Abstract
Salmonella enterica serovar Typhimurium is a versatile organism for the generation of live recombinant vaccines for mucosal immunization. Various strategies have been devised for the stable and efficient expression of heterologous antigens by attenuated S. enterica strains, but these methods often require complex manipulations. Use of phage λ Red recombinase has recently been devised for gene replacements in Escherichia coli and S. enterica after introduction of PCR products. Based on this method, we have developed an approach that allows the integration of recombinant expression cassettes for heterologous antigens in a single step. The recombinant construct is integrated into the chromosome and is devoid of any selective marker such as antibiotic resistance. We observed the stable expression of model antigens without selective pressure. In addition, the method allows the simultaneous generation of attenuating mutations by gene deletions. The novel “knock-in” approach allows the rapid and efficient construction of recombinant Salmonella strains as vaccine carriers.
Live attenuated bacteria delivered by the oral route are interesting tools for mucosal immunization. For this strategy, Salmonella enterica serotypes Typhimurium and Typhi are suitable organisms for the engineering of live recombinant vaccines (for a review, see reference 20). Genetic manipulation allows the generation of attenuated strains that are safe for application in vaccinees, as well as the introduction of heterologous antigens.
A common problem with recombinant bacterial vaccines is the generation of strains that allow stable expression of heterologous antigens in the absence of selective pressure. Several strategies have been developed to address this requirement. These include the balanced lethal plasmid stabilization approach, resulting in the complementation of an auxotrophy due to a mutation on the bacterial chromosome by a functional allele present on the plasmid that expresses the vaccine antigen (4, 8). Furthermore, one can utilize genetic switches to shift a part of the bacterial population to antigen expression (26). It was also possible to insert DNA fragments for the expression of foreign antigens into the chromosome of the carrier strain (22). Although these approaches resulted in the construction of vaccine carrier strains, the genetic manipulations required for the construction of such strains are rather laborious and time-consuming.
Over the last few years, bacteriophage-encoded recombinases have been used for engineering homologous recombination in the chromosomes of Escherichia coli and S. enterica serovar Typhimurium (5, 27). The Red recombinase technique was initially used for the construction of mutations and deletions in genes of interest but is also useful for the introduction of gene fusions (23) or reporter strains (7, 13).
Here, we introduce a novel approach that allows the stable integration of expression cassettes for heterologous antigens into the chromosome of S. enterica serotype Typhimurium. This technique also allows the simultaneous generation of deletions in the genome of Salmonella that can be used to attenuate virulence of the carrier strain. Due to the modular concept, an expression cassette can be inserted into various loci. We demonstrate that the chromosomal integration of expression cassettes allows the construction of strains that express foreign antigen in a stable and regulated manner. The “knock-in” approach could significantly accelerate the construction of recombinant vaccine carrier strains.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in the present study are listed in Table 1. S. enterica serovar Typhimurium strain 12023 was used as a wild-type strain. Bacteria were routinely cultured in Luria-Bertani (LB) broth and on LB agar plates. If required for the selection of recombinants or to maintain plasmids, carbenicillin (50 μg/ml) and/or kanamycin (50 μg/ml) were added. Minimal media with limiting (PCN-P medium) or nonlimiting (PCN medium) amounts of phosphate were used for the analyses of promoters under control of the SsrAB regulatory system in vitro and have been described before (6). For chromogenic tests of phosphatase activity, 30 μg of X-phosphate (BCIP [5-bromo-4-chloro-3-indolylphosphate], Biomol, Hamburg, Germany)/ml was added to LB agar plates. For growth of auxotrophic strains, synthetic media were supplemented with 40 μg each of l-tyrosine, l-tryptophan, and l-phenylalanine/ml and 10 μg each of 2,3-dihydrobenzoic acid and para-aminobenzoic acid/ml for aroA strains and 1 mM adenine for purD strains.
TABLE 1.
Strains and plasmids used in this study
| S. enterica serovar Typhimurium strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| NCTC 12023 | Wild type | NCTC, Colindale, United Kingdom |
| P8G12 | ssrB::mTn5 | 21 |
| HH104 | sseC::aphT; Kanr | 14, 19 |
| SL7207 | aroA; Smr | 15 |
| MvP440 | ΔphoN::aph Pro sseA::GFP; Kanr | This study |
| MvP453 | ΔgalE::aph Pro sseA::GFP; Kanr | This study |
| MvP455 | ΔrecA::aph Pro sseA::GFP; Kanr | This study |
| MvP458 | ΔpurD::aph Pro sseA::GFP; Kanr | This study |
| MvP459 | ΔhtrA::aph Pro sseA::GFP; Kanr | This study |
| MvP462 | ΔaroA::aph Pro sseA::GFP; Kanr | This study |
| MvP436 | ΔphoN Pro sseA::GFP | This study |
| MvP465 | ΔgalE Pro sseA::GFP | This study |
| MvP466 | ΔrecA Pro sseA::GFP | This study |
| MvP467 | ΔhtrA Pro sseA::GFP | This study |
| MvP468 | ΔaroA Pro sseA::GFP | This study |
| MvP472 | ΔpurD Pro sseA::GFP | This study |
| MvP442 | ΔaroA::aph Pro sseA::OVA; Kanr | This study |
| MVP444 | ΔphoN::aph Pro sseA::OVA; Kanr | This study |
| MvP454 | ΔgalE::aph Pro sseA::OVA; Kanr | This study |
| MVP456 | ΔrecA::aph Pro sseA::OVA; Kanr | This study |
| MvP460 | ΔpurD::aph Pro sseA::OVA; Kanr | This study |
| MvP461 | ΔhtrA::aph Pro sseA::OVA; Kanr | This study |
| MvP447 | ΔaroA Pro sseA::OVA | This study |
| MvP448 | ΔphoN Pro sseA::OVA | This study |
| MvP469 | ΔgalE Pro sseA::OVA | This study |
| MvP470 | ΔrecA Pro sseA::OVA | This study |
| MvP471 | ΔhtrA Pro sseA::OVA | This study |
| MvP483 | ΔpurD Pro sseA::OVA | This study |
| MvP457 | ΔpurD::aph Pro cat::OVA; Kanr | This study |
| MvP463 | ΔaroA::aph Pro cat::OVA; Kanr | This study |
| MvP464 | ΔphoN::aph Pro cat::OVA; Kanr | This study |
| MvP478 | ΔgalE::aph Pro cat::OVA; Kanr | This study |
| MvP482 | ΔpurD Pro cat::OVA | This study |
| Plasmids | ||
| pWSK29 | Low copy number; Ampr | 25 |
| pACYC184 | Mid copy number; Cmr Ampr | 3 |
| pKD4 | Template plasmid; Ampr Kanr | 5 |
| pKD46 | Expressing Red, ts; Ampr | 5 |
| pCP20 | Expressing FLP, ts; Ampr | 5 |
| pFPV25.1 | GFP constitutive expression | 24 |
| pLS824 | Pro sseA::GFP in pWSK29 | 16 |
| p2593 | Pro sseA OVA::M45 in pWSK | 15a |
| p2795 | aph FRT in pSK; Ampr Kanr | This study |
| p2811 | Pro sseA GFP::M45 in p2795 | This study |
| p2861 | Pro sseA OVA::M45 in p2795 | This study |
| p2883 | Pro cat OVA::M45 in p2795 | This study |
Smr, streptomycin resistance; Cmr, chloramphenicol resistance; Ampr, ampicillin resistance; ts, temperature sensitive.
DNA biochemistry.
The primers aph-For-KpnI and aph-Rev-KpnI were used to amplify the kanamycin resistance (Kanr) gene flanked by FLP sites from the plasmid pKD4 (5). KpnI sites were introduced in the primer sequences, and the DNA fragments obtained by PCR amplification were digested by KpnI and cloned into the KpnI site of pBluescript SK(+) (Stratagene, Heidelberg, Germany) to yield plasmid p2795.
Various expression cassettes were inserted into the polylinker of p2795 as described in Results in order to generate templates for the generation of targeting constructs. DNA constructs were routinely confirmed by DNA sequencing.
Targeting constructs were generated by PCR amplification of p2795 derivatives with the respective expression cassette by using the pairs xyz-knockin-left and xyz-knockin-right primers, with xyz standing for the respective target gene. The primers had 38 to 40 nucleotides complementary to the target genes phoN, aroA, purD, htrA, recA, or galE of the Salmonella chromosome, followed by 20 nucleotides complementary to the lacZ fragment in pBluescript SK(+) (Table 1).
Genetic manipulation of Salmonella strains.
DNA fragments resulting from amplification of targeting construct by using knock-in primer pairs were digested by DpnI to remove remaining plasmids DNA, purified on Qiagen columns, and used for electroporation of S. enterica serotype Typhimurium harboring pKD46 as previously described (5, 23). Kanr transformants were selected at 37°C and were further analyzed for sensitivity to carbenicillin and phenotypes resulting from the integration of the DNA cassettes into the respective target gene. If appropriate, the Kanr gene from chromosomally integrated cassettes was deleted by FLP-mediated recombination. For this purpose, plasmid pCP20 was introduced by electroporation or P22 transduction. Subsequently, the resulting clones were cured from pCP20 by growth at 42°C, and the absence of lysogenic P22 was confirmed according to standard methods (18) if appropriate.
Analysis of heterologous antigen expression.
To quantify expression levels of genes within chromosomally integrated expression cassettes, green fluorescent protein (GFP) fluorescence was quantified by flow cytometry of bacteria grown in vitro. Bacterial cultures grown in various media were harvested, and flow cytometry on a FACScalibur (BD, Heidelberg, Germany) was performed was previously described (16).
Expression levels of the model antigen ovalbumin (OVA) were determined by Western blot analysis of lysates of bacterial cultures grown in vitro. OVA in bacterial lysates was detected with rabbit antiserum to OVA (Chemicon, Hofheim, Germany) as primary antibody and a goat and rabbit-horseradish peroxidase conjugate as the secondary antibody and enhanced chemiluminescence detection (AP-Biotech, Freiburg, Germany).
Adoptive transfer model and analysis of T-cell proliferation after vaccination.
Ammonium chloride-treated splenocytes from 10- to 12-week-old female DO11.10 mice (JAX) were transferred by tail vein injection into sex- and age-matched BALB/c recipient mice (4 × 106 tgTCR CD4+ T cells per recipient mouse) and immunized 1 day later. Overnight cultures of the bacteria were harvested by centrifugation at 4,000 × g for 10 min, washed, and resuspended in phosphate-buffered saline. For intraperitoneal immunization, ca. 105 CFU per mouse in 200 μl of phosphate-buffered saline were injected. The infection dose used was always verified by serial dilution and plating on LB agar plates in the presence or absence of the appropriate antibiotic.
To measure OVA-specific T-helper-cell activation, mice were sacrificed 7 days after immunization with the bacterial strains. Single-cell suspensions of Peyer's patches, mesenteric lymph nodes, and spleens were stained with biotinylated anti-tgTCR clonotype antibody KJ1-26 (12), anti-CD4-allophycocyanin, and anti-B220-fluorescein, followed by streptavidin-PerCP (all from BD), and ca. 2 × 105 cells were analyzed by flow cytometry (BD). CD4+ tgTCR+ B220− lymphocytes were analyzed for their forward scatter (2).
RESULTS
Rationale of the knock-in approach.
The Red recombinase approach (5) was utilized for integration of expression cassettes into the chromosome of S. enterica serotype Typhimurium and the concomitant generation of deletions leading to the attenuation of virulence of the resulting strain.
Expression cassettes were constructed that contained either a constitutive promoter or an in vivo-activated promoter to control expression of the heterologous model antigen or GFP as reporter for expression. As an in vivo-activated promoter, we used Pro sseA derived from Salmonella pathogenicity island 2 (SPI2). This promoter is activated by Salmonella residing within the Salmonella-containing vacuole of infected host cells, and our previous work demonstrated that superior immune responses were induced if heterologous antigens were specifically expressed by intracellular Salmonella carrier strains (15a).
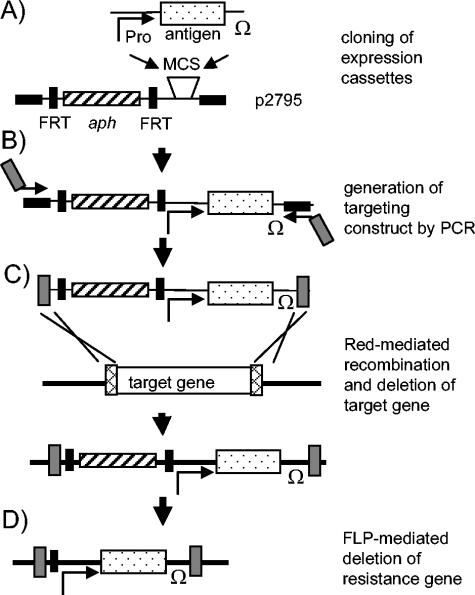
Expression cassettes were inserted into a plasmid that contained aph encoding kanamycin resistance flanked by FRT sites (Fig. 1A). The resulting targeting construct was amplified by PCR with primers complementary to (i) the flanks of the targeting constructs and (ii) the chromosomal gene selected for integration (Fig. 1B). By homologous recombination mediated by the Red recombinase, a chromosomal gene was replaced by the targeting construct (Fig. 1C). Finally, the aph gene was deleted by FLP-mediated recombination (Fig. 1D), resulting in strains that carried stable insertions of expression cassettes in the chromosome but were devoid a resistance markers.
FIG. 1.
Rationale for construction of targeting constructs and chromosomal integration of expression cassettes. (A) Expression cassettes consist of a constitutive or in vivo-activated promoter (Pro), a gene fragment encoding a model vaccine antigen (dotted areas), and a transcriptional terminator (Ω). Expression cassettes for expression of OVA as model antigen or GFP for analyses of expression were inserted into the multiple cloning site of p2795. p2795 contains the aph resistance gene (hatched areas) flanked by FRT sites and binding sites for primers (black symbols). (B) The targeting construct consisting of expression cassettes and resistance gene were amplified by knock-in primers containing sequences complementary to the chromosomal target gene (gray symbols). (C) S. enterica serovar Typhimurium harboring pKD46 for expression of Red recombinase was transformed with linear DNA of targeting construct, and recombinant colonies with replacements of a chromosomal target gene (open symbol) by the targeting construct were selected. (D) The resistance gene of the integrated targeting construct was removed by FLP-mediated recombination after transformation of recombinant strains with plasmid pCP20. The resulting strains were devoid of antibiotic resistance markers, and the targeted integration of the expression cassette was confirmed by PCR (not shown).
For the evaluation of the approach, we analyzed the chromosomal integration of targeting constructs into the phoN locus. phoN is not required for normal growth of S. enterica serovar Typhimurium and mutations of phoN do not result in the attenuation of virulence (10). However, the replacement of phoN by an expression cassette can be easily detected by a chromogenic plate test. Salmonella strains harboring the functional phoN gene form light blue colonies on agar plates containing the phosphatase substrate X-phosphate. phoN mutant strain could easily be detected by lack of color formation. After transformation of ca. 5 × 109 CFU of S. enterica serovar Typhimurium (pKD46) with a linear targeting construct generated by PCR, between 10 and 100 transformants were routinely obtained. To remove the Kanr marker, temperature-sensitive plasmid pCP20 expressing FLP recombinase was introduced. Resulting strains were cured from the plasmid and checked for sensitivity to kanamycin and ampicillin. On plate tests, all of the resulting strains were phosphatase negative (data not shown).
Stability of expression after chromosomal integration.
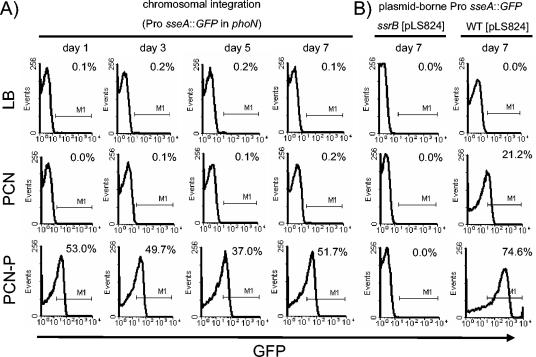
We next analyzed whether expression cassettes inserted into a chromosomal target gene were stably maintained in the absence of selective pressure. Furthermore, the regulated expression of a heterologous gene inserted by the expression cassette was monitored. Strain MvP436 harboring a replacement of the phoN by an expression cassette with GFP under the control of Pro sseA was cultured with repeated subcultures in the absence of selective pressure, and GFP expression was quantified by flow cytometry after various times of culture (Fig. 2A). We observed that expression of GFP was not affected by the length of culture in absence of selective pressure, suggesting that the majority of the bacteria were harboring the expression cassette.
FIG. 2.
Regulated expression and stability of chromosomally integrated expression cassettes. S. enterica serotype Typhimurium strain MvP436 harbors an expression cassette consisting of the intracellular activated promoter Pro sseA and GFP as reporter that was inserted into the phoN gene. The bacteria were cultured in LB medium without selective pressure, and subcultures were prepared each day over a period of 7 days, which corresponds to ca. 50 generations. Overnight cultures were used to inoculate LB medium, PCN minimal medium, or PCN-P minimal medium. Previous work showed that expression under the control of Pro sseA was not induced after bacterial growth in LB or PCN medium but that induction could be obtained by culture in PCN-P medium (6, 11). After growth in the indicated medium for 16 h, GFP fluorescence of bacterium-sized particles was analyzed by flow cytometry, and the percentage of GFP-expressing bacteria is indicated. For controls, S. enterica serotype Typhimurium wild type (WT) or a strain deficient in ssrB, each harboring plasmid pLS824 were cultured for 7 days with sequential subcultures in the presence of carbenicillin. pLS824 is a derivative of low-copy-number vector pWSK29 harboring the GFP expression cassette.
The specificity of regulation of the intracellular activated promoter Pro sseA remained identical throughout the course of the experiment. Compared to strains harboring plasmid-borne cassettes grown under selective pressure, GFP fluorescence of strains harboring chromosomally integrated cassettes was lower (Fig. 2B). Expression of chromosomal integrated cassettes under noninducing conditions (LB and PCN media) was not observed, indicating proper control by the SsrAB regulatory system. Furthermore, expression cassettes with Pro sseA in the background of an ssrB mutation were not induced under any culture conditions (Husseiny and Hensel, unpublished). These observations indicate that expression cassettes introduced by replacement of chromosomal loci allow the construction of stable carrier strains with regulated expression of heterologous antigens.
To test stability in vivo, a strain was used for oral immunization of mice in which the purD locus was replaced by an expression cassette with the aph gene. Analyses of feces indicated that this carrier strain was shed for up to 9 days. Plating on selective agar with or without kanamycin indicated that the recovered S. enterica serovar Typhimurium were Kanr (data not shown), supporting the stability of chromosomal integrated cassettes during vaccination.
Attenuation of virulence by knock-in mutagenesis.
Various genes were investigated as targets for integration of expression cassettes and simultaneous generation of attenuating mutations. We selected aroA, galE, recA, purD, or htrA as target sites, since mutant strains deficient in these genes have previously been described as attenuated vaccine strains or are known targets for attenuation (9, 17). The target specificity was determined by selection of knock-in primer set as listed in Table 2. Successful integration of expression cassettes into aroA or purD was phenotypically confirmed the auxotrophy of the resulting strain. Furthermore, all integrations were confirmed by PCR.
TABLE 2.
Oligonucleotides used in this study
| Designation | Sequence |
|---|---|
| aph-for-KpnI | 5′-GTTGGTACCGTGTAGGCTGGAGCTGCTTC-3′ |
| aph-rev-KpnI | 5′-ATCGGTACCATATGAATATCCTCCTTAG-3′ |
| phoN-knockin-left | 5′-GCTGTGGCCAGTTTGCGGGAAGACTTTCACCTTCAGTAATTAAGATACGACTCACTATAGGGCG-3′ |
| phoN-knockin-right | 5′-CTGTTTATTATTGCCTGATCCGGAGTGAGTCTTTATGAAAAGTTGACCATGATTACGCCAAGC-3′ |
| aroA-knockin-left | 5′-GTTGAGTTTCATGGAATCCCTGACGTTACAACCCATCGCGCGGATACGACTCACTATAGGGCG-3′ |
| aroA-knockin-right | 5′-CGCCAGCCCGTCGACTGGCGCAACAGAAGACTTAGGCAGGCGTTGACCATGATTACGCCAAGC-3′ |
| recA-knockin-left | 5′-TGCCCGCCCCACCATCACCTGATGATTAAAAATCTTCGTTGGTATACGACTCACTATAGGGCG-3′ |
| recA-knockin-right | 5′-ATCCGGTTCAATACCAAGTTGCATGACAGGAGTAATAATGGCTTGACCATGATTACGCCAAGC-3′ |
| htrA-knockin-left | 5′-ATGGCGGAAGGGGGACAAAGGTGATTACTGCATCAGCAAATAAATACGACTCACTATAGGGCG-3′ |
| htrA-knockin-right | 5′-GCGTTACCTGTTAATCGAGATTGAAACACATGAAAAAAACCACTGACCATGATTACGCCAAGC-3′ |
| purD-knockin-left | 5′-AAACGCTGCTGGCTAAACTCAGCCGTTAGTTTTGCTCACGCTCATACGACTCACTATAGGGCG-3′ |
| purD-knockin-right | 5′-TCACTGACATGCGCCACTTCCGCCATTAATGGAGCGAAACATGTGACCATGATTACGCCAAGC-3′ |
| galE-knockin-left | 5′-ATTAAATGGGGTCATAACAACGTCCTTAATCTGGGTATCCCTGATACGACTCACTATAGGGCG-3′ |
| galE-knockin-right | 5′-ATGGTTATTCCATACCATAGGCTTAACGGAGCGAATTATGAGATGACCATGATTACGCCAAGC-3′ |
In order to analyze the effect of the knock-in mutagenesis on the virulence of the resulting strains, infection studies were performed with mixtures of a new knock-in strain and the aroA strain SL7207 as a standard attenuated carrier strain. The competitive index (CI) as a sensitive value for the relative virulence of the strains was calculated (Table 3). As observed above, the attenuation of systemic virulence of strain SL7207 was similar to that of a strain deficient in sseC of SPI2. Furthermore, strain SL7207 and an aroA mutant generated by the knock-in approach (MvP462) were equally attenuated. Knock-in strains with galE, htrA, or purD deleted showed levels of attenuation similar to that of strain SL7207. However, strain MvP455 harboring a replacement of recA by the expression cassette was recovered from the spleens of infected mice 2 orders of magnitude more frequently than strain SL7207. This observation is in line with the previous observation that a recA mutant strain has a lower 50% lethal dose than an aroA-deficient strain (1). Attenuation of a recA strain might therefore be insufficient for the use as vaccine carrier strain.
TABLE 3.
Mixed infection experiments for analyses attenuation of virulencea
| Group | Strainb | Genotype | CI |
|---|---|---|---|
| a | HH104 | sseC | 2.43 |
| b | MvP453 | galE | 2.15 |
| c | MvP455 | recA | 108c |
| d | MvP458 | purD | 1.35 |
| e | MvP459 | htrA | 1.99 |
| f | MvP462 | aroA | 1.93 |
Groups of three mice were infected intraperitoneally with an inoculum consisting of a mixture of about 103 CFU of strain SL7207 (aroA) and about 103 CFU of the recombinant knock-in strains as indicated. For a control, a mixture of SL7207 and the SPI2-deficient strain HH104 was used for infection. The animals were sacrificed 48 h after infection, and the spleens were removed. Serial dilutions of spleen homogenates were plated onto agar plates containing streptomycin or kanamycin for the enumeration of CFU of strains SL7207 and/or knock-in strains, respectively. The CI was calculated as the output ratio of the CFU of knock-in strains to the CFU of SL7207 wild-type bacteria divided by the input ratio of knock-in to SL7203 bacteria. The CI is the geometric mean obtained from three mice for the mixed infections with the aroA mutant strain. The CI values of groups b to f were compared to group a by using the Student t test.
That is, a mixed infection with SL7207 aroA strain.
P < 0.05; the other CI values were not significantly different.
Expression cassettes are functional after insertion into various loci.
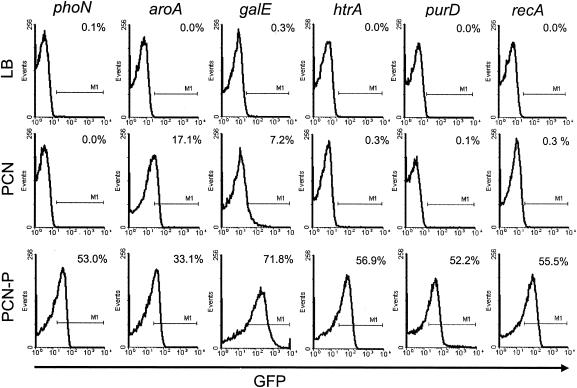
Various chromosomal loci were investigated for insertion of expression cassettes. Expression cassettes for GFP and OVA could be stable integrated into aroA, galE, htrA, purD, and recA. The regulated expression of knock-in cassettes with GFP under the control of the promoter Pro sseA was analyzed by flow cytometry of recombinant strains grown under conditions noninducing or inducing Pro sseA (Fig. 3). Induction of the GFP reporter was similar after integration of expression cassettes into phoN, htrA, purD, or recA. In contrast, an expression cassette inserted into aroA showed an aberrant pattern of regulation, i.e., low induction and high background expression. The expression cassettes inserted into the galE locus showed the highest induction under inducing growth conditions but also background expression under noninducing conditions.
FIG. 3.
Integration of expression cassettes into various chromosomal loci. Expression cassettes consisting of the intracellular activated promoter Pro sseA and GFP were integrated into various chromosomal loci of S. enterica serovar Typhimurium as indicated (phoN, aroA, galE, htrA, purA, or recA). The strains were grown in phosphate-limited minimal medium (PCN-P) known to induce expression under control of Pro sseA or rich (LB) or minimal (PCN) medium not inducing expression under control of Pro sseA. GFP fluorescence of bacterium-sized particles was analyzed by flow cytometry, and the percentage of GFP-expressing bacteria is indicated.
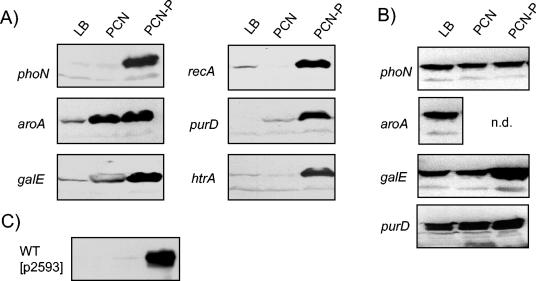
We also analyzed expression cassettes for the model antigen OVA under control of regulated or constitutive promoters after integration into various chromosomal loci (Fig. 4). Western blot analyses of bacterial cultures grown under noninducing conditions (LB and PCN medium) or inducing conditions (PCN-P) indicated regulated expression. Insertion of the Pro sseA OVA expression cassette into aroA or galE resulted in high background expression of the antigen (Fig. 4A). In general, the amounts of OVA expressed from genes on expression cassettes inserted into the chromosomes were lower than the amounts of OVA detected in strains harboring plasmids containing the expression cassettes (Fig. 4C). Introduction of expression cassettes with OVA under control of a constitutive promoter resulted in equal expression under various growth conditions as indicated by the similar amount of OVA in cell lysates (Fig. 4B and data not shown).
FIG. 4.
Regulated expression of the model antigen OVA after integration into various chromosomal loci. Strains harboring expression cassettes for OVA under control of the intracellular activated promoter Pro sseA (A) or the constitutive promoter Pro cat (B) integrated into various chromosomal loci (phoN, aroA, galE, recA, purD, or htrA) were grown in vitro in media inducing expression from SPI2 promoters. (C) For a control, S. enterica serovar Typhimurium wild type harboring low-copy-number plasmid p2593 expressing OVA under control of Pro sseA was grown in various media. Lysates containing equal amounts of bacteria were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses for the detection of OVA. For growth in PCN or PCN-P medium, aroA- or purD-deficient strains were supplemented as described in Materials and Methods. n.d., not determined.
These data demonstrate that expression cassettes can be inserted into various chromosomal locations and allow controlled expression of foreign antigens by attenuated Salmonella carrier strains.
Stimulation of immune response by strains harboring expression cassettes.
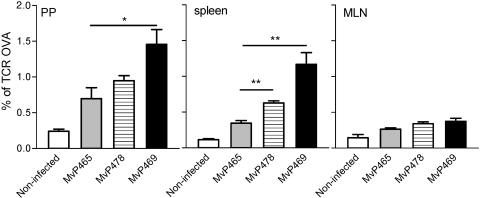
We analyzed whether strains harboring expression cassettes integrated in the chromosome were capable in triggering immune responses in experimental vaccination. The availability of transgenic DO11.10 mice expressing a T-cell receptor specific for an OVA epitope makes it possible to follow to the stimulation of T-cell proliferation after vaccination with OVA-expressing carrier strains. T cells from DO11.10 mice are transferred to naive BALB/c mice, which were subsequently infected with recombinant S. enterica serovar Typhimurium strains. We compared the numbers of OVA-specific T cells in various organs after vaccination with strains that were attenuated by replacement of the galE gene by various expression cassettes (Fig. 5). Compared to nonimmunized mice or mice immunized with a strain expressing an irrelevant antigen (GPF), higher numbers of OVA-specific T-cell were detected after vaccination with strains expressing OVA. T-cell proliferation was significantly enhanced in Peyer's patches and spleens by a carrier strain expressing OVA under control of the intracellular activated promoter Pro sseA. The observations indicate that recombinant strains expressing heterologous antigens from a cassette integrated into the chromosome can stimulate a specific immune response.
FIG. 5.
Proliferation of OVA-specific T cells after immunization with recombinant Salmonella carrier strains. Adoptive transfer of T cells from spleens of DO11.10 mice was performed as described in Materials and Methods. One day later, cohorts of five mice were immunized by intraperitoneal injection of recombinant Salmonella carrier strains attenuated by knock-in of expression cassettes into the galE locus. MvP465 harbors an expression cassette for GFP, whereas MvP478 and MvP469 harbor cassettes for expression of OVA under the control of a constitutive and intracellular activated promoter, respectively. Mice were sacrificed 7 days after immunization, and the amounts of CD4+ T cells expressing the transgenic TCR in cell suspensions for Peyer's patches (PP), mesenteric lymph nodes (MLN), and spleens were determined by flow cytometry. The data shown are representative for three independent experiments. Means ± standard errors of the mean are shown for the different groups, and the statistical significance was calculated by the Student t test. ✽, P < 0.05; ✽✽, P < 0.01.
DISCUSSION
In this report, we describe a novel approach for the generation of recombinant Salmonella vaccine strains. The approach is based on a modification of the Red recombinase technique that allows the simple, one-step construction of Salmonella strains that express heterologous antigens for vaccination strategies involving live carriers.
The Red recombinase technique has been introduced by Datsenko and Wanner (5) for the replacement of single genes or operons in E. coli by resistance genes that could be subsequently deleted by FLP-mediated recombination. We have modified the technique so that expression cassettes can be stably introduced into the chromosome of Salmonella. After removal of the antibiotic resistance marker, the expression of heterologous antigens by the cassettes was stable over many generations. We also observed that expression of chromosomally integrated cassettes containing a promoter specifically activated by intracellular Salmonella was tightly regulated.
Our approach has important advantages to construction of carrier strains for vaccination. Antibiotic resistance markers are only required during the construction of strains and can easily be eliminated. Since antibiotic resistance markers have to be avoided in recombinant strains that will ultimately be delivered to a vaccinee, maintenance of plasmid-based expression systems for heterologous antigens was often achieved by balanced lethal systems.
A further advantage of the system described here is the option of creating attenuating mutations concomitantly with introduction of the heterologous antigen. As demonstrated for targeting of expression cassettes into the aroA locus of Salmonella, a simple recombination event results in a strain that is attenuated, as well as expressing the heterologous antigen. We could also insert expression cassettes into purD, htrA, aroA, or galE, resulting in deletion of these loci. It was shown before that mutant strains deficient in these genes are highly attenuated in virulence and represent safe and efficient vaccine carrier strains. We also observed that deletions in aroA, htrA, purD, or galE generated by the knock-in approach were highly attenuated in mouse virulence. The construction of strains expressing a heterologous antigen with different attenuating mutations can be performed very rapidly by selecting the appropriate knock-in primer sets.
A possible limitation of the system might be the low amount of heterologous antigen due to the chromosomal expression cassettes in single copy in contrast to plasmid-based systems with multiple copies. In the present study, we used an intracellular activated promoter derived from SPI2 for regulated expression, but it is possible that other in vivo-activated promoters allow higher expression levels. Future work is necessary to determine the optimal promoters for high-level expression of heterologous antigens from chromosomally integrated cassettes.
In summary, we developed a versatile approach for vaccine carrier strain construction, since different targets for attenuation can be investigated just by design of a set of specific primers for amplification of the insertion cassette. It will be of future interest to use this approach also for the construction of recombinant vaccine strains of other bacterial species.
Acknowledgments
This study was supported by grants HE 1964/7-2 of the priority program Novel Vaccination Strategies of the Deutsche Forschungsgemeinschaft and QLK2-CT-1999-00310 of the European Commission. M.I.H. was a recipient of the long-term mission system of the embassy of the Arabic Republic Egypt.
We thank Daniela Jäckel for excellent technical support and Cédric Cheminay for assistance with the flow cytometry.
Editor: J. B. Bliska
REFERENCES
- 1.Buchmeier, N. A., C. J. Lipps, M. Y. So, and F. Heffron. 1993. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol. Microbiol. 7:933-936. [DOI] [PubMed] [Google Scholar]
- 2.Bumann, D. 2001. Regulated antigen expression in live recombinant Salmonella enterica serovar Typhimurium strongly affects colonization capabilities and specific CD4+-T-cell responses. Infect. Immun. 69:7493-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtiss, R., J. E. Galan, K. Nakayama, and S. M. Kelly. 1990. Stabilization of recombinant avirulent vaccine strains in vivo. Res. Microbiol. 141:797-805. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deiwick, J., T. Nikolaus, S. Erdogan, and M. Hensel. 1999. Environmental regulation of Salmonella Pathogenicity Island 2 gene expression. Mol. Microbiol. 31:1759-1764. [DOI] [PubMed] [Google Scholar]
- 7.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153-161. [DOI] [PubMed] [Google Scholar]
- 8.Galan, J. E., K. Nakayama, and R. Curtiss. 1990. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 94:29-35. [DOI] [PubMed] [Google Scholar]
- 9.Germanier, R., and E. Fuer. 1975. Isolation and characterization of Gal E mutant Ty21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J. Infect. Dis. 131:553-558. [DOI] [PubMed] [Google Scholar]
- 10.Groisman, E. A., M. H. Saier, Jr., and H. Ochman. 1992. Horizontal transfer of a phosphatase gene as evidence for mosaic structure of the Salmonella genome. EMBO J. 11:1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen-Wester, I., B. Stecher, and M. Hensel. 2002. Type III secretion of Salmonella enterica serovar Typhimurium translocated effectors and SseFG. Infect. Immun. 70:1403-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 157:1149-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hautefort, I., M. J. Proenca, and J. C. Hinton. 2003. Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl. Environ. Microbiol. 69:7480-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella Pathogenicity Island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 15.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 15a.Husseiny, M. I., and M. Hensel. Evaluation of an intracellular-activated promoter for the generation of live Salmonella recombinant vaccines. Vaccine, in press. [DOI] [PubMed]
- 16.Jantsch, J., C. Cheminay, D. Chakravortty, T. Lindig, J. Hein, and M. Hensel. 2003. Intracellular activities of Salmonella enterica in murine dendritic cells. Cell. Microbiol. 5:933-945. [DOI] [PubMed] [Google Scholar]
- 17.Lowe, D. C., T. C. Savidge, D. Pickard, L. Eckmann, M. F. Kagnoff, G. Dougan, and S. N. Chatfield. 1999. Characterization of candidate live oral Salmonella typhi vaccine strains harboring defined mutations in aroA, aroC, and htrA. Infect. Immun. 67:700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloy, S. R., V. L. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Medina, E., P. Paglia, T. Nikolaus, A. Müller, M. Hensel, and C. A. Guzman. 1999. Pathogenicity island 2 mutants of Salmonella typhimurium are efficient carriers for heterologous antigens and enable modulation of immune responses. Infect. Immun. 67:1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollenkopf, H., G. Dietrich, and S. H. Kaufmann. 2001. Intracellular bacteria as targets and carriers for vaccination. Biol. Chem. 382:521-532. [DOI] [PubMed] [Google Scholar]
- 21.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strugnell, R. A., D. Maskell, N. Fairweather, D. Pickard, A. Cockayne, C. Penn, and G. Dougan. 1990. Stable expression of foreign antigens from the chromosome of Salmonella typhimurium vaccine strains. Gene 88:57-63. [DOI] [PubMed] [Google Scholar]
- 23.Uzzau, S., N. Figueroa-Bossi, S. Rubino, and L. Bossi. 2001. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. USA 98:15264-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 25.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 26.Yan, Z. X., F. Reuss, and T. F. Meyer. 1990. Construction of an invertible DNA segment for improved antigen expression by a hybrid Salmonella vaccine strain. Res. Microbiol. 141:1003-1004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, Y., F. Buchholz, J. P. Muyrers, and A. F. Stewart. 1998. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20:123-128. [DOI] [PubMed] [Google Scholar]