Abstract
The presence of Porphyromonas gingivalis in the periodontal pocket and the high levels of gingipain activity detected in gingival crevicular fluid could implicate a role for gingipains in the destruction of the highly vascular periodontal tissue. To explore the effects of these proteases on endothelial cells, we exposed bovine coronary artery endothelial cells and human microvascular endothelial cells to gingipain-active extracellular protein preparations and/or purified gingipains from P. gingivalis. Treated cells exhibited a rapid loss of cell adhesion properties that was followed by apoptotic cell death. Cleavage of N- and VE-cadherin and integrin β1 was observed in immunoblots of cell lysates. There was a direct correlation between the kinetics of cleavage of N- and VE-cadherin and loss of cell adhesion properties. Loss of cell adhesion, as well as N- and VE-cadherin and integrin β1 cleavage, could be inhibited or significantly delayed by preincubation of P. gingivalis W83 gingipain-active extracellular extracts with the cysteine protease inhibitor Nα-p-tosyl-l-lysine chloromethylketone. Furthermore, purified gingipains also induced endothelial cell detachment and apoptosis. Apoptosis-associated events, including annexin V positivity, caspase-3 activation, and cleavage of the caspase substrates poly(ADP-ribose) polymerase and topoisomerase I (Topo I), were observed in endothelial cells after detachment. All of the effects observed were correlated with the different levels of cysteine-dependent proteolytic activity of the extracts tested. Taken together, these results indicate that gingipains from P. gingivalis can alter cell adhesion molecules and induce endothelial cell death, which could have implications for the pathogenicity of this organism.
Porphyromonas gingivalis is an asaccharolytic, black-pigmented, gram-negative anaerobe that is strongly associated with chronic periodontitis (13), a chronic inflammatory condition affecting tooth-supporting tissues that ultimately results in tooth loss, and may be involved in systemic illnesses, such as atherosclerosis (reviewed in references 28 and 44). P. gingivalis is well known for its cysteine proteases, designated gingipains, of two different specificities that are both extracellular and membrane bound. The rgpA gene encodes the arginine-specific protease isoforms HRgpA, RgpA(cat), and mt-RgpA, while rgpB encodes the RgpB and mt-RgpB isoforms. The lysine-specific protease Kgp is encoded by the kgp gene (55). Not only are the gingipains important in nutrition for P. gingivalis, they also have numerous other effects on host systems, including dysregulation of clotting and fibrinolytic pathways, activation of the kallikrein/kinin pathway, and modulation of host cytokine networks (reviewed in reference 41). It has also been established that gingipains can activate matrix metalloproteinases (MMPs) (16, 18, 31, 41; reviewed in reference 55). MMPs can cleave numerous cell surface proteins such as cytokine precursors, growth factors, cytokine receptors, and cell adhesion molecules, including cadherins, causing their shedding from the cell surface in a regular manner (3, 7, 19, 25, 26, 33, 48). However, the involvement of gingipains in periodontal tissue destruction is not clearly understood.
Studies of gingival crevicular fluid (GCF) have shown that it is a mixture of numerous proteases of both host and bacterial origin (reviewed in reference 55). The host-derived activities of both neutrophil elastase and MMPs, especially collagenase, have been shown to correlate with periodontal tissue destruction in vivo. Gingipain activity can be measured during disease progression in GCF, and its quantification can be used to predict attachment loss (20). It has been demonstrated that gingipains can cleave laminin, fibronectin, and collagen in vitro; however, this by itself does not seem to be sufficient to cause the destruction of the periodontal connective tissue. Because gingipains can activate host MMPs, the gingipains may be indirectly mediating the observed tissue destruction (55). Alternatively, the gingipains may harm cells of the periodontal pocket, thereby directly causing tissue damage.
Apoptosis, or programmed cell death, is a physiological form of cell death that has a characteristic morphology and set of associated biochemical events. Apoptotic cells are present in periodontal lesions (24, 40, 59, 67) along with an increased number of p53-positive cells (24, 67) and a decrease in Bcl-2-positive cells (59, 67), both important apoptosis-regulating molecules. It is well documented that proteases from P. gingivalis can induce cell death in different cell types in vitro, including fibroblasts, epithelial cells, and endothelial cells (5, 30, 37, 51, 62, 69). In addition, we have previously shown that gingipain-active extracts can induce apoptosis in KB epithelial cells (12). In that same study, we determined that prior to cell death the cell adhesion molecules (CAMs) neural cadherin (N-cadherin) and integrin β1 were cleaved and that KB cells lost adhesion to the culture surface. This finding is consistent with several reports demonstrating the ability of gingipains to cleave numerous CAMs in various cell types (4, 34, 39, 65). Since gingipains induce CAM cleavage and cell detachment, a special type of apoptosis may be induced by gingipains, called anoikis. It is triggered by cellular detachment and loss of integrin signaling and has not yet been fully characterized (22). Anoikis has been implicated in pathological processes such as cardiovascular disease (50).
Our previous report demonstrated that gingipain-active extracts from P. gingivalis W83 induced cell detachment and apoptosis in KB epithelial cells (12). Furthermore, extracts from strain FLL32, a recA-defective mutant with significantly reduced gingipain activity (1), and a W83 extract pretreated with Nα-p-tosyl-l-lysine chloromethylketone (TLCK), a cysteine protease inhibitor shown to inhibit both Rgp and Kgp activity (21, 54), exhibited no significant cell rounding and detachment (12). Because of the degradation of proteins of epithelial cell-cell junctional complexes, P. gingivalis may be able to invade and spread in deeper structures via a paracellular pathway (38). The periodontal pocket is a highly vascularized tissue in close proximity to the bacterial biofilm (15) that is separated from endothelial cells by one to two epithelial cells and a negligible layer of extracellular matrix (71). It has been suggested that in periodontitis, proapoptotic signals, such as direct toxic effects of bacterial products from the nearby plaque, at times overwhelm the usual antiapoptotic functional signals that maintain periodontal vessels (71). Because of the close proximity of epithelial cells and endothelial cells in the periodontal pocket, the damage of endothelial cells in periodontitis lesions (71), and the apoptosis that occurs in periodontitis (24, 40, 59, 67), we investigated if the gingipains would also induce loss of adhesion properties in two different types of endothelial cells, bovine coronary artery endothelial cells (BCAEC) and human microvascular endothelial cells (HMVEC). In patients with aggressive periodontal disease, total gingipain activity present in GCF ranged from 47.5 to 407.3 μU/μl (20). We demonstrate here that gingipain-active extracts with an enzyme activity similar to that of GCF of aggressive periodontal lesions induce apoptosis in both BCAEC and HMVEC. Prior to apoptosis, endothelial cells detached from the culture surface and N-cadherin, vascular endothelial cadherin (VE-cadherin), and integrin β1 were cleaved, suggesting that gingipains may induce cell death via anoikis.
MATERIALS AND METHODS
P. gingivalis strains and culture conditions.
P. gingivalis strains W83 and FLL32, a recA-defective mutant with reduced proteolytic activity that is nonvirulent in a mouse model (1), were grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% yeast extract (Difco Laboratories), hemin (5 μg/ml), vitamin K (0.5 μg/ml), and cysteine (0.1%) (all from Sigma-Aldrich, St. Louis, Mo.). All cultures were incubated at 37°C in an anaerobic chamber (Coy Manufacturing, Ann Arbor, Mich.) in 10% H2, 10% CO2, and 80% N2.
Gingipain extract preparation and protease assay.
P. gingivalis W83 and FLL32 were grown as previously described (12). Bacterial cultures were centrifuged (12,000 × g, 45 min, 4°C) to remove cells and then filtered through a 0.45-μm-pore filter (Millipore, Bellerica, Mass.). The extracellular culture fluid was acetone precipitated at −20°C in a 60:40 ratio of acetone (Fisher Scientific, Tustin, Calif.) to cell-free medium, with constant stirring. This precipitate was centrifuged (12,000 × g, 30 min, 4°C), and the pellet was resuspended in a solution containing 150 mM NaCl (VWR Scientific, Brisbane, Calif.), 20 mM Bis-Tris (Sigma-Aldrich), and 5 mM CaCl2 (VWR Scientific). The resuspended pellets were dialyzed at 4°C in Spectrapor 12,000- to 14,000-molecular-weight-cutoff dialysis tubing versus 4 liters of the same buffer with Aldrithiol-4 (Sigma-Aldrich), to stabilize the gingipains, overnight with three more changes of dialysis buffer without Aldrithiol-4. After dialysis, the sample was centrifuged (34,000 × g, 1 h, 4°C), and the resulting supernatant was concentrated in a pressurized stirring concentrator (Millipore) with a 10,000-molecular-weight-cutoff membrane at 4°C. This concentrated gingipain-active extract was clarified by centrifugation (192,000 × g, 1 h, 4°C) and stored in aliquots at −80°C.
To determine gingipain activity, 5 μl (for Rgp) and 15 μl (for Kgp) of concentrated extract were preincubated in a final volume of 150 μl of activated assay buffer, pH 7.6, containing 0.2 M Tris-HCl, 0.1 M NaCl, and 9 mM l-cysteine. The reaction was initiated by the addition of 50 μl of 4 mM N-α-benzoyl-dl-arginine-p-nitroanilide (BAPNA) (Sigma), for Rgp activity, or acetyl-lysine-p-nitroanilide (ALNA) (Bachem, King of Prussia, Pa.), for Kgp activity, which was added to the 150-μl reaction mixture, and the rate of enzymatic substrate hydrolysis was read with a microplate reader (Bio-Rad, Hercules, Calif.) at 405 nm using its kinetic measurement software program. One unit of gingipain activity is defined as the amount of enzyme releasing 1 pmol of p-nitroanilide per minute as calculated based on maximum velocity and the extinction coefficient of p-nitroanilide of 9,200 at 405 nm. For each sample used in this paper, gingipain activity was calculated based on the average of three measurements of the maximum velocity of the enzymatic reaction of specific substrate turnover and converted to units of gingipain activity.
Purification of gingipains from P. gingivalis strain HG66.
HRgpA, Kgp, and RgpB were purified from strain HG66 culture fluid as described previously (54, 56). Briefly, Kgp and HRgpA were purified by using gel filtration and arginine-Sepharose chromatography, while RgpB was purified by using a combination of gel filtration and anion-exchange chromatography on Mono Q (56). In this way, approximately 5 mg of each gingipain from 1 liter of bacterial culture was obtained in homogenous form as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Eukaryotic cell culture and gingipain treatments.
BCAEC and HMVEC were cultured according to the manufacturer's specifications and using their medium and reagents (Cambrex, Walkersville, Md.). Briefly, BCAEC (or HMVEC) were thawed and seeded at a density of 2,500 cells/cm2 (5,000 cells/cm2 for HMVEC) in Falcon T-75 flasks in 15 ml of EGM-MV medium (EGM-2 MV for HMVEC). The medium was changed on the cells every other day and doubled in volume when the cells were approximately 50% confluent. Cells from passages 6 through 9 were tested for viability prior to seeding in appropriate tissue culture dishes and allowed to reach near-confluency before gingipain treatment. Culture medium was removed from culture dishes and replaced with fresh medium containing 5 mM l-cysteine to activate the gingipains. Gingipain-active extracellular extracts or purified gingipains were added to the endothelial cells and allowed to incubate for the indicated times at 37°C in 5% CO2. Cells were also treated with 200 μg of P. gingivalis FLL32 extract/ml of medium (equivalent to 0.73 units of Rgp activity/ml of medium and 0.49 units of Kgp activity/ml of medium) as a negative control for gingipain activity and to exclude any other bacterial factors. To inhibit gingipain activity, purified gingipains or 200 μg of W83 extracts/ml of medium was pretreated for at least 30 min on ice with 10 mM TLCK (Sigma) and then added to the cells. Untreated cells did not have P. gingivalis extracts added to the cysteine-containing medium.
Caspase-3 (DEVDase) activity assay.
BCAEC were seeded in 250 μl of medium in black, clear-bottom, 96-well tissue culture plates and treated as described above with gingipain extracts in 100 μl of medium. Staurosporine (STR) (4 μM) was used as a positive control. Treatment was allowed to continue for the indicated times, and caspase-3 activity was determined by cleavage of the fluorescent substrate acetyl-Asp-Gly-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC; Alexis Biochemicals, San Diego, CA) according to the method of Carrasco and colleagues (8). One-step caspase-3/7 assay buffer (50 μl) containing dithiothreitol, Ac-DEVD-AMC, and phenylmethylsulfonyl fluoride was added to the wells and then incubated at 37°C in 5% CO2 for 1 h. The plate was then read at excitation and emission wavelengths of 380 and 460 nm, respectively, in a BIO-TEK Instruments (Winooski, Vermont) FLX800 microplate fluorescence reader. To calculate the increase in DEVDase activity, the average relative fluorescence intensity of three treated wells was divided by the average relative fluorescence intensity of three untreated wells. Standard error of the mean was calculated from seven independent trials, and significance was determined using the two-tailed, nonpaired Student's t test.
Quantification of apoptosis in BCAEC by flow cytometry.
Endothelial cells were seeded in duplicate onto 60-mm-diameter dishes and treated with P. gingivalis W83 extract as described above. After the indicated incubations, the cells were removed by pipette from both dishes, placed into a 15-ml tube, and centrifuged for 5 min at 2,500 rpm in a Labnet Hermle Z300 clinical centrifuge. The supernatant was removed, and the cell pellet was resuspended in 2 to 4 ml of 1× binding buffer (Annexin V-FITC Apoptosis Detection Kit I; BD Biosciences Pharmingen, San Diego, Calif.). One hundred microliters of cells was incubated for 15 min at room temperature in the dark with 5 μl of annexin V-fluorescein isothiocyanate and/or 5 μl of propidium iodide (PI) (both from the apoptosis detection kit). For each time point, a total of 10,000 unlabeled cells, cells stained with PI only, cells stained with annexin V only, and cells stained with annexin-V and PI were counted with a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, Calif.) using CellQuest software. The percentage of annexin V-positive cells was calculated from the quadrants as follows: percent annexin V-positive cells = (upper right + lower right in annexin V- and PI-stained cells) − (upper left + upper right + lower right from unstained cells) − (upper right + lower right from cells stained with PI only) − (upper left from cells stained with annexin V). The standard error of the mean was calculated from four independent trials.
Preparation of BCAEC lysates for SDS-PAGE.
Attached BCAEC were washed once with Dulbecco's phosphate-buffered saline (DPBS) and directly lysed in the plate with cell lysis buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 10% glycerol) containing complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, Ind.). Floating cells were collected by centrifugation and washed with DPBS plus complete protease inhibitor cocktail and combined with the lysed, attached cells. Lysates were heated to 95°C for 5 min and stored at −80°C until required. Prior to determination of the protein concentration (DC protein assay; Bio-Rad), lysates were sonicated to shear DNA.
SDS-PAGE and Western blot analysis.
Total protein in NuPAGE sample buffer and reducing agent were heated at 72°C for 10 min and separated on NuPAGE 4 to 12% Bis-Tris gels at 200 V for 60 min according to the supplier's instructions (Invitrogen, Carlsbad, Calif.). Gels were transferred to Optitran nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.) in NuPAGE XCell blot module at 30 V for 1 to 2 h according to the manufacturer's instructions. Nitrocellulose membranes were incubated with blocking buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20, 2% nonfat powdered milk) for 30 min with shaking. Membranes were probed with an appropriate dilution of primary antibody in blocking buffer for 1.5 h in a humidity chamber without shaking and washed for 5 min in blocking buffer with shaking. Membranes were incubated for 30 min with appropriate horseradish peroxidase-conjugated secondary antibodies diluted in blocking buffer in a humidity chamber without shaking. Membranes were washed with shaking in 1× phosphate-buffered saline (Sigma-Aldrich) with 0.1% Tween 20 five times for 5 min each. A 1:1 dilution of Western Lightning Chemiluminescence Plus (Perkin-Elmer Life Sciences, Boston, Mass.) reagents was added to the membrane and incubated for 3 min with shaking. Excess substrate was blotted from the membrane prior to exposure of the membrane to film.
The primary antibodies used in these experiments were N-cadherin clone 32 and integrin β1 clone 18 (BD Biosciences), VE-cadherin clone C-19 (Santa Cruz Biotechnology, Santa Cruz, Calif.), poly(ADP-ribose) polymerase (PARP) clone C2-10 (R & D Systems, Minneapolis, Minn.), and a highly specific human autoantibody to topoisomerase I (Topo I) (a generous gift from Eng M. Tan, The Scripps Research Institute, La Jolla, Calif.).
Immunoprecipitation and cleavage of cadherins.
BCAEC were seeded onto 60-mm-diameter dishes as described above and allowed to grow to confluency. Dishes were placed on ice and were washed once in cold 1× DPBS after medium was removed. One milliliter of cold native radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% IGEPAL CA-360 [equivalent to Triton X-100], 0.25% sodium deoxycholate) was added to the dishes and allowed to incubate for 10 to 20 min on ice. Lysed cells were collected by scraping, placed in 1.5-ml tubes, and centrifuged at 4°C for 10 min. The supernatants of 10 60-mm-diameter dishes were combined and placed into 15-ml screw-cap tubes. Four hundred microliters of a 50% slurry of protein G-agarose beads (Roche Applied Science) was added to 10 ml of lysed BCAEC and allowed to rotate at 4°C for 2.5 h. The beads were collected by centrifugation in a clinical centrifuge for 1 min, and the supernatants were transferred to new tubes. Twenty-five microliters of N-cadherin antibody or VE-cadherin antibody was added to the precleared supernatants and allowed to rotate at 4°C for 2 h. Four hundred microliters of a 50% slurry of protein G-agarose beads was added and allowed to rotate at 4°C overnight. After centrifugation, the supernatants were removed, and the beads were washed with native RIPA buffer and placed into a 1.5-ml tube. The beads were centrifuged at 6,000 × g for 1 min and then washed two times for 20 min with rotation at 4°C in native RIPA buffer. The beads were then washed two times in activated assay buffer (see above). The beads were finally resuspended in 400 μl of activated assay buffer.
For in vitro cleavage of immunoprecipitated N-cadherin and VE-cadherin, 25 μl of the resuspended bead mix was mixed with 0.125 μg of W83 extract (equivalent to 0.19 units of Rgp activity and 0.01 units of Kgp activity), with and without TLCK pretreatment, and 0.125 μg of FLL32 extract (0.0037 units of Rgp activity and 0.0025 units of Kgp activity) and incubated at 37°C for the indicated times. Control samples were incubated for the duration of the time course (20 min). After incubation, 10 mM TLCK and sample buffer were added to the reaction tube to a final volume of 50 μl, and the tube was immediately placed at −20°C. Half of the sample volume was separated by SDS-PAGE and immunoblotted versus N-cadherin and VE-cadherin as described above. For the VE-cadherin immunoblot, a secondary antibody designed to react only with the native rabbit antibody (True Blot; eBioscience, San Diego, Calif.) was used.
RESULTS
Endothelial cells lose adhesion properties after treatment with gingipain-active extracts.
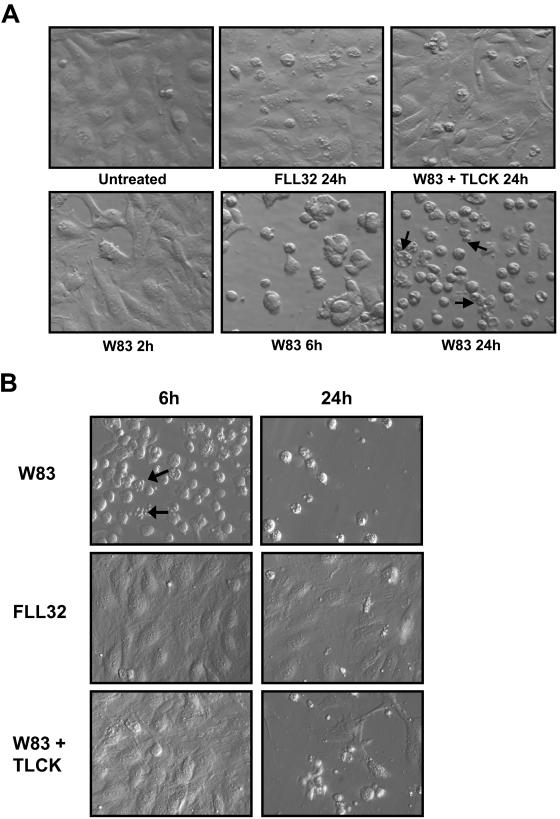
Endothelial cells rounded and detached from the culture surface and from each other in the presence of active gingipains and did not detach with TLCK-inactivated gingipains or extracts from P. gingivalis FLL32 (Fig. 1). A time course of gingipain treatment of BCAEC shows the beginning of detachment at 2 h, nearly complete detachment at 6 h, and apoptosis occurring by 24 h with no evidence of necrotic cells during these treatments (Fig. 1A). In contrast, BCAEC treated with extracts from FLL32 and W83 pretreated with TLCK to inhibit gingipain activity displayed minimal detachment and cell death by 24 h. HMVEC responded similarly to treatment with gingipain-active extracts but seemed to be more sensitive than BCAEC as evidenced by the higher levels of cells with apoptotic morphology present by 6 h (Fig. 1B). TLCK was not able to completely prevent the detachment and apoptosis induced by the gingipain-active extract treatment in HMVEC. It is possible that TLCK may be more toxic to HMVEC or that these cells were more stressed during the culturing process. Since both cell lines displayed similar morphological responses to gingipain-active extract treatment and BCAEC were healthier throughout culturing procedures, we chose BCAEC for subsequent experiments. Taken together, these results suggest that the gingipains are responsible for inducing the morphological changes exhibited by both BCAEC and HMVEC.
FIG.1.
Loss of adhesion and apoptosis in endothelial cells treated with P. gingivalis extracts. (A) BCAEC were treated with 200 μg of extracellular proteins from strains W83 (70 units of Rgp activity/ml of medium and 5.3 units of Kgp activity/ml of medium) and FLL32 (0.73 units of Rgp activity/ml of medium and 0.49 units of Kgp activity/ml of medium) per ml of medium in the presence of 5 mM l-cysteine for the indicated times. (B) HMVEC were treated with 200 μg of extracellular proteins from strains W83 (113 units of Rgp activity/ml of medium and 12.4 units of Kgp activity/ml of medium) and FLL32 (0.73 units of Rgp activity/ml of medium and 0.49 units of Kgp activity/ml of medium) per ml of medium in the presence of 5 mM l-cysteine for the indicated times. Extracellular extracts from W83 were preincubated on ice with 10 mM TLCK to inhibit both Rgp and Kgp activity before cell treatment. Arrows indicate cells with apoptotic morphology.
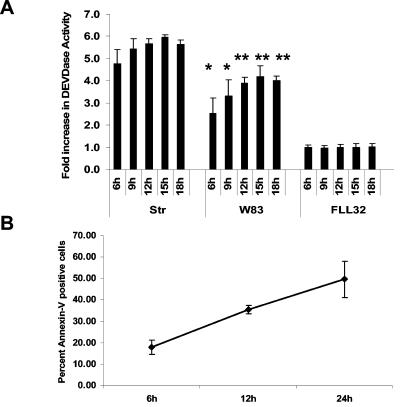
Gingipain-active extracts induce caspase-3 activation and annexin V positivity in BCAEC.
When cells are no longer attached to the extracellular matrix and integrin-mediated survival signals are lost, the cell can be committed to die via anoikis (22, 68). We have previously shown that apoptotic cell death in KB cells is induced by the proteolytic activity present in the P. gingivalis extracellular extracts and that their detachment precedes cell death (12). To verify that the gingipains induce apoptosis in endothelial cells, we measured the increase of DEVDase (caspase-3) activity fluorometrically (Fig. 2A) in BCAEC treated with P. gingivalis gingipain-active extracts. Measuring the activity of caspase-3 is normally used to assess the induction of apoptosis, irrespective of the apoptotic pathway activated. As a positive control, BCAEC were treated with 4 μM STR, a classic inducer of apoptosis. As expected with STR, caspase-3 was activated quickly, with maximal levels occurring between 12 and 15 h. In W83 extract treatments, there was a significant increase in DEVDase activity compared to that of FLL32 extract treatments (P < 0.005 for 6 and 9 h and P < 0.0004 for 12, 15, and 18 h of W83 extract treatment). There was no significant difference between early time points of TLCK pretreatment of W83 extract and FLL32 extract treatment (6 and 9 h; P > 0.12) (data not shown). Moreover, TLCK inhibition of gingipain activity significantly inhibited caspase-3 activation at 6 h compared to W83 treatment at 6 h (P < 0.04) (data not shown). These results confirmed that gingipains induce an apoptotic morphology and caspase-3 activation characteristic of apoptosis in BCAEC. The levels of apoptosis after detachment induced by W83 gingipain-active extracts were quantified by flow cytometry. There was an increase in the percentage of annexin V-positive cells from approximately 20% at 6 h to nearly 50% at 24 h (Fig. 2B). There was no evidence that gingipain extract treatment induced primary necrosis (cells positive for PI only) in BCAEC during the 24-h time course (data not shown). Collectively, our results indicate that gingipain-active extracts induce endothelial cell detachment and apoptosis.
FIG. 2.
Treatment of BCAEC with gingipain-active extracts induces activation of DEVDase activity and annexin V positivity. (A) BCAEC were treated with 4 μM STR or extracts (200 μg/ml) from W83 (113 units of Rgp activity/ml of medium and 12.4 units of Kgp activity/ml of medium, 76.2 units of Rgp activity/ml of medium and 5.2 units of Kgp activity/ml of medium, or 230 units of Rgp activity/ml of medium and 19.4 units of Kgp activity/ml of medium) and FLL32 (0.73 units of Rgp activity/ml of medium and 0.49 units of Kgp activity/ml of medium) for the indicated times and then assayed for cleavage of the fluorescent caspase-3 substrate Ac-DEVD-AMC. Error bars indicate standard errors of the means, with *P < 0.005 and **P < 0.0004. (B) BCAEC were treated with extracts (200 μg/ml) from W83 (230 units of Rgp activity/ml of medium and 19.4 units of Kgp activity/ml of medium). Beginning at 6 h, when endothelial cells detached, cells were collected and stained with annexin V and/or PI. Stained cells were then counted with a BD FACSCalibur flow cytometer using the CellQuest software. Error bars indicate standard errors of the means.
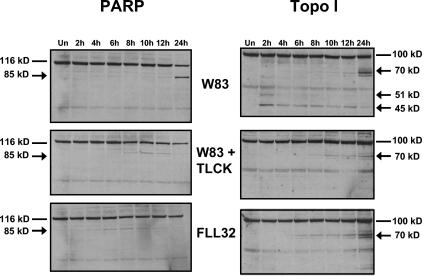
Gingipain-active extracts induce cleavage of PARP and Topo I.
Endothelial cell lysates were monitored by Western blotting for cleavage of PARP and Topo I. The cleavage products of these proteins that occur during cell death can be used to distinguish between apoptosis and necrosis. During apoptosis, caspases specifically cleave PARP and Topo I into 85- and 70-kDa products, respectively. However, in necrosis, PARP is cleaved into a 50-kDa product and Topo I is cleaved into 70- and 45-kDa products (9). In cell lysates from BCAEC treated with extract from W83 for 24 h, PARP was cleaved into its signature 85-kDa apoptotic fragment with no 50-kDa necrotic fragment, and Topo I was cleaved into its apoptotic 70-kDa product (Fig. 3). These cleavages were significantly inhibited by reducing the levels of gingipain activity. However, cells treated with extracts of FLL32 and extracts of W83 pretreated with TLCK, which have minimal gingipain activity and do not induce high levels of apoptosis, still showed some cleavage of PARP and Topo I. These results suggest that the residual amount of gingipain activity may be enough to induce low levels of apoptosis and limited caspase substrate cleavage.
FIG. 3.
Treatment of BCAEC with gingipain-active extracts induces cleavage of caspase substrates. Twenty micrograms of protein from BCAEC treated in the presence of 5 mM l-cysteine with W83 extract (70 units of Rgp activity/ml of medium and 5.3 units of Kgp activity/ml of medium) was separated by SDS-PAGE and immunoblotted with a monoclonal antibody to PARP (R & D Systems) and a highly specific human autoantibody to Topo I (9). Arrows indicate cleavage products. Un, untreated.
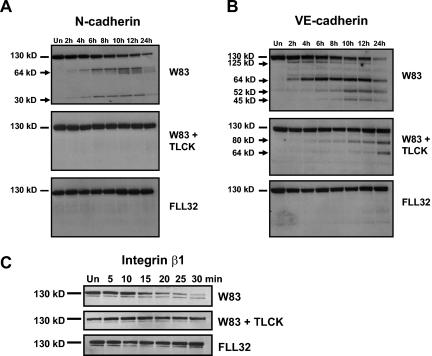
Gingipain-active extracts induce cleavage of N-cadherin, VE-cadherin, and integrin β1.
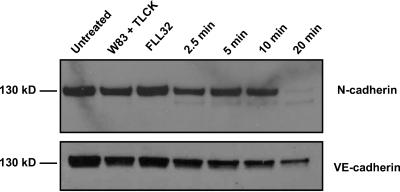
Cells treated with gingipain-active extracts detach quickly from the culture surface, suggesting the cleavage of CAMs. This would be consistent with other studies showing that gingipains can cleave different CAMs in several cell types (5, 30, 37, 51, 62, 69). Therefore, we expected gingipain-induced CAM cleavage in endothelial cells, which was confirmed by Western blot analysis of BCAEC lysates with different anti-CAM antibodies (Fig. 4). N-cadherin was cleaved into several fragments ranging from 62 to 65 kDa and into a single 30-kDa product (Fig. 4A). Cleavage of N-cadherin did not occur in the presence of inactivated gingipains or with FLL32 extract treatment. Only extracts from W83 caused the disappearance of full-length N-cadherin in HMVEC (data not shown), further establishing that these two endothelial cell types react similarly to treatment with gingipain-active extracts. In addition, VE-cadherin in BCAEC is also cleaved into four predominant fragments by gingipain-active extracts from P. gingivalis W83 (Fig. 4B). It is noteworthy that VE-cadherin was cleaved prior to N-cadherin in as little as 2 h after treatment. Surprisingly, TLCK pretreatment of W83 extracts was not able to completely inhibit VE-cadherin cleavage. VE-cadherin in the presence of TLCK was cleaved into two products, one of which was also present in W83-treated cells.
FIG. 4.
Cleavage of N-cadherin, VE-cadherin, and integrin β1 by gingipain-active extracts from strain W83. Ten micrograms of protein from BCAEC treated in the presence of 5 mM l-cysteine with W83 extracts (70 units of Rgp activity/ml of medium and 5.3 units of Kgp activity/ml of medium) and TLCK-treated W83 (W83 + TLCK) and FLL32 extracts (0.73 units of Rgp activity/ml of medium and 0.49 units of Kgp activity/ml of medium) for the indicated times was separated by SDS-PAGE and immunoblotted with a monoclonal antibody to N-cadherin (A) or a polyclonal antibody to VE-cadherin (B). (C) BCAEC treated with W83 extract (200 μg/ml) (230 units of Rgp activity/ml of medium and 19.4 units of Kgp activity/ml of medium), FLL32 extract (0.73 units of Rgp activity/ml of medium and 0.49 units of Kgp activity/ml of medium), and W83 extract pretreated with 10 mM TLCK were harvested after treatment for the indicated times, and 20 μg was separated by SDS-PAGE. Membranes were reacted with a monoclonal antibody to integrin β1. Arrows indicate cleavage products. Un, untreated.
Endothelial cells express several different members of the integrin β1 family along with αvβ3 and αvβ5 (58). Because of the predominance of integrin β1 on endothelial cells, we investigated the effects of the gingipains on this cell adhesion molecule. Integrin β1 appeared to be degraded even more rapidly and completely than VE-cadherin. By 2 h, there was no longer any full-length integrin β1 detectable by Western blotting, while the full-length protein remained in cells treated with FLL32 and TLCK-inactivated W83 extracts (data not shown). The amount of full-length integrin β1 was decreased at 15 min, with near-total disappearance at 30 min (Fig. 4C). Remarkably, complete integrin β1 degradation occurred considerably before the beginning of cell detachment at 2 h (Fig. 1A). Treatment of BCAEC with either FLL32 extracts or W83 extracts pretreated with TLCK did not result in integrin β1 degradation (Fig. 4C). These data show that gingipain-active extracts have significant multiple effects on endothelial cell adhesion to other cells and to the extracellular matrix and potentially on cell survival signaling, which may lead to detachment-induced apoptosis.
Gingipain-active extracts can directly cleave N-cadherin and VE-cadherin.
To determine if the cleavages of N-cadherin and VE-cadherin resulted from direct action of the gingipains, we performed the cleavage in a cell-free system devoid of endothelial cell factors. N-cadherin and VE-cadherin were immunoprecipitated from untreated BCAEC and then treated with extracts from W83, W83 pretreated with TLCK, and FLL32. Treatment with W83 extracts showed complete disappearance of the full-length N-cadherin band within 20 min and significant decrease of the VE-cadherin band in the same amount of time (Fig. 5). As expected, there was little change in the intensity of either cadherin band after treatment with inactivated gingipains or FLL32 extract. These results strongly suggest that the gingipains can directly cleave CAMs.
FIG. 5.
Gingipains can cleave N-cadherin and VE-cadherin immunoprecipitated from BCAEC. N-cadherin and VE-cadherin were immunoprecipitated from BCAEC lysates. The immunoprecipitated N-cadherin and VE-cadherin were incubated with 0.125 μg of W83 extract (equivalent to 0.19 units of Rgp activity and 0.01 units of Kgp activity), in the presence and absence of TLCK, and 0.125 μg of FLL32 extract (0.0037 units of Rgp activity and 0.0025 units of Kgp activity) for the indicated times. Control samples were incubated for the full time course. Samples were separated by SDS-PAGE, and Western blots were performed with antibodies to N-cadherin and VE-cadherin.
Purified gingipains induce loss of adhesion in BCAEC.
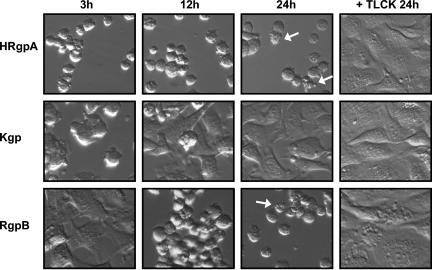
BCAEC were treated with purified HRgpA, Kgp, and RgpB to further demonstrate that the gingipains directly induce cell detachment and to ascertain which gingipain(s) may be responsible for the observed effects. All three gingipains caused the detachment of BCAEC that could be prevented by pretreatment with TLCK (Fig. 6). Interestingly, BCAEC treated with Kgp detached first (data not shown); but over the duration of the time course, most of the cells reattached to the culture substrate and continued to grow. Cells treated with RgpB showed a delay in detachment compared to cells treated with HRgpA and Kgp; but at 24 h, the only difference between HRgpA and RgpB was that RgpB-treated cultures had more clumps of detached cells, which may indicate differences in adhesion molecule cleavage. Furthermore, cell cultures treated with HRgpA and RgpB exhibited apoptosis following detachment. Collectively, these results confirmed that the gingipains are responsible for BCAEC detachment and suggest that they may have a synergistic function in inducing endothelial cell detachment and, subsequently, apoptosis.
FIG. 6.
Purified gingipains induce BCAEC detachment and apoptosis. BCAEC were treated with purified HRgpA (8 μg/ml of medium), Kgp (3 μg/ml), or RgpB (5.2 μg/ml of medium) (all equivalent to 113 units of Rgp activity/ml of medium or 12.4 units of Kgp activity/ml of medium) in the presence of 5 mM l-cysteine for the indicated times. Purified gingipains were preincubated on ice with 10 mM TLCK to inhibit both Rgp and Kgp activity before cell treatment. Arrows indicate cells with apoptotic morphology.
DISCUSSION
In this study, we have demonstrated that gingipains induce detachment and apoptosis in endothelial cells. This is consistent with reports of gingipain-induced apoptosis in several other cell types (5, 30, 37, 51, 62, 69). However, the kinetics of cell adhesion molecule cleavage appear to differ between epithelial and endothelial cells (12). While heat-stable molecules (such as butyric acid and lipopolysaccharide) produced by P. gingivalis can induce cell death (27, 32, 36, 42, 43), it is unlikely that the apoptosis induced in our experimental conditions was not gingipain dependent. Furthermore, there was no evidence indicating that the gingipains can induce primary necrosis in BCAEC. Interestingly, however, in BCAEC treated with W83 extract, there was the early, transient appearance of a 45-kDa cleavage product of Topo I, usually associated with necrosis (9). Since gingipains can traverse the plasma membrane and localize within the cell (60), it is tempting to speculate that the gingipains could directly cleave Topo I, which may have involvement in the cell death process. Surprisingly, TLCK pretreatment of W83 extracts was not able to completely inhibit VE-cadherin cleavage, most likely from incomplete gingipain inhibition. TLCK has been shown to more completely inhibit Kgp activity than Rgp activity (54). Alternatively, other bacterial or host factors could be responsible for this cleavage, or it could also be a by-product of apoptosis. This seems unlikely, since cells treated with FLL32 extracts show no VE-cadherin cleavage, gingipains can directly cleave immunoprecipitated cadherins, and there was no VE-cadherin cleavage when apoptosis was induced with staurosporine (data not shown). To our knowledge, this is the first demonstration of gingipain-induced cleavage of VE-cadherin, N-cadherin, and integrin β1 in endothelial cells.
Since the gingipain adhesin domains can modulate P. gingivalis adherence to epithelial cells (10, 11), it is not surprising that Kgp and HRgpA were able to induce morphological changes before RgpB. This would also be consistent with the report that HRgpA was more effective than RgpA(cat) in causing the loss of integrin β1 expression on human gingival fibroblasts (61). It is noteworthy that BCAEC treated with Kgp quickly detached and then over time reattached, with no significant loss in cell viability. It is possible that the purified Kgp was less stable than purified HRgpA or RgpB or the Kgp present in extracellular extracts from W83. Thus, in the continued presence of Kgp, with diminishing activity, the endothelial cells are able to recover, possibly through upregulation of adhesion molecules or cell survival proteins. There is evidence that Kgp can modulate gene expression in endothelial cells (52). We cannot rule out the possibility that in the periodontal pocket, where all gingipains would be present, they synergistically cause endothelial cell damage. Kgp and HRgpA could quickly adhere to endothelial cells and cause their detachment, while HRgpA and RgpB would be responsible for inducing cell death.
Because anoikis can be induced by loss of integrin signaling following cell detachment (23), integrin-extracellular matrix interactions are essential for cell survival and also protect endothelial cells from anoikis (2). The rapid degradation of integrin β1 suggests that the trigger for endothelial cell death induced by gingipains might be the loss of integrin signaling, which occurs prior to cell detachment and apoptosis. This would be consistent with a recent report demonstrating that disruption of focal adhesions and the actin cytoskeleton by overexpression of integrin β1 cytoplasmic domains preceded cell detachment and was, therefore, the cause rather than a consequence of endothelial cell detachment (53). Furthermore, it has been demonstrated that ligation of integrin β1 by antibodies protected fibroblasts from apoptosis through upregulation of phosphatidylinositol 3-kinase and Akt/protein kinase B activity (66) and that Akt is deactivated in both caspase-dependent and caspase-independent cell death in several cell types (46). It has even been suggested that integrins can mediate an apoptotic cell death distinct from anoikis, called “integrin-mediated death,” in which caspase-8 is recruited to unligated integrins (64) and triggers the caspase cascade of apoptosis.
Integrin β1 can directly interact with focal adhesion kinase (45) to transduce signals via Ras, phosphatidylinositol 3-kinase, and Akt, leading to upregulation of expression of bcl-2 (47), a prosurvival gene. If integrin signaling is prevented by gingipain-induced cleavage, it is likely that the levels of bcl-2 would decrease, sensitizing cells to die. Integrin β1 can also associate with Shc and signal survival through the mitogen-activated protein kinase pathway (70). Furthermore, since integrin β1 can regulate the proapoptotic protein Bim, it is also conceivable that loss of integrin function could release Bim and signal apoptosis through the Erk pathway (57). Integrins are associated with numerous survival pathways (reviewed in reference 63), and we are exploring the involvement of integrin β1 in gingipain-induced endothelial apoptosis-anoikis. In the periodontal pocket, integrin β1 is expressed within the gingival epithelium and junctional epithelium and in the endothelial cells of the connective tissue (35). Integrin β1 is consistently expressed in endothelial cells of all vessel types and sizes (49), and several different integrin β1 molecules are expressed on the luminal side of vessels (14). The tissue distribution of integrin β1 and the recent report that P. gingivalis invades aortic tissue in the ApoE−/− mouse model and accelerates atheroma development (29) raise the possibility that gingipains can cause endothelial cell dysfunction and apoptosis in both the periodontal pocket and the cardiovascular system.
It has been suggested that VE-cadherin, which is specific for endothelial cells of almost all types of vessels (17), promotes homotypic interactions with endothelial cells and that N-cadherin may be responsible for the anchorage of endothelial cells to other cell types. VE-cadherin acts as a seal at intercellular junctions, associating with β-catenin, plakoglobin, p120, and the actin cytoskeleton (17), and is a target of agents that increase vascular permeability. Inhibition of its function produced more damage to the endothelial monolayer in vivo than in vitro (6), suggesting that gingipain-induced disruption of VE-cadherin integrity may have a considerable role in the tissue damage that occurs in the periodontal pocket. It appears that the gingipains may be able to trigger endothelial cell detachment and cell death through degradation of several different molecules.
In conclusion, we have shown that gingipain-active extracts mediate the detachment and apoptosis of endothelial cells. BCAEC treated with gingipains become apoptotic, as determined by cell morphology, caspase-3 activation, annexin V positivity, and cleavage of PARP and Topo I. Gingipains cleave N-cadherin and VE-cadherin and degrade integrin β1, whereas extracts of FLL32 that have significantly reduced gingipain activity do not have cleaved CAMs. Gingipains directly cleave immunoprecipitated N-cadherin and VE-cadherin. Moreover, purified gingipains induce endothelial cell detachment and apoptosis, implicating the gingipains in vascular tissue destruction.
Acknowledgments
We thank Zhuo Chen for technical assistance in the initial stages of this work.
This work was supported by grant 3 PO4A 003 24 from the Committee of Scientific Research (KBN, Poland) (to J.P.), National Institutes of Health grant DE09761 (to J.T.), National Institutes of Health grant A144088 (to C.A.C.), Loma Linda University School of Dentistry, Loma Linda University School of Medicine Basic Research Support Grant, and Public Health Service grants DE13664 and DE13664-S1 from the National Institute of Dental and Craniofacial Research (to H.M.F.).
Editor: V. J. DiRita
REFERENCES
- 1.Abaibou, H., Q. Ma, G. J. Olango, J. Potempa, J. Travis, and H. M. Fletcher. 2000. Unaltered expression of the major protease genes in a non-virulent recA-defective mutant of Porphyromonas gingivalis W83. Oral Microbiol. Immunol. 15:40-47. [DOI] [PubMed] [Google Scholar]
- 2.Aoudjit, F., and K. Vuori. 2001. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-Flip and implications for anoikis. J. Cell Biol. 152:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arribas, J., L. Coodly, P. Vollmer, T. K. Kishimoto, S. Rose-John, and J. Massague. 1996. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem. 271:11376-11382. [DOI] [PubMed] [Google Scholar]
- 4.Baba, A., N. Abe, T. Kadowaki, H. Nakanishi, M. Ohishi, T. Asao, and K. Yamamoto. 2001. Arg-gingipain is responsible for the degradation of cell adhesion molecules of human gingival fibroblasts and their death induced by Porphyromonas gingivalis. Biol. Chem. 382:817-824. [DOI] [PubMed] [Google Scholar]
- 5.Baba, A., T. Kadowaki, T. Asao, and K. Yamamoto. 2002. Roles for Arg- and Lys-gingipains in the disruption of cytokine responses and loss of viability of human endothelial cells by Porphyromonas gingivalis infection. Biol. Chem. 383:1223-1230. [DOI] [PubMed] [Google Scholar]
- 6.Bazzoni, G., and E. Dejana. 2001. Pores in the sieve and channels in the wall: control of paracellular permeability by junctional proteins in endothelial cells. Microcirculation 8:143-152. [DOI] [PubMed] [Google Scholar]
- 7.Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitzner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385:729-733. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco, R. A., N. B. Stamm, and B. K. Patel. 2003. One-step cellular caspase-3/7 assay. BioTechniques 34:1064-1067. [DOI] [PubMed] [Google Scholar]
- 9.Casiano, C. A., R. L. Ochs, and E. M. Tan. 1998. Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 5:183-190. [DOI] [PubMed] [Google Scholar]
- 10.Chen, T., and M. J. Duncan. 2004. Gingipain adhesin domains mediate Porphyromonas gingivalis adherence to epithelial cells. Microb. Pathog. 36:205-209. [DOI] [PubMed] [Google Scholar]
- 11.Chen, T., K. Nakayama, L. Belliveau, and M. J. Duncan. 2001. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect. Immun. 69:3048-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Z., C. A. Casiano, and H. M. Fletcher. 2001. Protease-active extracellular protein preparations from Porphyromonas gingivalis W83 induce N-cadherin proteolysis, loss of cell adhesion, and apoptosis in human epithelial cells. J. Periodontol. 72:641-650. [DOI] [PubMed] [Google Scholar]
- 13.Christersson, L. A., J. J. Zambon, R. G. Dunford, S. G. Grossi, and R. J. Genco. 1989. Specific subgingival bacteria and diagnosis of gingivitis and periodontitis. J. Dent. Res. 68:1633-1639. [Google Scholar]
- 14.Conforti, G., C. Dominguez-Jimenez, A. Zanetti, M. A. Gimbrone, Jr., O. Cremona, P. C. Marchisio, and E. Dejana. 1992. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood 80:437-446. [PubMed] [Google Scholar]
- 15.Darveau, R. P., A. Tanner, and R. C. Page. 1997. The microbial challenge in periodontitis. Periodontol. 2000 14:12-32. [DOI] [PubMed] [Google Scholar]
- 16.DeCarlo, A. A., Jr., L. J. Windsor, M. K. Bodden, G. J. Harber, B. Birkedal-Hansen, and H. Birkedal-Hansen. 1997. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anaerobe Porphyromonas gingivalis. J. Dent. Res. 76:1260-1270. [DOI] [PubMed] [Google Scholar]
- 17.Dejana, E., G. Bazzoni, and M. G. Lampugnani. 1999. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp. Cell Res. 252:13-19. [DOI] [PubMed] [Google Scholar]
- 18.Ding, Y., V. J. Uitto, J. Firth, T. Salo, M. Haapasalo, Y. T. Konttinen, and T. Sorsa. 1995. Modulation of host matrix metalloproteinases by bacterial virulence factors relevant in human periodontal diseases. Oral Dis. 1:279-286. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers, M. R., and J. F. Riordan. 1991. Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry 30:10065-10074. [DOI] [PubMed] [Google Scholar]
- 20.Eley, B. M., and S. W. Cox. 1996. Correlation between gingivain/gingipain and bacterial dipeptidyl peptidase activity in gingival crevicular fluid and periodontal attachment loss in chronic periodontitis patients. A 2-year longitudinal study. J. Periodontol. 67:703-716. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher, H. M., H. A. Schenkein, and F. L. Macrina. 1994. Cloning and characterization of a new protease gene (prtH) from Porphyromonas gingivalis. Infect. Immun. 62:4279-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisch, S. M., and H. Francis. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frisch, S. M., and R. A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555-562. [DOI] [PubMed] [Google Scholar]
- 24.Gamonal, J., A. Bascones, A. Acevedo, E. Blanco, and A. Silva. 2001. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J. Periodontol. 72:517-525. [DOI] [PubMed] [Google Scholar]
- 25.Garton, K. J., P. J. Gough, J. Philalay, P. T. Wille, C. P. Blobel, R. H. Whitehead, P. J. Dempsey, and E. W. Raines. 2003. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). J. Biol. Chem. 278:37459-37464. [DOI] [PubMed] [Google Scholar]
- 26.Gearing, A. J., P. Beckett, M. Christodoulou, M. Churchill, J. Clements, A. H. Davidson, A. H. Drummond, W. A. Galloway, R. Gilbert, J. L. Gordon, T. M. Leber, M. Mangan, K. Miller, P. Nayee, K. Owen, S. Patel, W. Thomas, G. Wells, L. M. Wood, and K. Woolley. 1994. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 370:555-557. [DOI] [PubMed] [Google Scholar]
- 27.Geatch, D. R., J. I. Harris, P. A. Heasman, and J. J. Taylor. 1999. In vitro studies of lymphocyte apoptosis induced by the periodontal pathogen Porphyromonas gingivalis. J. Periodontal Res. 34:70-78. [DOI] [PubMed] [Google Scholar]
- 28.Gendron, R., D. Grenier, and L. Maheu-Robert. 2000. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2:897-906. [DOI] [PubMed] [Google Scholar]
- 29.Gibson, F. C., III, C. Hong, H. H. Chou, H. Yumoto, J. Chen, E. Lien, J. Wong, and C. A. Genco. 2004. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:2801-2806. [DOI] [PubMed] [Google Scholar]
- 30.Graves, D. T., M. Oskoui, S. Volejnikova, G. Naguib, S. Cai, T. Desta, A. Kakouras, and Y. Jiang. 2001. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J. Dent. Res. 80:1875-1879. [DOI] [PubMed] [Google Scholar]
- 31.Grayson, R., C. W. I. Douglas, J. Heath, A. Rawlinson, and G. S. Evans. 2003. Activation of human matrix metalloproteinase 2 by gingival crevicular fluid and Porphyromonas gingivalis. J. Clin. Periodontol. 30:542-550. [DOI] [PubMed] [Google Scholar]
- 32.Harris, J. I., R. R. B. Russell, M. A. Curtis, J. Aduse-Opoku, and J. J. Taylor. 2002. Molecular mediators of Porphyromonas gingivalis-induced T-cell apoptosis. Oral Microbiol. Immunol. 17:224-230. [DOI] [PubMed] [Google Scholar]
- 33.Herren, B., B. Levkau, E. W. Raines, and R. Ross. 1998. Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol. Biol. Cell 9:1589-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hintermann, E., S. K. Haake, U. Christen, A. Sharabi, and V. Quaranta. 2002. Discrete proteolysis of focal contact and adherens junction components in Porphyromonas gingivalis-infected oral keratinocytes: a strategy for cell adhesion and migration disabling. Infect. Immun. 70:5846-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hormia, M., J. Ylanne, and I. Virtanen. 1990. Expression of integrins in human gingiva. J. Dent. Res. 69:1817-1823. [DOI] [PubMed] [Google Scholar]
- 36.Isogai, E., H. Isogal, K. Kimura, N. Fujii, S. Takagi, K. Hirose, and M. Hayashi. 1996. In vivo induction of apoptosis and immune responses in mice by administration of lipopolysaccharide from Porphyromonas gingivalis. Infect. Immun. 64:1461-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson, A., and S. Kalfas. 1998. Characterization of the proteinase-dependent cytotoxicity of Porphyromonas gingivalis. Eur. J. Oral Sci. 106:863-871. [DOI] [PubMed] [Google Scholar]
- 38.Katz, J., V. Sambandam, J. H. Wu, S. M. Michalek, and D. F. Balkovetz. 2000. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect. Immun. 68:1441-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz, J., Q. B. Yang, P. Zhang, J. Potempa, J. Travis, S. M. Michalek, and D. F. Balkovetz. 2002. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect. Immun. 70:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koulouri, O., D. F. Lappin, M. Radvar, and D. F. Kinane. 1999. Cell division, synthetic capacity and apoptosis in periodontal lesions analysed by in situ hybridisation and immunohistochemistry. J. Clin. Periodontol. 26:552-559. [DOI] [PubMed] [Google Scholar]
- 41.Kuramitsu, H. K. 1998. Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol. Immunol. 13:263-270. [DOI] [PubMed] [Google Scholar]
- 42.Kurita-Ochiai, T., K. Ochiai, and K. Fukushima. 1998. Volatile fatty acid, metabolic by-product of periodontopathic bacteria, induces apoptosis in WEHI 231 and RAJI B lymphoma cells and splenic B cells. Infect. Immun. 66:2587-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurita-Ochiai, T., K. Ochiai, and K. Fukushima. 2001. Butyric acid-induced T-cell apoptosis is mediated by caspase-8 and -9 activation in a Fas-independent manner. Clin. Diagn. Lab. Immunol. 8:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, X., K. M. Kolltveit, L. Tronstad, and I. Olsen. 2000. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 13:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin, T. H., A. E. Aplin, Y. Shen, Q. Chen, M. Schaller, L. Romer, I. Aukhil, and R. L. Juliano. 1997. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J. Cell Biol. 136:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo, H. R., H. Hattori, M. A. Hossain, L. Hester, Y. Huang, W. Lee-Kwon, M. Donowitz, E. Nagata, and S. H. Snyder. 2003. Akt as a mediator of cell death. Proc. Natl. Acad. Sci. USA 100:11712-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matter, M. L., and E. Ruoslahti. 2001. A signaling pathway from the α5β1 and αvβ3 integrins that elevates bcl-2 transcription. J. Biol. Chem. 276:27757-27763. [DOI] [PubMed] [Google Scholar]
- 48.McGeehan, G. M., J. D. Becherer, R. C. Bast, Jr., C. M. Boyer, B. Champion, K. M. Connolly, J. G. Conway, P. Furdon, S. Karp, S. Kidao, A. B. McElroy, J. Nichols, K. M. Pryzwansky, F. Schoenen, L. Sekut, A. Truesdale, M. Verghese, J. Warner, and J. P. Ways. 1994. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature 370:558-561. [DOI] [PubMed] [Google Scholar]
- 49.Mechtersheimer, G., T. Barth, W. Hartschuh, T. Lehnert, and P. Moller. 1994. In situ expression of beta 1, beta 3 and beta 4 integrin subunits in non-neoplastic endothelium and vascular tumours. Virchows Arch. 425:375-384. [DOI] [PubMed] [Google Scholar]
- 50.Michel, J. B. 2003. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler. Thromb. Vasc. Biol. 23:2146-2154. [DOI] [PubMed] [Google Scholar]
- 51.Morioka, M., D. Hinode, A. Nagata, H. Hayashi, S. Ichimiya, M. Ueda, R. Kido, and R. Nakamura. 1993. Cytotoxicity of Porphyromonas gingivalis toward cultured human gingival fibroblasts. Oral Microbiol. Immunol. 8:203-207. [DOI] [PubMed] [Google Scholar]
- 52.Nassar, H., H. H. Chou, M. Khlgatian, F. C. Gibson III, T. E. Van Dyke, and C. A. Genco. 2002. Role for fimbriae and lysine-specific cysteine proteinase gingipain K in expression of interleukin-8 and monocyte chemoattractant protein in Porphyromonas gingivalis-infected endothelial cells. Infect. Immun. 70:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oguey, D., P. W. George, and C. Ruegg. 2000. Disruption of integrin-dependent adhesion and survival of endothelial cells by recombinant adenovirus expressing isolated beta integrin cytoplasmic domains. Gene Ther. 7:1292-1303. [DOI] [PubMed] [Google Scholar]
- 54.Pike, R., W. McGraw, J. Potempa, and J. Travis. 1994. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 269:406-411. [PubMed] [Google Scholar]
- 55.Potempa, J., A. Banbula, and J. Travis. 2000. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol. 2000 24:153-192. [DOI] [PubMed] [Google Scholar]
- 56.Potempa, J., J. Mikolajczyk-Pawlinska, D. Brassell, D. Nelson, I. B. Thogersen, J. J. Enghild, and J. Travis. 1998. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J. Biol. Chem. 273:21648-21657. [DOI] [PubMed] [Google Scholar]
- 57.Reginato, M. J., K. R. Mills, J. K. Paulus, D. K. Lynch, D. C. Sgroi, J. Debnath, S. K. Muthuswamy, and J. S. Brugge. 2003. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 5:733-740. [DOI] [PubMed] [Google Scholar]
- 58.Rupp, P. A., and C. D. Little. 2001. Integrins in vascular development. Circ. Res. 89:566-572. [DOI] [PubMed] [Google Scholar]
- 59.Sawa, T., F. Nishimura, H. Ohyama, K. Takahashi, S. Takashiba, and Y. Murayama. 1999. In vitro induction of activation-induced cell death in lymphocytes from chronic periodontal lesions by exogenous Fas ligand. Infect. Immun. 67:1450-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scragg, M. A., A. Alsam, M. Rangarajan, J. M. Slaney, P. Shepherd, D. M. Williams, and M. A. Curtis. 2002. Nuclear targeting of Porphyromonas gingivalis W50 protease in epithelial cells. Infect. Immun. 70:5740-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scragg, M. A., S. J. Cannon, M. Rangarajan, D. M. Williams, and M. A. Curtis. 1999. Targeted disruption of fibronectin-integrin interactions in human gingival fibroblasts by the RI protease of Porphyromonas gingivalis W50. Infect. Immun. 67:1837-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah, H. N., S. E. Gharbia, and C. M. O'Toole. 1992. Assessment of the relative cytotoxicity of Porphyromonas gingivalis cells, products, and components on human epithelial cell lines. J. Periodontol. 63:44-51. [DOI] [PubMed] [Google Scholar]
- 63.Stupack, D. G., and D. A. Cheresh. 2002. Get a ligand, get a life: integrins, signaling and cell survival. J. Cell Sci. 115:3729-3738. [DOI] [PubMed] [Google Scholar]
- 64.Stupack, D. G., X. S. Puente, S. Boutsaboualoy, C. M. Storgard, and D. A. Cheresh. 2001. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tada, H., S. Sugawara, E. Nemoto, T. Imamura, J. Potempa, J. Travis, H. Shimauchi, and H. Takada. 2003. Proteolysis of ICAM-1 on human oral epithelial cells by gingipains. J. Dent. Res. 82:796-801. [DOI] [PubMed] [Google Scholar]
- 66.Tian, B., K. Lessan, J. Kahm, J. Kleidon, and C. Henke. 2002. Beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J. Biol. Chem. 277:24667-24675. [DOI] [PubMed] [Google Scholar]
- 67.Tonetti, M. S., D. Cortellini, and N. P. Lang. 1998. In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingiva. Infect. Immun. 66:5190-5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vitale, M., T. Di Matola, M. Bifulco, A. Casamassima, G. Fenzi, and G. Rossi. 1999. Apoptosis induced by denied adhesion to extracellular matrix (anoikis) in thyroid epithelial cells is p53 dependent but fails to correlate with modulation of p53 expression. FEBS Lett. 462:57-60. [DOI] [PubMed] [Google Scholar]
- 69.Wang, P. L., S. Shirasu, M. Shinohara, M. Daito, M. Oido, Y. Kowashi, and K. Ohura. 1999. Induction of apoptosis in human gingival fibroblasts by a Porphyromonas gingivalis protease preparation. Arch. Oral Biol. 44:337-342. [DOI] [PubMed] [Google Scholar]
- 70.Wary, K. K., F. Mainiero, S. J. Isakoff, E. E. Marcantonio, and F. G. Giancotti. 1996. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87:733-743. [DOI] [PubMed] [Google Scholar]
- 71.Zoellner, H., C. C. Chapple, and N. Hunter. 2002. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc. Res. Tech. 56:15-31. [DOI] [PubMed] [Google Scholar]