Abstract
By measuring phosphate uptake by Mycobacterium tuberculosis strains with the pstS1 and pstS2 genes genetically inactivated, we showed that these pstS genes encode high-affinity phosphate binding proteins. In a mouse infection model, both mutants were attenuated in virulence, suggesting that M. tuberculosis encounters limiting phosphate concentrations during its intracellular life span.
As inorganic phosphate is an essential but often limiting nutrient in the environment, its import in bacteria is important and can be accomplished through the phosphate-specific transporter (Pst) (7, 8, 19, 25, 28, 31). Pst is a membrane-associated complex that belongs to the superfamily of ABC transporters (1, 6, 15). In Escherichia coli (12, 30) and other procaryotes (26), it is composed of four distinct subunits encoded by the pstS, pstA, pstC, and pstB genes arranged in an operon. PstS is the periplasmic phosphate binding protein, PstA and PstC are integral inner membrane proteins, and the PstB subunit provides energy for transport through ATP hydrolysis. Interestingly, in Mycobacterium tuberculosis, three putative pst operons have been identified (7, 8, 10), which probably constitutes a subtle biochemical adaptation of this microorganism for its growth and survival under different phosphate-limiting conditions during its infectious cycle (19). It has been shown that PstS1 from M. tuberculosis is able to bind phosphate with an affinity similar to that of PstS from E. coli (9, 29) and that the production of the different PstS proteins is induced under phosphate starvation in M. tuberculosis (3, 19).
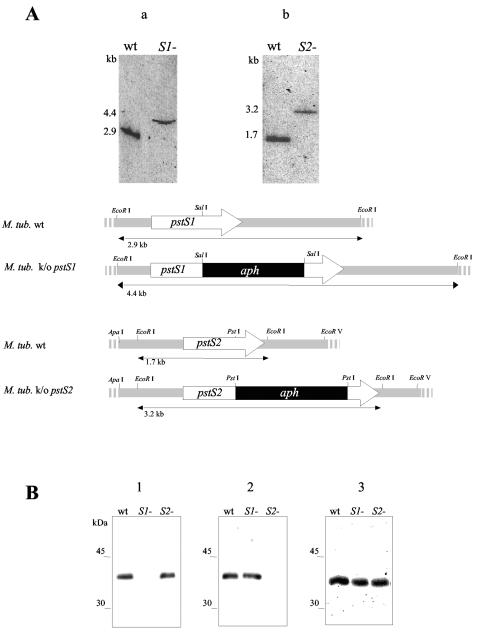
To further investigate the importance of the pstS1 and pstS2 genes for the phosphate uptake and virulence of M. tuberculosis, we created M. tuberculosis pstS1 and pstS2 knockout strains using genes isolated from an M. tuberculosis H37Rv cosmid library. A kanamycin resistance cassette (the aph gene from pYUB53) (18) was cloned into pstS1 and pstS2, yielding pstS1::aph and pstS2::aph, respectively. These genes and the xylE gene (from pXYL4 carrying the xylE colored marker gene from Pseudomonas putida) were cloned into pPR27; transformed into M. tuberculosis H37Rv, where the knockout mutants were selected by a two-step counterselection strategy (24); and further analyzed by Southern hybridization (Fig. 1A) and immunoblot analysis (Fig. 1B). Anti-PstS1-reactive material was lacking in the pstS1 knockout mutant but present in the parental strain and in the pstS2 knockout mutant. Conversely, anti-PstS2-reactive material was lacking in the pstS2 knockout mutant but present in the other strains. In this pstS2 knockout mutant, we observed that the expression of the pknD (mbk) gene (22, 23), located downstream of the pstS2 gene, is also abolished (data not shown). The PstS3 subunit is present in all strains (Fig. 1B). The different strains exhibited similar apparent growth rates in Middlebrook 7H9 albumin-dextrose-catalase (ADC) liquid medium in a 14-day experiment, suggesting that the two PstS proteins are not essential for growth in this phosphate-rich medium (25 mM Pi).
FIG. 1.
(A) Southern blot analysis of the M. tuberculosis (M. tub.) H37Rv pstS1 and pstS2 knockout (k/o) mutants. Genomic DNA was digested with EcoRI, subjected to electrophoresis, blotted onto membranes, and probed with the pstS1 (an NaeI-SalI fragment of the pstS1gene) (a) or the pstS2 (a SacI-PstI fragment of the pstS2 gene) (b) probe. The probes were labeled with [α-32P]dCTP using the Megaprime random-primed labeling kit (Amersham). The hybridization and washing protocols were carried out under high-stringency conditions as described previously (5). The sizes of the hybridizing bands, indicated on the left, were determined from the migration distance of the DNA molecular marker Smartladder (Eurogentec). The arrows depict the lengths and transcriptional orientations of the pstS1 and pstS2 genes. The black boxes represent the aph gene, and the hatched boxes show the pstS1 and pstS2 gene flanking regions used for allelic exchange. Only the relevant restriction sites are indicated. wt, wild type. (B) Immunoblot analysis of the lysates of the pstS1 and pstS2 knockout mutants and the wild-type M. tuberculosis strain. Total cell extracts of the wild-type and the pstS1 (S1−) and pstS2 (S2−) knockout mutant strains were probed with anti-PstS1 (HBT12) (2, 8) (blot 1), anti-PstS2 (2A1-2) (17, 19) (blot 2), and anti-PstS3 (2F-8) (7, 17) (blot 3), and goat alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Sigma). Bound antibodies were detected using BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium visualization solution (Promega). The electrophoretic mobilities of the rainbow-colored protein molecular mass markers (Amersham Pharmacia Biotech) as observed on the blots are indicated on the left.
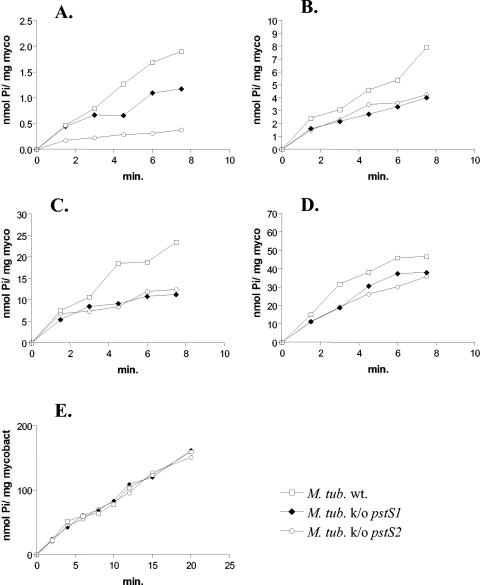
To assess the involvement of PstS1 and PstS2 in phosphate uptake, the different strains were grown in Middlebrook 7H9 ADC medium to an optical density at 600 nm of 0.3. The cells were then washed in 7H9 ADC medium without phosphate (4) and further cultivated in this medium for 24 h at 37°C to induce maximal phosphate uptake by the high-affinity Pst system (11). The bacteria were then washed twice in the uptake buffer [50 mM Tris-HCl (pH 6.9), 15 mM KCl, 10 mM (NH4)2SO4, and 1 mM MgSO4] and incubated in the uptake buffer supplemented with 0.5, 2, 5, 10, or 25 μM Pi and 33Pi (25 nM; 10 μCi/ml). The rate of uptake of orthophosphate was measured as described previously (8). At 0.5 μM Pi, the rates of phosphate uptake by the pstS1 and pstS2 knockout mutants were reduced compared to that of the wild-type (Fig. 2A). The reduced phosphate uptake by the pstS2 knockout strain is due to the absence of the PstS2 protein and not to the absence of the PknD protein kinase, since the rate of phosphate uptake by a pknD knockout mutant is not reduced compared to that of the parental strain (results not shown). These results indicate that PstS1 and PstS2 are involved in phosphate uptake from this medium. Increasing the phosphate concentration resulted in less pronounced differences in phosphate uptake between the parental and mutant strains (Fig. 2B, C, and D). At 25 μM Pi, no difference in phosphate uptake was observed among the three strains (Fig. 2E), suggesting that PstS1 and PstS2 can substitute for each other and/or that phosphate uptake may be mediated by PstS3 or the putative Pit transporter (14, 27, 32).
FIG. 2.
Phosphate uptake rates of the pstS1 and pstS2 knockout (k/o) mutant and the wild-type M. tuberculosis (M. tub. wt.) strains. The uptake rate of orthophosphate (10 mCi/mmol; Amersham-Pharmacia) (expressed in nanomoles of Pi per milligram of mycobacterial protein extract) of the parental wild type and the pstS1 and pstS2 mutant derivatives of M. tuberculosis H37Rv were measured at 0.5 (A), 2 (B), 5 (C), 10 (D), and 25 (E) μM Pi.
PstS1 and PstS2 may contribute to the intracellular survival of M. tuberculosis, since both PstS1 and PstS2 appear to be involved in phosphate uptake from media with low phosphate concentrations. This concentration is similar to what has been found within macrophages infected with Salmonella enterica serovar Typhimurium (20). Therefore, mouse peritoneal macrophages were infected with the three M. tuberculosis strains, and we observed that both pstS knockout mutant strains showed significantly reduced multiplication within the macrophages compared to the parental strain (results not shown).
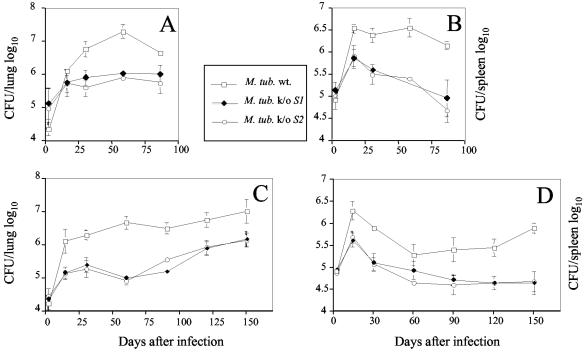
To further investigate the roles of the two PstS proteins in tuberculosis virulence, we used an in vivo infection model. BALB/c and C57BL/6 mice were infected intravenously with either the mutant or wild-type strain, and growth in lungs and spleens was monitored over time (Fig. 3). In both mouse strains, the pstS1 and pstS2 mutants were attenuated (10- to 30-fold lower CFU numbers). In the spleen (Fig. 3B and D), this reduction was observed throughout the entire 3 and 5 months in the BALB/c and C57BL/6 mice, respectively. However, in the lungs (Fig. 3A and C), attenuation was strong for the first 3 months, but in the C57BL/6 mice, the CFU numbers of both mutant strains started to increase at later time points. The observed effect on the multiplication of the pstS2 knockout mutant strain is most probably due to the inactivation of the pstS2 gene and not to the disruption of the pknD gene, since in mice, a pknD knockout mutant does not seem to be attenuated compared to the parental strain (preliminary results).
FIG. 3.
Growth of pstS1 (M. tub S1−) and pstS2 (M. tub S2−) knockout mutant and wild-type (M. tub wt.) M. tuberculosis strains in lungs (A and C) and spleens (B and D) of infected mice. The bacteria were grown as a surface pellicle on synthetic Sauton medium for 14 days at 37°C and then harvested and homogenized by ball mill as previously described (16). The M. tuberculosis H37Rv wild-type and pstS1 and pstS2 knockout mutant strains were used to infect BALB/c (A and B) and C57BL/6 (C and D) mice intravenously with 2 × 105 CFU from the different M. tuberculosis H37Rv strains. At the indicated time points, the spleen and lungs from individual mice were homogenized in phosphate-buffered saline, and serial threefold dilutions were plated in duplicate onto Middlebrook 7H11 oleic acid-albumin-dextrose-catalase medium and incubated at 37°C for 3 to 4 weeks. The bacteria were then counted visually, and the numbers of CFU per organ were determined. The results represent the mean log10 values ± standard deviations of at least four mice per group. The mice (3 to 4 months old at the time of infection) were bred in the animal facilities of the Pasteur Institute of Brussels from breeding pairs obtained from Bantin and Kingman (Grimston, United Kingdom).
The reduced multiplication of the two pstS mutants observed in infected macrophages and mice suggests that PstS1 and PstS2 are functional in vivo during infection and cannot be replaced by each other, by PstS3, by the putative Pit transporter, or by any other phosphate transporter. In addition, our results suggest that during intracellular growth, M. tuberculosis encounters low phosphate concentrations. M. tuberculosis preferentially resides within macrophages; little is known about the biochemical environment in the phagosomes harboring M. tuberculosis (21), and restrictions in phosphate availability for M. tuberculosis have not been shown in vivo. Our results suggest that low phosphate concentrations in intracellular vacuoles of phagocytic cells may stimulate bacteria to differentially express genes so as to survive and replicate within the host.
The M. tuberculosis complex is unusual in having three phosphate binding proteins and four membrane-spanning proteins organized in three operons. There is only one pstB gene encoding an ATP-binding subunit from the transporter in these operons, but another gene, called phoT, located 130 kb from pstB on the chromosome also encodes ATP-binding protein from the transporter. In fact, this protein has even higher homology to PstB in some other prokaryotes (http://genolist.pasteur.fr/TubercuList) than does PstB of M. tuberculosis. Sequencing of the Mycobacterium bovis genome has revealed that in this member of the M. tuberculosis complex, the pstB gene is frameshifted (13). It has been shown that PhoT is necessary for growth at low phosphate concentrations (11) and that PhoT is a virulence gene, since an M. bovis phoT knockout strain was significantly less virulent than its parental strain in different animal models (11). These results, together with our observation that the phosphate concentration is restricted to the intracellular vacuoles of phagocytic cells, lead to the hypothesis that the high-affinity phosphate-specific transporters are virulence factors of M. tuberculosis and M. bovis.
Acknowledgments
We thank B. Giquel for the plasmids pPR27 and pXyl4, Douglas B. Young (Imperial College, London, United Kingdom) for M. tuberculosis H37Rv containing pSMT1, and W. R. Jacobs for the M. tuberculosis H37Rv cosmid library. The excellent technical assistance of Fabienne Jurion and Kamiel Palfliet is gratefully acknowledged.
This work was supported by grant G.0266.00 from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, by EEC (TB Vaccine Cluster QLK2-CT-1999-01093), by the Brussels Hoofdstedelijk Gewest, by the Damiaanaktie Belgium, and by grants from “Les Amis de L'Institut Pasteur de Bruxelles (ASLB)-De Vrienden van het Instituut Pasteur van Brussel (VZW).”
Editor: F. C. Fang
REFERENCES
- 1.Ames, G. F.-L. 1993. Bacterial periplasmic permeases as model systems for the superfamily of traffic ATPases, including the multidrug resistance protein and the cystic fibrosis transmembrane conductance regulator. Int. Rev. Cytol. 137:1-35. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, A. B., and E. B. Hansen. 1989. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect. Immun. 57:2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, A. B., L. Ljungqvist, and M. Olsen. 1990. Evidence that protein antigen b of Mycobacterium tuberculosis is involved in phosphate metabolism. J. Gen. Microbiol. 136:477-480. [DOI] [PubMed] [Google Scholar]
- 4.Braibant, M., and J. Content. 2001. The cell surface associated phosphatase activity of Mycobacterium bovis BCG is not regulated by environmental inorganic phosphate. FEMS Microbiool. Lett. 195:121-126. [DOI] [PubMed] [Google Scholar]
- 5.Braibant, M., L. De Wit, P. Peirs, M. Kalai, J. Ooms, A. Drowart, K. Huygen, and J. Content. 1994. Structure of the Mycobacterium tuberculosis antigen 88, a protein related to the Escherichia coli PstA periplasmic phosphate permease subunit. Infect. Immun. 62:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braibant, M., P. Gilot, and J. Content. 2000. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 24:449-467. [DOI] [PubMed] [Google Scholar]
- 7.Braibant, M., P. Lefèvre, L. De Wit, J. Ooms, P. Peirs, K. Huygen, R. Wattiez, and J. Content. 1996. Identification of a second Mycobacterium tuberculosis gene cluster encoding proteins of an ABC phosphate transporter. FEBS Lett. 394:206-212. [DOI] [PubMed] [Google Scholar]
- 8.Braibant, M., P. Lefevre, L. De Wit, P. Peirs, J. Ooms, K. Huygen, A. B. Andersen, and J. Content. 1996. A Mycobacterium tuberculosis gene cluster encoding proteins of a phosphate transporter homologous to the Escherichia coli Pst system. Gene 176:171-176. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Z., A. Choudhary, R. Lathigra, and F. A. Quiocho. 1994. The immunodominant 38-kDa lipoprotein of M. tuberculosis is a phosphate binding protein. J. Biol. Chem. 269:1956-1958. [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Collins, D. M., R. P. Kawakami, B. M. Buddle, B. J. Wards, and G. W. de Lisle. 2003. Different susceptibility of two animal species infected with isogenic mutants of Mycobacterium bovis identifies phoT as having roles in tuberculosis virulence and phosphate transport. Microbiology 149:3203-3212. [DOI] [PubMed] [Google Scholar]
- 12.Cox, G. B., H. Rosenberg, J. A. Downie, and S. Silver. 1981. Genetic analysis of mutants affected in the Pst inorganic phosphate transport system. J. Bacteriol. 148:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris, R. M., D. C. Webb, S. M. Howitt, and G. B. Cox. 2001. Characterization of PitA and PitB from Escherichia coli. J. Bacteriol. 183:5008-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 16.Huygen, K., D. Abramowicz, P. Vandenbussche, F. Jacobs, J. De-Bruyn, A. Kentos, A. Drowart, J. P. Van-Vooren, and M. Goldman. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 60:2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen, K., A. Drowart, M. Harboe, R. ten-Berg, J. Cogniaux, and J. P. Van-Vooren. 1993. Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect. Immun. 61:2687-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalpana, G. V., B. R. Bloom, and W. Jacobs, Jr. 1991. Insertional mutagenesis and illegitimate recombination in mycobacteria. Proc. Natl. Acad. Sci. USA 88:5433-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefèvre, P., M. Braibant, L. De Wit, M. Kalai, D. Röeper, J. Grötzinger, J.-P. Delville, P. Peirs, J. Ooms, K. Huygen, and J. Content. 1997. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome and are present at the surface of Mycobacterium bovis BCG. J. Bacteriol. 179:2900-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas, R. L., and C. A. Lee. 2000. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol. Microbiol. 36:1024-1033. [DOI] [PubMed] [Google Scholar]
- 21.McKinney, J. D., and J. E. Gomez. 2003. Life on the inside for Mycobacterium tuberculosis. Nat. Med. 9:1356-1357. [DOI] [PubMed] [Google Scholar]
- 22.Peirs, P., L. De Wit, M. Braibant, K. Huygen, and J. Content. 1997. A serin/threonine protein kinase from Mycobacterium tuberculosis. Eur. J. Biochem. 244:604-612. [DOI] [PubMed] [Google Scholar]
- 23.Peirs, P., B. Parmentier, L. De Wit, and J. Content. 2000. The Mycobacterium bovis homologous protein of the Mycobacterium tuberculosis serine/threonine protein kinase Mbk (PknD) is truncated. FEMS Microbiol. Lett. 188:135-139. [DOI] [PubMed] [Google Scholar]
- 24.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi, Y., Y. Kobayashi, and M. Hulett. 1997. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the Pho regulon. J. Bacteriol. 179:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quentin, Y., G. Fichant, and F. Denizot. 1999. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J. Mol. Biol. 287:467-484. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg, H., R. G. Gerdes, and K. Chegwidden. 1977. Two systems for the uptake of phosphate in Escherichia coli. J. Bacteriol. 131:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surin, B. P., H. Rosenberg, and G. B. Cox. 1985. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J. Bacteriol. 161:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vyas, N. K., M. N. Vyas, and F. A. Quiocho. 2003. Crystal structure of M. tuberculosis ABC phosphate transport receptor: specificity and charge compensation dominated by ion-dipole interactions. Structure 11:765-774. [DOI] [PubMed] [Google Scholar]
- 30.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 31.Wanner, B. L. 1996. Signal transduction in the control of phosphate-regulated genes of Escherichia coli. Kidney Int. 49:964-967. [DOI] [PubMed] [Google Scholar]
- 32.Willsky, G. R., and M. H. Malamy. 1980. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J. Bacteriol. 144:356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]