously found that in synergism with neutrophils, sera from patients with complicated malaria damage endothelial cells in vitro (23). In clinical cases, endothelial damage is indicated by high levels in plasma of thrombomodulin, a nonsecretable membrane protein of resting endothelial cells. In P. falciparum malaria, high thrombomodulin levels in plasma correlate with high levels in plasma of elastase, a serine protease secreted by activated neutrophils (23).
Neutrophils secrete proteolytic enzymes and reactive oxygen species, both of which can trigger endothelial cell apoptosis at low concentrations and necrosis at high concentrations (4, 7, 48, 57). Apoptosis is a genetically controlled form of cell suicide characterized by surface blebbing, contraction of cells and their nuclei, proteolysis, and DNA digestion. It is distinct from necrosis, where physical or chemical injury leads to cell swelling, organelle disruption, and membrane rupture (20).
In P. falciparum malaria, apoptosis as a possible mechanism of endothelial cell death is suggested by elevated levels in plasma of Fas ligand (31), which triggers apoptosis by binding to Fas, its receptor on the target cell. In addition, Dürck's granulomas (aggregates of astrocytes and glial cells), seen in cerebral malaria, contain large amounts of endostatin, a collagen XVIII fragment known to induce endothelial cell apoptosis (11).
Apoptosis of endothelial cells can also be caused by P. falciparum-parasitized erythrocytes in vitro (39). However, vascular leakage in P. falciparum malaria often develops several days after the initiation of antiparasitic therapy (27), even though antimalarial drugs reduce the endothelial adherence of parasitized erythrocytes (50). Therefore, interaction of parasitized erythrocytes with the vascular endothelium is probably not the only mechanism that leads to endothelial cell apoptosis in P. falciparum malaria.
This study shows that sera from patients with P. falciparum malaria—together with neutrophils—induce endothelial cell apoptosis, which can be prevented by antioxidants and inhibitors of proteolytic enzymes in vitro.
MATERIALS AND METHODS
Patients.
Serum samples from two patients with fatal P. falciparum malaria, five patients with severe nonfatal P. falciparum malaria, five patients with mild P. falciparum malaria, six patients with P. vivax malaria, and six healthy controls were investigated (Table 1). All patients were nonimmune European travelers. Informed consent for taking blood samples was obtained from patients and healthy control subjects. Approval for this study was granted by the Ethics Committees of the State Medical Boards of Hamburg and Mecklenburg-Vorpommern.
TABLE 1.
Endothelial apoptosis after incubation with serum and neutrophils
| Serum source | % Apoptosis (range)a
|
Median (range)
|
|||
|---|---|---|---|---|---|
| Before therapy | After start of antiparasitic therapy
|
No. of parasitized erythrocytes | Serum TNF-α level (pg/ml) | ||
| Day 7 | Day 30 | ||||
| Fatal P. falciparum malaria (n = 2) | 61 (54-68) | 705 (369-1,041) | 343 (285-400) | ||
| Severe P. falciparum malaria (n = 5) | 55 (37-49) | 22 (16-28) | 13 (12-14)b | 651 (200-848) | 175 (175-350) |
| Mild P. falciparum malaria (n = 5) | 38 (26-42) | 24 (20-25) | 60 (2.7-221) | 40 (38-108) | |
| P. vivax malaria (n = 6) | 25 (22-40) | 18 (15-21) | 20 (<1-47) | 96 (60-891) | |
| Healthy controls (n = 6) | 15 (9-20) | 0 | <15 | ||
Median proportions of TUNEL-positive endothelial cells in relation to those of all endothelial cells.
For two patients.
Reagents and test kits.
All chemicals (analytical grade) were purchased from Sigma (Munich, Germany) unless otherwise indicated. Endothelial cell growth supplement was obtained from Intracel Corporation, Rockville, Md.; injectable preparations of ascorbic acid were obtained from Jenapharm, Jena, Germany; human urinary trypsin inhibitor (ulinastatin) was obtained from Mochida Co., Tokyo, Japan; and gabexate mesilate was obtained from Ono Pharmaceutical Co., Osaka, Japan. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) kits (in situ cell death detection kit) and phosphatidylserine staining kits (Annexin V-Fluos) were obtained from Roche Diagnostics (Mannheim, Germany), neutralizing anti-tumor necrosis factor alpha (TNF-α) immunoglobulin G antibody was obtained from R&D Systems (Wiesbaden, Germany), and transwell tissue culture inserts with porous membranes were obtained from Nunc (Wiesbaden, Germany) and Costar-Corning (Schiphol, The Netherlands).
Endothelial cell cultures.
Endothelial cells were cultured from human umbilical veins from healthy donors as described in detail previously (23, 45). Briefly, the veins of human umbilical cords were cannulated, with endothelial cells then obtained by mild collagenase digestion and seeded on gelatin-coated tissue culture flasks. For our experiments, second- to third-passage endothelial cells were grown to confluence in 96- or 48-well plates by using RPMI medium with 20% fetal bovine serum and 35-mg/liter endothelial cell supplement.
Isolation of neutrophils.
Before each experiment, EDTA blood was obtained from a healthy donor and centrifuged at 500 × g and 4°C for 10 min. Neutrophils were isolated by Percoll (Sigma) density centrifugation (13). For this purpose, a discontinuous gradient with densities of 1.081, 1.090, and 1.098 was formed of Percoll, which had been made isotonic with 10× phosphate-buffered saline (PBS) at pH 7.4. All cells were collected at the interface between the densities 1.098 and 1.090 and washed three times in ice-cold PBS. More than 95% of the leukocytes were neutrophils, and the other cells were lymphocytes or monocytes, as analyzed by using a fluorescence-activated cell sorter. This method was chosen because it avoids neutrophil activation during cell separation (28).
Endothelial cell experiments.
Each experiment was carried out in duplicate on one occasion and replicated on two other occasions. Sera from patients with malaria or from healthy controls were diluted 1:10 in endothelial cell medium without endothelial cell growth supplement and without fetal bovine serum. Freshly isolated neutrophil granulocytes from a healthy volunteer were added to the diluted serum to obtain a final cell count of 1,000 neutrophils per μl. Cultured endothelial cells were incubated with this mixture for 1 h at 37°C, rinsed three times with HEPES-buffered saline at pH 7.4, incubated with RPMI medium containing 20% fetal bovine serum and endothelial cell growth supplement (35 mg/ml) for 4.5 h, and rinsed again three times with HEPES-buffered saline. At this time, practically all adhering cells displayed endothelial cell morphology with round nuclei. Granulocytes, which were differentiated from endothelial cells by the shape and size of the cells and their nuclei, comprised less than 1% of the adhering cells.
Endothelial cells were tested for apoptosis by the TUNEL method or annexin V staining. Both staining procedures were performed according to the manufacturer's instructions.
Briefly, endothelial cells were fixed with methanol-acetone (50:50, vol/vol) for TUNEL staining. Cell membranes and nuclei were permeabilized with 0.1% Triton X-100. To label the double-strand breaks of nuclear DNA, fixed endothelial cells were incubated with fluorescein isothiocyanate-conjugated UDP and terminal deoxynucleotidyltransferase for 30 min and rinsed with PBS. To stabilize the fluorescence of stained endothelial cell nuclei, 100 μl of 0.6% 1,4-diazabicyclo[2.2.2]octane (DABCO) in PBS-glycerol (50:50, vol/vol) was added before microscopy. Fluorescent nuclei were counted in 100 cells in each well with an inversion fluorescence microscope (Axiovert; Zeiss, Jena, Germany). The results were expressed as the percentages of fluorescent (i.e., apoptotic) nuclei among all nuclei.
Apoptosis was also demonstrated by staining unfixed cells with fluorescein isothiocyanate-conjugated annexin V because after fixation, membrane-associated phosphatidylserine residues are no longer able to bind annexin V. Propidium iodide was added to identify damaged endothelial cells which had lost their capacity to eliminate this dye in the course of apoptotic cell death. In endothelial cells, this tends to happen soon after the induction of apoptosis (56). Fluorescence microscopy was performed immediately after staining. Endothelial cells incubated with hydrogen peroxide (3 mmol/liter) or with neutrophils and sera from healthy subjects served as positive and negative controls, respectively. The negative control is shown in the inset of Fig. 1B.
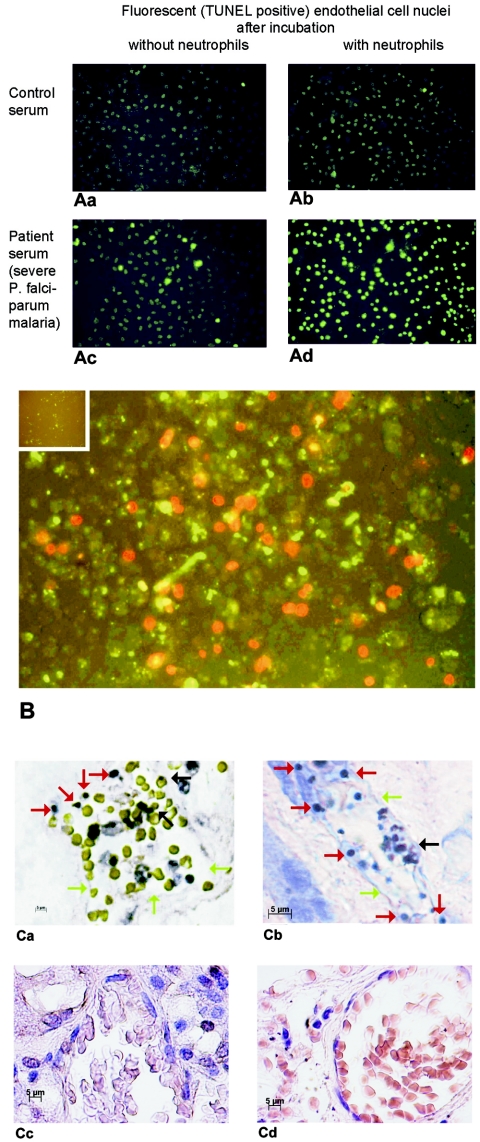
FIG. 1.
(A) TUNEL-stained nuclei from endothelial cells incubated with serum from a patient with fatal P. falciparum malaria (Table 1, patient 2) and from a healthy control, with or without neutrophils. (B) Annexin V-stained endothelial cells incubated with neutrophils plus serum from a patient with fatal P. falciparum malaria (green fluorescence). Necrotic cells were stained with propidium iodide (red fluorescence). The inset shows endothelial cells incubated with serum from a healthy person as a negative control. (C) TUNEL-stained kidney (a) and lung (b) sections from two patients who had died from P. falciparum malaria. The capillaries contain erythrocytes and malaria pigment (black arrows). Much of the endothelial cell lining is missing (green arrows). The endothelial cell nuclei are round, condensed, and TUNEL positive, indicating apoptosis (red arrows). Normal capillary endothelial cells in kidney (c) and lung (d) sections from a traffic accident victim are also shown.
In order to test whether endothelial cell apoptosis requires direct contact with neutrophils, endothelial cells were kept separate from neutrophils by porous membranes (transwell inserts) during incubation with patient serum and endothelial cells.
Inhibition experiments.
To prevent apoptotic damage, the antioxidants ascorbic acid (10−6 to 10−3 M) and tocopherol (10−5 to 10−3 M), the serine protease inhibitor ulinastatin (10−10 to 10−5 M), or the antisecretory substances gabexate mesilate (10−7 to 10−4 M) or pentoxifylline (10−6 to 10−4 M) was added to patient sera with neutrophils before incubation of endothelial cells. Ascorbic acid and tocopherol were chosen for their capacity to neutralize reactive oxygen species, ulinastatin was chosen for its capacity to inhibit neutrophil elastase, and gabexate mesilate and pentoxifylline (which neutralize neither reactive oxygen species nor elastase) were chosen for their capacity to inhibit neutrophil secretory activity. The concentrations of these inhibitors were chosen to reflect levels in serum that can be achieved by administering these drugs under clinical conditions. Additional experiments were performed in the presence of a neutralizing anti-TNF-α antibody (1 μg/ml).
For all inhibition experiments, endothelial cells were incubated with serum from a patient with fatal malaria (Table 1, patient 1 or 2) plus neutrophils as a positive control or with sera from healthy volunteers plus neutrophils as a negative control.
Histopathology.
Thin sections from paraffin-embedded blocks of kidney tissue from two patients and lung tissue from another patient who had died of complicated P. falciparum malaria were stained with hematoxylin and eosin stain. Thin lung and kidney sections from a traffic accident victim were used as negative controls. Apoptotic nuclei were labeled by the TUNEL method.
Statistics.
Correlations were calculated as Spearman rank correlations.
RESULTS
Incubation of endothelial cells with serum.
Incubation of endothelial cells with serum from healthy controls with or without neutrophils resulted in a low number of TUNEL-positive apoptotic cells (without neutrophils, median of 10% and range of 0.5 to 14.5%; with neutrophils, median of 15% and range of 5 to 19.5%; n = 6). In contrast, incubation of endothelial cells with sera from malaria patients together with neutrophils resulted in a markedly higher number of apoptotic endothelial cells than incubation without neutrophils (Fig. 1A). In these coincubation experiments, sera from patients with fatal P. falciparum malaria showed the highest apoptosis rates (median of 61% and range of 54 to 68 for two patients), followed by sera from patients with severe P. falciparum, mild P. falciparum, and P. vivax malaria (Table 1). Sera from patients with fatal malaria plus neutrophils induced large defects in the endothelial cell layers, a phenomenon that is consistently seen secondary to apoptosis (58).
All sera obtained before antimalarial therapy induced a higher rate of TUNEL-positive endothelial cell nuclei than reconvalescent sera obtained 7 days later (48 versus 21% of sera from patients with severe malaria, 40 versus 21% of sera from patients with mild P. falciparum malaria, and 25 versus 19% of sera from patients with P. vivax malaria [Table 1]).
Parasitemia levels showed a significant correlation with the percentage of TUNEL-positive endothelial cell nuclei (r = 0.751, P < 0.001), but no correlation was seen for TNF-α concentrations in serum (r = 0.216, P > 0.1).
Annexin V staining confirmed the elevated rates of apoptotic endothelial cells after incubation with serum from a patient with fatal malaria and neutrophils (48% [Fig. 1B]) compared to that with control serum (10% [Fig. 1B, inset]). In fatal malaria, 28% of the annexin V-positive cells retained propidium iodide, reflecting the fact that apoptotic endothelial cells progress to necrosis more quickly than other cell types (56). The propidium iodide-retaining nuclei were round and condensed, indicating that apoptosis had been induced before the cells died. Cells negative for annexin and positive for propidium iodide were not seen, probably because dead endothelial cells detach from their matrix (23, 58).
Transwell experiments.
In order to test whether the impact of malaria patient serum and neutrophils on endothelial apoptosis depends upon direct contact between neutrophils and endothelial cells, neutrophils were separated from endothelial cells by porous membranes during incubation. Sera from the two patients with fatal malaria led to apoptosis rates of 49 and 42%, with neutrophils in direct contact with endothelial cells; 25 and 29%, with neutrophils separated from endothelial cells by porous membranes; and 24 and 33% in the absence of neutrophils. Serum from the patient with severe nonfatal malaria led to an endothelial apoptosis rate of 43%, with neutrophils in direct contact with endothelial cells; 29%, with neutrophils separated from endothelial cells by a porous membrane; and 28% in the absence of neutrophils.
Inhibition experiments.
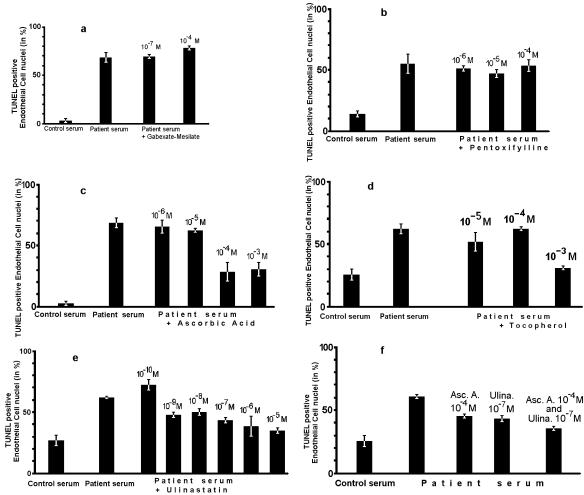
The water-soluble antioxidant ascorbic acid and the urinary trypsin inhibitor ulinastatin inhibited the apoptotic effect of sera from patients with severe malaria in a concentration-dependent manner. The apoptosis rate was 68% without either substance. The apoptosis rate was reduced to 62% with 10−5 M, to 28% with 10−4 M, and to 32% with 10−3 M ascorbic acid, while 10−6 M ascorbic acid had no effect (Fig. 2c). The rank correlation between the concentrations of ascorbic acid and the apoptosis rates was 0.85 (P = 0.006). The apoptosis rate was reduced to 42% with 10−7 M ulinastatin and to 35% at a concentration of 10−5 M, while 10−10 M ulinastatin had no effect (Fig. 2e). The rank correlation between the concentrations of ulinastatin and the apoptosis rates was 0.85 (P < 0.001). Likewise, the lipid-soluble antioxidant tocopherol reduced the apoptosis rate from 62 to 29% at a concentration of 10−3 M, while lower concentrations had no effect (Fig. 2d).
FIG. 2.
Prevention of endothelial damage induced by serum (severe P. falciparum malaria) and neutrophils. Shown are results with TUNEL-positive endothelial cell nuclei after incubation with gabexate mesilate (a), pentoxifylline (b), ascorbic acid (c), tocopherol (d), ulinastatin (e), and ascorbic acid plus ulinastatin (f). Each of these graphs shows one representative experiment out of three. Means (bars) and individual results (whiskers) of duplicate assays are shown.
Reduction of endothelial apoptosis by 10−4 M ascorbic acid and by 10−7 M ulinastatin has been confirmed with sera from two patients with fatal malaria, one patient with severe nonfatal malaria, and three patients with mild P. falciparum malaria.
Since neither ascorbic acid nor ulinastatin alone was able to suppress apoptotic endothelial cell damage completely, both substances were combined. At concentrations of 10−4 M ascorbic acid and 10−7 M ulinastatin, the apoptosis rate of 35% was lower than that with either substance alone at the respective concentration (ascorbic acid, 45%; ulinastatin, 43% [Fig. 2f]).
Since the effect of malaria patient serum on endothelial cells may depend upon the TNF-α present in the patient serum, a neutralizing anti-TNF-α antibody was tested with sera from two patients with fatal malaria. In the absence of neutrophils, the anti-TNF-α antibody reduced the endothelial apoptosis rate from 33 to 24% and from 33 to 26%, while in the presence of neutrophils, the antibody reduced the apoptosis rate from 50 to 30% and from 47 to 35% in the first and second sera, respectively.
Since the contents of neutrophil granules may cause endothelial apoptosis, the experiments were performed in the presence or absence of gabexate mesilate or pentoxifylline, both of which block secretion from neutrophil granulocytes (6, 36). However, addition of gabexate mesilate (0.1 to 100 μM) or pentoxifylline (1 to 100 μM) did not reduce the percentage of TUNEL-positive endothelial cell nuclei (Fig. 2a and b).
Histopathology.
Observation of capillaries in kidney and lung tissues from two patients who had died from P. falciparum malaria showed TUNEL-positive endothelial cells with round and condensed nuclei, indicating that they were undergoing apoptosis (Fig. 1C, panels a and b). Much of the capillary endothelial cell layer was missing, suggesting malaria-induced endothelial damage or cell detachment, as previously suggested from in vitro experiments (23).
DISCUSSION
This study indicates that sera from patients with malaria induces endothelial apoptosis, which is amplified by neutrophils, and that endothelial apoptosis can be prevented by the neutralization of reactive oxygen species or proteolytic enzymes in vitro.
Neutrophils can be activated by TNF-α (23, 43, 44) and by P. falciparum-specific products (32), both present in significant amounts in the sera of patients with P. falciparum malaria before therapy (3, 10, 18, 30, 34, 37). Sera obtained 7 days after the initiation of therapy, when TNF-α levels and parasitemia had decreased, induced less endothelial apoptosis than sera obtained before therapy. Earlier, we had shown that anti-TNF-α antibodies protect endothelial cells from damage induced by patient serum (severe malaria) and neutrophils (23). In the present study, we show that anti-TNF-α antibodies reduce the apoptosis-inducing capacity of patient serum both in the presence and in the absence of neutrophils. TNF-α not only activates neutrophils and endothelial cells but also triggers endothelial cell apoptosis in a concentration- and time-dependent manner (41). This may explain why malaria patient serum can induce endothelial cell apoptosis independently of neutrophils, albeit at a lower level. However, the proapoptotic effect of patient serum correlates better with parasitemia than with TNF-α concentrations in serum. Therefore, parasite-derived products may also play a role in neutrophil-mediated endothelial apoptosis.
Reactive oxygen species and elastase, which are secreted by neutrophils, induce apoptosis of endothelial cells at low concentrations and necrosis at high concentrations (4, 7, 48, 57). In our experiments, staining with annexin V and propidium iodide confirmed that most of the endothelial damage was due to apoptosis. Likewise, we found apoptotic, rather than necrotic, endothelial cells in the renal and pulmonary blood vessels of patients with P. falciparum malaria who had died of multiorgan failure (Fig. 1C, panels a and b).
To prevent multiorgan failure in malaria, several intervention strategies have been studied in simian and murine models, including the administration of heparin (12), anti-TNF-α antibodies (17), pentoxifylline (33), or dexamethasone (14). However, in all these studies, the intervention had to be applied before the onset of disease to prevent organ failure and death. This is also true for neutrophil depletion, which protects mice from cerebral complications of P. berghei malaria only if performed before symptoms appear (8). Later, when death from cerebral malaria is imminent, only administration of an antibody against leukocyte function antigen 1, which blocks binding of neutrophil granulocytes to the vascular endothelium via intercellular adhesion molecule 1, offers protection in this animal model (19). This is in agreement with our observation that direct contact between neutrophils and the vascular endothelium is essential for neutrophil-mediated endothelial apoptosis in the presence of malaria patient serum.
In human P. falciparum malaria, heparin (21), anti-TNF-α antibodies (52), and pentoxifylline (24, 35) have failed to provide any therapeutic benefit, while dexamethasone (which is known to induce endothelial apoptosis in vivo [15, 53]) was even deleterious (25, 54). Activated or apoptotic endothelial cells become procoagulant, reflected by decreased protein C and elevated plasma thrombin-antithrombin III levels in P. falciparum malaria (5, 22, 44). Heparin is beneficial in simian malaria (P. knowlesi) but not in human malaria (P. falciparum), where it fails to reverse these procoagulant alterations (12, 21).
Thus, one explanation for the failure of these intervention strategies is that they may come too late to inhibit the harmful pathophysiological events. Another reason may be that these intervention strategies cannot prevent endothelial damage and increased capillary permeability, which is a key to organ impairment in severe malaria (23, 27, 38). In contrast, an antiapoptotic strategy should prevent damage even when applied after activation of the host response.
Our study demonstrates that apoptosis of endothelial cells was reduced by the antioxidants ascorbic acid and tocopherol and by the protease inhibitor ulinastatin. In combination, ascorbic acid and ulinastatin were more effective than either substance alone but did not block endothelial apoptosis completely (Fig. 2f). The incremental effect of this combination over each individual substance is not proven to have biological significance. However, induction of endothelial damage by the joint action of reactive oxygen species and elastase has been demonstrated in an isolated rat lung model (2). This may be due to the fact that reactive oxygen species inactivate protease inhibitors which otherwise might protect the endothelium from the elastase-induced damage (46).
As shown in Fig. 2d, endothelial cell apoptosis was also significantly inhibited by tocopherol. The antioxidant tocopherol prevents apoptosis by inhibiting caspases (51). In murine P. berghei malaria, administration of the antioxidants butylhydroxyanisol or tocopherol prevents death from cerebral complications (49), suggesting that inhibition of apoptosis by this antioxidant may indeed be operative in vivo. However, while ascorbic acid and ulinastatin protected endothelial cells at concentrations that are achieved in humans, tocopherol was effective only at much higher concentrations.
None of the substances tested provided complete protection from apoptosis. This may be explained by the fact that substances directed against neutrophil-derived reactive oxygen species and proteases would not necessarily block neutrophil-independent apoptosis by TNF-α or other serum ingredients.
Nevertheless, the clinical benefit of an antiapoptotic strategy for endothelial protection has been illustrated in cardiovascular diseases. Just like the serum malaria samples in this study, serum samples from patients with cardiopulmonary bypass (1) or congestive heart failure (42) cause endothelial apoptosis in vitro. In agreement with this study, ascorbic acid prevents endothelial apoptosis in congestive heart failure in vitro and in vivo (42). The multivalent enzyme inhibitor ulinastatin neutralizes human neutrophil elastase in vitro (29) and improves pulmonary function (lower alveolar arterial oxygen gradient, or AaDO2) in vivo (47).
The antiapoptotic strategy derived from our in vitro model may be relevant for P. falciparum malaria, since this disease is associated with both elevated levels of human neutrophil elastase in plasma (23, 26, 40) and a decreased serum antioxidative status with abnormally low levels of ascorbic acid (9). Thus, antioxidants and protease inhibitors may offer clinical benefit by preventing organ complications due to endothelial apoptosis even though they may not accelerate parasite clearance or defervescence.
Acknowledgments
The help of Volker Briese (Department of Gynecology and Obstetrics, Rostock University Medical School) and Eckhard Koepcke (Department of Gynecology and Obstetrics, Rostock City Hospital “Suedstadtklinikum”) in providing umbilical cords is greatly appreciated. We thank Rudolf Wegener (Department of Forensic Medicine, Rostock University Medical School) and Horst Nizze (Department of Pathology, Rostock University Medical School) for technical assistance. We also thank Joachim Rychly (Department of Experimental Medicine, Rostock University Medical School) for permitting us to use the inversion fluorescence microscope and other facilities in his department.
Financial support was received from the German Research Foundation (Deutsche Forschungsgemeinschaft grant He 3137/2-1), the B. Braun Foundation, Melsungen, Germany (grant Hemmer 2004), and the Research Support Program, University of Rostock Medical School (grants FORUN 2002 and 2004).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aebert, H., S. Kirchner, A. Keyser, D. E. Birnbaum, E. Holler, R. Andreesen, and G. Eissner. 2000. Endothelial apoptosis is induced by serum of patients after cardiopulmonary bypass. Eur. J. Cardiothorac. Surg. 18:589-593. [DOI] [PubMed] [Google Scholar]
- 2.Baird, B. R., J. C. Cheronis, R. A. Sandhaus, E. M. Berger, C. W. White, and J. E. Repine. 1986. O2 metabolites and neutrophil elastase synergistically cause edematous injury in isolated rat lungs. J. Appl. Physiol. 61:2224-2229. [DOI] [PubMed] [Google Scholar]
- 3.Bate, C. A., J. Taverne, E. Roman, C. Moreno, and J. H. Playfair. 1992. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology 75:129-135. [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme, M. W., P. Galle, and W. Stremmel. 2002. Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals. Immunology 107:340-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bombeli, T., A. Karsan, J. F. Tait, and J. M. Harlan. 1997. Apoptotic vascular endothelial cells become procoagulant. Blood 89:2429-2442. [PubMed] [Google Scholar]
- 6.Boogaerts, M. A., S. Malbrain, P. Meeus, L. van Hove, and G. E. Verhoef. 1990. In vitro modulation of normal and diseased human neutrophil function by pentoxifylline. Blut 61:60-65. [DOI] [PubMed] [Google Scholar]
- 7.Burlacu, A., V. Jinga, A. V. Gafencu, and M. Simionescu. 2001. Severity of oxidative stress generates different mechanisms of endothelial cell death. Cell Tissue Res. 306:409-416. [DOI] [PubMed] [Google Scholar]
- 8.Chen, L., Z. Zhang, and F. Sendo. 2000. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin. Exp. Immunol. 120:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, B. S., J. K. Patnaik, S. Mohanty, S. K. Mishra, D. Mohanty, S. K. Satpathy, and T. K. Bose. 1993. Plasma antioxidants and lipid peroxidation products in falciparum malaria. Am. J. Trop. Med. Hyg. 49:720-725. [DOI] [PubMed] [Google Scholar]
- 10.de Dominguez, N., and A. Rodriguez-Acosta. 1996. Glutamate dehydrogenase antigen detection in Plasmodium falciparum infections. Korean J. Parasitol. 34:239-246. [DOI] [PubMed] [Google Scholar]
- 11.Deininger, M. H., B. Fimmen, P. G. Kremsner, R. Meyermann, and H. J. Schluesener. 2002. Accumulation of endostatin/collagenXVIII in brains of patients who died with cerebral malaria. J. Neuroimmunol. 131:216-221. [DOI] [PubMed] [Google Scholar]
- 12.Dennis, L. H., and M. E. Conrad. 1968. Anticoagulant and antimalarial action of heparin in simian malaria. Lancet i:769-771. [DOI] [PubMed] [Google Scholar]
- 13.Dooley, D. C., J. F. Simpson, and H. A. T. Meryman. 1982. Isolation of large numbers of fully viable human neutrophils: a preparative technique using percoll density gradient centrifugation. Exp. Hematol. 10:591-599. [PubMed] [Google Scholar]
- 14.Franz, D. R., T. S. Lim, W. B. Baze, S. Arimbalam, M. Lee, and G. E. Lewis, Jr. 1988. Pathologic activity of Plasmodium berghei prevented but not reversed by dexamethasone. Am. J. Trop. Med. Hyg. 38:249-254. [DOI] [PubMed] [Google Scholar]
- 15.Gaytan, F., C. Morales, C. Bellido, and J. E. Sanchez-Criado. 2002. Selective apoptosis of luteal endothelial cells in dexamethasone-treated rats leading to ischemic necrosis of luteal tissue. Biol. Reprod. 66:232-240. [DOI] [PubMed] [Google Scholar]
- 16.Golenser, J., M. Kamyl, A. Tsafack, E. Marva, A. Cohen, N. Kitrossky, and M. Chevion. 1992. Correlation between destruction of malarial parasites by polymorphonuclear leucocytes and oxidative stress. Free Radic. Res. Commun. 17:249-262. [DOI] [PubMed] [Google Scholar]
- 17.Grau, G. E., L. F. Fajardo, P. F. Piguet, B. Allet, P. H. Lambert, and P. Vassalli. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210-1212. [DOI] [PubMed] [Google Scholar]
- 18.Grau, G. E., T. E. Taylor, M. E. Molyneux, J. J. Wirima, P. Vassalli, M. Hommel, and P. H. Lambert. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 320:1586-1591. [DOI] [PubMed] [Google Scholar]
- 19.Grau, G. E., P. Pointaire, P. F. Piguet, C. Vesin, H. Rosen, I. Stamenkovic, F. Takei, and P. Vassalli. 1991. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur. J. Immunol. 21:2265-2267. [DOI] [PubMed] [Google Scholar]
- 20.Hasnain, S. E., R. Begum, K. V. Ramaiah, S. Sahdev, E. M. Shajil, T. K. Taneja, M. Mohan, M. Athar, N. K. Sah, and M. Krishnaveni. 2003. Host-pathogen interactions during apoptosis. J. Biosci. 28:349-358. [DOI] [PubMed] [Google Scholar]
- 21.Hemmer, C. J., P. Kern, F. G. Holst, P. P. Nawroth, and M. Dietrich. 1991. Neither heparin nor acetylsalicylic acid influence the clinical course in human Plasmodium falciparum malaria: a prospective randomized study. Am. J. Trop. Med. Hyg. 45:608-612. [DOI] [PubMed] [Google Scholar]
- 22.Hemmer, C. J., P. Kern, F. G. E. Holst, K. P. Radtke, R. Egbring, A. Bierhaus, P. P. Nawroth, and M. Dietrich. 1991. Activation of the host response in human Plasmodium falciparum malaria: relation of parasitemia to tumor necrosis factor/cachectin, thrombin-antithrombin III, and protein C levels. Am. J. Med. 91:37-44. [DOI] [PubMed] [Google Scholar]
- 23.Hemmer, C. J., A. Bierhaus, J. von Riedesel, S. Gabat, B. Liliensiek, P. Pitronik, J. Lin, A. Grauer, J. Amiral, R. Ziegler, S. Schieffer, P. Kern, R. Seitz, R. Egbring, M. Dietrich, and P. P. Nawroth. 1994. Elevated thrombomodulin plasma levels as a result of endothelial involvement in Plasmodium falciparum malaria. Thromb. Haemost. 72:457-464. [PubMed] [Google Scholar]
- 24.Hemmer, C. J., G. Hort, C. B. Chiwakata, R. Seitz, R. Egbring, W. Gaus, J. Hoegel, M. Hassemer, P. P. Nawroth, P. Kern, and M. Dietrich. 1997. Supportive pentoxifylline in falciparum malaria: no effect on tumor necrosis factor alpha levels or clinical outcome: a prospective, randomized, placebo-controlled study. Am. J. Trop. Med. Hyg. 56:397-403. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman, S. L., D. Rustama, N. H. Punjabi, B. Surampaet, B. Sanjaya, A. J. Dimpudus, K. T. McKee, Jr., F. P. Paleologo, J. R. Campbell, H. Marwoto, and L. Laughlin. 1988. High-dose dexamethasone in quinine-treated patients with cerebral malaria: a double-blind, placebo-controlled trial. J. Infect. Dis. 158:325-331. [DOI] [PubMed] [Google Scholar]
- 26.Holst, F. G., C. J. Hemmer, C. Foth, R. Seitz, R. Egbring, and M. Dietrich. 1999. Low levels of fibrin-stabilizing factor (factor XIII) in human Plasmodium falciparum malaria: correlation with clinical severity. Am. J. Trop. Med. Hyg. 60:99-104. [DOI] [PubMed] [Google Scholar]
- 27.Horstmann, R. D., J. H. Ehrich, J. Beck, and M. Dietrich. 1985. Toedliche Komplikationen der Malaria tropica. Eine retrospektive klinisch-pathologische Untersuchung von 25 Faellen. Dtsch. Med. Wochenschr. 110:1651-1656. [DOI] [PubMed] [Google Scholar]
- 28.Jackson, M. H., A. M. Millar, J. Dawes, and D. Bell. 1989. Neutrophil activation during cell separation procedures. Nucl. Med. Commun. 10:901-904. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson, B. M., C. Loeffler, and K. Ohlsson. 1982. Human granulocyte elastase is inhibited by the urinary trypsin inhibitor. Hoppe-Seyler's Z. Physiol. Chem. 363:1167-1175. [DOI] [PubMed] [Google Scholar]
- 30.Kern, P., C. J. Hemmer, J. Van Damme, H. J. Gruss, and M. Dietrich. 1989. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 87:139-143. [DOI] [PubMed] [Google Scholar]
- 31.Kern, P., M. Dietrich, C. Hemmer, and N. Wellinghausen. 2000. Increased levels of soluble Fas ligand in serum in Plasmodium falciparum malaria. Infect. Immun. 68:3061-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharazmi, A., S. Jepsen, and B. J. Andersen. 1987. Generation of reactive oxygen radicals by human phagocytic cells activated by Plasmodium falciparum. Scand. J. Immunol. 25:335-341. [DOI] [PubMed] [Google Scholar]
- 33.Kremsner, P. G., H. Grundmann, S. Neifer, K. Sliwa, G. Sahlmuller, B. Hegenscheid, and U. Bienzle. 1991. Pentoxifylline prevents murine cerebral malaria. J. Infect. Dis. 164:605-608. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowski, D., A. V. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. R. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 35.Looareesuwan, S., P. Wilairatana, S. Vannaphan, V. Wanaratana, C. Wenisch, M. Aikawa, G. Brittenham, W. Graninger, and W. H. Wernsdorfer. 1998. Pentoxifylline as an ancillary treatment for severe falciparum malaria in Thailand. Am. J. Trop. Med. Hyg. 58:348-353. [DOI] [PubMed] [Google Scholar]
- 36.Mikawa, K., H. Akamatsu, N. Maekawa, K. Nishina, H. Obara, and Y. Niwa. 1994. Inhibitory effect of gabexate mesilate on human neutrophil function. J. Int. Med. Res. 22:245-254. [DOI] [PubMed] [Google Scholar]
- 37.Parra, M. E., C. B. Evans, and D. W. Taylor. 1991. Identification of Plasmodium falciparum histidine-rich protein 2 in the plasma of humans with malaria. J. Clin. Microbiol. 29:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patnaik, J. K., B. S. Das, S. K. Mishra, S. Mohanty, S. K. Satpathy, and D. Mohanty. 1994. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 51:642-647. [PubMed] [Google Scholar]
- 39.Pino, P., I. Vouldoukis, J. P. Kolb, N. Mahmoudi, I. Desportes-Livage, F. Bricaire, M. Danis, B. Dugas, and D. Mazier. 2003. Plasmodium falciparum-infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J. Infect. Dis. 187:1283-1290. [DOI] [PubMed] [Google Scholar]
- 40.Pukrittayakamee, S., R. Clemens, C. Pramoolsinsap, H. E. Karges, S. Vanijanonta, D. Bunnag, and N. J. White. 1992. Polymorphonuclear leucocyte elastase in Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 86:598-601. [DOI] [PubMed] [Google Scholar]
- 41.Robaye, B., R. Mosselmans, W. Fiers, J. E. Dumont, and P. Galand. 1991. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am. J. Pathol. 138:447-453. [PMC free article] [PubMed] [Google Scholar]
- 42.Rossig, L., J. Hoffmann, B. Hugel, Z. Mallat, A. Haase, J. M. Freyssinet, A. Tedgui, A. Aicher, A. M. Zeiher, and S. Dimmeler. 2001. Vitamin C inhibits endothelial apoptosis in congestive heart failure. Circulation 104:2182-2187. [DOI] [PubMed] [Google Scholar]
- 43.Shalaby, M. R., B. B. Aggarwal, E. Rinderknecht, L. P. Svedersky, B. S. Finkle, and M. A. Palladino, Jr. 1985. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J. Immunol. 135:2069-2073. [PubMed] [Google Scholar]
- 44.Shalaby, M. R., M. A. Palladino, Jr., S. E. Hirabayashi, T. E. Eessalu, G. D. Lewis, H. M. Shepard, and B. B. Aggarwal. 1987. Receptor binding and activation of polymorphonuclear neutrophils by tumor necrosis factor-alpha. J. Leukoc. Biol. 41:196-204. [DOI] [PubMed] [Google Scholar]
- 45.Stern, D. M., I. Bank, P. P. Nawroth, J. Cassimeris, W. Kisiel, J. W. Fenton II, C. Dinarello, L. Chess, and E. A. Jaffe. 1985. Self-regulation of procoagulant events on the endothelial cell surface. J. Exp. Med. 162:1223-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stief, T. W., and N. Heimburger. 1988. Inactivation of serine proteinase inhibitors (serpins) in human plasma by reactive oxidants. Biol. Chem. Hoppe-Seyler 369:1337-1342. [DOI] [PubMed] [Google Scholar]
- 47.Sugita, T., S. Watarida, K. Katsuyama, Y. Nakajima, R. Yamamoto, and A. Mori. 2002. Effect of a human urinary protease inhibitor (ulinastatin) on respiratory function in pediatric patients undergoing cardiopulmonary bypass. J. Cardiovasc. Surg. (Torino) 43:437-440. [PubMed] [Google Scholar]
- 48.Suhara, T., K. Fukuo, T. Sugimoto, S. Morimoto, T. Nakahashi, S. Hata, M. Shimizu, and T. Ogihara. 1998. Hydrogen peroxide induces up-regulation of Fas in human endothelial cells. J. Immunol. 160:4042-4047. [PubMed] [Google Scholar]
- 49.Thumwood, C. M., N. H. Hunt, W. B. Cowden, and I. A. Clark. 1989. Antioxidants can prevent cerebral malaria in Plasmodium berghei-infected mice. Br. J. Exp. Pathol. 70:293-303. [PMC free article] [PubMed] [Google Scholar]
- 50.Udomsangpetch, R., B. Pipitaporn, S. Krishna, B. Angus, S. Pukrittayakamee, I. Bates, Y. Suputtamongkol, D. E. Kyle, and N. J. White. 1996. Antimalarial drugs reduce cytoadherence and rosetting Plasmodium falciparum. J. Infect. Dis. 173:691-698. [DOI] [PubMed] [Google Scholar]
- 51.Uemura, M., H. Manabe, N. Yoshida, N. Fujita, J. Ochiai, N. Matsumoto, T. Takagi, Y. Naito, and T. Yoshikawa. 2002. Alpha-tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 456:29-37. [DOI] [PubMed] [Google Scholar]
- 52.van Hensbroek, M. B., A. Palmer, E. Onyiorah, G. Schneider, S. Jaffar, G. Dolan, H. Memming, J. Frenkel, G. Enwere, S. Bennett, D. Kwiatkowski, and B. Greenwood. 1996. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J. Infect. Dis. 174:1091-1097. [DOI] [PubMed] [Google Scholar]
- 53.Vogt, C. J., and G. W. Schmid-Schönbein. 2001. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation 8:129-139. [PubMed] [Google Scholar]
- 54.Warrell, D. A., S. Looareesuwan, M. J. Warrell, P. Kasemsarn, R. Intaraprasert, D. Bunnag, and T. Harinasuta. 1982. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N. Engl. J. Med. 306:313-319. [DOI] [PubMed] [Google Scholar]
- 55.Westlin, W. F., and M. A. Gimbrone, Jr. 1993. Neutrophil-mediated damage to human vascular endothelium. Role of cytokine activation. Am. J. Pathol. 142:117-128. [PMC free article] [PubMed] [Google Scholar]
- 56.Wolbers, F., P. Buijtenhuijs, C. Haanen, and I. Vermes. 2004. Apoptotic cell death kinetics in vitro depend on the cell types and the inducers used. Apoptosis 9:385-392. [DOI] [PubMed] [Google Scholar]
- 57.Yang, J. J., R. Kettritz, R. J. Falk, J. C. Jennette, and M. L. Gaido. 1996. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am. J. Pathol. 149:1617-1626. [PMC free article] [PubMed] [Google Scholar]
- 58.Zoellner, H., M. Hofler, R. Beckmann, E. Bielek, E. Vanyek, I. Kumabashiri, and B. Binder. 1998. Fibrinolytic proteins in apoptotic human umbilical vein endothelial cells. Thromb. Res. 915:209-219. [DOI] [PubMed] [Google Scholar]