Abstract
Pseudomonas aeruginosa utilizes a number of distinct pathways to secrete proteins that play various roles during infection. These include the type II secretion system, which is responsible for the secretion of the majority of exoproducts into the surrounding environment, including toxins and degradative enzymes. In contrast, the type III secretion system mediates the delivery of protein effectors directly into the cytoplasm of the host cell. Using tissue culture assays and a mouse acute-pneumonia model, we have determined the contribution of each of the type III effectors during infection. In strain PAK, ExoS is the major cytotoxin required for colonization and dissemination during infection. ExoT confers protection of tissue culture cells from type III-dependent lysis, while ExoY seemed to have little effect on cytotoxicity. ExoU is over 100-fold more cytotoxic than ExoS. The cytotoxicity of type II secretion was determined following deletion of the genes for the more toxic type III secretion system. The participation of these secretion systems during lifelong colonization of cystic fibrosis (CF) patients is unclear. By comparing clonal strains from the same patient isolated at the initial onset of P. aeruginosa infection and more than a decade later, after chronic colonization has been established, we show that initial strains are more cytotoxic than chronic strains that have evolved to reduce type III secretion. Constitutive expression of genes for the type III secretion system restored ExoS secretion but did not always reestablish cytotoxicity, suggesting that CF strains accumulate a number of mutations to reduce bacterial toxicity to the host.
Pseudomonas aeruginosa is an opportunistic human pathogen that is responsible for a range of infections in individuals with a variety of predisposing immune-compromising conditions. It is also the major pathogen of individuals with cystic fibrosis (CF), where it is the key contributor to the destructive lung disease (45). The ability of P. aeruginosa to colonize a large number of organs, in a variety of clinical settings, is largely attributable to the coding capacity of its large genome, which contains genes for a significant number of virulence factors (43).
The major mechanism that allows P. aeruginosa to proliferate in infected hosts and to overcome host defense mechanisms is the export of various protein factors via specialized secretion machineries. The genome of P. aeruginosa harbors genes for the type II secretion system and the type III secretion system (TTSS) (43). The xcp gene cluster encodes the functional type II secretion machinery (1, 29), which is responsible for the extracellular secretion of several well-characterized virulence factors, including exotoxin A (ExoA) (32), elastase (LasA) (26), and phospholipase C (23). The expression of these type II-secreted factors, as well as the xcp genes themselves, is controlled by quorum sensing, and the factors are expressed only at high bacterial density (31, 39, 49). ExoA is a potent toxin that ADP ribosylates eukaryotic elongation factor 2, causing an arrest of translation and eventually cell death (16). Elastase and phospholipase C degrade proteins and lipids, respectively, allowing P. aeruginosa to sequester nutrients from host cell components. These factors are thought to promote the dissemination of the organism from the site of infection. P. aeruginosa is also capable of utilizing a TTSS to directly inject effector proteins into the contacted host cell (55). Once inside the host cell, effector molecules modulate host functions important in cytoskeletal organization and signal transduction. Four type III-secreted effectors, ExoS, ExoT, ExoY, and ExoU, have been identified in P. aeruginosa. The majority of P. aeruginosa strains carry exoT and exoY; however, the presence of exoS and that of exoU appear to be mutually exclusive (5, 51). ExoS and ExoT are bifunctional enzymes that have an N-terminal small G-protein activating protein (GAP) domain (10, 18, 19) and a C-terminal ADP ribosylation domain (24, 44). Both ExoS and ExoT act on a number of host small G proteins, thus altering the cytoskeleton and signaling pathways (9, 10, 13, 18, 19). ExoY is an adenylate cyclase (56), and ExoU is a phospholipase that is a potent cytotoxin once injected inside a host cell (37). The type III effectors act rapidly, immediately following contact of the bacteria with the host cell, while the type II-secreted exoproducts act more slowly, after significant proliferation of the bacteria and induction of the quorum-sensing regulatory network.
The functions of these secretion systems during acute and chronic infections have been investigated. Using animal infection models that mimic acute human infections, such as burn wound, acute pneumonia, and corneal infections, it was shown that type III secretion is an important virulence mechanism (12, 15, 20, 22, 38). The conclusions from studies evaluating the contribution of the type II secretion system and its substrate to virulence in burn, corneal, and chronic lung infection models were inconsistent (6, 8, 28, 54). The relative contribution of each of these secretion systems and the virulence factors that utilize them during infections of CF patients are not completely understood. Analysis of mRNA isolated from sputum of CF patients revealed that genes for several of the type II-secreted products are transcribed (42). Additional evidence indicating that both type II and type III secretion systems are active during prolonged infection in CF patients was obtained by demonstrating that most CF patients harbor antibodies against proteins exported by type II and type III secretion mechanisms (2, 27, 33). Clinical isolates from CF patients have also been evaluated for expression of functional secretion systems (41). Most isolates secrete type II proteins, albeit at low levels (30, 53); however, most CF isolates lack the ability to secrete type III effector molecules (3, 36). These studies suggest that proteins secreted via the type II and type III pathways could play a role in infection only during initial colonization, not during the chronic stage of the disease. These assertions have not been formally tested by comparing clinical isolates that initially colonize the CF patient lung to genotypically identical strains isolated during chronic colonization from the same patient.
We sought to determine the contribution of each of the type III secretion effector molecules as well as the type II secretion system to cytotoxicity using various infection models. A classic plating assay was used to determine the level of toxicity induced by each effector and the long-term effect of injection during infection of tissue culture cells. ExoS and ExoU were the major cytotoxins in both in vitro and in vivo assays. ExoT protected cells in vitro from type III machinery-dependent cytotoxicity. However, ExoT and ExoY had a slightly more subtle effect in a mouse model of acute pneumonia. To assess the ability of P. aeruginosa to mediate type III secretion and cytotoxicity over the course of a chronic CF infection, we obtained clinical strains isolated from the same CF patients after being initially colonized and also more than a decade into the chronic infection (35). The ability of these strains to secrete type III effectors and intoxicate CHO cells was determined. Secretion of effectors and cytotoxicity were higher in the strains isolated first than in strains isolated later, suggesting that the environment in CF patient lungs selects for P. aeruginosa with attenuated cytotoxicity. Secretion of exoenzymes could be restored in the majority of the strains by overexpression of the TTSS transcriptional regulator ExsA. However, only a subset of those strains were cytotoxic, suggesting that persistence in the CF patient lung has caused additional genetic defects that prevent the activity or delivery of TTSS effectors.
MATERIALS AND METHODS
Media and growth condition.
P. aeruginosa PAK and derivatives were routinely grown in Luria-Bertani broth (LB). Antibiotics for selection were used at the following concentrations: gentamicin, 75 mg/ml; carbenicillin, 150 mg/ml. The pMMB-exsA plasmid (52) was maintained with 50 mg of carbenicillin/ml, and exsA expression was induced with 1 mM isopropylthiogalactose (IPTG).
Bacterial strains.
P. aeruginosa strain PAK and derivatives harboring various in-frame deletions were used in this study. Deletion of an internal fragment from each exoenzyme gene and the xcp operon was carried out as previously described (52). Briefly, 800- to 1,200-bp fragments upstream and downstream of the deleted gene were amplified by PCR. The two fragments were joined and tailed with attB1 and attB2 by a modified PCR termed splicing by overlap extension (50). The AttB extensions allow for Gateway cloning into pEXGmGW (52). Mutant alleles were introduced onto the chromosome of PAK as described previously (14). Deletion of exoS, exoT, exoY, pscC, and the xcp operon are designated in the paper as ΔS, ΔT, ΔY, ΔC, and ΔX, respectively. Additional strains that contained deletions in various combinations of the effectors were constructed. The pMMB-exsA plasmid was described previously (52). Clinical strains were isolated and characterized as described previously (35).
Low-calcium fractionation.
P. aeruginosa PAK and derivatives were grown overnight in LB. Bacteria were subcultured 1:1,000 in LB supplemented with 5 mM EGTA and grown for 6 h at 37°C with aeration. Bacterial densities were determined by optical density measurements at 600 nm. Bacteria were sedimented by centrifugation at 3,220 × g for 15 min at 4°C. Culture supernatant was collected, and proteins were precipitated with trichloroacetic acid and washed with acetone. Proteins were resuspended according to culture density and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were either stained with Coomassie or transferred onto polyvinylidene difluoride (PVDF) membranes for detection by antibodies against ExoS or ExoU.
LDH release assay.
Chinese hamster ovary (CHO) cells were routinely grown in F-12 medium supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine (full F-12). Prior to infection, confluent CHO cells were washed and covered with F-12 containing 1% FBS and 2 mM glutamine. P. aeruginosa was grown overnight in LB, subcultured into fresh LB, and grown to mid-log phase. CHO cells were infected with mid-log-phase P. aeruginosa at an initial multiplicity of infection (MOI) of 10. Culture supernatants were collected at the times indicated in the figure legends and centrifuged for 10 min at 3,220 × g to sediment bacteria and CHO cells. Lactose dehydrogenase (LDH) in the supernatant was measured with a Roche LDH kit in accordance with the manufacturer's instructions. Percent LDH release was calculated relative to that of the uninfected control, which was set at 0% LDH release, and that of cells lysed with Triton X-100, which was set at 100% LDH release.
CHO plating assay.
CHO cells were grown to confluence (1.2 × 106 cells) in six-well plates. Cells were infected with P. aeruginosa PAK and derivatives at a starting MOI of 10 for various times. Gentamicin, at a final concentration of 300 μg/ml, was added to the infection media as well as all subsequent solutions to kill P. aeruginosa. Culture media were aspirated, and cells were gently washed with 1 ml of phosphate-buffered saline. Cells were trypsinized with 250 μl of 0.05% trypsin-EDTA and resuspended in 750 μl of full F-12 containing 150 μg of gentamicin/ml. Cells were diluted serially and seeded into new six-well plates and grown for 5 days, thus allowing each viable cell to form foci, which were visualized by staining with 1% Gentian Violet in 70% ethanol.
Mouse infections.
Mouse infections were performed as previously described (40). Briefly, female BALB/c mice were anesthetized and infected intranasally with 2 × 106 to 5 × 107 CFU as described for each experiment. At 16 h postinfection, animals were sacrificed and dissected to obtain lung, liver (300 mg), and spleen. Bacteria in each organ were enumerated by plating serial dilutions of tissue homogenates on L-agar plates.
RESULTS
Generation and characterization of strains of P. aeruginosa with in-frame deletions of genes for type III effector proteins.
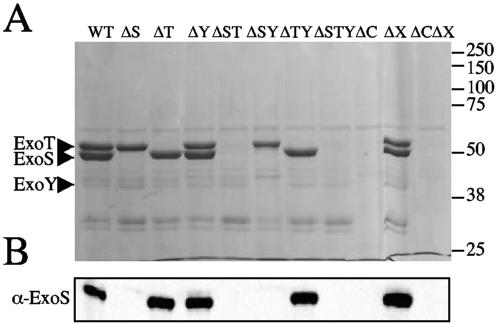
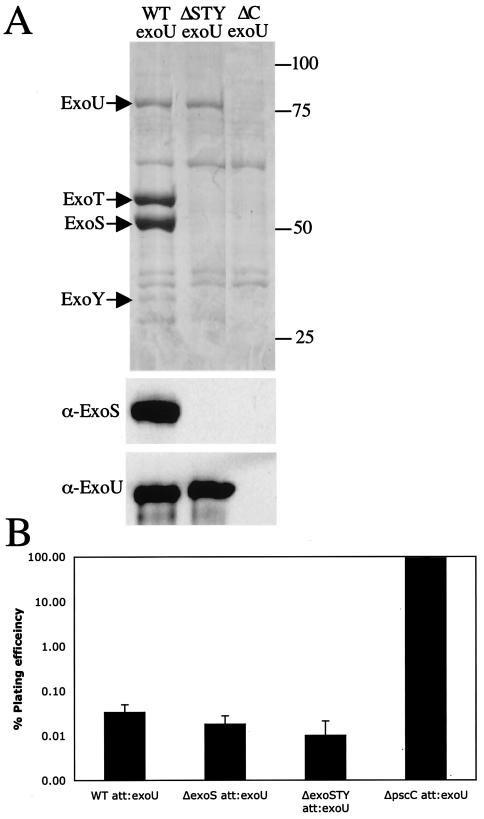
The genomes of most P. aeruginosa strains harbor genes for three of the four type III secreted effectors: ExoT, ExoY, and ExoS or ExoU. We used allelic exchange to generate in-frame deletions of the coding sequence of each effector, resulting in strains expressing these individually or in several combinations. Secreted proteins from TTSS-inducing, calcium-depleted culture supernatants were precipitated and separated by SDS-PAGE. ExoS and ExoT are the major secreted proteins of P. aeruginosa strain PAK, with mobilities corresponding to their predicted sizes (49 and 53 kDa, respectively) (Fig. 1A). ExoY is a minor secreted protein that is 37 kDa (Fig. 1A; see Fig. 5A). The secretion of these three effector proteins by wild-type P. aeruginosa PAK was dependent on the functional type III secretion machinery, since mutant PAKΔC, lacking the secretin component of the type III apparatus, did not secrete any of the effectors (Fig. 1, lane 9). Deletion of each gene encoding a type III effector resulted in the absence of the corresponding protein in culture supernatant (Fig. 1A). The production of ExoS was also confirmed by immunoblotting with anti-ExoS antibodies (Fig. 1B). Deletion of the xcp gene cluster (ΔX), which encodes the components of the type II secretion system, abolished secretion of the hemolytic phospholipase C and elastase (data not shown) but had no effect on the secretion of TTSS effectors (Fig. 1, lane 11). Certain strains of P. aeruginosa also carry a gene for another type III effector protein, ExoU, which is a cytotoxin with a phospholipase D activity (37). To compare the effects of all type III effectors in the same genetic background, the exoU-spcU operon from P. aeruginosa PA103 was cloned and integrated in a single copy at the CTX phage site in wild-type PAK and the ΔSTY mutant. These resulting strains expressed and secreted ExoU, as detected by staining with Coomassie or immunoblotting with anti-ExoU antibodies (see Fig. 5A).
FIG. 1.
Secretion profiles of P. aeruginosa strain PAK and isogenic derivatives induced for type III secretion. (A) Secreted proteins were separated by 12% PAGE and revealed by staining with Coomassie. The positions of ExoS, ExoT, and ExoY on the gel are indicated. WT, wild type. (B) Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS (α-ExoS). In-frame deletion mutation of each exoenzyme gene resulted in the loss of that specific protein from the induced cultures.
FIG. 5.
ExoU secretion enhances cytotoxicity of PAK and ΔSTY strains. (A) Secreted proteins from low-calcium-induced cultures were separated by SDS-PAGE. Total proteins were revealed by staining with Coomassie. Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS or ExoU. WT, wild type. (B) Plating efficiency of CHO cells after 2 h of infection with PAK strains harboring exoU.
Cytotoxic effect of P. aeruginosa type III effector mutants.
Delivery of effectors into target cells utilizing the TTSS has been implicated as a key virulence mechanism during acute infections by P. aeruginosa. Two common host cell types encountered by P. aeruginosa during infection are epithelial cells, which line the various mucosal surfaces, and cells mobilized by the innate host defenses that migrate to the site of infection. We utilized cultured CHO cells as a model for epithelial cells since they are sensitive to P. aeruginosa type III-mediated cytotoxicity (46). To determine the contribution of each of the type III effectors to the killing of cultured mammalian cells in vitro, we infected CHO cells with P. aeruginosa strains that have deletions of various type III effector molecules and determined the cytotoxic effect by measuring the release of LDH. This assay allowed the detection of cytotoxicity resulting from the disruption of plasma membrane integrity. We also developed a second assay based on the plating efficiency of CHO cells following exposure to P. aeruginosa (17). In this assay, the ability of CHO cells to replicate and form foci postinfection allows an evaluation of the long-term effects of each effector molecule on the intoxicated cell. The advantage of the plating assay is twofold. First, it is possible to determine the cytotoxicity and disruption in CHO cell replication at earlier infection times. Second, this assay provides a quantitative measure for the cytotoxic effect of each of the effectors.
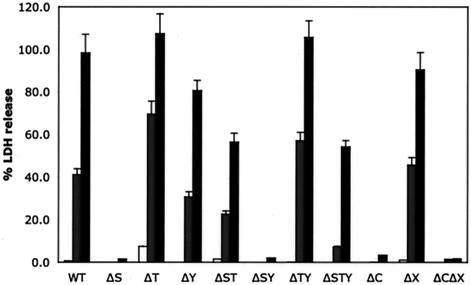
The LDH assay was used to measure the cytotoxicity of the CHO cells during a time course of infection. Supernatant samples were collected and analyzed at 3, 4, and 5 h postinfection. Levels of released LDH in the medium at 3 h postinfection were similar to those for uninfected cells (Fig. 2) even though cells were found to have a rounding morphology when examined by phase microscopy (data not shown). At 4 h postinfection, many of the CHO cells infected with wild-type P. aeruginosa strain PAK were lysed and had released approximately 40% of LDH compared to a Triton X-100-lysed sample (Fig. 2). At 5 h postinfection, greater than 90% of wild-type-infected CHO cells were lysed (Fig. 2). The cytotoxicity induced by PAK is type III secretion dependent, as a mutant lacking the type III secretion component PscC (PAKΔC) is noncytotoxic. Deletion of the type II secretion system (mutant PAKΔX) had no effect on LDH release. A mutant with a deletion of the exoS gene (PAKΔS) exhibited a reduced cytotoxicity for CHO cells. A mutant lacking ExoT (PAKΔT) showed enhanced cytotoxicity at all time points, whereas ΔY mutants had levels of toxicity similar to those of the parental PAK strain. The availability of the various combinations of ΔS, ΔT, and ΔY single, double, and triple mutants allows multiple pairwise comparisons between isogenic strains with and without each exoenzyme. These comparisons allow verification of the effect of each gene product. For ExoS, pairwise comparisons between the wild type and PAKΔS, PAKΔT, and PAKΔST, between PAKΔY and PAKΔSY, and between PAKΔTY and PAKΔSTY revealed that strains expressing ExoS induce a 3- to 10-fold increase in CHO cell membrane permeability at the later infection time points. Four similar pairwise comparisons of isogenic strains that harbor or lack the exoT gene revealed that, when strains containing exoT were tested, there was a two- to fivefold decrease in the toxicity for infected CHO cells. However, comparisons of isogenic strains with and without exoY show only minor differences, suggesting that exoY has no role in cytotoxicity to CHO cells. Mutants lacking both exoS and exoT (PAKΔST and PAKΔSTY) have features of both the exoS mutant and the exoT mutant. These mutants are less toxic than strains harboring exoS (PAKΔT and PAKΔTY) but more toxic than strains harboring exoT, (PAKΔS and PAKΔSY), and thus they appear to be as toxic as wild-type PAK (Fig. 2). These results suggest that ExoS is the major cytotoxin, while ExoT confers protection against cell lysis and ExoY appears to have no effect on CHO cells in this assay.
FIG. 2.
Contribution of each exoenzyme to the cytotoxicity of CHO cells infected with PAK or mutant derivatives. CHO cells were infected with PAK at a MOI of 10, and supernatants were collected at 3 (white bars), 4 (gray bars), and 5 h (black bars) postinfection. Cleared supernatants were analyzed for LDH release as a measure of cell lysis. WT, wild type.
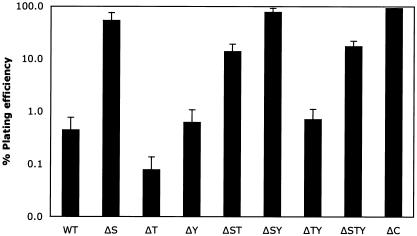
Cell rounding induced by P. aeruginosa TTSS effectors was observed by microscopy as early as 1 h after infection and well before the detection of LDH in culture supernatants. We sought to determine if the early delivery of the type III effectors caused any effect on CHO cell viability by utilizing the modified plating assay. The plating efficiency of wild-type PAK-infected CHO cells at 3 h postinfection was 0.4% (Fig. 3). However, the plating efficiency of type III secretion-defective mutant PAKΔC was greater than 90%. These results suggest that the replicative ability of CHO cells is disrupted well before disintegration of the plasma membrane and release of cytoplasmic proteins. Exposure of CHO cells to the ΔS mutant results in a plating efficiency of greater than 50%, suggesting that injection of ExoS by wild-type PAK results in a 100-fold decrease in CHO survival (Fig. 3). The plating efficiency of CHO cells infected with the ΔT mutant was 0.1%, showing that the delivery of ExoT provided a fourfold protection to these cells. The plating efficiency of mutant PAKΔY is similar to that of wild-type PAK. Pairwise comparisons of the isogenic strains harboring or lacking the exoS gene further demonstrated that ExoS reduces CHO cell survival by 100-fold. Pairwise comparison of isogenic strains with and without exoT supports the fourfold protective effect of ExoT. These results demonstrate the relative contribution of each P. aeruginosa effector protein to the cytotoxicity of in vitro-infected cells. Mutants lacking both exoS and exoT (PAKΔST and PAKΔSTY) displayed a phenotype intermediate between the toxicities of wild-type PAK and the ΔC strain. Both strains are less toxic as a result of the exoS deletion but more toxic as a result of the exoT deletion. In these cytotoxicity assays, each exoenzyme appears to act independently of the other effector, suggesting that the total cytotoxic effect of any PAK strain on CHO cells could be determined to be the added effect of the exoenzymes ExoS, -T, and -Y expressed by each strain.
FIG. 3.
Survival of CHO cells after infection with PAK or mutant derivatives. CHO cells were infected with PAK at a MOI of 10 for 3 h. Cells were trypsinized, serially diluted, and seeded into media containing gentamicin. The foci that formed from CHO cells infected with various strains of PAK were enumerated. The relative plating efficiency was compared to that for uninfected cells, which was set to 100%. WT, wild type.
Effect of mutations of type III effectors in an acute pneumonia infection model.
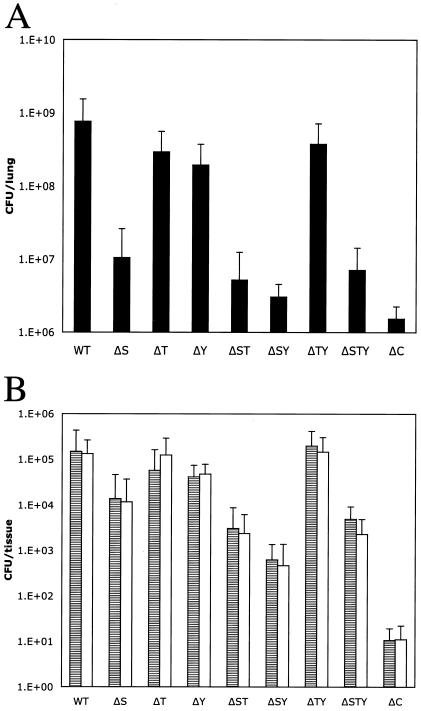
Previously we used a mouse model of acute pneumonia to demonstrate the role of regulators of TTSS on P. aeruginosa virulence (40). We used the same model to assess the in vivo contribution of each effector during infection. Mice were infected intranasally with 4 × 107 CFU of P. aeruginosa strain PAK or various deletion mutants. At 16 h postinfection, bacteria in lungs, livers (300 mg), and spleens were enumerated. Lungs from mice infected with wild-type PAK showed a more-than-10-fold increase in the number of bacteria relative to the initial inoculum (Fig. 4A). In contrast, the number of bacteria recovered from the lungs of mice infected with the type III-defective mutant PAKΔC was reduced, with a yield of bacteria almost 400-fold lower than that observed for the wild type. All mutants lacking exoS alone or as a multiple deletion with the other effector genes were recovered from the lungs in numbers reduced from the initial inoculum. The absence of the exoT or exoY gene (ΔT or ΔY mutant) results only in minor effects on the number of bacteria recovered from the lung. Despite lacking genes for all type III effectors, the ΔSTY triple mutant is recovered in higher number than PAKΔC, suggesting that the TTSS machinery alone could have basal activity in vivo, as was noted in the in vitro CHO cell cytotoxicity assay (Fig. 2 and 3) (4). Bacterial dissemination to the liver and the spleen followed a similar trend, suggesting that dissemination either requires functional ExoS or is simply a reflection of the bacterial load in the lungs (Fig. 4B). The presence of an TTSS that is intact but that lacks any known effector (the ΔSTY mutant) resulted in an increase in the number of bacteria recovered from peripheral organs compared to that found with PAKΔC infections, indicating residual, effector-independent functions of the type III machine during infections.
FIG. 4.
Colonization and dissemination of PAK in a mouse acute pneumonia model are attenuated in strains lacking exoS. Mice were inoculated intranasally with 5 × 107 CFU of PAK or derivatives. At 16 h postinfection, the lungs (A), 300 mg of livers (B; gray bars), and spleens (B; white bars) were harvested and the number of CFU of P. aeruginosa was determined by serial dilution and plating on L agar. Error bars indicate standard deviations.
Effect of ExoU in vitro and in vivo.
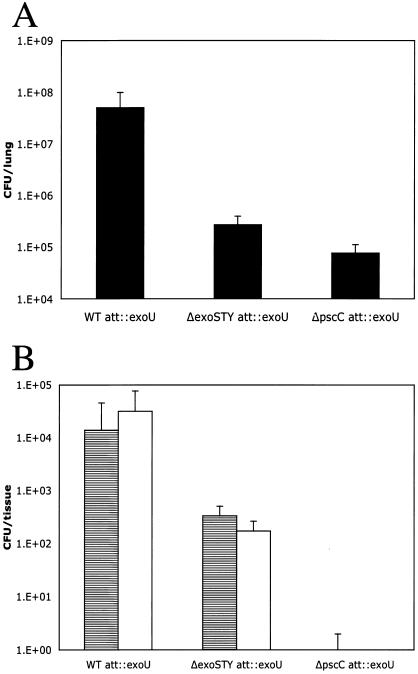
ExoU has been shown to be a highly cytotoxic type III effector protein with phospholipase A2 activity that disrupts eukaryotic membranes following its delivery into the cytoplasm (34, 37). The exoU-spcU operon from strain PA103 was integrated into the PAK chromosome at the CTX site. This engineered PAK exoU strain secreted all four effectors. In a TTSS-defective background (PAKΔC) none of the four effectors were secreted (Fig. 5A). The same operon was introduced into a mutant lacking its endogenous effector genes (PAKΔSTY), resulting in strain PAKΔSTY exoU, capable of only ExoU secretion (Fig. 5A). The toxicity of exoU-containing strains was determined by the same assays as those described above. In the CHO plating assay, infection with PAK exoU resulted in the reduction of the plating efficiency of CHO cells to 0.06% in 2 h (Fig. 5B). The effector-independent toxicity observed previously is also independent of ExoU, as the ΔSTY triple mutant expressing exoU from the chromosome also had a reduced plating efficiency of 0.01%. This result also suggests that ExoT mediates a minor level of protection even in the presence of a highly toxic ExoU. The effect of ExoU is absolutely dependent on type III secretion, as mutant PAKΔC exoU showed no demonstrable cytotoxicity. These results show that injection of ExoU by P. aeruginosa PAK is more than 100-fold more toxic than injection by PAK of ExoS.
In the mouse infection model, the strains harboring exoU were also more virulent than the parental PAK strain. Two of seven mice infected with PAK exoU at a low initial inoculum (4 × 106 CFU) died. At the same inoculum, PAKΔSTY exoU produced 100% mortality, with all seven mice succumbing to infection. With a further-reduced initial inoculum of 2 × 106 CFU, the PAK exoU strain colonized the lung and replicated to 10 times the initial inoculum (Fig. 6A) and was able to disseminate into the liver and spleen (Fig. 6B). Infection with PAKΔSTY exoU at the same inoculum resulted in a fivefold reduction in the number of bacteria recovered from the lung, suggesting that the bacteria were being cleared (Fig. 6A). Type III-defective PAKΔC exoU is avirulent and is cleared from the mouse. This result suggests that the high levels of CHO cell cytotoxicity observed for strains harboring exoU correlates with their enhanced virulence. Expression of exoS, exoT, and exoY somewhat attenuates the virulence of the strains expressing exoU, allowing mice to survive the course of the infection. In the absence of the other effectors, interaction between P. aeruginosa harboring only exoU and the host shows a threshold phenomenon, in which either the host contains the infection and clears the bacteria or dies from the infection.
FIG. 6.
Colonization of PAK exoU strains in vivo requires the presence of exoS, exoT, and exoY. Mice were inoculated intranasally with 2 × 106 CFU of PAK or derivatives. At 16 h postinfection, the lungs (A), 300 mg of livers (B; gray bars), and spleens (B; white bars) were harvested and the number of CFU of P. aeruginosa was determined by serial dilution and plating on L agar. Error bars indicate standard deviations. WT, wild type.
Detection of type III secretion and cytotoxicity from serially derived clinical isolates from the same patients.
The contribution of type III secretion in chronic infections by P. aeruginosa, such as in CF patients, has not been firmly established due to the lack of suitable CF animal models. Serum from both adult and adolescent CF patients harbors antibodies against TTSS components, suggesting that P. aeruginosa must express these proteins during infection (2, 27). However, when a number of clinical isolates from CF patients were tested for type III secretion in low-calcium-induced culture, the majority of such strains were lacking a functional TTSS (3, 36). It has been suggested that the secretion defect is regulatory since overexpression of ExsA, the TTSS transcription factor, resulted in the secretion of type III effectors (3). These studies further imply that, during chronic colonization, the strains undergo pathoadaptive regulatory mutations, leading to the loss of functional TTSS; however, these studies miss the link between P. aeruginosa strains that initially colonize the CF lung and those isolated later during the chronic phase of infection. To address this issue, we have analyzed CF isolates from longitudinal studies where the strains of P. aeruginosa were isolated shortly after initial colonization of the lungs and were contiguously collected over the course of the subsequent 15 years. The chromosomal DNA of these strains shows identical restriction fragment length polymorphism patterns (data not shown), suggesting that these strains are of clonal descent (35). Genomic arrays reveal that all of the strains contain genes encoding the type II and type III secretion systems. Additionally, all of the strains carry the exoS gene, except strains isolated from patient E, which have exoU instead of exoS. Four additional clinical CF isolates that have been previously subjected to genomic DNA microarray analysis (52) were also included in this analysis.
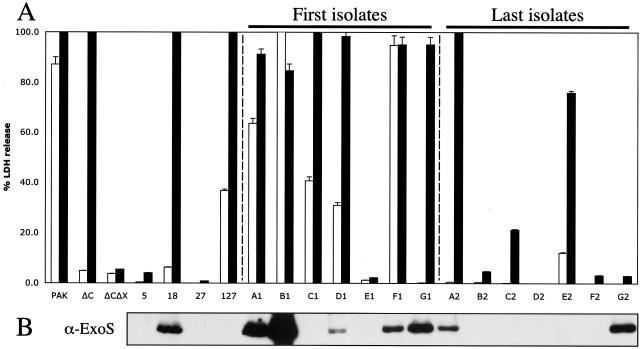
Growth of clinical isolates from CF patients in TTSS-inducing low-calcium media allowed the detection of ExoS secretion in five of seven of the early CF isolates (Fig. 7B and Table 1). Only two of the seven late isolates secreted detectable amounts of ExoS (Fig. 7B). The late isolate from patient A secreted a reduced amount of ExoS, while both early and late isolates from patient G showed similar levels of ExoS in the culture media. Strains from patient E did not secrete detectable levels of ExoU (data not shown). Only one of the four other isolates, CF18, secreted detectable amounts of ExoS. CF18 was isolated from a 24-month-old patient, suggesting that CF18 is an early isolate. These results suggest that in CF isolates there is a general trend toward loss of the ability to secrete type III effectors over the course of the chronic phase of infection.
FIG. 7.
CF P. aeruginosa isolates lose the ability to secrete ExoS and induce cytotoxicity over the course of chronic colonization. (A) CHO cells were infected with PAK, PAKΔC (pscC deletion), PAKΔCΔX (pscC and xcp operon deletions), or CF isolates at an initial MOI of 10 for either 4.5 (white bars) or 7.5 h (black bars). Cleared supernatants were analyzed for LDH release as a measure for cell lysis. (B) Secreted proteins from low-calcium-induced cultures were separated by SDS-PAGE. Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS (α-ExoS). Patients were designated A to G; early strains are labeled 1, and late strains are labeled 2.
TABLE 1.
| Strain | Secretion of:
|
No pMMB-exsA
|
pMMB-exsA, no IPTG
|
pMMB-exsA with IPTG
|
Reference or source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −Ca2+ secretiona | Cytotoxicity at:
|
−Ca2+ secretion | Cytotoxicity at:
|
−Ca2+ secretion | Cytotoxicity at:
|
|||||||
| ExoS | ExoU | 4.5 h | 7.5 h | 4.5 h | 7.5 h | 4.5 h | 6 h | |||||
| CF5 | + | − | − | − | − | NAb | NA | NA | NA | NA | NA | 51 |
| CF18 | + | − | + | − | + | + | − | − | ++ | + | + | 51 |
| CF27 | + | − | − | − | − | − | − | − | ++ | − | − | 51 |
| CF127 | + | − | − | + | + | NA | NA | NA | NA | NA | NA | 51 |
| A1 | + | − | + | + | + | NA | NA | NA | NA | NA | NA | This study |
| B1 | + | − | ++ | + | + | + | + | + | ++ | + | + | This study |
| C1 | + | − | − | + | + | − | − | + | + | + | + | This study |
| D1 | + | − | + | + | + | NA | NA | NA | NA | NA | NA | This study |
| E1 | + | − | − | − | − | NA | NA | NA | NA | NA | NA | This study |
| F1 | + | − | + | + | + | NA | NA | NA | NA | NA | NA | This study |
| G1 | + | − | + | + | + | NA | NA | NA | NA | NA | NA | This study |
| A2 | + | − | + | − | + | − | − | − | + | − | + | This study |
| B2 | + | − | − | − | − | NA | NA | NA | NA | NA | NA | This study |
| C2 | + | − | − | − | + | NA | NA | NA | NA | NA | NA | This study |
| D2 | + | − | − | − | − | NA | NA | NA | NA | NA | NA | This study |
| E2 | − | + | + | − | + | − | + | + | + | + | + | This study |
| F2 | + | − | − | − | − | − | − | − | + | − | − | This study |
| G2 | + | − | + | − | − | NA | NA | NA | NA | NA | NA | This study |
−Ca2+ secretion, secretion from cultures induced with low calcium. ++, high level of secretion; +, detectable secretion; −, no detectable secretion.
NA, not applicable.
The same isolates were tested for their ability to cause cytotoxicity on CHO cells. The wild-type PAK parental strain, type III null strain PAKΔC, and double mutant PAKΔCΔX, which lacks the structural genes encoding both type II and type III secretion systems, were utilized as controls for toxicity induced by each secretion system. At the 4.5-h time point, killing of CHO cells by wild-type PAK is mediated by effectors utilizing the TTSS, as the ΔC strain is noncytotoxic (Fig. 2 and 7A and Table 1). At 7.5 h postinfection, CHO cell toxicity is mediated by proteins secreted by the type II secretion system, as a TTSS mutant PAKΔC is as cytotoxic as the wild-type PAK strain; however the mutant strain PAKΔCΔX, lacking both type II and type III secretion systems, is nontoxic to CHO cells at this time point. Five of the seven early CF strains caused some cytotoxicity at 4.5 h, and all of these but one (from patient E) were cytotoxic at 7.5 h (Fig. 7A). All of the later strains other than those from patient E showed reduced cytotoxicity at 4.5 h. Four of seven later strains had no effect on CHO cells, even after 7.5 h. CF18 and CF127 also caused cytotoxicity at the later time point, whereas CF5 and CF27 were not toxic. Two of the nontoxic strains, CF27 and E1, were highly mucoid, suggesting that hyperproduction of alginate prevents type III secretion. Together, these results suggest that early isolates of P. aeruginosa harbor a functional TTSS and are more cytotoxic than the same strains isolated years later from the same patient.
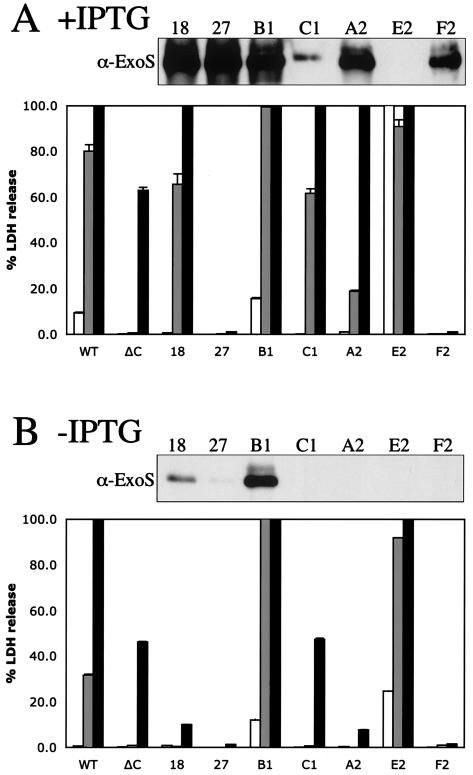
In a previous study, a number of CF isolates were unable to secrete type III effectors, and this defect could be restored by the expression of the gene encoding the TTSS activator ExsA, which also reestablished the cytotoxicity of the majority of these strains to human blood polynuclear monocytes (3). In this study, a plasmid expressing exsA under the control of the IPTG-inducible tac promoter was introduced into the CF strains by conjugation. Two of the early strains, three of the late strains, and two of the other CF strains were able to maintain the exsA-expressing plasmid. In the absence of IPTG, the strains containing the exsA plasmid behaved as the parental strain and only the early strain from patient B secreted ExoS (Fig. 8B). Induction of the expression of exsA by the addition of 1 mM IPTG resulted in the secretion of ExoS or ExoU from all strains (Fig. 8A and data not shown).
FIG. 8.
Overexpression of exsA results in the secretion of ExoS or ExoU in vitro but does not correlate with cytotoxicity to CHO cells. Secreted proteins from low-calcium-induced cultures were separated by SDS-PAGE. Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS (α-ExoS) or ExoU. CHO cells were infected with PAK, PAKΔC (pscC deletion), or CF isolates harboring pMMB-exsA at an initial MOI of 10 for either 3 (white bars), 4.5 (gray bars), or 6 h (black bars). Cleared supernatants were analyzed for LDH release as a measure of cell lysis. (A) Cultures induced with IPTG. WT, wild type. (B) Culture without IPTG addition.
Strains harboring the exsA plasmid were also tested for cytotoxicity during the infection of CHO cells. Without the induction of exsA, only the late strain from patient E was more toxic than the parental strain (Fig. 7A and 8A and Table 1). Upon induction of exsA, strains CF18, C1, A2, and E2 showed increased cytotoxicity. Strain B1 secreted ExoS with and without induction of exsA expression and was cytotoxic in both conditions. However, strains CF27 and F2 did not show any change in cytotoxicity with exsA expression. These results indicate that both early and late CF isolates contain genes encoding a functional TTSS. The late strains are likely carrying mutations in their genomes in either exsA or genes that encode other regulators that act in a pathway upstream of exsA that prevent expression of TTSS. Interestingly some strains seem to carry additional mutations that abolish ExoS-mediated cytotoxicity despite having the ability to robustly secrete ExoS when induced with exsA (Fig. 8A, strains F2 and 27).
DISCUSSION
The results of the studies presented here examine the association between the activities of various type III effectors secreted by P. aeruginosa strain PAK in in vitro and in vivo infection models. Particularly noteworthy is a comparison of these data with the works of Vallis et al., Miyata et al., and Lee et al., who examined the effect of TTSS expressed by P. aeruginosa strains harboring exoU. Vallis et al. investigated the infection of CHO cells utilizing the strain PA103, which expresses the effectors ExoU and ExoT (46). Miyata et al. evaluated the effects of ExoU, ExoT, and ExoY expressed by PA14 in a Galleria mellonella infection and various tissue culture intoxication and invasion assays (25). Lee et al. investigated the TTSSs of PA103 and PAK in the mouse corneal-infection model (22). In all these studies, the TTSS contributed significantly to the virulence of P. aeruginosa strains harboring exoU; however, significant differences were observed when the outcomes of interactions among bacteria, epithelial cells, and bacterial host organisms were analyzed. It is conceivable that the effects of TTSS are manifested differently in various models of infection; however, it is also likely that the genetic background of the three P. aeruginosa strains can influence the activities of the various effectors. In our studies, we used strain PAK, which naturally expresses three type III-dependent effectors, ExoS, ExoT, and ExoY, while PA103 expresses ExoU and ExoT and PA14 produces ExoU, ExoT, and ExoY.
We demonstrated that in our cell culture systems and in the mouse pneumonia model the most significant attenuation of virulence was exhibited by a mutant lacking the pscC gene, which encodes the secretin component of the type III secretion machinery. Similarly a TTSS-defective PA14 mutant (carrying pscD) was shown to be 1,000-fold less virulent in the G. mellonella infection model and noncytotoxic in various cell culture assays (25). PA103 lacking the type III activator exsA was noncytotoxic to CHO cells (46) and in corneal infections (22). In the corneal-infection model, PAK strains invaded corneal cells irrespective of TTSS, suggesting that neither ExoS nor ExoT of PAK functions as their counterparts in strain PA103 (22). We examined deletion strain PAKΔS, lacking the gene for ExoS but expressing the other two effectors, ExoT and ExoY, for its ability to kill CHO cells using two different cytotoxicity assays. The inability of P. aeruginosa strain PAK to produce ExoS correlated with the nearly complete lack of cytotoxic activity of the mutant. Interestingly, deletion of the gene for the cytotoxin ExoU from the strain PA14 genome gave a somewhat attenuated phenotype; however, the residual virulence and cytotoxicity were significantly higher than those observed for a TTSS null mutant (25). In contrast, PA103 lacking exoU, although noncytotoxic for CHO cells, had a minimal effect on virulence in a corneal-infection model (46). Surprisingly, the effect of the expression of ExoS and ExoY in strain PA103 during infection caused altered CHO cell morphology, but not cytotoxicity, as was observed for strain PAK (46).
Another paradoxical result of comparing P. aeruginosa strains PAK, PA103, and PA14 was the observed protective effect of ExoT. We demonstrate that deletion of exoT resulted in a strain (PAKΔT) that was even more cytotoxic than the wild-type PAK, while the deletion of exoT alone had no effect on the cytotoxicity or virulence of strains PA14 and PA103 (22, 25). ExoT-dependent protection also occurred in the absence of ExoS. Strain PAKΔST caused a fivefold increase in virulence compared to strain PAKΔS, which lacked only ExoS. In contrast, the analogous combination of mutations in strains PA14 and PA103 (ΔU versus ΔUT) produced an opposite effect. The absence of ExoU and ExoT attenuated the virulence of PA14 by an additional 2 orders of magnitude in the G. mellonella model, and a corresponding decrease in cytotoxicity was observed in the CHO cell plaque assay (25). The corneal colonization of the PA103ΔUT mutant strain was also reduced by 3 orders of magnitude compared to that of PA103 and the PA103ΔU and PA103ΔT mutants (22). Thus, ExoT contributes to the virulence of ExoU-expressing strains. The effect of ExoT in PAK is also independent of ExoY. Strain PAKΔSTY, lacking all effectors, is more toxic than strain PAKΔSY, expressing only ExoT. These findings therefore strongly suggest that introduction of ExoT into the host cytoplasm counteracts the potentially adverse effects of the damage resulting from the insertion of the needle complex component of the type III secretion apparatus. The protection of cells against damage from the type III secretion apparatus by ExoT is analogous to the effects observed with YopE expression by Yersinia spp. in a variety of cellular infection models (47). Since both YopE and ExoT possess GAP activity, the reversal of TTSS-induced damage may be attributable to this effect. Both ExoS and ExoT have the same N-terminal GAP activities; thus either protein should enable protection (10, 18, 19). However, the C-terminal ADP ribosylation activities of the two proteins in substrate selection differ drastically, and this may explain the dramatic difference between the observed cytotoxic effect of ExoS and the protective effect of ExoT on cells (44). The protective property of ExoT is not universal, since a comparable effect was not observed with the analogous PA14 mutants in the G. mellonella model and the plaquing assay or with PA103 strains in CHO cytotoxicity assays and the corneal-infection model. Although it is difficult to precisely correlate the differences observed in the functions of the four effectors in various strains of P. aeruginosa with different models, the observed differences in the effects of ExoS and ExoT may also be due to variations in the genetic background of the bacteria. The sequences of ExoS from strains PAK and PA103 are nearly identical, suggesting that the differences may be due to the expression levels of each effector molecule. PAK expresses high levels of ExoS, while PA103 expresses ExoS to a much lower level than ExoT (46). ExoT from PA103, although not cytotoxic to CHO cells, appears to be important in in vivo infection models, where the bacteria may encounter many more host cell types. Perhaps some of the differences in the phenomenology of ExoS and ExoT observed in these studies are due to strain-specific effects in the expression of each effector molecule as well as the effects of different host environments.
Studies of P. aeruginosa in different model systems have revealed a large number of virulence mechanisms in addition to type III secretion. The contribution of type II-secreted enzymes delivered by P. aeruginosa to eukaryotic cell cytotoxicity during in vitro infection is only detectable in the absence of the fast-acting TTSS. PAKΔC, lacking type III secretion, causes cytotoxicity in CHO cells upon prolonged incubation. Strain PAKΔCΔX, lacking both type III and type II secretion systems, did not cause cytotoxicity in CHO cells even after 8 h, suggesting the importance of these two pathways for PAK to interact with mammalian cells (Fig. 7A).
While the killing of epithelial and phagocytic cells may be an important feature of acute infections, the same virulence mechanisms appear to be incompatible with chronic colonization of CF patients (11). We therefore examined P. aeruginosa obtained from seven patients soon after their initial colonization and then again more than a decade later, after the establishment of chronic lung infections. Early isolates were typically cytotoxic, the exception being the highly mucoid strains. The same strains, isolated years later from the same patients, were found to be nontoxic, suggesting that there has been a selection for loss of cytotoxicity (Fig. 7A). This attenuation in cytotoxicity may be caused by several factors, including defects in the regulation of the TTSS, mutations in structural components of the type III secretion machinery, and mutations in other factors, such as the effectors themselves. We have demonstrated that in many cases restoration of type III regulation, through the expression of the ExsA regulator, was able to reestablish ExoS secretion and cytotoxicity (Fig. 8). However, this is clearly not the only mechanism of attenuation since expression of ExsA was not able to restore ExoS secretion in all of the clinical isolates. In some cases, as seen with strains CF27 and F2, ExoS secretion could be restored with ExsA expression; however, cytotoxicity was still attenuated (Fig. 8). These data suggest that there may be additional mutations in other factors required for injection or in the effectors, resulting in inactive enzymes. This was also observed when comparing strains G1 and G2. Both strains secrete ExoS; however, only G1 was cytotoxic (Fig. 7). What is also apparent from the pairwise comparisons of early and late isolates is that the phenomenon of delayed cytotoxicity is also lost in later isolates. The change in toxicity observed in CF isolates also correlated with reports demonstrating that proteins secreted by the type II secretion system are produced at reduced levels in mucoid CF isolates (30, 53). The respiratory tracts of CF patients therefore provide a strongly selective environment for the accumulation of pathoadaptive mutations, which favor a chronic existence that often necessitates the elimination of a potent cytotoxic mechanism.
Another common P. aeruginosa mutation that accumulates in CF patients results in the overproduction of alginate and conversion to a mucoid phenotype (11). Mucoid bacteria grow as microcolonies in the CF patient lung and have the ability to inhibit host phagocytosis (21). The inability of the immune system to clear mucoid strains may reduce the selective pressure to maintain the secretion of toxins and effectors normally used to combat the host defenses. It is not known whether alginate overproduction also provides a physical barrier to the secretion of effectors and toxins or whether other mutations accumulate during CF infection to prevent secretion. A physical barrier due to alginate overproduction is unlikely, as the mucoid strain CF27 can secrete type III proteins upon overexpression of TTSS regulator exsA. Microarray analysis of global transcription in strains with mutant proteins involved in alginate production, TTSS regulation, and quorum sensing has not provided a direct link between the two phenotypes (7). Adaptation of P. aeruginosa during chronic colonization of the CF patient lung likely results in the accumulation of multiple mutations, which results in mucoid bacteria that are unable to export proteins via type III and type II secretion systems. Recently, it has also been shown that certain CF small-colony isolates may revert to TTSS-active, cytotoxic strains (48). The presence of such strains indicates a complex host-pathogen-dependent evolution and accumulation of mutations that either inactivate or reactivate bacterial virulence factors.
Acknowledgments
This work was supported by a grant from the Cystic Fibrosis Foundation. S. L.’s laboratory was supported by NIH grant AI21451. V.T.L. was supported by a NIAID postdoctoral research fellowship. R.S.S. was supported by a Cystic Fibrosis Foundation postdoctoral research fellowship.
We thank R. Vance, A. Rietsch, J. Mekalonos, K. Metcalf, and C. Ordonez for sharing unpublished results.
Editor: V. J. DiRita
REFERENCES
- 1.Bally, M., A. Filloux, M. Akrim, G. Ball, A. Lazdunski, and J. Tommassen. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6:1121-1131. [DOI] [PubMed] [Google Scholar]
- 2.Banwart, B., M. L. Splaingard, P. M. Farrell, M. J. Rock, P. L. Havens, J. Moss, M. E. Ehrmantraut, D. W. Frank, and J. T. Barbieri. 2002. Children with cystic fibrosis produce an immune response against exoenzyme S, a type III cytotoxin of Pseudomonas aeruginosa. J. Infect. Dis. 185:269-270. [DOI] [PubMed] [Google Scholar]
- 3.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dacheux, D., J. Goure, J. Chabert, Y. Usson, and I. Attree. 2001. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 40:76-85. [DOI] [PubMed] [Google Scholar]
- 5.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 6.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 7.Firoved, A. M., and V. Deretic. 2003. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J. Bacteriol. 185:1071-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraylick, J. E., E. A. Rucks, D. M. Greene, T. S. Vincent, and J. C. Olson. 2002. Eukaryotic cell determination of ExoS ADP-ribosyltransferase substrate specificity. Biochem. Biophys. Res. Commun. 291:91-100. [DOI] [PubMed] [Google Scholar]
- 10.Goehring, U. M., G. Schmidt, K. J. Pederson, K. Aktories, and J. T. Barbieri. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369-36372. [DOI] [PubMed] [Google Scholar]
- 11.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser, A. R., P. J. Kang, and J. N. Engel. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 13.Henriksson, M. L., C. Sundin, A. L. Jansson, A. Forsberg, R. H. Palmer, and B. Hallberg. 2002. Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities towards small GTPases in vivo. Biochem. J. 367:617-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 15.Holder, I. A., A. N. Neely, and D. W. Frank. 2001. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns 27:129-130. [DOI] [PubMed] [Google Scholar]
- 16.Iglewski, B. H., and D. Kabat. 1975. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc. Natl. Acad. Sci. USA 72:2284-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao, F. T., and T. T. Puck. 1967. Genetics of somatic mammalian cells. IV. Properties of Chinese hamster cell mutants with respect to the requirement for proline. Genetics 55:513-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazmierczak, B. I., and J. N. Engel. 2002. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect. Immun. 70:2198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krall, R., G. Schmidt, K. Aktories, and J. T. Barbieri. 2000. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68:6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudoh, I., J. P. Wiener-Kronish, S. Hashimoto, J. F. Pittet, and D. Frank. 1994. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am. J. Physiol. 267:L551-L556. [DOI] [PubMed] [Google Scholar]
- 21.Lam, J., R. Chan, K. Lam, and J. W. Costerton. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28:546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, E. J., B. A. Cowell, D. J. Evans, and S. M. Fleiszig. 2003. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Investig. Ophthalmol. Vis. Sci. 44:3892-3898. [DOI] [PubMed] [Google Scholar]
- 23.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J. Infect. Dis. 116:112-116. [DOI] [PubMed] [Google Scholar]
- 24.Liu, S., S. M. Kulich, and J. T. Barbieri. 1996. Identification of glutamic acid 381 as a candidate active site residue of Pseudomonas aeruginosa exoenzyme S. Biochemistry 35:2754-2758. [DOI] [PubMed] [Google Scholar]
- 25.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morihara, K. 1964. Production of elastase and proteinase by Pseudomonas aeruginosa. J. Bacteriol. 88:745-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss, J., M. E. Ehrmantraut, B. D. Banwart, D. W. Frank, and J. T. Barbieri. 2001. Sera from adult patients with cystic fibrosis contain antibodies to Pseudomonas aeruginosa type III apparatus. Infect. Immun. 69:1185-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicas, T. I., and B. H. Iglewski. 1985. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can. J. Microbiol. 31:387-392. [DOI] [PubMed] [Google Scholar]
- 29.Nunn, D. N., and S. Lory. 1992. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc. Natl. Acad. Sci. USA 89:47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohman, D. E., and A. M. Chakrabarty. 1982. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect. Immun. 37:662-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 32.Pavlovskis, O. R., and F. B. Gordon. 1972. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J. Infect. Dis. 125:631-636. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen, S. S., N. Hoiby, G. H. Shand, and T. Pressler. 1989. Antibody response to Pseudomonas aeruginosa antigens in cystic fibrosis. Antibiot. Chemother. 42:130-153. [DOI] [PubMed] [Google Scholar]
- 34.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 35.Romling, U., B. Fiedler, J. Bosshammer, D. Grothues, J. Greipel, H. von der Hardt, and B. Tummler. 1994. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis. 170:1616-1621. [DOI] [PubMed] [Google Scholar]
- 36.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 37.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO. J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawa, T., M. Ohara, K. Kurahashi, S. S. Twining, D. W. Frank, D. B. Doroques, T. Long, M. A. Gropper, and J. P. Wiener-Kronish. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, R. S., M. C. Wolfgang, and S. Lory. 2004. An adenylate cyclase-controlled signaling network regulates Pseudomonas aeruginosa virulence in a mouse model of acute pneumonia. Infect. Immun. 72:1677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storey, D. G., E. E. Ujack, I. Mitchell, and H. R. Rabin. 1997. Positive correlation of algD transcription to lasB and lasA transcription by populations of Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Infect. Immun. 65:4061-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storey, D. G., E. E. Ujack, H. R. Rabin, and I. Mitchell. 1998. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 66:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 44.Sun, J., and J. T. Barbieri. 2003. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J. Biol. Chem. 278:32794-32800. [DOI] [PubMed] [Google Scholar]
- 45.Tummler, B., J. Bosshammer, S. Breitenstein, I. Brockhausen, P. Gudowius, C. Herrmann, S. Herrmann, T. Heuer, P. Kubesch, F. Mekus, U. Romling, K. D. Schmidt, C. Spangenberg, and S. Walter. 1997. Infections with Pseudomonas aeruginosa in patients with cystic fibrosis. Behring Inst. Mitt. 1997:249-255. [PubMed] [Google Scholar]
- 46.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO. J. 20:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Gotz, F., S. Haussler, D. Jordan, S. S. Saravanamuthu, D. Wehmhoner, A. Strussmann, J. Lauber, I. Attree, J. Buer, B. Tummler, and I. Steinmetz. 2004. Expression analysis of a highly adherent and cytotoxic small colony variant of Pseudomonas aeruginosa isolated from a lung of a patient with cystic fibrosis. J. Bacteriol. 186:3837-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warrens, A. N., M. D. Jones, and R. I. Lechler. 1997. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene 186:29-35. [DOI] [PubMed] [Google Scholar]
- 51.Wolfgang, M. C., B. R. Kulasekara, X. Liang, D. Boyd, K. Wu, Q. Yang, C. G. Miyada, and S. Lory. 2003. Conservation of genome content and virulence determinants among clinical and environmental isolates of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 100:8484-8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253-263. [DOI] [PubMed] [Google Scholar]
- 53.Woods, D. E., M. S. Schaffer, H. R. Rabin, G. D. Campbell, and P. A. Sokol. 1986. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J. Clin. Microbiol. 24:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wretlind, B., and O. R. Pavlovskis. 1983. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev. Infect. Dis. 5(Suppl. 5):S998-S1004. [DOI] [PubMed] [Google Scholar]
- 55.Yahr, T. L., J. Goranson, and D. W. Frank. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22:991-1003. [DOI] [PubMed] [Google Scholar]
- 56.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]