Abstract
LT-IIa and LT-IIb, the type II heat-labile enterotoxins of Escherichia coli, are closely related in structure and function to cholera toxin and LT-I, the type I heat-labile enterotoxins of Vibrio cholerae and E. coli, respectively. Recent studies from our group demonstrated that LT-IIa and LT-IIb are potent systemic and mucosal adjuvants. To determine whether binding of LT-IIa and LT-IIb to their specific ganglioside receptors is essential for adjuvant activity, LT-IIa and LT-IIb enterotoxins were compared with their respective single-point substitution mutants which have no detectable binding activity for their major ganglioside receptors [e.g., LT-IIa(T34I) and LT-IIb(T13I)]. Both mutant enterotoxins exhibited an extremely low capacity for intoxicating mouse Y1 adrenal cells and for inducing production of cyclic AMP in a macrophage cell line. BALB/c female mice were immunized by the intranasal route with the surface adhesin protein AgI/II of Streptococcus mutans alone or in combination with LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I). Both LT-IIa and LT-IIb potentiated strong mucosal and systemic immune responses against AgI/II. Of the two mutant enterotoxins, only LT-IIb(T13I) had the capacity to strongly potentiate mucosal anti-AgI/II and systemic anti-AgI/II antibody responses. Upon boosting with AgI/II, however, both LT-IIa(T34I) and LT-IIb(T13I) enhanced humoral memory responses to AgI/II. Flow cytometry demonstrated that LT-IIa(T34I) had no affinity for cervical lymph node lymphocytes. In contrast, LT-IIb(T13I) retained binding activity for T cells, B cells, and macrophages, indicating that this immunostimulatory mutant enterotoxin interacts with one or more unknown lymphoid cell receptors.
Mucosal surfaces represent the major entry route of many microbial pathogens. Hence, it is important that prospective vaccines stimulate maximal immune response at these sites. The mucosal immune system usually requires the aid of immune-stimulating agents (i.e., adjuvants) to generate robust immunity and long-lived memory responses to an antigen. The type I heat-labile enterotoxins produced by Vibrio cholerae and Escherichia coli (CT and LT-I, respectively) have been extensively characterized as mucosal adjuvants in a variety of animals (22). Recently, the immunomodulatory activities of a second class of heat-labile enterotoxins of E. coli were described. This second class consists of LT-IIa and LT-IIb, two heat-labile enterotoxins which can be distinguished from LT-I by a variety of antigenic and genetic differences (17, 18). Murine experiments demonstrated that certain immunomodulatory activities of LT-IIa and LT-IIb are equivalent to or greater than those of CT (8, 33).
LT-IIa, LT-IIb, CT, and LT-I belong to the AB5 superfamily of bacterial enterotoxins. Members of this superfamily are related in structure and function (17, 18, 58, 60). Each of these enterotoxins is an oligomeric protein composed of an A polypeptide which is noncovalently coupled to a pentameric array of B polypeptides. The A polypeptide is enzymatically active and upregulates adenylyl cyclase by catalyzing the ADP-ribosylation of the Gsα regulatory protein. This modification of Gsα promotes accumulation of intracellular adenosine 3′,5′ cyclic monophosphate (cAMP), which indirectly induces the intoxicated cell to secrete chloride ions and likely modulates other processes for which cAMP is a signaling molecule (4, 23, 35-37). The B pentamer mediates binding of LT-IIa, LT-IIb, CT, and LT-I to gangliosides, a heterogeneous family of glycolipids located on the surface of mammalian cells (56). CT and LT-I bind with high affinity to GM1 and with lower affinity to ganglioside GD1b; LT-IIa binds specifically, in descending order of avidity, to gangliosides GD1b, GM1, GT1b, GQ1b, GD2, GD1a, and GM3; LT-IIb binds most avidly to GD1a and binds to GM2 and GM3 with much lower affinities (15).
LT-IIa, LT-IIb, CT, and LT-I are potent mucosal and systemic adjuvants capable of eliciting strong immune responses to themselves and to unrelated coadministered antigens (8, 13, 33, 34, 48, 57). Use of these enterotoxins as mucosal adjuvants in human vaccines has been inhibited by their toxic activity. Several strategies have been developed to reduce or eliminate the intrinsic toxicity of the type I enterotoxins to facilitate their use as human adjuvants. In early attempts, recombinant B pentamers, due to the absence of the A polypeptide, were used as potential adjuvants. Results of those experiments have been varied, in that the pentamer sometimes potentiated immune responses to a coadministered antigen and at other times was ineffective (1, 24, 39, 64, 67). Others have attempted to use recombinant chimeras in which the toxic A polypeptide is genetically replaced with the antigen (21, 26, 32). It is likely that these chimeras increase immune responses to the fused antigen not only via adjuvant stimulation, but also by facilitating antigen uptake and processing. Detoxified CT and LT-I enterotoxins have also been engineered by genetically replacing amino acids in the A polypeptides which are critical for enzymatic activity with alternative amino acids. Those mutant enterotoxins, including CT(S61F), CT(E112K), LT(R7K), LT(H44A), LT(S63K), LT(A72R), LT(E112K), and LT(R192G), have little or no detectable enzymatic activity, yet retain adjuvant activity (2, 5, 9, 10, 12, 19, 25, 40, 45, 46, 50, 59, 66). CT(S61F) and CT(E112K) harbor single amino acid substitutions in the ADP-ribosyltransferase active center and lack ADP-ribosyltransferase activity and diarrheagenicity (66). Both mutant enterotoxins are effective adjuvants and are comparable to their wild-type (wt) CT when given parenterally (65) or nasally (20). On the other hand, it was first shown that the LT(E112K) mutant enterotoxin, which contains a single amino acid substitution in the ribosyltransferase active center (54), was nontoxic and also lacked adjuvanticity when administered orally (30). A recent study suggested that this mutant enterotoxin is an effective adjuvant when administered by the nasal route (25). In addition, LT(R7K) (12) and LT(R192) (44) were reported to be nontoxic or to display limited toxicity and retain adjuvant properties when administered by the nasal or oral routes, respectively (10, 12, 25). In addition, another LT mutant enterotoxin, designated LT(S61K), was shown to be without toxicity and a poor mucosal adjuvant (11, 44). CT and LT-I have also been detoxified by engineering amino acid substitutions at sites within the B polypeptides that are essential for GM1 binding. In these cases, it was shown that binding to GM1 was essential for immunomodulation, since these mutant enterotoxins did not exhibit adjuvant activity (16, 51).
Although several mutant enterotoxins with appropriate nontoxic phenotypes have been engineered (6, 7), the requirements of toxicity or ganglioside-binding activity for adjuvant activity have not been evaluated for LT-IIa or LT-IIb. Replacement of threonine by isoleucine at amino acid position 34 of LT-IIa and replacement of threonine by isoleucine at position 13 of LT-IIb abolishes the binding activities of the enterotoxins for their specific ganglioside receptors (6, 7). In this investigation, the adjuvant activities of LT-IIa and LT-IIb and of their respective single-point substitution mutants [e.g., LT-IIa(T34I) and LT-IIb(T13I)] were evaluated. The results of these experiments indicate that binding of LT-IIa and LT-IIb to their major ganglioside receptors is not essential for their immunomodulatory activities.
MATERIALS AND METHODS
Engineering and purification of His-tagged enterotoxins.
To facilitate subsequent cloning, a redundant HindIII restriction site in pTDC100 (7) and a SacI restriction site in pTDC200 (6) were removed by partial digestion of the plasmids with SacI or HindIII followed by blunting the digested sites with Klenow fragment and religation with T4 DNA ligase. The modified plasmids were denoted pTDC100ΔH and pTDC200ΔS.
To engineer a His-tagged version of LT-IIa, a fragment encoding a portion of the A polypeptide and the B polypeptide was PCR amplified from pTDC400 (6) by using the synthetic oligonucleotides 5′-GATGGGATCCTTGGTGTGCATGGAGAAAG-3′ and 5′-AAATAAACTAGTTTAGTGGTGGTGGTGGTGGTGTGACTCTCTATCTAATTCCAT-3′ (BcuI site is underlined; His codons are double underlined) as primers. PCR conditions were the following: 30 cycles of denaturation at 95°C for 45 s, annealing at 44°C for 45 s, and extension at 72°C for 2 min. After digestion with BcuI and with SacI (internal to the gene encoding the A polypeptide), the PCR fragment was substituted for the SacI/BcuI fragment of pTDC200ΔS. The new plasmid encoding the LT-IIa holotoxin with a His-tagged B polypeptide was denoted pHN4. A similar process was performed, with the exception that pTDC400 (T34I) was used as the PCR template, to produce pHN6, a plasmid encoding the mutant LT-IIa(T34I) holotoxin containing His-tagged B polypeptide harboring a threonine-to-isoleucine substitution at amino acid position 34 (6).
To engineer a His-tagged version of LT-IIb, a fragment encoding the genes for A and B polypeptides, was PCR amplified from pTDC100 by using the synthetic oligonucleotides 5′-CGGGATCCATGCTCAGGTGAG-3′ (BamHI site underlined) and 5′-GGAATTCTTAGTGGTGGTGGTGGTGGTGTTCTGCCTCTAACTCGA-3′ (EcoRI site underlined and His codons double underlined). PCR conditions were the following: 30 cycles of denaturation at 95°C for 45 s, annealing at 44°C for 45 s, and extension for 2 min. After digestion with BamHI/EcoRI, the PCR fragment was ligated into pBluescript II KS(+) (Stratagene, La Jolla, Calif.) at the BamHI/EcoRI sites to produce pHN1, which encoded LT-IIb holotoxin with a His-tagged B polypeptide. To engineer the His-tagged version of the T13I mutant of LT-IIb, a fragment was PCR amplified from pTDC700 (T13I) (2) using a synthetic KS primer (Stratagene) and the synthetic oligonucleotide 5′-GGAATTCTTAGTGGTGGTGGTGGTGGTGTTCTGCCTCTAACTCGA-3′ (EcoRI site underlined) as primers. After digestion of the PCR fragment with HindIII/EcoRI, the fragment was substituted for the HindIII/EcoRI fragment of PTDC100ΔH to produce pHN2 encoding the mutant LT-IIb holotoxin containing a His-tagged B polypeptide with a threonine-to-isoleucine substitution at amino acid 13.
All plasmids were introduced into E. coli DH5αF′Kan (Life Technologies, Inc., Gaithersburg, Md.). Expression of recombinant holotoxins was induced by isopropyl-β-d-thiogalactoside, and the proteins were extracted from the periplasmic space by polymyxin B treatment as previously described (33). Periplasmic protein extracts were precipitated by addition of ammonium sulfate to 60% saturation (390 g/liter). The precipitate was collected by centrifugation and dissolved in phosphate-buffered saline (PBS; pH 7.4). The dissolved precipitate was dialyzed overnight in PBS to remove ammonium sulfate, after which the recombinant proteins were purified by means of affinity chromatography using a His·Bind resin column (Novagen, Madison, Wis.) according to a protocol provided by the manufacturer. Analysis by gel filtration chromatography of the peak fractions indicated that the holotoxins were contaminated with less than 5% free B pentamer. After the fractions from nickel affinity chromatography were passed through a 0.45-μm-pore-size syringe filter, holotoxin was separated from the residual amounts of contaminating B pentamer by means of preparative gel filtration chromatography (Sephacryl-100; Pharmacia, Piscataway, N.J.) using an ÄKTA-FPLC apparatus (Pharmacia). After concentration of the peak fractions with Vivaspin concentrators (Viva Science, Hanover, Germany), recombinant proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for purity and by quantitative Limulus amebocyte lysate assay (Charles River Endosafe, Charleston, S.C.) to measure incidental endotoxin contamination. All holotoxin preparations were essentially free of lipopolysaccharide (LPS; <0.03 ng/μg of protein). AgI/II used in this study was purified from the culture supernatants of Streptococcus mutans as previously described (52). CT was purchased from List Biological Laboratories (Campbell, Calif.).
Ganglioside-dependent ELISA.
Binding of LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I) to their ganglioside receptors was measured as previously described (6, 7) with some modifications. Briefly, polyvinyl 96-well enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at 4°C with 10 ng of GT1b, GQ1b, GM2, GM3, GM1, GD1a, GD1b, or GD2, or with a ganglioside mixture (Matreya, State College, Pa., and Sigma Chemical Company, St. Louis, Mo.), or with 3.0 μg of goat anti-LT-IIa or goat anti-LI-IIb antibody/ml. After washing and blocking of nonspecific binding with 10% horse serum, 50 μl of a 1.0-μg/ml solution of LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I) was added to wells and plates were incubated for 3 h at 37°C. Unbound enterotoxins were washed away and 50 μl of rabbit anti-LT-IIa or LT-IIb (diluted 1:5,000 in PBS plus 10% horse serum) was added to the wells. Plates were incubated for another 2 h at 37°C and washed to remove unbound antibodies. Fifty microliters of 1.0-μg/ml alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary antibody was added to each well. Plates were incubated for 1 h at 37°C, after which wells were washed and immediately developed with nitrophenyl phosphate (Amresco, Solon, Ohio) diluted in diethanolamine buffer (100 ml of diethanolamine, 1 mM MgCl2, and deionized H2O to 1 liter; pH 9.8). Color reactions were terminated by adding 50 μl of 2.0 M NaOH to each well. The optical density of the color reaction was measured at 405 nm.
Toxicity bioassay.
The toxicity of purified enterotoxins was measured using Y1 adrenal cells (ATCC CCL-79), a cell line which is acutely sensitive to heat-labile enterotoxins. Briefly, mouse Y1 adrenal cells were cultured to 50% confluence in 96-well tissue culture plates in F-12 medium supplemented with 30% horse serum and 10% fetal bovine serum at 37°C and in an atmosphere of 5% CO2. One microgram of CT, LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I) per well was added to the Y1 cell cultures and diluted in a twofold dilution series across the plate. Plates were incubated at 37°C in an atmosphere of 5% CO2 and examined for 48 h to monitor rounding of cells, which is an indicator of toxicity. One unit of toxicity was defined as the smallest concentration of enterotoxin that induced rounding of 75 to 100% of the cultured mouse Y1 adrenal cells.
Animals and immunizations.
Female BALB/c mice, 11 to 12 weeks of age, were immunized by the intranasal (i.n.) route. Groups of eight mice were immunized three times at 10-day intervals with AgI/II (10 μg) alone or with AgI/II in combination with 1 μg of CT, LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I). Immunizations were administered in a standardized volume of 10 μl, applied slowly to both external nares. At day 203 after initial immunization, all groups were reimmunized i.n. with 5 μg of AgI/II alone. All animal experiments were approved by the Institutional Animal Care and Use Committee at the State University of New York at Buffalo.
Collection of secretions and sera.
Samples of serum, saliva, and vaginal washes were collected from individual mice 2 days before the initial immunization (day zero) and at 18, 28, 42, 60, and 175 days after the primary immunization. Saliva samples were collected with a micropipetter after stimulation of salivary flow by injecting each mouse intraperitoneally with 5 μg of carbachol (Sigma). Vaginal washes were collected by flushing the vaginal vault three times with 50 μl of sterile PBS. Serum samples were obtained following centrifugation of blood collected from the tail vein by use of a calibrated capillary tube. Mice were sacrificed at day 217, and blood was collected after cardiac puncture using 20-gauge syringe needles. Mucosal secretions and serum samples were stored at −70°C until assayed for antibody activity.
Antibody analysis.
Levels of isotype-specific antibodies in saliva, sera, and vaginal washes were measured by ELISA. Polystyrene microtiter plates (96-well; Nunc, Roskilde, Denmark) were coated overnight at 4°C with AgI/II (5 μg/ml), LT-IIa (3 μg/ml), LT-IIb (3 μg/ml), or CT (3 μg/ml). To determine total Ig isotype concentrations, plates were coated with goat anti-mouse Ig isotype-specific antibodies (Southern Biotechnology Associates, Birmingham, Ala.). Serial twofold dilutions of serum or secretion samples were added in duplicate, and plates were incubated overnight at 4°C. Plates were washed with PBS containing 0.1% Tween 20 (PBS-Tween) and incubated at room temperature (RT) with the appropriate alkaline phosphatase-conjugated goat anti-mouse Ig isotype-specific antibodies (Southern Biotechnology). Plates were washed and developed with nitrophenyl phosphate, as described previously. Concentrations of antibodies and total IgA levels were calculated by interpolation of calibration curves generated by using a mouse Ig reference serum (ICN Biomedicals, Aurora, Ohio). Mucosal IgA responses are reported as the percentage of specific antibody IgA in total IgA to compensate for variations arising from salivary flow rate and dilution of secretions. All enterotoxins were able to induce antienterotoxin serum IgG. LT-IIa(T34I) induced lower levels of serum IgG than its wt, while LT-IIb(T13I) induced an equivalent level of serum IgG as its wt (data not shown).
Isolation of lymphoid cells.
Superficial cervical lymph nodes (CLN) were excised as previously described (33). CLN and spleens were teased apart with syringe pistons, dispersed through a 70-μm nylon mesh screen, and passed twice through a 26-gauge syringe needle to obtain a single-cell suspension. Cell suspensions were filtered through nylon mesh to remove tissue debris and centrifuged through Ficoll-Hypaque 1083 (Sigma) to remove erythrocytes and dead cells. All preparations were washed twice and suspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Total cell yield and viability were enumerated in a hemacytometer using trypan blue (Sigma) staining.
Cytokine assays.
Spleen and CLN lymphoid cells were plated in triplicate at 5 × 105 cells per well in flat-bottomed, 96-well tissue culture plates (Nunc) and cultured for 4 days in the presence of concanavalin A (2.5 μg/ml) or AgI/II (5 μg/ml) or in the absence of stimulus. Supernatants were collected after centrifugation and stored at −70°C until assayed for the presence of cytokines. The levels of interleukin-4 (IL-4) and gamma interferon (IFN-γ) in culture supernatants were determined by a cytokine-specific ELISA according to the manufacturer's protocol (Pharmingen, San Diego, Calif.). Briefly, 96-well culture plates were coated with monoclonal anti-IL-4 or anti-IFN-γ (2 μg/ml) and incubated overnight at 4°C. Plates were washed with PBS-Tween and blocked to limit nonspecific binding with 10% FBS in PBS for 1 h at RT. After washing the plates, supernatants were serially diluted in 10% FBS in PBS and added to the wells. A standard curve was generated by using serial dilutions of recombinant IL-4 (500 pg/ml) or IFN-γ (2,000 pg/ml). All serial dilutions were incubated at 37°C for 3 h followed by washing with PBS-Tween. Secondary antibodies consisted of peroxidase-labeled anti-IL-4 or biotinylated anti-IFN-γ. In assays using biotinylated antibodies, a 1:1,000 dilution of horseradish peroxidase-conjugated streptavidin in 10% FBS in PBS was added to the appropriate wells. After incubation at RT for 2 h, reactions were developed for 20 min with o-phenylenediamine-H2O2 substrate and terminated by addition of 1.0 M H2SO4. The color reaction was measured at 490 nm.
Binding of enterotoxins to CLN lymphoid cells.
A total of 106 cells obtained from CLN of naïve mice were treated in vitro with 1.0 μg of LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I). After incubation on ice for 10 min, cells were washed and subsequently incubated on ice for 10 min with a pretitration concentration of polyclonal rabbit antibody to LT-IIa or LT-IIb. After washing, cells were treated with phycoerythrin-conjugated goat anti-rabbit IgG (0.5 μg/ml) and with fluorescein isothiocyanate-conjugated monoclonal antibody to CD3, CD4, CD8, B220, or CD11b. After incubation for 10 min on ice, cells were washed and then incubated with 1.0 μg of propidium iodide/ml. CD16/CD32 antibodies were used to block Fc receptor following the manufacturer's instructions. Enterotoxin-binding mutants (1.0 μg), isotype-matched fluorochrome-labeled antibodies, and specific antienterotoxin rabbit sera were used as controls to set detection limits. Data acquisition and analysis were performed using a FACScalibur flow cytometer (Beckton-Dickinson, Franklin Lakes, N.J.) and the CellQuest software (Beckton-Dickinson).
Detection of cAMP.
cAMP production was measured in mouse macrophage RAW264.7 cells (ATCC TIB-71) as a relevant lymphoid cell type. Briefly, mouse macrophage RAW264.7 cells (5 × 107 per well) were cultured in triplicate for 24 h in 24-well tissue culture plates at 37°C and in an atmosphere of 5% CO2 in Dulbecco's modified Eagle medium supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 10% fetal bovine serum. Culture medium was removed and replaced with fresh culture medium with or without 1.0 μg of CT, LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I)/ml. After incubation at 37°C for 4 h, enterotoxin-treated cells were extracted with 200 μl of 0.1 M HCl for 20 min at RT, scraped from the wells, and centrifuged to clear the extracts of cells and cell debris. Levels of cAMP in the extracts were measured twice using a cAMP enzyme immunoassay kit (Cayman Chemical Co., Ann Arbor, Mich.) according to the manufacturer's protocols.
Statistical analysis.
Analysis of variance and the Tukey multiple-comparison test were used for multiple comparisons. Unpaired t tests with Welch correction were performed to analyze differences between two groups. Statistical analyses were performed using InStat (GraphPad, San Diego, Calif.). Statistical differences were considered significant at the P < 0.05 level.
RESULTS
Purification of wt and mutant LT-IIa and LT-IIb.
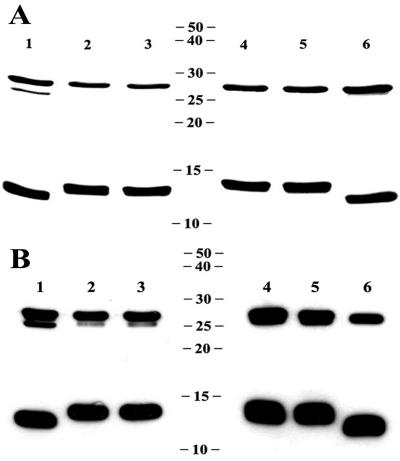
To facilitate their purification, recombinant LT-IIa, LT-IIa(T34I), LT-IIb, and LT-IIb(T13I) holotoxins were engineered with His tags fused to the carboxyl end of the B pentamers. His-tagged holotoxins were purified from periplasmic extracts of recombinant E. coli by a two-step chromatographic protocol. In the first step, holotoxins and B pentamers were isolated from periplasmic extracts by using nickel affinity chromatography. Holotoxins were separated from the contaminating B pentamers by subsequent gel filtration chromatography. Recombinant wt and mutant holotoxins were examined by SDS-PAGE and immunoblotting using polyclonal antibodies directed toward LT-IIa or LT-IIb to demonstrate that each enterotoxin was purified to apparent homogeneity (Fig. 1). Since experiments to investigate adjuvant properties would be confounded by inadvertent LPS contamination of the purified holotoxins, Limulus amebocyte lysate assays were used to confirm that the purified wt and mutant holotoxins contained less than 0.03 ng of LPS per μg of protein, a level at which LPS-associated immune effects are undetectable in the mouse model (64).
FIG. 1.
(A) SDS-PAGE of purified non-His-tagged LT-IIa, His-tagged LT-IIa, and His-tagged LT-IIa(T34I) (lanes 1, 2, and 3, respectively), and His-tagged LT-IIb, His-tagged LT-IIb(T13I), and non-His-tagged LT-IIb (lanes 4, 5, and 6) dissociated into the A subunit (∼28 kDa) and B subunit monomers (∼12.5 and 13.5 kDa for non-His-tagged and His-tagged B subunits, respectively). (B) Western blot of non-His-tagged LT-IIa, His-tagged LT-IIa, and His-tagged LT-IIa(T34I) (lanes 1, 2, and 3, respectively), and His-tagged LT-IIb, His-tagged LT-IIb(T13I), and non-His-tagged LT-IIb (lanes 4, 5, and 6, respectively) probed with rabbit polyclonal antibodies to LT-IIa and LT-IIb, respectively. Molecular masses are noted in kilodaltons.
Binding of wt and mutant LT-IIa and LT-IIb to gangliosides.
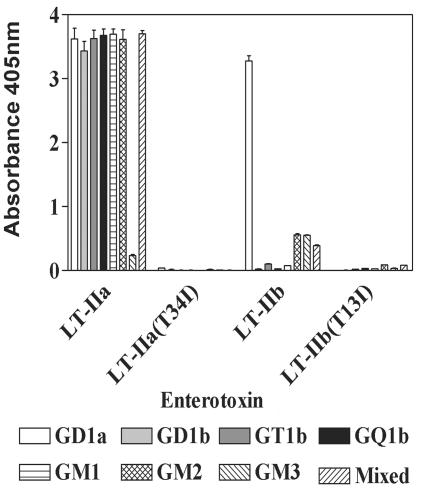
Inhibition of binding of LT-IIa(T34I) and LT-IIb(T13I) to gangliosides was originally defined using periplasmic extracts from recombinant strains of E. coli as crude sources of the mutant enterotoxins (15). To confirm that the ganglioside-binding activities of the purified mutant enterotoxins were equivalent to those of the mutant enterotoxins in the crude extracts, binding of the purified wt and mutant enterotoxins for various gangliosides was measured by ganglioside-specific ELISA (6, 7) (Fig. 2). As expected, LT-IIa bound to gangliosides GD1b, GM1, GT1b, GQ1b, GD2, GD1a, and GM3 (15). LT-IIa(T34I), however, exhibited no detectible affinity for those gangliosides (6). LT-IIb bound strongly to GD1a and with lower affinity to GM2 and GM3 (7). In contrast, LT-IIb(T13I) had no detectable binding affinity above background for GD1a, GM2, or GM3.
FIG. 2.
Binding of LT-IIa, LT-IIa(T34I), LT-IIb, and LT-IIb(T13I) to various gangliosides. Polyvinyl plates were coated with 10 ng of purified ganglioside or a mixture of gangliosides. Enterotoxins were allowed to bind to ganglioside-coated plates followed by probing with rabbit polyclonal antibodies. Plates were developed using alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibody and nitrophenyl phosphate.
Toxicity of LT-IIa(T34I) and LT-IIb(T13I).
Prior results using crude periplasmic extracts from recombinants expressing LT-IIa(T34I) and LT-IIb(T13I) indicated that LT-IIa(T34I) and LT-IIb(T13I) were severely attenuated in toxicity (6, 7). To confirm those results using purified wt and mutant holotoxins, Y1 adrenal cell toxicity assays were repeated. Comparisons of the toxicities revealed that CT was the most toxic of the five enterotoxins. Only 0.49 ng of CT was sufficient to induce rounding of 100% of Y1 adrenal cells within a test well. LT-IIa was 16-fold less toxic, requiring 15.65 ng of enterotoxin to cause the same effect. LT-IIa(T34I) exhibited no detectible toxic activity at levels up to 1.0 μg of enterotoxin. Only after 24 h of incubation with LT-IIa(T34I) was any toxicity detected, i.e., 10% of the cells in the well containing 1.0 and 0.5 μg of enterotoxin demonstrating a rounding morphology. Y1 adrenal cells had to be incubated with eight times the amount of LT-IIb (0.49 versus 3.91 ng) to elicit the same degree of toxicity for Y1 adrenal cells as by CT. In comparison, LT-IIb(T13I) was 256-fold less toxic than LT-IIb. In conclusion, the LT-IIa and LT-IIb were significantly less toxic than CT by the Y1 adrenal cell bioassay, and each of the respective mutant enterotoxins was significantly less toxic than its wt parent enterotoxin.
Mucosal adjuvant activities of LT-IIa(T34I) and LT-IIb(T13I).
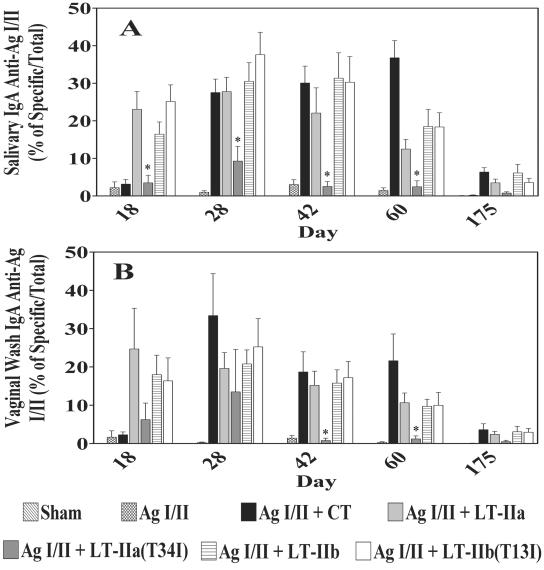
To compare the adjuvant activities of the mutant enterotoxins with the wt enterotoxins, mice were immunized i.n. with AgI/II, a protein antigen from S. mutans (52), in the presence or absence of LT-IIa or LT-IIb. CT was utilized as an external control, as the mucosal adjuvant activities of this enterotoxin for AgI/II have been well established (33, 64). Initial immunizations were followed by booster immunizations at day 10 and at day 20. Saliva and vaginal secretions, obtained at intervals up to 175 days after the initial immunization, were analyzed for AgI/II-specific IgA antibodies as a measure of mucosal adjuvant activity of the enterotoxins.
Immunization with AgI/II alone did not elicit a strong salivary IgA response to the antigen (Fig. 3A). In contrast, in mice immunized with AgI/II in the presence of LT-IIa, LT-IIb, or CT high levels of AgI/II-specific IgA were detected in the saliva after the second immunization (day 18), peaked at day 28, and persisted, yet declined, through day 175. At all time points, AgI/II-specific salivary IgA levels were 5- to 25-fold higher in mice administered AgI/II in the presence of either LT-IIa or LT-IIb. These results confirmed prior observations that LT-IIa and LT-IIb were strong mucosal adjuvants (33) with capacities for potentiating mucosal anti-AgI/II responses.
FIG. 3.
Salivary IgA (A) and vaginal IgA (B) antibody responses to AgI/II from mice after i.n. immunization with AgI/II alone or with LT-IIa, LT-IIa(T34I), LT-IIb, LT-IIb(T13I), or CT as adjuvant. Results are reported as the arithmetic means ± standard errors of means obtained from immunized mice (n = 6 to 8 mice per group). *, significant difference at P < 0.05 compared to LT-IIa.
When the salivary anti-AgI/II IgA responses of mice immunized with AgI/II plus LT-IIa(T34I) were measured, it was found that the mutant enterotoxin was capable of inducing a higher mean of anti-AgI/II IgA antibodies at day 28, but those values were not statistically significant (P > 0.05) from mice immunized with AgI/II alone due to high variation among mice (Fig. 3A). Salivary anti-AgI/II IgA responses of those mice were significantly different from the salivary anti-AgI/II IgA of mice immunized with AgI/II plus LT-IIa at days 18, 28, 42, and 60 (P < 0.05) but not at day 175 (P > 0.05). On the other hand, the adjuvant activity was unaffected by the mutation LT-IIb(T13I), which altered its ganglioside-binding activities. The salivary IgA responses to AgI/II for mice immunized with AgI/II plus LT-IIb and for mice immunized with AgI/II plus LT-IIb(T13I) were strong and statistically equivalent at all time points (P > 0.05) (Fig. 3A).
LT-IIa and LT-IIb when used as i.n. adjuvants were also capable of inducing strong immune responses to a coadministered antigen at distal mucosa (33). To determine whether mucosal adjuvant responses were potentiated at distal sites in these experiments, levels of AgI/II-specific IgA were measured in samples taken from the vaginal mucosa (Fig. 3B). Immunization with AgI/II in the absence of enterotoxin did not induce significant amounts of vaginal anti-AgI/II IgA at any time point. In all cases, however, mice administered AgI/II in the presence of LT-IIa, LT-IIb, or CT produced high levels of AgI/II-specific vaginal IgA in comparison to mice receiving only AgI/II (P < 0.05) (Fig. 3B) at days 28, 42, and 60. Vaginal IgA responses to AgI/II in those mice receiving an enterotoxin adjuvant peaked at day 28, slowly diminished at later time points, but persisted through day 60 and declined somewhat by day 175. As observed for salivary anti-AgI/II IgA, use of LT-IIa(T34I) as an i.n. mucosal adjuvant induced a higher mean vaginal anti-AgI/II IgA level than mice immunized solely with Ag I/II, indicating that the mutant enterotoxin retained some mucosal adjuvant activity. In contrast, mice immunized with AgI/II in the presence of LT-IIb(T13I) exhibited a level of vaginal anti-AgI/II which was equivalent to the levels of antigen-specific IgA induced by use of the wt LT-IIb as a mucosal adjuvant (Fig. 3B).
From these results, it was clear that the mucosal adjuvant activity of LT-IIa(T34I) was diminished by the loss of binding affinity for its known ganglioside receptors (e.g., GD1b, GM1, GT1b, GQ1b, GD2, GD1a, and GM3). In the case of LT-IIb(T13I), however, the mutation had little or no effect on mucosal adjuvant activity. The mucosal adjuvant activities of LT-IIb(T13I) for inducing antigen-specific IgA, surprisingly, were indistinguishable from the mucosal adjuvant activities of wt LT-IIb.
Systemic adjuvant activity of LT-IIa(T34I) and LT-IIb(T13I).
i.n. administration of LT-IIa, LT-IIb, and CT also induces strong circulating antibody responses to coadministered antigens (8, 33). To examine whether mucosal immunomodulatory activities of LT-IIa(T34I) and LT-IIb(T13I) had the capacity to potentiate serum antibody responses, antigen-specific IgA and antigen-specific IgG were measured in serum samples taken at various time points from mice immunized i.n. with AgI/II in the presence or absence of mutant or wt enterotoxins.
As expected, both LT-IIa and LT-IIb potentiated anti-AgI/II serum IgA after i.n. administration with AgI/II (Fig. 4). As observed for secretory IgA in saliva and vaginal washes, serum IgA (Fig. 4A) responses to AgI/II in mice receiving LT-IIa or LT-IIb as mucosal adjuvants peaked on day 28 and persisted through day 175. In comparison to the serum IgA levels in mice immunized solely with AgI/II, serum IgA responses in mice immunized with AgI/II plus LT-IIa (P < 0.01), AgI/II plus LT-IIb (P < 0.001), AgI/II plus LT-IIb(T13I) (P < 0.001), and AgI/II plus CT (P < 0.001) were significantly elevated at day 28. Mice receiving LT-IIa(T34I) as a mucosal adjuvant had only a slight elevation in serum IgA level in comparison to mice administered only AgI/II (P < 0.05), but this elevation was also significantly diminished from that induced by wt LT-IIa at day 28 (P < 0.01) and at days 42, 60, and 175 (P < 0.05). The conclusion from these experiments was that LT-IIa(T34I) was a weak adjuvant for eliciting serum IgA after i.n. application. In contrast, and similar to the patterns observed for salivary and vaginal IgA production, wt LT-IIb and LT-IIb(T13I) had equivalent capacities to induce antigen-specific serum IgA (P > 0.05) when used as i.n. adjuvants at all time points.
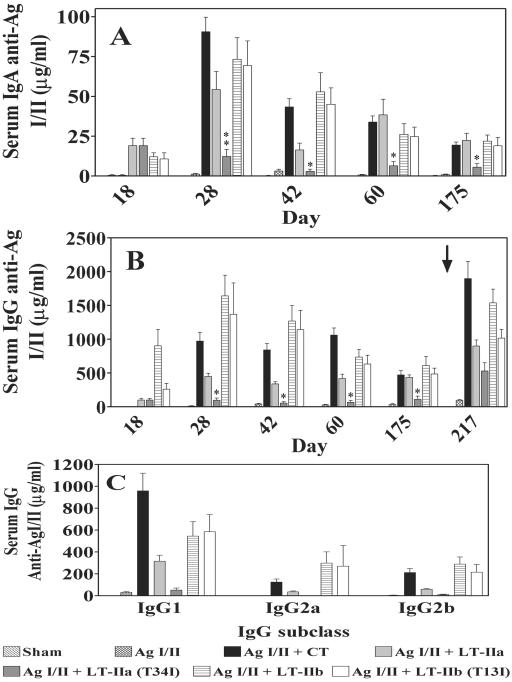
FIG. 4.
Serum IgA (A), IgG (B), and IgG (C) subclass antibody responses to AgI/II after i.n. immunization of mice with AgI/II alone or with LT-IIa, LT-IIa(T34I), LT-IIb, LT-IIb(T13I), or CT as adjuvant. Results are reported as the arithmetic means ± standard errors of means of immunized mice (n = 6 to 8 mice per group). IgG subclasses were examined from mice at day 28. The arrow indicates the time point at which the third booster immunization with 5 μg of AgI/II was administered (day 203). * and **, significant differences at P < 0.05 and < 0.01, respectively, compared to LT-IIa.
At all time points tested, serum IgG responses to AgI/II were also elevated in mice immunized with AgI/II plus LT-IIa (P < 0.05), AgI/II plus LT-IIb (P < 0.001), and AgI/II plus LT-IIb(T13I) (P < 0.001) compared to mice immunized with AgI/II alone (Fig. 4B). No significant differences in serum IgG responses were observed between mice immunized with AgI/II and mice immunized with AgI/II plus LT-IIa(T34I), although the mean value of the antibody responses was higher in mice immunized with LT-IIa(T34I) as an adjuvant. Boosting with 5 μg of AgI/II alone at day 203 i.n. induced two- to fivefold increases in serum IgG to AgI/II at day 217 in mice administered LT-IIa and LT-IIb compared to the levels of anti-AgI/II IgG at day 175, demonstrating that these enterotoxins stimulated antigen-specific memory responses. When the mice receiving mutant enterotoxins were examined, it was found that there were no significant differences in serum IgG to AgI/II at day 217 between mice immunized with AgI/II plus LT-IIb and mice immunized with AgI/II plus LT-IIb(T13I). More surprisingly, there was also no statistical difference in AgI/II-specific serum IgG produced in mice immunized with AgI/II plus LT-IIa and mice immunized with AgI/II plus LT-IIa(T34I). Thus, while LT-IIa(T34I) had only a minor ability to potentiate anti-AgI/II immune responses shortly after the initial series of immunizations, this mutant enterotoxin was capable of priming for the recall of antigen-specific immune responses at later time points after boosting.
Serum IgG subclass responses.
Based on IgG subclass distribution, LT-IIb stimulates a more balanced T helper 1 (Th1)/Th2 immune response than either CT or LT-IIa (33). To determine if the mutant enterotoxins stimulated IgG subclass distribution similarly or differently from those stimulated by their wt parent enterotoxins, the concentrations of AgI/II-specific IgG1, IgG2a, and IgG2b were determined in the serum obtained at day 28. Immunization with AgI/II alone induced low levels of IgG1, IgG2a, and IgG2b (Fig. 4C). Levels of IgG subclasses to AgI/II were elevated when AgI/II was coadministered with LT-IIa, LT-IIb, and LT-IIb(T13I), but not when AgI/II was coadministered with LT-IIa(T34I). Consistent with those results, the level of IgG1 was significantly increased in mice immunized with AgI/II plus CT in comparison to the levels of IgG2a and IgG2b in mice immunized with AgI/II alone. LT-IIa induced a pattern of AgI/II-specific IgG subclass elevation similar to that with CT, although the levels were much reduced. IgG1 was the most abundant IgG subclass in mice immunized with AgI/II plus LT-IIa, while IgG2a and IgG2b levels were considerably lower. When AgI/II was coadministered with LT-IIb or with LT-IIb(T13I), the levels of IgG1, IgG2a, and IgG2b were significantly increased over those observed in mice immunized solely with AgI/II (Fig. 4C). These data indicate that LT-IIb(T13I) induced a more balanced Th1/Th2 immune response than either LT-IIa or CT and that was similar to the pattern observed when LT-IIb was used as an i.n. adjuvant.
Cytokine production.
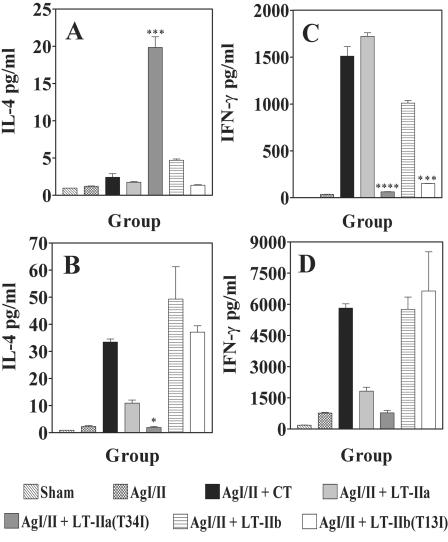
To complement the IgG subclass distribution experiments, expression patterns for IFN-γ and IL-4 were measured in lymphoid cells obtained from the draining superficial CLN and from the spleens of immunized mice after in vitro AgI/II stimulation (Fig. 5). Only low levels of IL-4 were detected in culture supernatants of CLN lymphoid cells of all groups, with the exception of culture supernatants of CLN lymphoid cells isolated from mice in which LT-IIa(T34I) was used as the i.n. adjuvant (P < 0.001) (Fig. 5A). In contrast, IL-4 was detectable in significantly higher concentrations in culture supernatants of splenic lymphoid cells isolated from mice immunized with AgI/II plus LT-IIa (P < 0.05), AgI/II plus LT-IIb (P < 0.001), AgI/II plus LT-IIb(T13I) (P < 0.01), or with AgI/II plus CT (P < 0.01) compared to splenic cells from mice immunized with AgI/II without adjuvant or with LT-IIa(T34I) as an adjuvant (Fig. 5B). Very high concentrations of IFN-γ were detected in culture supernatants of CLN lymphoid cells isolated from mice receiving LT-IIa, LT-IIb, or CT as adjuvants compared to mice immunized with AgI/II alone (P < 0.0001) (Fig. 5C). IFN-γ concentrations were significantly higher in culture supernatants of CLN lymphoid cells isolated from mice immunized with AgI/II in the presence of LT-IIa (P < 0.0001) and LT-IIb (P < 0.001) than in mice immunized with AgI/II in the presence of LT-IIa(T34I) or LT-IIb(T13I), respectively (Fig. 5C). Higher levels of IFN-γ were also detected in culture supernatants of splenic lymphoid cells isolated from mice immunized with AgI/II and CT, LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(I13I) (Fig. 5D). IFN-γ concentrations were significantly higher in culture supernatants of splenic lymphoid cells isolated from mice administered LT-IIa (P < 0.05), LT-IIb (P < 0.001), LT-IIb(T13I) (P < 0.001), or CT (P < 0.001) as adjuvant. There was no significant difference between IFN-γ concentrations in culture supernatants of splenic lymphoid cells isolated from mice administered LT-IIa and those from mice administered LT-IIa(T34I) as adjuvants, or between IFN-γ concentrations in culture supernatants of splenic lymphoid cells isolated from mice immunized with LT-IIb and those from mice immunized with LT-IIb(T13I) (Fig. 5D).
FIG. 5.
Production of IFN-γ and IL-4 by AgI/II-specific lymphoid cells isolated from CLN (A and C) and spleens (B and D) of BALB/c mice immunized i.n. with AgI/II alone or with LT-IIa, LT-IIa(T34I), LT-IIb, LT-IIb(T13I), or CT at a point 40 days after the third immunization (day 60). Cells were stimulated in vitro for 4 days with 5 μg of AgI/II. Results are reported as the arithmetic means ± standard errors of means (n = 3). (A) ***, significant difference at P < 0.001 compared to LT-IIa. (B) *, significant difference at P < 0.05 compared to LT-IIa. (C) ****, significant difference at P < 0.0001 compared to LT-IIa; ***, significant difference at P < 0.001 compared to LT-IIb.
Binding of wt and mutant LT-IIa and LT-IIb to lymphocytes.
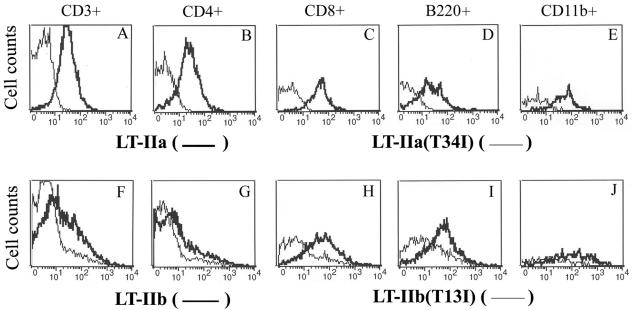
In vitro binding experiments revealed that LT-IIb(T13I) had little or no detectable binding affinity for ganglioside receptors. Furthermore, LT-IIb(T13I) exhibits extremely low toxicity for Y1 adrenal cells (7), indicating that the mutant enterotoxin is incapable of inducing production of cAMP, a potent intracellular messenger for a variety of metabolic processes. Thus, it was difficult to envision how LT-IIb(T13I) stimulated lymphoid cells to produce potent anti-AgI/II responses without interacting in some manner with lymphoid cells. It was hypothesized, therefore, that LT-IIb(T13I) did, indeed, interact with one or more types of lymphoid cells. To determine whether LT-IIb(T13I) had residual binding affinity for lymphoid cells, cells from the CLN of naïve mice were incubated with wt LT-IIb or with LT-IIb(T13I) and subsequently examined by flow cytometry for bound enterotoxin (Fig. 6). LT-IIb bound to 44.9% of total T cells, 25.3% of CD4+ T cells, 83.2% of CD8+ T cells, 84.0% of B cells, and 91.5% of macrophages (Fig. 6F to J). Lesser numbers of all four lymphoid cell types were bound by LT-IIb(T13I), i.e., 13% of total T cells, 8.6% of CD4+ T cells, 20.9% of CD8+ T cells, 38.4% of B cells, and 44.4% of macrophages (Fig. 6F to J). In contrast, there was no detectable binding of LT-IIa(T34I) to lymphoid cells (Fig. 6A to E). The binding of the wt enterotoxins to different lymphocytes could be inhibited by preincubating the enterotoxins with high concentrations of their known ganglioside receptors. Preincubation of LT-IIb(T13I) had no effect on its ability to bind to lymphocytes (data not shown).
FIG. 6.
Binding of wt and mutants LT-IIa and LT-IIb to lymphoid cells isolated from CLN of naïve BALB/c mice. Histograms were gated on CD3+ (total T cells), CD4+ (helper T cells), CD8+ (cytotoxic T cells), B220+ (B cells), or CD11b+ (macrophages). Dead cells were excluded by propidium iodide staining. Light lines, binding patterns of LT-IIa(T34I) and LT-IIb(T13I); bold lines, binding patterns of LT-IIa and LT-IIb. A shift to the left in fluorescent intensity indicates a decrease or absence of binding of an enterotoxin to the cells.
These cytometric results can be explained by hypothesizing that LT-IIb(T13I) retains binding activity for one or more unknown gangliosides (or glycoproteins) on the surface of some lymphoid cells. Our current hypothesis is that binding of LT-IIb to this unknown receptor on T cells, B cells, and/or macrophages initiates the cellular and molecular events that are required for potentiated immune responsiveness. Experiments are ongoing to identify that putatively functional receptor.
cAMP production in macrophages treated with LT-IIa(T34I) and LT-IIb(T13I).
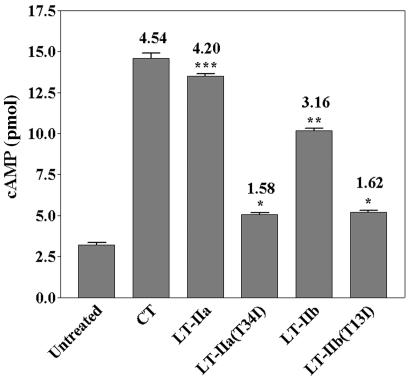
Although LT-IIa(T34I) and LT-IIb(T13I) had no detectable binding in vitro to their major ganglioside receptors (Fig. 2) and exhibited extremely low toxicity for Y1 adrenal cells, our observations that LT-IIb(T13I) bound to lymphoid cells prompted us to determine whether LT-IIb(T13I) and LT-IIa(T34I) retained the capacity to induce cAMP in lymphocytes. Binding assays demonstrated that LT-IIa(T34I) and LT-IIb(T13I), and their respective wt enterotoxins, bound to RAW 264.7 cells, a mouse macrophage cell line (data not shown) in a similar pattern to CLN macrophages (Fig. 6). To measure cAMP, 5.0 × 107 cells were incubated for 4 h in the presence or absence of each enterotoxin. The endogenous level of cAMP in untreated RAW264.7 cells was 3.22 ± 0.13 pmol. As expected, after incubation with enterotoxins, it was found that LT-IIa, LT-IIb, and CT induced intracellular accumulation of cAMP in RAW 264.7 cells (13.51 ± 0.17, 10.16 ± 0.20, and 14.59 ± 0.42 pmol, respectively), levels which were 3.2- to 4.5-fold higher than those observed in untreated cells (Fig. 7). Cells treated with either of the mutant enterotoxins, however, exhibited only slightly elevated amounts of cAMP (1.6-fold) in comparison to the amount of cAMP in untreated cells. The amount of cAMP in cells treated with LT-IIa(T34I) was significantly less than the amount of cAMP induced by treatment of the macrophages with wt LT-IIa (5.20 ± 0.15 versus 13.51 ± 0.17 pmol; P < 0.001). LT-IIb(T13I), which is unable to bind in vitro to its known ganglioside receptors and which exhibited little detectable binding to T cells, B cells, or to macrophages from the CLN (Fig. 6), retained a minor capacity to induce production of cAMP in RAW264.7 cells. LT-IIb(T13I) induced significantly less cAMP production than that induced by treatment with wt LT-IIa (5. 07 ± 0.16 versus 10.16 ± 0.20 pmol; P < 0.01) (Fig. 7). These data indicated that the capacity of the two mutant enterotoxins to elevate cAMP levels in RAW 264.7 cells was significantly reduced from the capacities of their respective wt enterotoxins and from CT.
FIG. 7.
Induction of cAMP production in macrophages after treatment with enterotoxin. cAMP was measured in RAW264.7 cells (5 × 107) after incubation for 4 h with LT-IIa, LT-IIa(T34I), LT-IIb, LT-IIb(T13I), or CT. Results are reported as the arithmetic mean values ± standard errors of means (n = 3). *, significant difference at P < 0.05 compared to untreated cells; **, significant difference at P < 0.01 compared to LT-IIb(T13I); ***, significant difference at P < 0.001 compared to LT-IIa(T34I). The fold increase of cAMP in the treated cells over the untreated cells is denoted at the top of the respective bars.
DISCUSSION
The results of this investigation demonstrate that the adjuvant activity of LT-IIa(T34I) was dramatically reduced in comparison to the adjuvant activity of wt LT-IIa. LT-IIa(T34I) was incapable of stimulating either mucosal IgA or systemic IgA and IgG responses at levels comparable to those induced when LT-IIa was used as an adjuvant. Moreover, the mucosal antigen-specific IgA and systemic IgG levels were somewhat higher at day 28 but virtually equivalent at days 18, 42, and 60 to those observed in mice administered AgI/II in the absence of adjuvant. These results are consistent with those obtained with LT-I in which a mutant [LT-IB(G33D)] with no binding affinity for GM1 ganglioside had little or no capacity for stimulating adjuvant activity, thus confirming the dependence of LT-I on GM1 binding for immunomodulation (16, 39). It is likely that loss of binding to GD1b, GD1a, and GM1 had a negative effect on the adjuvant activities of LT-IIa(T34I). In contrast, the adjuvant activity of LT-IIb(T13I) was not significantly altered by loss of binding affinity for GD1b, GM2, and GM3. It has been established that LT-IIb(T13I) has no significant in vitro toxic activity for several nonlymphoid cell lines (7). Experiments are being performed to determine if the mutant enterotoxin will intoxicate T cells, B cells, or macrophages. Nevertheless, the present results suggest that the ganglioside-binding and ADP-ribosylation activities of LT-IIb may not be required for LT-IIb or LT-IIb(T13I) to potentiate immune responses. There are, however, alternative models. The cytometry experiments show that LT-IIb(T13I) retains some binding activity for a receptor(s) on lymphoid cells, presumably other than GD1a, GM2, and GM3. In addition to GM1, LT-I binds to cell surface glycoproteins (41). If LT-IIb(T13I) does interact with receptors other than GD1a, GM2, and GM3, it is feasible that binding to those alternative receptors is the event which initiates enterotoxin-dependent immunomodulatory responses. In that case, the LT-IIb(T13I) mutant enterotoxin will be an important molecular tool for identifying and characterizing those functional receptors.
In a previous investigation of the adjuvant activities of LT-IIa and LT-IIb, LT-IIa was shown to induce an IgG1-biased distribution pattern of IgG anti-AgI/II subclasses; a more balanced IgG1/IgG2b response pattern was induced with LT-IIb as a mucosal adjuvant (33). In this study, administration of LT-IIa(T34I) as a mucosal adjuvant did not alter the IgG subclass distribution of anti-AgI/II responses from those observed in mice immunized solely with AgI/II. This pattern of responses elicited by LT-IIa(T34I) is not surprising, since this mutant enterotoxin did not exhibit significant adjuvant activity. In comparison, the adjuvant activities of wt LT-IIb and LT-IIb(T13I) were similar for essentially all parameters measured. Thus, it was not surprising that the IgG subclass distribution induced with LT-IIb(T13I) as a mucosal adjuvant was also similar to the pattern elicited by wt LT-IIb. Cytokines have a major role in isotype selection and isotype switching during an immune response. Although no single cytokine appears to regulate IgG subclass responses in vivo, IL-4 and IFN-γ are known to enhance production of IgG1 and IgG2a, respectively (43, 55). LT-IIb enhances IgG2a by stimulating more IFN-γ production in both mucosal and systemic compartments (33). The results reported herein show that LT-IIb(T13I) induced IFN-γ at levels similar to those induced by wt LT-IIb. It is likely, therefore, that the equivalent capacities of LT-IIb and LT-IIb(T13I) to stimulate IFN-γ explain their abilities to induce similar patterns of IgG subclass expression.
Robust immunity and long-lived memory responses to an antigen often require the aid of an adjuvant to generate antigen-specific memory T and B cells, which persist after immunization in various lymphoid compartments. After i.n. immunization, T cells migrate and reside in the CLN (63). Use of LT-IIa and LT-IIb as i.n. adjuvants has been correlated with the establishment of AgI/II-specific cells in both CLN and spleen (33). Results from the present experiments were consistent with those prior investigations. LT-IIa and LT-IIb stimulated robust IgG responses to AgI/II after reimmunization at 203 days post-initial immunization with AgI/II. An apparent disparity in immune responsiveness was observed for LT-IIa(T34I). Although this mutant enterotoxin had little significant capacity to potentiate anti-AgI/II immune responses at earlier time points prior to reimmunization (with the exception of serum IgA), there was a significant enhancement of the memory response to AgI/II in those mice. This pattern of delayed responsiveness may be a result of stimulated production of IL-4. IL-4 profoundly regulates the activation, growth, and differentiation of many cell types, most notably T cells and B cells (42, 43). Immunization with LT-IIa(T34I) as adjuvant induces a dramatic production of IL-4 in lymphoid cells of the CLN.
CT and LT-I bind to B cells, T cells, macrophages, and dendritic cells. Binding of these type I enterotoxins to these cells is associated with apoptosis of CD8+ T cells and with polyclonal B-cell activation (61). LT-I-associated B-cell activation is GM1 dependent and promotes upregulated expression of major histocompatibility complex class II, B7, CD40, ICAM-1, and IL-2Rα (38). CT-dependent upregulation of major histocompatibility complex class II, costimulatory molecules and adhesins on B cells likely enhances the roles of those cells in antigen presentation. The adjuvant activity of LT-IIb(T13I) may be a result of signal transduction events initiated by binding of the enterotoxin to B cells or to other cells which, directly or indirectly, enhance the capacity of B cells to function as antigen-presenting cells. Further investigations will be required to identify the signal transduction pathways and the initiating receptors that control this process. Binding of CT to GM1 is proposed to augment protein-lipid modifications on lymphoid cells. These modifications are believed to promote association of those complexes with lipid rafts and trafficking of those complexes into secretory pathways (14, 29, 62). In the case of LT-IIb, and to a lesser extent LT-IIa, the results of these immunization experiments using the mutant enterotoxins suggest that binding of enterotoxins to ganglioside receptors and any subsequent association of those enterotoxin-ganglioside complexes with lipid rafts may be an essential property for adjuvant activity. Although LT-IIb(T13I) did not bind to its major ganglioside receptors, it retained binding activity for some receptor (or receptors) on the lymphoid cell surface. It will be interesting to determine whether LT-IIb(T13I) binds to lipid rafts and if binding stimulates certain signal transduction pathways associated with immunostimulation (28, 31, 47, 53). Additional nonbinding mutant enterotoxins of LT-IIa and LT-IIb (6, 7) are being evaluated for adjuvant activity in the hopes of identifying one or more mutant enterotoxins which have lost all adjuvant activity, with the goal of separating enterotoxin-binding activity from immunomodulation. Such a mutant enterotoxin will be invaluable in characterizing the molecular mechanisms by which LT-IIb(T13I) and LT-IIa(T34I) enhance potentiated immune responses.
CT, LT-I, LT-IIa, and LT-IIb are capable of activating the adenylyl cyclase by catalyzing the ADP-ribosylation of the Gsα regulatory protein, which promotes accumulation of intracellular cAMP. cAMP has been shown to serve as a second messenger and modulates a variety of cellular processes (4, 23, 35-37), including modulation of IL-4 and IL-5 production by activated CD4+ T cells (27) and transcriptional activation of the human IL-10 gene in monocytic cells (3, 49). It was intriguing to observe that production of cAMP was induced in macrophages which had been treated with LT-IIa(T34I), albeit at a much lower level than the level induced by either wt LT-IIa or CT, since the mutant enterotoxin exhibited little if any detectable binding to lymphoid cells. The ability of LT-IIa(T34I) to induce intracellular accumulation of cAMP in macrophages suggests that this mutant enterotoxin, indeed, interacts with lymphocytes at some level which is, nevertheless, sufficient for intoxification. On a functional level, this residual toxic capacity might explain the ability of LT-IIa(T34I) to induce IL-4 secretion by CLN lymphoid cells (27). LT-IIb(T13I) also induced intracellular accumulation of cAMP. Whether the interaction that promotes cAMP production by LT-IIb(T13I) is due to binding of the mutant enterotoxin to the unknown receptor on macrophages or to an alternative receptor involved only in toxicity has yet to be determined. It should be noted, however, that LT-IIa(T34I) does not augment Ag-specific immune responses, although the mutant enterotoxin stimulates cAMP production by macrophages. These data indicate that the capacity LT-IIa and LT-IIb to stimulate production of cAMP is either not the sole factor required by the enterotoxins for modulation of immune cells or is not required, at all, for their adjuvant responses.
In conclusion, these experiments demonstrate that the adjuvant activities of LT-IIa are altered by the amino acid substitution at T34. Binding of LT-IIa to its known ganglioside receptors is essential for its early immunostimulation properties, since little augmentation of antibody responses was observed. The mutation, however, did not affect later immune responses, since LT-IIa(T34I) enhanced Ag-specific memory responses. While we believe that the effects are due to the loss of binding to its known ganglioside receptors, we cannot, however, discount the possibility that LT-IIa(T34I) has some residual but undetectable binding to those receptors and that the immunomodulatory effects would be reestablished if the dosage of the mutant enterotoxin were increased. If, however, one were to hypothesize that the ability of an enterotoxin to intoxicate cells is a correlate with the propensity of that enterotoxin to interact with cells, we would need to increase the dosage of LT-IIa(T34I) over 256-fold. In the case of LT-IIb, immunostimulation does not require binding to its known ganglioside receptor, since the adjuvant effects of LT-IIb and LT-IIb(T13I) are equivalent. We surmise that immunostimulation by LT-IIb(T13I) is a result of binding of the mutant enterotoxins to an alternative, albeit unknown, surface receptor(s) located on the surface of one or more immunocompetent cell subtypes. The current hypothesis is that this alternative receptor(s) is the functional costimulatory molecule which, in an enterotoxin-dependent manner, potentiates immune responses to coadministered antigens. Both mutant enterotoxins LT-IIa(T34I) and LT-IIb(T13I) undoubtedly will be powerful reagents for investigating the molecular and cellular pathways by which enterotoxin-dependent mucosal and systemic adjuvant effects are elicited.
Acknowledgments
This investigation was supported by the National Institutes of Dental and Craniofacial Research under the auspices of Public Health Service grants DE13833 and DE014357 awarded to T.D.C. and Public Health Service grant DE06746 awarded to M.W.R.
We are indebted to Swasti Majumdar and Daniel J. Metzger for their technical help and to George Hajishengallis for his critical comments and advice.
Editor: J. D. Clements
REFERENCES
- 1.Aman, A. T., S. Fraser, E. A. Merritt, C. Rodigherio, M. Kenny, M. Ahn, W. G. Hol, N. A. Williams, W. I. Lencer, and T. R. Hirst. 2001. A mutant cholera toxin B subunit that binds GM1-ganglioside but lacks immunomodulatory or toxic activity. Proc. Natl. Acad. Sci. USA 98:8536-8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baudner, B. C., M. M. Giuliani, J. C. Verhoef, R. Rappuoli, H. E. Junginger, and G. D. Giudice. 2003. The concomitant use of the LTK63 mucosal adjuvant and of chitosan-based delivery system enhances the immunogenicity and efficacy of intranasally administered vaccines. Vaccine 21:3837-3844. [DOI] [PubMed] [Google Scholar]
- 3.Brenner, S., S. Prosch, K. Schenke-Layland, U. Riese, U. Gausmann, and C. Platzer. 2003. cAMP-induced interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 278:5597-5604. [DOI] [PubMed] [Google Scholar]
- 4.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, E., L. Cardenas-Freytag, and J. D. Clements. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38-49. [DOI] [PubMed] [Google Scholar]
- 6.Connell, T., and R. Holmes. 1992. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect. Immun. 60:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell, T. D., and R. K. Holmes. 1995. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol. Microbiol. 16:21-31. [DOI] [PubMed] [Google Scholar]
- 8.Connell, T. D., D. Metzger, C. Sfintescu, and R. T. Evans. 1998. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol. Lett. 62:117-120. [DOI] [PubMed] [Google Scholar]
- 9.de Haan, L., I. K. Feil, W. R. Verweij, M. Holtrop, W. G. Hol, E. Agsteribbe, and J. Wilschut. 1998. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur. J. Immunol. 28:1243-1250. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Tommaso, A., G. Saletti, M. Pizza, R. Rappuoli, G. Dougan, S. Abrignani, G. Douce, and M. T. De Magistris. 1996. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect. Immun. 64:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douce, G., C. Turcotte, I. Cropley, M. Roberts, M. Pizza, M. Domenghini, R. Rappuoli, and G. Dougan. 1995. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Natl. Acad. Sci. USA 92:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elson, C. O. 1989. Cholera toxin and its subunits as potential oral adjuvants. Curr. Top. Microbiol. Immunol. 146:29-33. [DOI] [PubMed] [Google Scholar]
- 14.Fujinaga, Y., A. A. Wolf, C. Rodighiero, H. Wheeler, B. Tsai, L. Allen, M. G. Jobling, T. Rapoport, R. K. Holmes, and W. I. Lencer. 2003. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulm. Mol. Biol. Cell 14:4783-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuta, S., J. L. Magnani, E. M. Twiddy, R. K. Holmes, and V. Ginsburg. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 56:1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidry, J., L. Cardenas, E. Cheng, and J. Clements. 1997. Role of receptor binding in toxicity, immunogenicity, and adjuvanticity of Escherichia coli heat-labile enterotoxin. Infect. Immun. 65:4943-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth, B. E., C. L. Pickett, E. M. Twiddy, R. K. Holmes, T. A. Gomes, A. A. Lima, R. L. Guerrant, B. D. Franco, and L. R. Trabulsi. 1986. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 54:587-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guth, B. E., E. M. Twiddy, L. R. Trabulsi, and R. K. Holmes. 1986. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect. Immun. 54:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwar, Y., T. Tsuji, T. Iwasaki, S. Kadowaki, H. Asanuma, Z. Chen, K. Komase, Y. Suzuki, C. Aizawa, T. Kurata, and S. Tamura. 2001. Effectiveness and safety of mutant Escherichia coli heat-labile enterotoxin (LT H44A) as an adjuvant for nasal influenza vaccine. Vaccine 19:2071-2079. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara, Y., K. Komase, Z. Chen, K. Matsuo, Y. Suzuki, C. Aizawa, T. Kurata, and S. Tamura. 1999. Mutants of cholera toxin as an effective and safe adjuvant for nasal influenza vaccine. Vaccine 17:2918-2926. [DOI] [PubMed] [Google Scholar]
- 21.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 22.Harandi, A. M., J. Sanchez, K. Eriksson, and J. Holmgren. 2003. Recent developments in mucosal immunomodulatory adjuvants. Curr. Opin. Investig. Drugs 4:156-161. [PubMed] [Google Scholar]
- 23.Holmes, R. K., M. G. Jobling, and T. D. Connell. 1995. Cholera toxin and related enterotoxins of gram-negative bacteria, p. 225-255. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease, vol. 8. Marcel Dekker, Inc., New York, N.Y.
- 24.Isaka, M., Y. Yasuda, T. Taniguchi, S. Kozuka, K. Matano, J. Maeyama, K. Morokuma, K. Ohkuma, N. Goto, and K. Tochikubo. 2003. Mucosal and systemic antibody responses against an acellular pertussis vaccine in mice after intranasal co-administration with recombinant cholera toxin B subunit as an adjuvant. Vaccine 21:1165-1173. [DOI] [PubMed] [Google Scholar]
- 25.Komase, K., S. Tamura, K. Matsuo, K. Watanabe, N. Hattori, A. Odaka, Y. Suzuki, T. Kurata, and C. Aizawa. 1998. Mutants of Escherichia coli heat-labile enterotoxin as an adjuvant for nasal influenza vaccine. Vaccine 16:248-254. [DOI] [PubMed] [Google Scholar]
- 26.Kweon, M. N., M. Yamamoto, F. Watanabe, S. Tamura, F. W. Van Ginkel, A. Miyauchi, H. Takagi, Y. Takeda, T. Hamabata, K. Fujihashi, J. R. McGhee, and H. Kiyono. 2002. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J. Infect. Dis. 186:1261-1269. [DOI] [PubMed] [Google Scholar]
- 27.Lacour, M., J. F. Arrighi, K. M. Muller, C. Carlberg, J. H. Saurat, and C. Hauser. 1994. cAMP up-regulates IL-4 and IL-5 production from activated CD4+ T cells while decreasing IL-2 release and NF-AT induction. Int. Immunol. 6:1333-1343. [DOI] [PubMed] [Google Scholar]
- 28.Leitinger, B., and N. Hogg. 2002. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 115:963-972. [DOI] [PubMed] [Google Scholar]
- 29.Lencer, W. I., T. R. Hirst, and R. K. Holmes. 1999. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450:177-190. [DOI] [PubMed] [Google Scholar]
- 30.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 31.Manes, S., R. A. Lacalle, C. Gomez-Mouton, G. del Real, E. Mira, and C. Martinez-A. 2001. Membrane raft microdomains in chemokine receptor function. Semin. Immunol. 13:147-157. [DOI] [PubMed] [Google Scholar]
- 32.Martin, M., G. Hajishengallis, D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4+ T cells. Infect. Immun. 69:252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2000. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect. Immun. 68:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCluskie, M. J., R. D. Weeratna, J. D. Clements, and H. L. Davis. 2001. Mucosal immunization of mice using CpG DNA and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine 19:3759-3768. [DOI] [PubMed] [Google Scholar]
- 35.Moss, J., S. J. Stanley, and M. C. Lin. 1979. NAD glycohydrolase and ADP-ribosyltransferase activities are intrinsic to the A1 peptide of choleragen. J. Biol. Chem. 254:11993-11999. [PubMed] [Google Scholar]
- 36.Moss, J., and M. Vaughan. 1979. Activation of adenylate cyclase by choleragen. Annu. Rev. Biochem. 48:581-600. [DOI] [PubMed] [Google Scholar]
- 37.Moss, J., and M. Vaughan. 1977. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J. Biol. Chem. 252:2455-2457. [PubMed] [Google Scholar]
- 38.Nashar, T. O., T. R. Hirst, and N. A. Williams. 1997. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology 91:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nashar, T. O., H. M. Webb, S. Eaglestone, N. A. Williams, and T. R. Hirst. 1996. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. USA 93:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicollier-Jamot, B., A. Ogier, L. Piroth, P. Pothier, and E. Kohli. 2004. Recombinant virus-like particles of a norovirus (genogroup II strain) administered intranasally and orally with mucosal adjuvants LT and LT(R192G) in BALB/c mice induce specific humoral and cellular Th1/Th2-like immune responses. Vaccine 22:1079-1086. [DOI] [PubMed] [Google Scholar]
- 41.Orlandi, P. A., D. R. Critchley, and P. H. Fishman. 1994. The heat-labile enterotoxin of Escherichia coli binds to polylactosaminoglycan-containing receptors in CaCo-2 human intestinal epithelial cells. Biochemistry 33:12886-12895. [DOI] [PubMed] [Google Scholar]
- 42.Paul, W. E. 1987. Interleukin 4/B cell stimulatory factor 1: one lymphokine, many functions. FASEB J. 1:456-461. [DOI] [PubMed] [Google Scholar]
- 43.Peschel, C., I. Green, J. Ohara, and W. E. Paul. 1987. Role of B cell stimulatory factor 1/interleukin 4 in clonal proliferation of B cells. J. Immunol. 139:3338-3347. [PubMed] [Google Scholar]
- 44.Pizza, M., M. Domenighini, W. Hol, V. Giannelli, M. R. Fontana, M. M. Giuliani, C. Magagnoli, S. Peppoloni, R. Manetti, and R. Rappuoli. 1994. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol. Microbiol. 14:51-60. [DOI] [PubMed] [Google Scholar]
- 45.Pizza, M., M. M. Giuliani, M. R. Fontana, G. Douce, G. Dougan, and R. Rappuoli. 2000. LTK63 and LTR72, two mucosal adjuvants ready for clinical trials. Int. J. Med. Microbiol. 290:455-461. [DOI] [PubMed] [Google Scholar]
- 46.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: nontoxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 47.Pizzo, P., E. Giurisato, A. Bigsten, M. Tassi, R. Tavano, A. Shaw, and A. Viola. 2004. Physiological T cell activation starts and propagates in lipid rafts. Immunol. Lett. 91:3-9. [DOI] [PubMed] [Google Scholar]
- 48.Plant, A., and N. A. Williams. 2004. Modulation of the immune response by the cholera-like enterotoxins. Curr. Top. Med. Chem. 4:509-519. [DOI] [PubMed] [Google Scholar]
- 49.Platzer, C., E. Fritsch, T. Elsner, M. H. Lehmann, H. D. Volk, and S. Prosch. 1999. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 29:3098-3104. [DOI] [PubMed] [Google Scholar]
- 50.Rappuoli, R., G. Douce, G. Dougan, and M. Pizza. 1995. Genetic detoxification of bacterial toxins: a new approach to vaccine development. Int. Arch. Allergy Immunol. 108:327-333. [DOI] [PubMed] [Google Scholar]
- 51.Rodighiero, C., Y. Fujinaga, T. R. Hirst, and W. I. Lencer. 2001. A cholera toxin B-subunit variant that binds ganglioside GM1 but fails to induce toxicity. J. Biol. Chem. 276:36939-36945. [DOI] [PubMed] [Google Scholar]
- 52.Russell, M. W., L. A. Bergmeier, E. D. Zanders, and T. Lehner. 1980. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 28:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 54.Sixma, T. K., S. E. Pronk, K. H. Kalk, B. A. van Zanten, A. M. Berghuis, and W. G. Hol. 1992. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature 355:561-564. [DOI] [PubMed] [Google Scholar]
- 55.Snapper, C. M., C. Peschel, and W. E. Paul. 1988. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 140:2121-2127. [PubMed] [Google Scholar]
- 56.Sonnino, S., D. Acquotti, L. Riboni, A. Giuliani, G. Kirschner, and G. Tettamanti. 1986. New chemical trends in ganglioside research. Chem. Phys. Lipids 42:3-26. [DOI] [PubMed] [Google Scholar]
- 57.Sougioultzis, S., C. K. Lee, M. Alsahli, S. Banerjee, M. Cadoz, R. Schrader, B. Guy, P. Bedford, T. P. Monath, C. P. Kelly, and P. Michetti. 2002. Safety and efficacy of E. coli enterotoxin adjuvant for urease-based rectal immunization against Helicobacter pylori. Vaccine 21:194-201. [DOI] [PubMed] [Google Scholar]
- 58.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens, L. A., J. Moss, M. Vaughan, M. Pizza, and R. Rappuoli. 1999. Effects of site-directed mutagenesis of Escherichia coli heat-labile enterotoxin on ADP-ribosyltransferase activity and interaction with ADP-ribosylation factors. Infect. Immun. 67:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Akker, F., S. Sarfaty, E. M. Twiddy, T. D. Connell, R. K. Holmes, and W. G. Hol. 1996. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure 4:665-678. [DOI] [PubMed] [Google Scholar]
- 61.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 62.Wolf, A. A., M. G. Jobling, S. Wimer-Mackin, M. Ferguson-Maltzman, J. L. Madara, R. K. Holmes, and W. I. Lencer. 1998. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J. Cell Biol. 141:917-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, H. Y., E. B. Nikolova, K. W. Beagley, J. H. Eldridge, and M. W. Russell. 1997. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect. Immun. 65:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, H. Y., and M. W. Russell. 1998. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16:286-292. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto, S., H. Kiyono, M. Yamamoto, K. Imaoka, K. Fujihashi, F. W. Van Ginkel, M. Noda, Y. Takeda, and J. R. McGhee. 1997. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 94:5267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yasuda, Y., M. Isaka, T. Taniguchi, Y. Zhao, K. Matano, H. Matsui, K. Morokuma, J. Maeyama, K. Ohkuma, N. Goto, and K. Tochikubo. 2003. Frequent nasal administrations of recombinant cholera toxin B subunit (rCTB)-containing tetanus and diphtheria toxoid vaccines induced antigen-specific serum and mucosal immune responses in the presence of anti-rCTB antibodies. Vaccine 21:2954-2963. [DOI] [PubMed] [Google Scholar]