Abstract
Two groups of four calves each were immunized either intramuscularly (i.m. vaccinated) or intranasally (i.n. vaccinated) at 2 and 6 weeks of age with ca. 109 CFU of a derivative of P. multocida serotype B:2 strain 85020 containing a deletion in the aroA gene (strain JRMT12). Both groups of calves and three unvaccinated control calves were challenged subcutaneously at 8 weeks of age with ca. 107 CFU of the wild-type 85020 strain. The first and second vaccinations caused a significant pyrexia and increase in the mean demeanor score (P < 0.05) in i.m. but not i.n. vaccinated calves. Serum agglutinating activity against whole cells of P. multocida strain 85020 and immunoglobulin G antibody concentrations increased after the second vaccination in i.m. but not in i.n. vaccinated animals, and this difference was statistically significant (P < 0.05). Concentrations of serum amyloid A (SAA) increased significantly 3 h after both the primary (P < 0.05) and booster (P < 0.001) i.m. vaccinations, but not in i.n. vaccinated calves. All four i.m. vaccinated calves were solidly immune to challenge with wild-type P. multocida B:2. However, the mean rectal temperatures, demeanor scores, and serum SAA concentrations of i.n. vaccinated and control calves increased significantly (P < 0.01). Three i.n. vaccinated and two control calves were killed for humane reasons within 14 h postchallenge, and postmortem examination revealed pathological lesions consistent with hemorrhagic septicemia. These data showed that the aroA mutant strain, given i.m. as two doses 4 weeks apart, acted as an effective live-attenuated vaccine strain to protect calves against challenge with the virulent parent strain.
Hemorrhagic septicemia (HS) caused by infection with Pasteurella multocida serotype B:2 is a commonly fatal systemic disease of cattle and buffaloes in countries of South and Southeast Asia (3, 22). The disease is peracute, having a short clinical course involving severe depression, pyrexia, submandibular edema, and dyspnea, followed by recumbency and death. Some control is achieved with alum-precipitated or oil adjuvant broth bacterins injected subcutaneously, but these vaccines have the disadvantage of providing only short-term immunity (4 to 6 months and up to 1 year for alum adjuvant and oil adjuvant vaccines, respectively) (21), and the high viscosity of oil adjuvant vaccines makes them unpopular among field users. The disease remains a significant obstacle to sustainable agriculture in the region, and in attempts to elicit longer-term immunity, live vaccines have been developed (14), although they have been ill defined and of questionable safety. On the other hand, a marker-free aroA deletion derivative (strain JRMT12) of a virulent field isolate of P. multocida B:2 (strain 85020) obtained from Sri Lanka has been shown to be attenuated and to confer a high degree of protection when used as a live vaccine in a mouse model of hemorrhagic septicemia (19). The construct is particularly suited to be a live vaccine candidate, as it possesses no antibiotic-resistance-encoding genes and a deletion of the aroA gene avoids possible reversion to the wild type. The objectives of this work were to determine the safety and efficacy of the attenuated JRMT12 strain as a vaccine in cattle, given either intramuscularly (i.m.) or intranasally (i.n.) prior to subcutaneous challenge with the virulent wild-type parent 85020 strain using a model system established for studies of septicemic disease in sheep (7). The two routes of inoculation were compared in order to define the more efficacious route of vaccination. For field use, i.m. vaccination is the route of choice for ease of administration, but i.n. vaccination can also prime systemic responses (23) and mimics the likely oronasal route of infection in the natural disease. In the longer term, this work in a natural host species aims to produce an effective vaccine with which to control HS and thereby improve the health and welfare of farm animals throughout the affected regions and the prosperity and welfare of communities dependent upon them.
MATERIALS AND METHODS
Animal procedures.
Infection of the upper respiratory tract of calves with P. multocida serogroup A is common in the United Kingdom (5), and only P. multocida-free animals were selected to reduce the possibility of an active humoral immune response compromising the vaccination studies. Nasal swabs (CultureSwab Plus; BD Biosciences, Oxford, United Kingdom) were obtained from selected calves within 24 h of birth and assessed for growth of P. multocida after being plated on sheep blood agar (blood agar base [Oxoid] containing 5% [vol/vol] sheep blood) (SBA) containing vancomycin (1 mg/ml) to prevent the growth of gram-positive bacteria and to enrich for the presence of P. multocida. Suspect colonies were confirmed as P. multocida by PCR using genus-specific primers (20). Twelve calves that had been shown to be free of P. multocida infection were treated at 2 days of age with enrofloxacin at 2.5 mg/kg of body weight (Baytril Max; Bayer Animal Health, Newbury, United Kingdom) by subcutaneous injection, housed in air-filtered isolation pens, offered milk, and at 7 weeks old, weaned onto a diet of calf creep feed and hay. Throughout the work, the calves were maintained at a high standard of individual care and sanitation with their health and well-being assessed daily; veterinary assistance was available at all times, and feed and water were provided ad libitum. The conditions for containment and handling of calves inoculated with either the mutant or parent strain of P. multocida B:2 (absent from the United Kingdom cattle population) were set at containment category 2 (Health and Safety Executive Advisory Committee on Dangerous Pathogens). The animal accommodation used for the work provided this level of containment, but as added safety precautions to prevent the escape of P. multocida B:2 into the environment, ventilation was adjusted so that the rooms were under negative pressure, drains were blocked, matting and absorbent bedding were provided and renewed regularly, and all waste was bagged, surface disinfected, and incinerated.
The calves were allocated randomly to three groups of four calves each, restricting group size to the minimum likely to generate a statistically significant result. All experimental protocols were approved by the Moredun Research Institute Animal Experiments Committee and authorized under the United Kingdom Animals (Scientific Procedures) Act of 1986. Two groups were immunized either i.m. or i.n. when they were 2 (day 0) and 6 (day 30) weeks old with a mean of 7 × 108 and 9.5 × 108 CFU, respectively, of P. multocida aroA mutant strain JRMT12 in 5 ml of phosphate-buffered saline (PBS) as two 2.5-ml doses either injected into both hind limb muscles (semitendinosus muscle) or given as a fine spray into the nostrils (18). A third group consisted of unvaccinated calves used as challenge controls. On day 38, the control group was reduced to three due to the necessary euthanasia of one calf that failed to thrive despite veterinary treatment. On day 42, at 8 weeks of age, all calves were given subcutaneous injections of 1.2 × 107 CFU of P. multocida wild-type strain 85020 in 5 ml of PBS as two 2.5-ml doses, one over each prescapular region. A subcutaneous route of administration was employed to standardize the challenge dose and to avoid the variability encountered with aerosol challenge procedures. Experienced observers, with access to veterinary advice and care at all times, monitored the rectal temperatures and general demeanor (normal, dull, depressed, or recumbent as a score of 0, 1, 2, or 3, respectively) of all calves at intervals of 4 h between 0800 and 2400 in order to characterize clinical responses to immunization or challenge and to identify any calf requiring veterinary treatment. Any calf showing pyrexia was treated with intravenous flunixin meglumine (Finadyne; Schering-Plough Animal Health, Welwyn Garden City, United Kingdom), a nonsteroidal anti-inflammatory drug (NSAID), at 2.2 mg/kg. Postmortem examination was performed 7 days after challenge or earlier, as dictated by clinical assessment, using increases in the demeanor score and temperature that were not reduced by treatment with an NSAID as the decision criteria. The calves were killed within the containment facility by intravenous injection of 25 ml of Pentobarbitone sodium B.P. (200 mg/ml; Animal Care, York, United Kingdom), surface disinfected, and bagged before transport to the postmortem suite for gross pathological examination and removal of tissues for bacteriology and histopathology.
Preparation of vaccination and challenge doses.
To ensure the uniformity of vaccination and challenge, aliquots (1 ml) of a single batch of a stock bacterial suspension containing either the P. multocida JRMT12 mutant or the 85020 wild-type strain were resuscitated from −70°C storage, inoculated into 9 ml of brain heart infusion (BHI) broth, and incubated overnight (16 h static) at 37°C, and 3 ml of the resulting culture was transferred to 27 ml of BHI broth. The flasks were incubated at 37°C with shaking at 200 rpm for ∼3 (strain JRMT12) or 2.5 (strain 85020) h to give cultures containing ca. 109 CFU/ml as determined by previous experiments. Cells were collected by centrifugation, resuspended in PBS, and diluted in PBS to provide vaccination or challenge doses of ∼109 or 107 CFU in 5 ml, respectively, confirmed by viable counts on SBA.
Blood analysis.
Periodic jugular blood samples (10 ml) were collected into plain and heparinized Vacutainers (Becton Dickinson, Meylan, France) from all calves ≤3 days before challenge, at 3- to 4-h intervals on the day of vaccination or challenge, and at weekly intervals in between. Serum or plasma was separated and stored at −40°C until it was analyzed for the acute-phase protein (APP) serum amyloid A (SAA) and endotoxin, using methods described previously (4, 8) and antibody to P. multocida B:2 envelope antigens, by enzyme-linked immunosorbent assay (ELISA).
ELISA for antibody to P. multocida B:2 envelope.
The ELISA for antibody to P. multocida B:2 envelope was based on that of Pati et al. (15) with modifications. High-binding, 96-well ELISA plates (Greiner Bio-One Ltd., Stonehouse, United Kingdom) were coated with an envelope preparation from the wild-type 85020 strain. For this, 1 ml of frozen stock culture was thawed, inoculated into 9 ml of BHI broth, and incubated statically at 37°C for 18 h. Cells were collected by centrifugation at 3,000 × g for 30 min at 4°C and washed three times with PBS, and a suspension of ca. 7 × 109 CFU/ml was prepared. The suspension was lysed by sonication using a Vibra Cell ultrasonic processor (Jencons-PLS, Leighton Buzzard, United Kingdom) for three 60-s bursts with intermittent cooling on ice. The broken-cell suspension was centrifuged at 3,000 × g for 30 min at 4°C, and the supernatant was filtered through a 0.2-μm-pore-size membrane (Sartorius). The filtrate was diluted 1 in 8 with carbonate-bicarbonate buffer, pH 9.6 (Sigma), to give a final concentration of 60 μg of protein/ml. Plates were filled with 50 μl per well and left overnight at 4°C, after which the plates were washed three times with PBS-T (0.05% [vol/vol] Tween 20 in PBS) and then incubated for 2 h at room temperature with 200 μl of 0.1% (wt/vol) gelatin in PBS per well as a blocking buffer. Following further washing with PBS, 50 μl of 1-in-100 dilutions of serum samples were added to the wells, and doubling dilutions were prepared in duplicate in PBS. The plates were incubated at 37°C for 1 h and then washed three times with PBS-T, followed by incubation with 50 μl of a 1-in-10,000 dilution of sheep anti-bovine immunoglobulin G (IgG) or IgM-horseradish peroxidase (HRP) conjugate (Serotec Ltd., Kidlington, United Kingdom) at 37°C for 1 h. After a final three-wash step with PBS-T, bound conjugate was detected using 50 μl of the peroxidase substrate Fast OPD (Sigma) per well in the dark for 30 min. The reaction was stopped by the addition of 50 μl of 0.25 M sulfuric acid per well. Optical density values were measured at 492 nm in an Anthos ELISA reader. Antibody titers were determined as the number of ELISA units of undiluted calf serum per milliliter by comparison with values obtained for sera with intermediate IgG or IgM titers (from the survivor of the i.n. vaccinated group at 7 days postchallenge for IgG and from an i.m. vaccinated calf on the day of challenge for IgM), each arbitrarily assigned the reference value of 1,000 ELISA units/ml. The antibody titers (in ELISA units per milliliter) of all other samples were calculated as the ratio of test serum titer to reference serum titer × 1,000.
Agglutination test.
Serum samples (25 μl) were placed in Nunclon 96-well round-bottom cell culture plates, and doubling dilutions were made in PBS. Aliquots (25 μl) of a dense bacterial suspension (2.5 McFarland units) prepared from an overnight culture of P. multocida B:2 85020 were then added to all the wells, and the plate was rotated for 90 min and then incubated at 4°C overnight. Agglutination titers were calculated as the reciprocal of the highest dilution of serum antibody able to cause clumping of the bacterial cells. All sera were tested in duplicate, and each plate contained negative controls with PBS in place of serum.
Bacteriological examination.
Small samples of tissue (∼1-cm cubes; ∼1 g) taken from lung, kidney, heart, spleen, liver, brain, and lymph nodes were homogenized in 9 ml of peptone-water, and aliquots (10 μl) were spread on SBA containing vancomycin (1 mg/ml) and incubated at 37°C for 16 to 20 h prior to phenotypic and numerical analysis. Blood samples (10 μl) were cultured in the same way.
Necropsy.
All calves were subjected to postmortem examination. Gross pathology was assessed, and representative samples from lung, liver, kidney, spleen, brain, tonsil, thymus, and lymph nodes (prescapular, bronchial, mid- and caudal mediastinal, mesenteric, submandibular, axillary, retropharyngeal, and prefemoral) were placed in saline containing 4% (wt/vol) formaldehyde: formol-saline and prepared for histopathological examination using standard techniques (embedded in paraffin wax, sectioned, mounted on microscope slides, and stained with hematoxylin and eosin).
Statistical methods.
The data for rectal temperature and SAA measurements were taken at interval after each vaccination and postchallenge and analyzed separately for each period by fitting linear mixed models with calf fitted as a random effect. The effects of treatment on temperatures were analyzed as differences from the baseline temperature at time zero. The data for SAA were positively skewed, and therefore, the analyses were carried out using differences from the baseline measurement on a log scale. For the analysis of clinical scores, both the mean and maximum score attained by each calf during each treatment were calculated, and the difference in the proportion of calves from the different experimental groups that had maximum scores of either 0 or >0 was investigated using Fisher's exact test. The differences in mean scores over each 12-h surveillance period, and also the differences in agglutination titers (log dilutions) between i.m. and i.n. vaccinated calves, were investigated using either the Mann-Whitney U test (for a comparison between two groups) or the Kruskal-Wallis test (for three groups); in these tests, allowance was made for the small sample sizes. All analyses were carried out using the appropriate procedures in Genstat version 7.
RESULTS
Clinical responses to vaccination with aroA mutant strain JRMT12.
At 3 and 7 h after the first vaccination with the JRMT12 strain, the mean rectal temperatures (± pooled standard errors of the mean [SEM]) of i.m. vaccinated animals showed an average rise of 0.7°C (±0.17). By comparison, i.n. vaccinated animals showed no significant change from a mean (± SEM) rectal temperature of 39.2°C (±0.14) at time zero, and this difference between the groups was statistically significant (P = 0.011). At 4 and 6 h after the booster vaccination, the mean rectal temperatures (± pooled SEM) of i.m. and i.n. vaccinated animals showed an average rise of 1.6 and 0.3 °C (±0.13°C), respectively, and this difference between the groups was statistically significant (P < 0.001).
During the period 0 to 11 h after the first vaccination, the mean demeanor score for i.m. vaccinated animals was 0.8 (range, 0 to 2), whereas that for i.n. vaccinated animals was 0, and this difference was significant (P < 0.05). The corresponding value for i.m. vaccinated animals after the second vaccination was 0.6 (range, 0 to 1), whereas demeanor scores of 0 were assigned to all i.n. vaccinated animals, apart from one calf that had a score of 2 at 11 h postvaccination. Because of this individual, the difference between the mean demeanor scores for the two groups was not statistically significant. Within 4 to 5 h of treating i.m. vaccinated calves with NSAID (7 h after the first vaccination and 6 h after the second vaccination), rectal temperatures returned to pretreatment (time zero) values and demeanor scores returned to 0.
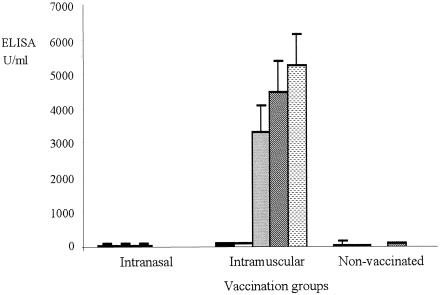
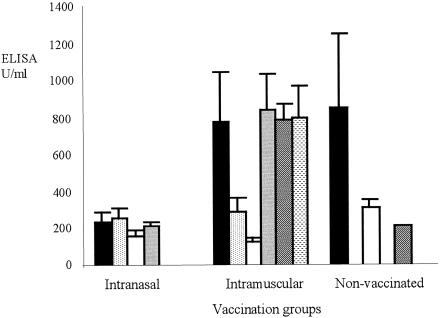
The mean agglutination titers of sera taken preimmunization from animals in the i.m. and i.n. vaccinated groups showed no significant difference between the groups (median values, 0.070 and 0.125, respectively; range, 0.016 to 0.250), but the mean titer from i.m. vaccinated animals 7 days after the second vaccination (day 37) was significantly (P < 0.05) lower (median value, 0.003; range, 0.001 to 0.007) than that from i.n. vaccinated animals (median value, 0.063; range, 0.016 to 0.063). The median value of sera from control animals taken on day 37 (0.063; range 0.004 to 0.125) was similar to those observed in i.m. and i.n. vaccinated animals preimmunization. ELISA data for IgG showed little response 28 days after the first vaccination (Fig. 1), but at day 37, in agreement with the agglutination data, sera from i.m. vaccinated calves had markedly increased IgG titers, which had increased further by day 42 (the day of challenge) and continued to increase until termination of the experiment (day 49). There was no evidence of an increase in IgG titer in i.n. vaccinated animals. Data for IgM in prevaccinated calves showed a wide range of titers, from 67 to 1,548 U/ml in individual animals, but by 14 and 28 days postvaccination, these values had fallen (Fig. 2). As with IgG, the IgM titers had increased markedly by 7 days after the second vaccination in the i.m. vaccinated animals but remained low in the i.n. vaccinated and control calves.
FIG. 1.
Total IgG titers in sera from vaccinated and control calves. Titers were assessed by ELISA from sera collected 5 days before the first vaccination (day −5; black column), 28 days after the first vaccination (day 28; white column), 7 days after the second vaccination (day 37; gray column), the day of challenge (day 42; hatched column), and 1 week postchallenge (day 49; stippled column). Results are mean values, with error bars indicating 1 SEM.
FIG. 2.
Total IgM titers in sera from vaccinated and control calves. Titers were assessed by ELISA from sera collected 5 days before the first vaccination (day −5; black column), 14 days after the first vaccination (day 14; dotted column), 28 days after the first vaccination (day 28; white column), 7 days after the second vaccination (day 37; gray column), the day of challenge (day 42; hatched column), and 1 week postchallenge (day 49; stippled column). Results are means, with error bars indicating 1 SEM.
The patterns of response in SAA concentrations after the first vaccination (Table 1) were different in i.m. and i.n. vaccinated calves: the concentrations increased significantly (P < 0.05) in i.m. vaccinated calves but showed no significant changes in i.n. vaccinated calves. The SAA concentrations declined in i.m. vaccinated calves after 24 h but increased again significantly (P < 0.001) in response to the second vaccination. No significant changes due to the second vaccination were noted for i.n. vaccinated calves.
TABLE 1.
SAA levels after vaccinationa
| Time after vaccination (h) | Mean SAA (μg/ml) ± SEM in calves vaccinated:
|
|
|---|---|---|
| i.m. | i.n. | |
| First vaccination | ||
| 0 | 51.7 ± 47.0 | 52.9 ± 67.1 |
| 3 | 71.1 ± 86.8 | 46.2 ± 58.4 |
| 8 | 144.0 ± 128.0 | 31.4 ± 24.9 |
| 24 | 392.5 ± 220.2 | 25.6 ± 16.8 |
| 48 | 189.3 ± 225.8 | 26.3 ± 15.0 |
| 144 | 43.5 ± 36.5 | 66.0 ± 91.5 |
| Second vaccination | ||
| 0 | 39.3 ± 25.3 | 21.3 ± 15.9 |
| 24 | 660.0 ± 65.5 | 14.4 ± 3.5 |
Two groups of four calves each were vaccinated twice, at a 4-week interval, either i.m. or i.n., with the aroA strain. SAA levels were determined in sera collected on the day of first vaccination (day 0), and at intervals thereafter, and on the day of second vaccination (day 30) and 24 h later.
Responses to challenge with wild-type strain 85020.
Between 3 and 12 h after challenge with the wild-type 85020 strain, the rectal temperatures for i.n. vaccinated and control calves showed similar patterns of response, during which the mean (± pooled SEM) values rose by 1.2 and 0.9°C (±0.12°C), respectively. By comparison, i.m. vaccinated animals showed no significant change from a mean (± SEM) rectal temperature of 39.1°C (±0.16°C) at time zero, and the difference between this group and the i.n. vaccinated and control groups was statistically significant (P < 0.001).
Mean demeanor scores during this same period (3 to 12 h postchallenge) were 0.8 (range, 0 to 2) and 1.0 (range, 0 to 2) for i.n. vaccinated and control animals, respectively, whereas that for i.m. vaccinated animals was 0.1 due to a score of 1 on one occasion, and this difference was significant (P < 0.01). Animals that were dull and febrile were treated with NSAID between 9 and 12 h postchallenge, but in three i.n. vaccinated and two control calves, temperatures and demeanor scores continued to increase and, for humane reasons, they were killed within 14 h postchallenge. The temperatures of the two remaining calves from these groups returned to normal within 2 h of NSAID administration, and the demeanor scores returned to 0 within 24 to 48 h postchallenge. None of the calves in the i.m. vaccinated group required treatment with NSAID, and all survived challenge.
ELISA antibody titers obtained from individual calves varied considerably, but mean (± SEM) data (Fig. 1 and 2) showed that IgG titers (in units per milliliter) in i.m. vaccinated calves at 7 days postchallenge (5,285 ± 878.6) had increased compared to the values obtained on the day of challenge (4,522 ± 900.5). IgM titers (in units per milliliter) had not altered at 7 days postchallenge (795 ± 172.2) compared to the values obtained on the day of challenge (783 ± 90.1). Only one calf remained in each of the i.n. vaccinated and control groups at 7 days postchallenge, so the ELISA data postchallenge are not included in the figures, but the surviving calf in each group had IgG titers of 1,000 and 263 U/ml and IgM titers of 617 and 631 U/ml, respectively.
The concentrations of SAA in individual calves varied considerably, and the means before challenge were highest in the control calves, followed by i.m. vaccinated and then by i.n. vaccinated calves (Table 2). The mean SAA concentration in i.n. vaccinated animals increased 13-fold by 10 h after challenge but by only 66 and 11% in i.m. vaccinated and control calves, respectively, and this difference between i.n. vaccinated calves and the others was statistically significant (P < 0.01). At 23 h postchallenge, SAA values exceeded 158 μg/ml in the two calves, one each from the i.n. vaccinated and control groups, that had survived, whereas the maximum increase observed for calves in the i.m. vaccinated group was only threefold (to 37 ± 15.6 μg/ml). High SAA concentrations took 6 to 8 days to return to prechallenge levels in the surviving calves.
TABLE 2.
SAA levels after challenge of vaccinated and control calves with wild-type strain 85020a
| Time after challenge (h) | Mean SAA (μg/ml) ± SEM (no. of surviving calves)
|
||
|---|---|---|---|
| Vaccinated i.m. | Vaccinated i.n. | Controls | |
| 0 | 13.1 ± 7.3 (4) | 2.2 ± 0.7 (4) | 65.2 ± 37 (3) |
| 10 | 21.8 ± 8.1 (4) | 28.8 ± 3.1 (4) | 72.3 ± 17 (3) |
| 23 | 37.0 ± 15.6 (4) | >157.6 (1) | >157.6 (1) |
| 47 | 29.2 ± 12.8 (4) | >157.6 (1) | 91.1 (1) |
| 71 | 22.7b (4) | >157.6 (1) | −c |
| 143 | 12.6 ± 8.1 (4) | − | 12.5 (1) |
| 167 | 4.5b (4) | 5.9 (1) | − |
Two groups of four calves each were vaccinated twice, at a 4-week interval, either i.m. or i.n., with the aroA mutant strain, and a group of three calves were left unvaccinated as controls. Two weeks after the second vaccination, the animals were challenged with the wild-type strain. SAA levels were determined in sera collected on the day of challenge (day 42) and at intervals thereafter.
Only one serum sample was assayed.
No sample available.
Endotoxin concentrations, expressed as endotoxin units per milliliter by reference to a control standard endotoxin solution (ACC International, Inc., Liverpool, United Kingdom), in sera taken between 6 and 11 h postchallenge in all calves and at 1, 2, 4, 6, and 9 days postchallenge in surviving calves showed mean (± standard deviation) values of 0.17 ± 0.04 (control calves), 0.19 ± 0.04 (i.n. vaccinated calves), and 0.17 ± 0.06 (i.m. vaccinated calves), with no statistically significant differences between groups (P > 0.05).
Direct plating out of calf blood samples onto SBA at various times postchallenge, including immediately prior to necessary or scheduled euthanasia, provided no evidence of bacteremia in any of the animals.
Postmortem examination revealed a range of gross and histopathological lesions in the two control and three i.n. vaccinated calves that were killed within 14 h of challenge that were consistent with a systemic hemorrhagic and endotoxemic disease (Table 3). Histopathological examination of the lymphoreticular system (prescapular, prefemoral, bronchial, mid- and caudal mediastinal, mesenteric, submandibular, axillary, and retropharyngeal lymph nodes, along with tonsil, spleen, and thymus) showed little difference among the three groups except in the prescapular lymph nodes. Calves vaccinated i.m. showed no hemorrhage in the paracortex of these lymph nodes and only slight infiltration by polymorphonuclear leukocytes compared to the often severe changes in those of the other two groups (Table 3). In addition, the more apparent cell depletion in the paracortex of the lymph nodes of the i.m. vaccinated calves suggested that they were mounting a more vigorous immunological response to antigenic stimulation. Bacteriological examination postmortem found P. multocida in the pleural fluid of one and in the tonsilar tissue of all i.n. vaccinated calves (range, 3.6 × 105 to 2 × 107 CFU/g). Additionally, P. multocida was present in prescapular and/or retropharyngeal lymph nodes in both control and two i.n. vaccinated calves (range, 1 × 103 to 1.4 × 105 CFU/g) that developed early signs of disease and were killed for humane reasons.
TABLE 3.
Occurrence and severity of gross and histopathological lesions at postmortema
| Lesionb | Lesionsc in treatment group:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| i.m. (calf no. 1-4)d
|
i.n. (calf no. 1-4)
|
Control (calf no. 1-3)
|
|||||||||
| 1d | 2d | 3d | 4d | 1 | 2 | 3 | 4d | 1 | 2 | 3d | |
| Serosanguinous peritoneal fluid | − | − | − | − | − | − | + | − | + | + | − |
| Serosanguinous pericardial fluid | − | − | − | − | + | − | − | − | + | +/− | − |
| Serosanguinous pleural fluid | − | − | − | − | + | − | − | − | − | − | − |
| Enlarged prescapular lymph nodes | +/− | +/− | +/− | +/− | +/− | ++ | ++ | + | − | + | ++ |
| Grossly edematous/hemorrhagic lymph nodes/tissues | − | − | − | − | − | ++ | + | − | + | ++ | − |
| Enlarged liver | − | + | − | − | +++ | − | − | − | + | ++ | − |
| CNS perivascular hemorrhages | + | − | +/− | +/− | + | + | +/− | + | ++ | + | + |
| CNS serum leakage | − | − | − | − | − | − | + | + | − | + | − |
| Hemorrhage, prescapular LN | − | − | − | − | − | +++ | +++ | − | − | +++ | − |
| PMN infiltration, prescapular LN | +/− | +/− | + | +/− | +++ | +++ | +++ | + | +++ | ++ | + |
| Cell depletion, paracortex of prescapular LN | ++ | +++ | +++ | ++ | +/− | + | + | − | ++ | + | +++ |
Two groups of four calves each were vaccinated twice, at a 4-week interval, either i.m. or i.n., with the aroA mutant strain, and a group of three calves were left unvaccinated as controls. Two weeks after the second vaccination, the animals were challenged with the wild-type strain. Postmortem examination was done 7 days after challenge or earlier as dictated by clinical assessment.
LN, lymph node; PMN, polymorphonuclear leukocyte; CNS, central nervous system.
−, not present; +/−, slight; +, mild; ++, moderate; +++, severe.
Survived 7 days postchallenge.
DISCUSSION
Although HS is ranked as the primary fatal disease in buffaloes and is a cause of major economic losses in cattle in Asian countries, the nature of the immune response to P. multocida is poorly understood. Current vaccines are administered parenterally, require repeated administration, and are not sufficiently efficacious. Vaccine development was highlighted as a major area for investigation at the last International Workshop on HS (Proceedings of the FAO/APHCA Workshop on Haemorrhagic Septicaemia, February 1991, Colombo, Sri Lanka). A live-attenuated vaccine, which would mimic the early stages of the natural infection, might be expected to confer more solid and long-term protective immunity.
Our results showed that i.m., but not i.n., vaccination of calves with two doses of ∼109 CFU of an aroA deletion derivative of P. multocida B:2 prevented clinical signs of infection after parenteral challenge with the virulent parent strain and conferred solid immunity against disease. There was a clear difference between the temperature responses of calves to the two routes of vaccination: i.m. vaccinated calves showed a transient febrile response after each dose, possibly caused by associated bacterial endotoxin, which was controlled by a single injection of NSAID, whereas those vaccinated i.n. showed little, if any, response. It will be important to assess whether lower doses given via the i.m. route will be less reactogenic while providing equivalent protection.
Endotoxic shock has been shown to play a major role in the pathology and death associated with HS (11, 12, 16). However, in the present work, serum endotoxin concentrations in samples taken postchallenge were low and showed no statistically significant differences among the three treatment groups. This is in line with previous observations that the onset of endotoxemia is from ∼20 to 24 h postchallenge (11), whereas in the present work, the disease was not allowed to progress beyond the initial clinical stage (up to 14 h postchallenge) for welfare and ethical considerations. Nevertheless, the similarity of the gross and histopathological lesions observed, although not as severe or extensive as in field cases, indicated that the present experimental approach may be used to induce disease consistent with HS.
One calf from each of the otherwise vulnerable i.n. vaccinated and control groups did not develop disease. The reasons why not all animals succumb to infection are not clear, although it has been suggested that they may be related to a reduced endotoxemia and a gradual but sustained rise in APP that may play a part in helping to control disease (11). Thus, the large increase in SAA concentrations after challenge observed in these particular calves may have contributed to their survival. On the other hand, changes in the concentrations of SAA may also be used to indicate the progress and severity of infection (10). In the present work, the large increase in SAA concentrations observed in i.n. vaccinated animals upon challenge, compared with the moderate response of the i.m. group, may well have reflected a less well-developed immune protection in the former group. However, there was no clear indication that a higher SAA level prechallenge might represent a marker for subsequent survival, as the i.n. vaccinated calf that subsequently survived challenge had a low SAA level of 4.3 μg/ml while the control calf that survived had a high level of 128 μg/ml at the point of challenge.
The APP response to the initial i.m. vaccination paralleled the clinical response (increased rectal temperature and demeanor score), indicating that the attenuated bacteria persisted long enough to promote an inflammatory response. However, there was no corresponding increase in either the serum IgG or IgM concentration during the days following primary vaccination, in agreement with the observations of others (2, 9, 17), who reported only a gradual increase in serum IgG titers over a 6- to 10-week period after vaccination with oil-adjuvant vaccine (which may allow a slow release of antigen). The reasons for this in the present work may have been that the aroA derivative was too attenuated and cleared too rapidly to have stimulated a clear humoral response to the primary vaccination, and it may be beneficial in future to test the efficacy of other attenuating mutations using the animal model described in this study. Alternatively, in the case of i.m. vaccinated calves in which initially high IgM concentrations declined following vaccination, maternally derived IgM may have cross-reacted with and neutralized the live vaccine, thereby minimizing any humoral response, a phenomenon reported previously (1). In this regard, it will be important to test the protective efficacy of a single i.m. vaccination of the aroA strain in older calves, as for field use, a single inoculation would be preferable to having to use a booster inoculation. The situation was markedly different following the second i.m. vaccination. Concentrations of serum IgG rose 50-fold within 7 days (day 37) and had risen further by the day of challenge (day 42). Similarly, concentrations of serum IgM rose markedly by 7 days following the second i.m. vaccination, and this concentration was maintained until termination of the experiment on day 49. Horadagoda et al. (9) also reported a more rapid and significant increase in IgG response by 14 days after a booster injection, which parallels the findings reported here. Also, the lack of an IgG response to i.n. vaccination does not preclude the induction of mucosal IgA antibody, which was not assessed.
The relative contributions of cellular and humoral immunity to long-term protection have not been established, but a strong correlation between the induction of humoral immunity and active protection in buffaloes vaccinated with killed whole-cell vaccines has been reported (2), and thus, the humoral response to vaccination was the focus of the present work. The principal antibody response in buffaloes vaccinated with oil-adjuvant vaccines or formalin-killed bacteria was the IgG class, with only mild IgM responses reported (2, 17). Our data were essentially in agreement with this, although a clear IgM response to the i.m. booster vaccination was noted. The strong correlation between ELISA IgG antibody titers to bacterial cells of an HS strain and active protection in buffaloes vaccinated with oil-adjuvant vaccines or formalin-killed bacteria (2) prompted these authors to suggest that there may be a minimum threshold of antibody necessary for protection. It was of interest that IgM titers were high in some prevaccinated animals but then declined, a feature not apparent with the IgG titers. This may indicate that these calves possessed some cross-reacting antibodies, perhaps maternally derived, from previous infection in the mother by United Kingdom serotypes of P. multocida. Infection of the upper respiratory tract of cattle with P. multocida serogroup A is common in the United Kingdom (5), and in keeping with this, cross-reaction of a P. multocida B:2 envelope antigen preparation with antiserum raised to a P. multocida A3 strain using anti-bovine IgM HRP conjugate (3,981 U/ml) was high, whereas cross-reaction when anti-bovine IgG HRP conjugate was used was minimal (87 U/ml). Horadagoda et al. (9) showed that, in newborn buffalo calves, HS-specific antibody increased markedly after suckling and then declined over a period of 28 days, indicating the transfer of colostrum-derived maternal antibodies. It seems likely, therefore, that the high IgM titers in some prevaccinated animals in this study were due to cross-reacting maternal antibodies.
Overall, the results provided a realistic bovine model of HS and showed that i.m. vaccination of calves twice, at an interval of 4 weeks, with a live-attenuated aroA derivative of P. multocida B:2 was 100% effective in preventing clinical disease following parenteral challenge with the virulent parent strain. In these experiments, supplementary treatment with an anti-inflammatory agent was necessary to offset the moderate febrile response that occurred after vaccination. The lack of protection observed in i.n. vaccinated calves may have been due to poor retention of the two 2.5-ml doses directed into both nares. Vaccination of pigs by exposure to an aerosol of a live temperature-sensitive mutant strain of P. multocida serotype A did not produce statistically significant changes in serum IgG levels compared to unvaccinated controls but produced a significant rise in IgA and IgG antibodies in lung lavage fluid (13). Work with goats (6, 23) demonstrated that intranasal exposure to formalin-killed Mannheimia haemolytica A:2 not only led to the local production of IgG and IgA but also stimulated a systemic antibody response. Future work will investigate the safety and efficacy of intramuscular vaccination using lower doses of vaccine and a one-dose, instead of two-dose, vaccination regime.
Acknowledgments
The expert assistance of L. Gibbard and staff, Clinical Division, MRI, with care and maintenance of animals, of J. Finlayson with histological preparation, of J. Sales, Biomathematics and Statistics Scotland, Edinburgh, Scotland, with statistical help, and of P. D. Eckersall, Glasgow University, with serum amyloid A assays is gratefully acknowledged.
The work was funded by the Biotechnology and Biological Sciences Research Council and the Scottish Executive Environment and Rural Affairs Department.
Editor: J. T. Barbieri
REFERENCES
- 1.Black, L., M. J. Francis, and M. J. Nicholls. 1985. Protecting young domesticated animals from infectious disease. Vet. Ann. 25:46-61. [Google Scholar]
- 2.Chandrasekaran, S., L. Kennett, P. C. Yeap, N. Muniandy, B. Rani, and T. K. S. Mukkur. 1994. Characterisation of immune response and duration of protection in buffaloes immunised with haemorrhagic septicaemia vaccines. Vet. Microbiol. 41:213-219. [DOI] [PubMed] [Google Scholar]
- 3.De Alwis, M. C. 1995. Haemorrhagic septicaemia in cattle and buffaloes, p. 9-24. In W. Donachie, F. A. Lainson and J. C. Hodgson (ed.), Haemophilus, Actinobacillus and Pasteurella. Plenum, New York, N.Y.
- 4.Dowling, A., J. C. Hodgson, A. Schock, W. Donachie, P. D. Eckersall, and I. J. Mckendrick. 2002. Experimental induction of pneumonic pasteurellosis in calves by intratracheal infection with Pasteurella multocida biotype A:3. Res. Vet. Sci. 73:37-44. [DOI] [PubMed] [Google Scholar]
- 5.Dowling, A., J. C. Hodgson, M. Dagleish, P. D. Eckersall, and J. Sales. 2004. Pathophysiological and immune cell responses in calves prior to and following lung challenge with formalin-killed Pasteurella multocida biotype A:3 and protection studies involving subsequent homologous live challenge. Vet. Immunol. Immunopathol. 100:197-207. [DOI] [PubMed] [Google Scholar]
- 6.Effendy, A. W. M., M. Zamri-Saad, R. Puspa, and S. Rosiah. 1998. Efficacy of intranasal administration of formalin-killed Pasteurella haemolytica A:2 against intratracheal challenge in goats. Vet. Rec. 142:428-431. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson, J. C., G. M. Moon, M. Quirie, and W. Donachie. 1993. Biochemical signs of endotoxaemia in lambs challenged with T10 strain of Pasteurella haemolytica and the effect of vaccination on the host response. Proc. Sheep Vet. Soc. 17:201-204. [Google Scholar]
- 8.Hodgson, J. C., G. R. Barclay, L. A. Hay, G. M. Moon, and I. R. Poxton. 1995. Prophylactic use of human endotoxin-core hyperimmune gammaglobulin to prevent endotoxaemia in colostrum-deprived, gnotobiotic lambs challenged orally with Escherichia coli. FEMS Immunol. Med. Microbiol. 11:171-180. [DOI] [PubMed] [Google Scholar]
- 9.Horadagoda, N. U., T. G. Wijewardana, I. S. Mulleriyawa, H. M. R. Ramya Kumari, and A. A. Vipulasiri. 1994. Development of an enzyme-linked immunosorbent assay for detection of serum antibodies to haemorrhagic septicaemia, p. 185-193. In Strengthening research on animal reproduction and disease diagnosis in Asia through the application of immunoassay techniques. IAEA publication no. IAEA-TECDOC-736. International Atomic Energy Agency, Vienna, Austria.
- 10.Horadagoda, N. U., K. M. Knox, H. A. Gibbs, S. W. Reid, A. Horadagoda, S. E. Edwards, and P. D. Eckersall. 1999. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet. Rec. 144:437-441. [DOI] [PubMed] [Google Scholar]
- 11.Horadagoda, N. U., J. C. Hodgson, G. M. Moon, T. G. Wijewardana, and P. D. Eckersall. 2001. Role of endotoxin in the pathogenesis of haemorrhagic septicaemia in the buffalo. Microb. Pathol. 30:171-178. [DOI] [PubMed] [Google Scholar]
- 12.Horadagoda, N. U., J. C. Hodgson, G. M. Moon, T. G. Wijewardana, and P. D. Eckersall. 2002. Development of a clinical syndrome resembling haemorrhagic septicaemia in the buffalo following intravenous inoculation of Pasteurella multocida serotype B:2 endotoxin and the role of tumour necrosis factor-alpha. Res. Vet. Sci. 72:194-200. [DOI] [PubMed] [Google Scholar]
- 13.Muller, G., H. Kohler, R. Diller, and W. Erler. 2001. Antibody reactions after aerogenous or subcutaneous immunization of pigs with Pasteurella multocida antigens. Vaccine 19:751-757. [DOI] [PubMed] [Google Scholar]
- 14.Myint, A., and G. R. Carter. 1989. Prevention of haemorrhagic septicaemia in buffaloes and cattle with a live vaccine. Vet. Rec. 124:508-509. [DOI] [PubMed] [Google Scholar]
- 15.Pati, U. S., S. K. Srivastava, S. C. Roy, and T. More. 1996. Immunogenicity of outer membrane protein of Pasteurella multocida in buffalo calves. Vet. Microbiol. 52:301-311. [DOI] [PubMed] [Google Scholar]
- 16.Rhoades, K. R., K. L. Heddleston, and P. A. Rebers. 1967. Experimental hemorrhagic septicemia: gross and microscopic lesions resulting from acute infections and from endotoxin administration. Can. J. Comp. Med. Vet. Sci. 31:226-233. [PMC free article] [PubMed] [Google Scholar]
- 17.Shah, N. H., N. H. Shah, and F. K. De Graaf. 1997. Protection against haemorrhagic septicaemia induced by vaccination of buffalo calves with an improved oil adjuvant vaccine. FEMS Microbiol. Lett. 155:203-207. [DOI] [PubMed] [Google Scholar]
- 18.Stanley, A. C. 2003. Mucosal immunisation using the ovine nasopharyngeal route. Ph.D. thesis. University of Edinburgh, Edinburgh, Scotland.
- 19.Tabatabaei, M., Z. Liu, A. Finucane, R. Parton, and J. G. Coote. 2002. Protective immunity conferred by attenuated aroA derivatives of Pasteurella multocida B:2 strains in a mouse model of hemorrhagic septicemia. Infect. Immun. 70:3355-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend, K. M., A. J. Frost, C. W. Lee, J. M. Papadimitriou, and H. J. Dawkins. 1998. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. J. Clin. Microbiol. 36:1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma, R., and T. N. Jaiswal. 1998. Haemorrhagic septicaemia vaccines. Vaccine 16:1184-1192. [DOI] [PubMed] [Google Scholar]
- 22.Wijewardana, T. G. 1992. Haemorrhagic septicaemia. Rev. Med. Microbiol. 3:59-63. [Google Scholar]
- 23.Zamri-Saad, M., A. W. M. Effendy, D. A. Israf, and M. L. Azmi. 1999. Cellular and humoral responses in the respiratory tract of goats following intranasal stimulation using formalin-killed Pasteurella haemolytica A2. Vet. Microbiol. 65:233-240. [DOI] [PubMed] [Google Scholar]