Abstract
Helicobacter pylori is known to induce a local immune response, which is characterized by activation of lymphocytes and the production of IFN-γ in the stomach mucosa. Since not only T cells, but also natural killer (NK) cells, are potent producers of gamma interferon (IFN-γ), we investigated whether NK cells play a role in the immune response to H. pylori infection. Our results showed that NK cells were present in both the gastric and duodenal mucosae but that H. pylori infection did not affect the infiltration of NK cells into the gastrointestinal area. Furthermore, we could show that NK cells could be activated directly by H. pylori antigens, as H. pylori bacteria, as well as lysate from H. pylori, induced the secretion of IFN-γ by NK cells. NK cells were also activated without direct contact when separated from the bacteria by an epithelial cell layer, indicating that the activation of NK cells by H. pylori can also occur in vivo, in the infected stomach mucosa. Moreover, the production of IFN-γ by NK cells was greatly enhanced when a small amount of interleukin-12 (IL-12) was added, and this synergistic effect was associated with increased expression of the IL-12 receptor β2. It was further evident that bacterial lysate alone was sufficient to induce the activation of cytotoxicity-related molecules. In conclusion, we demonstrated that NK cells are present in the gastroduodenal mucosa of humans and that NK cells produce high levels of IFN-γ when stimulated with a combination of H. pylori antigen and IL-12. We propose that NK cells play an active role in the local immune response to H. pylori infection.
Natural killer (NK) cells are important components of the innate immune system and are phenotypically identified by the expression of an adhesion molecule, CD56. It has been shown in peripheral blood that an abundant NK cell subset expresses low levels of CD56 (and is therefore referred to as CD56dim NK cells), while ∼6% of the NK cells express CD56 at high density (CD56bright) (8, 11). The main function of NK cells has been described as acting as effective killers of virus-infected cells early in the course of infection, before the specific CD8+ T cells have emerged (34, 45). NK cells can also kill certain tumor cells and thereby suppress cancer development (16, 24). Apart from their cytotoxic capacity, NK cells are important producers of gamma interferon (IFN-γ), a cytokine that can activate many parts of the immune system, including phagocytosis and antigen presentation (6, 55). Classically, NK cells have been regarded as activated either by virus-infected or cancer-transformed cells that lack major histocompatibility complex class I expression or by activating cytokines, like interleukin-12 (IL-12) or type I interferons produced during acute infections (9, 34, 44). Recently, however, evidence has accumulated showing that NK cells also can be activated in bacterial infections, preferentially by phagocyte-derived IL-12 (29) but possibly also by direct action of bacterial products (30).
Helicobacter pylori is a gram-negative bacterium that colonizes the human gastric and duodenal mucosa and causes life-long infection. H. pylori infects the stomachs of >50% of the human population worldwide. The infection invariably induces a chronic and active inflammation of the antral mucosa, with influx of B cells, T cells, and neutrophils. By as yet unknown mechanisms, the infection is also a risk factor for the development of peptic ulcer disease and gastric adenocarcinoma (5, 15, 37). It is known that the local immune response to H. pylori in both humans and experimental animals is characterized by increased production of Th1-type cytokines, such as IFN-γ and IL-12 (36, 46). In a mouse model, it was recently demonstrated that IL-12 and IFN-γ are crucial for H. pylori-specific protective immunity (1, 25). Furthermore, it appears that IL-12 responses with IFN-γ predominate and play a role in the pathogenesis of H. pylori infection and the development of ulceration (25). Since not only T cells but also NK cells are potent producers of IFN-γ, we have investigated whether NK cells may play a role in the immune response to H. pylori infection.
MATERIALS AND METHODS
Bacterial preparation.
H. pylori (strain Hel305, isolated from a duodenal ulcer patient; cagA+ vacA+) from −70°C stock cultures was grown on Columbia isoagar plates, followed by brucella broth liquid culture. The bacteria were diluted in appropriate cell culture medium to an optical density at 600 nm of 1 (equal to 5 × 109 bacteria/ml) and used for further experiments. Lysates of H. pylori strain Hel305 and Escherichia coli (E11881/9; ST+ LT+) were prepared as previously described (40), and lipopolysaccharides (LPS) were purified using phenol-water extraction (52). The protein contents were determined by spectrometry. Each lysate was snap frozen in liquid nitrogen and stored in aliquots at −70°C until it was used.
Purification of NK cells.
To purify peripheral blood NK cells, buffy coats enriched in leukocytes were obtained from healthy blood donors. Mononuclear cells were isolated from the buffy coats by using a Histopaque-1077 gradient, and NK cells (CD56+ CD3−) were isolated and purified by negative selection using a magnetic bead isolation kit (Human NK isolation kit; Miltenyi Biotech, Bergisch Gladbach, Germany) according to the recommendations of the manufacturer. These semipurified (>90% pure) NK cells were further purified, after being stained with anti-CD56 and anti-CD3 antibodies, using a flow cytometry cell sorter (FACSVantage SE; BD Biosciences, San Jose, Calif.). The sorted NK cells were routinely >99% pure as estimated by fluorescence-activated cell sorter analysis. The H. pylori infection status of each blood donor was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (24).
NK cell stimulation.
To stimulate NK cells, 50,000 viable NK cells were initially cultured in the absence or presence of antigenic stimulants (bacterial lysates in 0.2, 2, or 20 μg/ml concentrations or inactivated or live bacteria at 104, 106, or 108/well) with or without IL-12 (50, 500, or 1,000 pg/ml) in X-vivo 15 (BioWhittaker, Verviers, Belgium) in round-bottom 96-well plates. In experiments in which the synergistic effects of lysate and IL-12 were studied, the NK cells were stimulated with bacterial lysate (2 μg/ml) and IL-12 (50 pg/ml). Antibiotic-free X-vivo 15 was used when NK cells were stimulated with live bacteria. Supernatants were taken at designated time points and were kept at −80°C until they were analyzed for cytokine content.
AGS cells (a human gastric carcinoma cell line) were obtained from the American Type Culture Collection and used in transwell coculture experiments with NK cells. AGS cells were placed in filter inserts (pore size, 3 μm) of six-well plates and cultured to confluence in X-vivo 15. Then, the cells were given fresh medium and stimulated with 107 live H. pylori bacteria. At the same time, NK cells (106/well) were added to the bottom wells. In control wells, either AGS cells, H. pylori, or NK cells were omitted. All of the cells were cultured for 48 additional hours, supernatants were taken from the top and bottom wells, and the concentration of IFN-γ was measured using ELISA.
Human volunteers and collection of samples.
For analysis of the presence of NK cells in the gastroduodenal mucosa, six adult Swedish volunteers infected with H. pylori but without any subjective symptoms (Hp+; 23 to 58 years old) and six healthy, uninfected volunteers (Hp−; 24 to 40 years old) were recruited for the study. The study was approved by the Ethical Committee for Human Research, Göteborg University, and informed consent was obtained from each volunteer before participation.
The asymptomatic carriers were identified by screening of healthy blood donors for elevated immunoglobulin G (IgG) antibody titers against H. pylori by using an in-house ELISA (22), and their H. pylori infection status was confirmed either by culturing of H. pylori bacteria from antral biopsy specimens or by a urea breath test. None of the volunteers had a previous history of gastrointestinal symptoms or illnesses or was under medication during the 3 weeks before recruitment for the study. Gastroduodenal endoscopies were performed, and biopsy specimens were obtained from the antrum and duodenum. The epithelium and the intraepithelial lymphocytes were removed by stirring the biopsy specimens four (duodenal specimens) or six (antral specimens) times for 15 min each time in Hank's balanced salt solution without calcium or magnesium and containing 1 mM EDTA and 1 mM dithiothreitol, followed by two incubations in Hank's balanced salt solution without EDTA at room temperature. Lamina propria lymphocytes were isolated from the remaining tissue by stirring the biopsy specimens in 5 ml of collagenase-DNase solution (100 U of collagenase/ml [Sigma C-0255] and 0.1 mg of DNase/ml [Sigma D-5025]) for 2.5 h at 37°C. The resulting suspension was filtered through a nylon mesh, and the cells were counted under the microscope. Preliminary experiments showed that this cell isolation regimen gave the maximal yield of cells with very little of the epithelium remaining in the lamina propria fraction.
Cytokine assays.
Supernatants from duplicate or triplicate samples were frozen at −80°C until the cytokine content was determined using ELISA for IFN-γ, as previously described (31), and for IL-12p40 (R&D Systems) according to the instructions of the manufacturer. Samples were thawed only once for the analysis. The minimum detectable cytokine concentrations were 3 pg/ml for IFN-γ and 25 pg/ml for IL-12.
Flow cytometry analysis.
Fluorescein isothiocyanate-conjugated anti-CD3 and phycoerythrin (PE)-conjugated anti-CD56 antibodies were used for the detection of NK cells in biopsy samples and for the purification and confirmation of the purity of NK cells. PE-conjugated anti-CD25 and PE-conjugated anti-CD69 were used to study the changes of cell surface expression in the NK cells with or without stimulation with bacterial antigens and/or IL-12. The flow cytometry analysis was performed with a FACSCalibur (BD Biosciences), using the analysis software FlowJo (Treestar Inc., San Carlos, Calif.). All antibodies were obtained from BD Biosciences.
Quantification of mRNA levels using reverse transcription (RT)-PCR analysis.
Total RNA was isolated from NK cells using a Total RNA Extraction kit (Sigma Aldrich, St. Louis, Mo.) and was then subjected to DNase treatment with the DNase Amp grade kit (Invitrogen, Carlsbad, Calif.). The concentration and integrity of the RNA were measured spectrophotometrically at 260 and 280 nm and by gel electrophoresis in 1% agarose gels. Thereafter, cDNA was synthesized by using an oligo(dT) primer and the Omniscript RT-PCR kit (QIAGEN, Hilden, Germany), as described by the manufacturer, and adjusted to a concentration of 0.2 or 1 μg/μl. PCR was performed in 25-μl reaction volumes containing 200 nM (each) primer sets for IFN-γ, granzyme B, perforin, or granulysin coamplified with 200 nM (each) primer for the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene, 1 U of Taq polymerase, 1× PCR buffer and 2 mM MgCl2 (Sigma Aldrich), 200 nM deoxynucleoside triphosphate (Roche, Mannheim, Germany), and 1 μl of cDNA. All PCR products were initially denatured at 94°C for 5 min before amplification. The PCRs of granzyme B, perforin, and granulysin were amplified at 94 (30 s), 55 (30 s), and 72°C (30 s) for 22 or 25 cycles, followed by final elongation of the products at 72°C for 5 min. IFN-γ was amplified as described for the other genes but with 22 or 27 cycles and an elongation time of 90 s. The primer set used for PCR is shown in Table 1. The PCR products were run on 3% agarose gels, stained with ethidium bromide, and visualized under UV light. Quantification of the PCR products was performed with ScionImage software (Scion Corp., Frederick, Md.). Similar results were obtained when the PCR was performed with 22 and 25 or 27 cycles, and also using different concentrations of input cDNA (0.2 and 1.0 μg/μl).
TABLE 1.
Primer pairs used in RT-PCR
| Gene | Accession no. | Forward primer | Reverse primer | Size (bp) |
|---|---|---|---|---|
| GAPDH | M17851 | TCACCATCTTCCAGGAGCGA | AGTGATGGCATGGACTGTGG | 325 |
| IFN-γ | X01992 | GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC | CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG | 427 |
| Granzyme B | BC030195 | AGGAAGATCGAAAGTGCGAA | AGGTGTTTCATTACAGCGGG | 282 |
| Granulysin | NT015805 | GAAGATGGTGGATAAGCCCA | ACAAGGTGAGAGGGCTCAGA | 223 |
| Perforin | X13224 | ACTCACAGGCAGCCAACTTT | GGGTGCCGTAGTTGGAGATA | 213 |
Statistical analysis.
Comparative data were analyzed using Student's t test, with a P value of <0.05 considered statistically significant.
RESULTS
NK cells are present in the gastrointestinal mucosa.
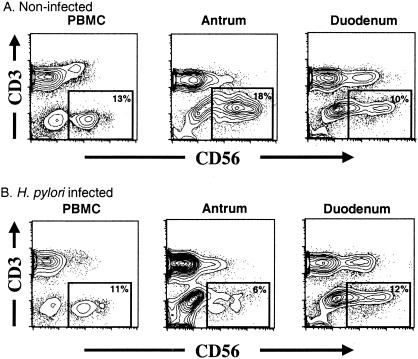
In order to analyze whether NK cells are present in the gastrointestinal mucosa, biopsy specimens were collected by gastroduodenal endoscopy from H. pylori-infected and uninfected volunteers, and the presence of CD3− CD56+ NK cells was analyzed by flow cytometry. In uninfected individuals, there was a substantial percentage of NK cells in the gastric mucosal area, with ∼15% of the lymphocytes being NK cells (Table 2 and Fig. 1), whereas in H. pylori-infected individuals, ∼6% of the lymphocytes were NK cells.
TABLE 2.
Percentages of NK cells and CD4+ and CD8+ T cells in gastric biopsy specimens from H. pylori-positive or -negative subjects
| Subject | % T Cellsa
|
||
|---|---|---|---|
| NKb | CD4+c | CD8+d | |
| H. pylori+ (n = 6) | 5.9 ± 2.0 | 59.7 ± 8.7 | 36.4 ± 9.1 |
| H. pylori− (n = 6) | 14.6 ± 9.3 | 40.3 ± 17.0 | 51.2 ± 18.7 |
Data are expressed as mean percentage of lymphocytes ± standard error.
P = 0.04.
P = 0.03.
P = 0.11.
FIG. 1.
NK cells in peripheral blood mononuclear cells (PBMC) and in the gastrointestinal mucosa. Cells were obtained from biopsies of the antrum and duodenum of noninfected healthy (A) or H. pylori-infected (B) volunteers. Lamina propria cells were obtained by treatment with EDTA and collagenase, and the cells were stained with anti-CD56 and anti-CD3 antibodies and analyzed for the presence of NK cells. Each plot shows at least 5,000 lymphocytes gated based on forward and side scatter characteristics.
NK cells produce IFN-γ after stimulation with H. pylori.
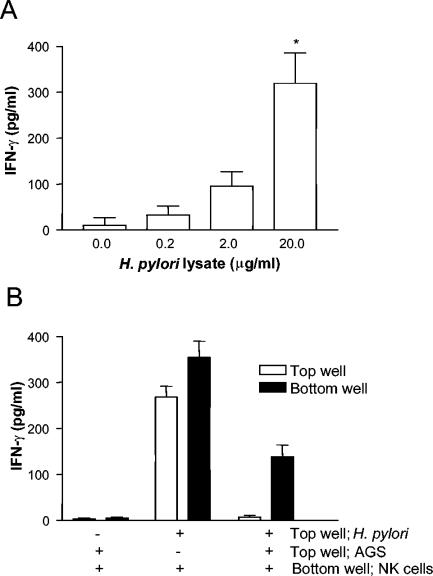
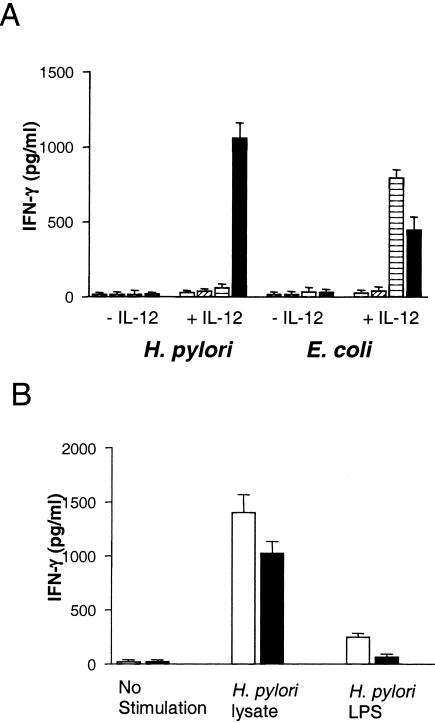
In order to further investigate whether NK cells could be activated by H. pylori, NK cells were purified from peripheral blood mononuclear cells and stimulated with different doses of H. pylori lysate. The results showed that NK cells produced IFN-γ after stimulation with H. pylori, and the secretion of IFN-γ increased in a dose-dependent manner with increasing concentrations of H. pylori lysate (Fig. 2A). The levels of IFN-γ produced in NK cells obtained from H. pylori-infected and uninfected individuals were similar (data not shown). In an attempt to investigate whether NK cells can be activated by H. pylori in a more in vivo-like situation, NK cells and the gastric epithelial cell line AGS were cocultured in a transwell system, with AGS cells with or without live H. pylori in the insert filter (top well) and NK cells in the bottom well. Using this system, it could be shown that although the H. pylori bacteria and the NK cells were separated by an epithelial cell layer, as in the infected gastric mucosa in vivo, the NK cells could respond with IFN-γ production to H. pylori lysate (Fig. 2B).
FIG. 2.
IFN-γ secretion by NK cells stimulated with H. pylori antigens. (A) NK cells were stimulated with different concentrations of H. pylori lysate (0, 0.2, 2, or 20 μg/ml) for 48 h, the supernatants were removed, and the concentration of IFN-γ was measured using ELISA. The results are expressed as the mean plus standard error of the mean of six independent experiments. *, P < 0.05 compared to unstimulated cells. (B) NK cells were stimulated in an epithelial cell transwell system. AGS cells were placed in the top wells of six-well plates and cultured to confluence. Then, the cells were fed with new medium and stimulated with 107 live H. pylori bacteria. At the same time, NK cells (106/well) were added to the bottom wells. The cells were cultured for 48 additional hours, the supernatants were taken from the top and bottom wells, and the concentrations of IFN-γ were measured using ELISA. The results are expressed as means of quadruplicates plus the standard error of the mean. The experiment was performed four times with similar results. +, present; −, absent.
Synergistic activation of NK cells by H. pylori antigens and IL-12.
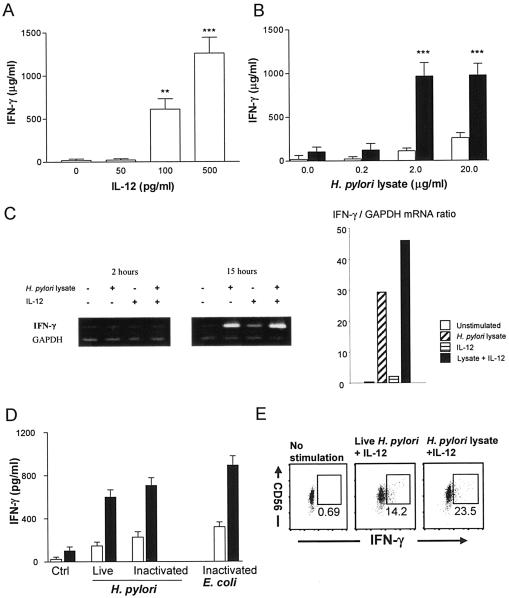
In the H. pylori-infected mucosa, there is chronic inflammation and production of different cytokines that activate cellular immunity, such as IL-12 (1). Since IL-12 is known to induce IFN-γ production by NK cells, we wanted to investigate whether IL-12 could influence the H. pylori-induced activation of NK cells. Therefore, NK cells were stimulated with a combination of low levels of H. pylori lysate (2 μg/ml) and IL-12 (50 pg/ml)—neither of these concentrations induced any significant production of IFN-γ when used separately (Fig. 2A and 3A). Under these conditions, there was a strong synergistic effect of IL-12 on the H. pylori-induced IFN-γ production by the cells. Approximately ninefold-higher IFN-γ production by NK cells stimulated with H. pylori lysate and IL-12 than by cells stimulated with lysate alone was observed (Fig. 3B). The synergistic effect was also confirmed by mRNA analysis (Fig. 3C). Similar synergistic effects were also seen in NK cells stimulated with live or inactivated H. pylori bacteria, together with IL-12 (Fig. 3D).
FIG. 3.
IFN-γ secretion from NK cells stimulated with H. pylori antigens. NK cells were stimulated with different concentrations of IL-12 (0, 50, 100, or 500 pg/ml) (A), with H. pylori lysate alone (open bar) or together with 50 pg of IL-12/ml (closed bar) (B), or with live or formalin-inactivated H. pylori or E. coli (106/well) in the absence (open bars) or presence (closed bars) of IL-12 (50 pg/ml) (D) for 48 h, and the supernatants were removed and assayed for IFN-γ concentration using ELISA. The results are expressed as the mean plus standard error of the mean of six independent experiments (A and B) or shown as representative of four experiments (D). **, P < 0.01; ***, P < 0.001 compared to relevant control wells. (C) NK cells were stimulated (+) for 2 or 15 h with H. pylori lysate and/or IL-12. No stimuli were added in the control. Total RNA was extracted from NK cells, reverse transcribed, and amplified using an IFN-γ-specific primer set. Amplified products were separated by electrophoresis and stained with ethidium bromide. On the left panel are the gel images; on the right, the ratio of staining intensity between IFN-γ and GAPDH is shown. The results shown are representative of three independent experiments. (E) NK cells were stimulated with H. pylori antigens and 50 pg of IL-12/ml for 24 h. The cells were stained with anti-CD56 antibody, followed by intracellular anti-IFN-γ antibody staining. The numbers show the percentages of CD56+ IFN-γ+ double-positive cells. The experiment was performed four times with similar results.
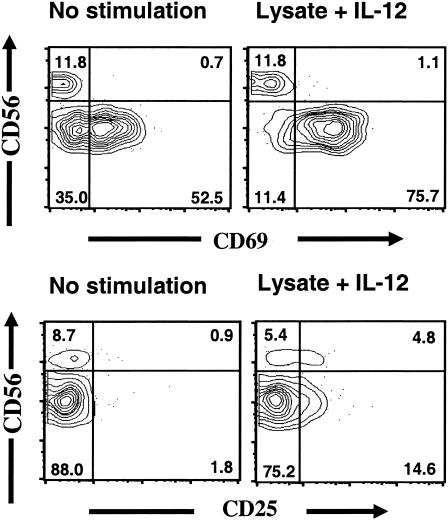
There was no detectable level of IL-12 in wells containing NK cells stimulated with H. pylori lysate only (data not shown), showing that the synergistic effect was not due to lysate-induced IL-12 production by NK cells or contaminating cells. Analysis using intracellular flow cytometry showed that a large fraction of the NK cells (ranging from 12 to 25%) were producing IFN-γ after H. pylori stimulation and that the cells producing the highest levels of IFN-γ were NK cells expressing high levels of CD56 (CD56bright NK cells) (Fig. 3E). In addition to IFN-γ secretion, there was a marked increase in the expression of the activation markers CD25 and CD69 after stimulation with H. pylori lysate and IL-12 (Fig. 4). CD69 was predominantly expressed by CD56dim NK cells (∼85%), and CD25 was preferentially expressed by CD56bright NK cells.
FIG. 4.
Flow cytometry analysis of activation markers on NK cells stimulated with H. pylori lysate and IL-12. NK cells were purified from buffy coats and stimulated with H. pylori lysate and IL-12 for 15 h. Expression of surface molecules (CD25 and CD69) was analyzed using flow cytometry. The figure shows composite contour-dot plots, in which areas of infrequent events are shown as individual dots and higher-density areas are shown as concentric probability contours with each successive layer depicting an increased frequency of events. The numbers in the boxed areas show the percentages of events contained within the boxes.
Increase in cytotoxicity-related molecules after stimulation with H. pylori lysate.
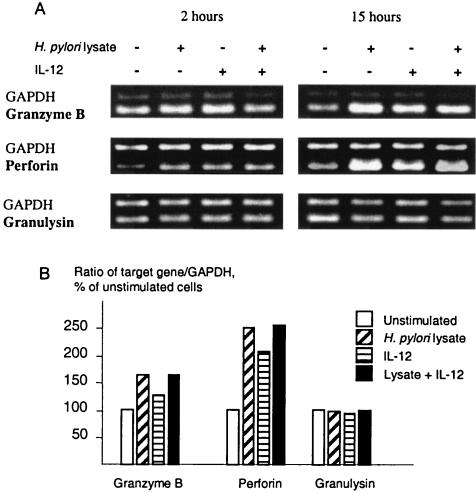
Since there was an increase in cellular activation markers and in production of IFN-γ following stimulation of NK cells with bacterial antigens and IL-12, the expression of cytotoxicity-related molecules after stimulation was also studied. Flow cytometry analysis of the IgG receptor CD16, which is involved in antibody-dependent cellular cytotoxicity, did not reveal any increase after stimulation of the NK cells (data not shown). On the other hand, there were increased levels of mRNA for granzyme B and perforin, but not granulysin, in NK cells stimulated with bacterial lysate, IL-12, and lysate plus IL-12 (Fig. 5). However, no synergistic effect of IL-12 on granzyme B or perforin expression could be seen.
FIG. 5.
RT-PCR analysis of granzyme B, perforin, and granulysin mRNA expression in NK cells stimulated with H. pylori lysate and/or IL-12. (A) NK cells were stimulated (+) for 2 or 15 h with H. pylori lysate, IL-12, or H. pylori lysate and IL-12. No stimuli were added in the control. Total RNA was isolated from NK cells, reverse transcribed, and amplified using specific primer sets. Amplified products were separated by electrophoresis and stained with ethidium bromide. (B) The intensity of staining was analyzed using computer software, and the ratio between the target genes and GAPDH was calculated. The ratios of the different stimulations were then divided by the ratio of unstimulated cells. The results shown are representative of three independent experiments.
The activation of NK cells by H. pylori is independent of LPS.
One possible mechanism by which NK cells recognize H. pylori lysate is by binding of LPS to toll-like receptors on the surfaces of target cells. In order to investigate whether such recognition of H. pylori-related molecules takes place, we stimulated NK cells with different concentrations (10 pg, 10 ng, or 10 μg/ml) of purified LPS from both H. pylori and E. coli. The results showed that in contrast to lysate, neither H. pylori LPS nor E. coli LPS could activate NK cells in the absence of IL-12 (Fig. 6A). When IL-12 was added to the cultures, H. pylori LPS was ∼1,000-fold less effective than E. coli LPS in inducing IFN-γ. In order to get IFN-γ production in the presence of IL-12 similar to 2 μg of protein/ml of H. pylori lysate, LPS from H. pylori had to be added in a concentration of 10 μg/ml. Furthermore, when high concentrations (2 μg/ml) of polymyxin B, an inhibitor of LPS, were used to block the effects of LPS in the different antigen preparations, only partial (30%) inhibition of lysate-induced IFN-γ could be seen, while the inhibition of pure LPS-induced IFN-γ was more profound (80%) (Fig. 6B). Taken together, these results indicate that the activation of NK cells by H. pylori is largely independent of LPS.
FIG. 6.
Induction of IFN-γ in NK cells by LPS from H. pylori or E. coli. (A) NK cells were stimulated with different concentrations (0, open bars; 10 pg/ml, hatched bars; 10 ng/ml, horizontally lined bars; 10 μg/ml, closed bars) of pure LPS with (+) or without (−) IL-12 (50 pg/ml) for 48 h. The supernatants were taken, and the amount of IFN-γ was measured by ELISA. (B) Ten micrograms of H. pylori lysate or H. pylori LPS per milliliter was pretreated with 2 μg of polymyxin B/ml for 2 h. Then, NK cells were stimulated with bacterial antigen (untreated [open bars] or pretreated [closed bars] with polymyxin B) with IL-12 for 48 h. The supernatants were taken, and the amount of IFN-γ was measured by ELISA. The data are expressed as the mean plus standard error of the mean of four wells. The experiment was repeated six times with similar results.
Activation of NK cells by H. pylori lysate precedes the synergistic effect of IL-12.
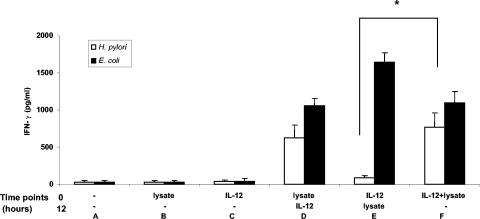
To investigate the mechanism behind the synergistic effects of H. pylori antigens and IL-12 in further detail, time delay experiments were performed in which IL-12 or lysate was first added to the cultures, followed by the reciprocal stimulus 15 h later. The results showed that in order to get the synergistic effect, H. pylori lysate had to be added before IL-12 (Fig. 7). Thus, while the protein level of IFN-γ was high in cultures where lysate was added before IL-12, it was very low in cultures where IL-12 had been added first, followed by lysate stimulation. However, this was not the case for E. coli lysate, since there was high IFN-γ production regardless of whether IL-12 or E. coli lysate was added first (Fig. 7).
FIG. 7.
IFN-γ secretion in NK cells stimulated with bacterial lysate and/or IL-12 at different time points. NK cells were stimulated with medium (A), 2 μg of bacterial lysates/ml (B and D), or 50 pg of IL-12 (C and E) or IL-12 plus bacterial lysates (F)/ml for 15 h, and then the same amounts of IL-12 or bacterial lysates were added (D) and (E) and all the cells were incubated for another 58 h. The supernatants were removed and assayed for IFN-γ concentration using ELISA. The data are expressed as the mean plus standard error of the mean of four experiments. *, P < 0.05.
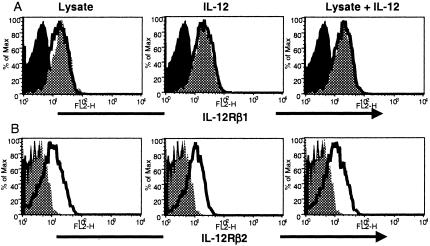
H. pylori lysate up-regulates IL-12Rβ2 expression on NK cells.
Since we observed that activation of NK cells by H. pylori lysate precedes the synergistic effect of IL-12, we analyzed whether H. pylori lysate induced any changes in IL-12 receptor expression on NK cells. IL-12Rβ1 was highly expressed in both resting CD56bright (Fig. 8A) and resting CD56dim (data not shown) NK cells, and the pattern did not change over time with H. pylori and/or IL-12 treatment. In contrast, there was no detectable expression of the IL-12Rβ2 chain in either resting CD56bright or CD56dim NK cells prior to stimulation (Fig. 8B). However, after stimulation with H. pylori lysate or IL-12, there was a marked increase in the expression of this receptor by CD56bright cells (Fig. 7). This IL-12Rβ2 expression in CD56bright NK cells could be detected ∼24 h after stimulation with H. pylori lysate alone, while it was detected in <12 h with IL-12 treatment.
FIG. 8.
Flow cytometry analysis of IL-12 receptor expression in NK cells stimulated with H. pylori lysate and/or IL-12. Purified NK cells were stimulated with H. pylori lysate and/or IL-12 for 15 h. Expression of IL-12 receptors was analyzed using flow cytometry. The figure depicts the histograms for the expression of IL-12 receptors (IL-12Rβ1 and IL-12Rβ2) in CD56bright NK cells. The solid black shaded area shows isotype control, the gray shaded area shows unstimulated control, and the bold line shows the indicated stimulation.
DISCUSSION
We demonstrated in the present study that NK cells are present in the gastroduodenal mucosa of humans, in both H. pylori-infected and uninfected individuals. The percentage of NK cells in the antrum in infected individuals was lower than in infected subjects. However, it is important to note that the absolute number of cells in the antra of infected individuals is higher than in the tissues of uninfected subjects (47). This was due to a significantly higher infiltration of B cells and CD4+ T cells in the antra of infected patients than in those of healthy individuals (data not shown). Therefore, the relative decrease in NK cell frequency was due to the influx of other cells and not to an actual decrease in absolute numbers of NK cells. The present study further shows that NK cells from peripheral blood produced high levels of IFN-γ when stimulated with a combination of H. pylori antigens and IL-12. Although there are two other reports of IFN-γ production in NK cells after stimulation with H. pylori antigens in lymphocyte cultures (21, 50), our study is the first to demonstrate that highly purified NK cells can be activated by H. pylori antigens to produce IFN-γ and that H. pylori and IL-12 can cooperate synergistically to increase the IFN-γ production. Although for practical reasons the present study was performed by stimulating peripheral blood NK cells with H. pylori antigens, we argue that the results also hold true for mucosal NK cells. As shown in Fig. 1, gastrointestinal NK cells are enriched in CD56bright cells, which are the primary source of IFN-γ (Fig. 3E) (2). Preliminary data from our laboratory also indicate that NK cells purified from gastric biopsy specimens indeed produce IFN-γ after stimulation with H. pylori lysate and IL-12 (data not shown). Furthermore, previous studies have shown that CD56+ NK cells purified from human intestinal mucosa exhibit functions similar to those of peripheral blood NK cells (51).
One could argue that the effect of H. pylori stimulation on the NK cells was caused by IL-12 production by contaminating myeloid cells in the cultures. However, we ruled out this possibility by using flow cytometry-sorted NK cells with very high purity (>99%). We also analyzed the supernatants from lysate-treated cells and were not able to detect any IL-12 production. Therefore, we are confident that H. pylori antigen and IL-12 act synergistically in stimulating NK cells to produce IFN-γ. Our results are well in line with the recent study by Hafsi et.al., which showed that IFN-γ production was induced in NK cells stimulated by H. pylori-pulsed dendritic cells; these pulsed dendritic cells were shown to produce large amounts of IL-12 (21).
A number of studies have shown synergistic effects of cytokines, such as IL-12 plus IL-2 (13), IL-12 plus IL-4 (7), IL-12 plus IL-18 (18, 38), or combinations of other cytokines (14, 17), on the activation of NK cells, mainly on the induction of IFN-γ and enhancement of cytotoxicity. All these studies, however, demonstrated synergistic effects by combinations of different cytokines and not by combinations of cytokines and microbial antigens. We showed that bacterial lysate from H. pylori alone induced moderate levels of IFN-γ from highly purified NK cells. Stimulation of NK cells with E. coli lysate showed a similar pattern, indicating that IFN-γ production by NK cells is not specific for stimulation with H. pylori but can be induced by other bacterial species. The ability of NK cells to be activated by a combination of different cytokines allows them to react early in the course of infection without the need for direct pattern recognition. However, since the present study shows that highly purified (>99%) NK cells do respond to bacterial lysate alone or in combination with IL-12, NK cells probably possess the ability to recognize the bacterial products directly as well.
Other studies (12, 39, 41) have shown that human NK cells express different densities of CD56, CD56bright, and CD56dim. These subpopulations possess distinct phenotypic properties, such as different CD16 (FCγ receptor III) and CD62L (l-selectin) expression levels. The CD56bright NK cells are the major IFN-γ-producing populations, while CD56dim NK cells have more cytotoxic activity (11, 28). Here, we studied the changes in the expression of surface molecules by these NK cell populations after stimulation with H. pylori lysate and IL-12. Our studies have shown that CD25 was up-regulated in both CD56dim and CD56bright NK cell populations, whereas CD69 was up-regulated only in CD56dim NK cells, with no change in CD69 expression by CD56bright cells. By intracellular IFN-γ staining of the activated NK cells, we could confirm the previous studies by showing that the cells that produce the highest IFN-γ levels were indeed CD56bright cells. Therefore, one should be cautious in using CD69 as an activation marker, since CD69 is not up-regulated in CD56bright NK cells, yet these cells actively produce IFN-γ both in response to the bacterial lysate, as shown in the present study, and in response to cytokines (11, 12).
Classically, NK cells have been known to be activated in two different ways apart from cytokine activation: via the IgG receptor CD16 (32), which can bind antibody-coated target cells, or via a combination of the lack of inhibitory signals detected by major histocompatibility complex class I-binding inhibitory receptors and of activating signals, such as NKG2D, which can bind ligands presented by stressed, virus-infected, or tumor-transformed cells (4, 19, 23, 48). However, it is unlikely that any of these interactions is involved in the recognition of H. pylori lysate and the further enhancement by IL-12, since our system was devoid of both antibodies and target cells. Instead, it is more plausible that the activation of NK cells by H. pylori is mediated by the engagement of pattern recognition receptors, such as toll-like receptors (TLRs). This is supported by a recent study, which demonstrated that resting NK cells express mRNA for several TLRs (26, 35). Since we showed that the activation was not mediated by H. pylori LPS, TLRs other than TLR4 are likely involved. This is in line with the results from another study, which showed that the response to H. pylori stimulation by epithelial cells is dependent on TLR2 and TLR5 but not TLR4 (43). However, studies of the activation of different cell types by H. pylori are contradictory (20), and so far the molecular mechanisms of NK cell activation have not been studied.
Although the details of how the recognition of H. pylori components takes place are still unknown, our results suggest that the mechanism behind the synergistic effect of H. pylori lysate and IL-12 involves IL-12 receptors. Since we demonstrated that the H. pylori lysate-NK cell interaction needs to precede the NK cell-IL-12 interaction in order to produce enhanced IFN-γ production, it could be hypothesized that bacterial lysate up-regulates the IL-12 receptors on NK cells. Flow cytometric analysis showed that IL-12Rβ1 is highly expressed in both CD56bright and CD56dim resting cells, and the pattern did not change after stimulation. However, the expression of IL-12Rβ2 was undetectable in resting cells but increased in the CD56bright cells after stimulation with H. pylori lysate and/or IL-12, whereas no changes were found in the CD56dim NK cells. This increase in receptor expression might allow CD56bright NK cells to respond more vigorously to a given IL-12 concentration, which would lead to a high level of production of IFN-γ. Since IL-12Rβ1 is shared with IL-23 (33) and both heterodimer chains (IL-12Rβ1 and IL-12Rβ2) are required to produce high affinity to IL-12, it is likely that up-regulation of IL-12Rβ2 will induce a specific increase in affinity to IL-12. This notion was supported by findings in a mouse model, where IL-12Rβ2-deficient mice were defective in IL-12-mediated signaling (10, 54). Our results, combined with those of others (10, 53), show that although IL-12Rβ1 is the subunit primarily responsible for the binding of IL-12, IL-12Rβ2 plays an essential role in mediating the biological functions of IL-12.
Our study further shows that IL-12-activated NK cells respond to E. coli lysate but not to H. pylori products. Thus, in contrast to stimulation with H. pylori lysate, E. coli lysate enhanced IFN-γ production by NK cells when IL-12 was added to the cells first. One possibility that could explain this intriguing finding is that IL-12 induces an up-regulation of some receptors that can interact with E. coli lysate but not with H. pylori lysate. Since E. coli LPS is 1,000-fold more potent than H. pylori LPS in inducing IFN-γ production by NK cells, we believe that the receptors that might be up-regulated by IL-12 are TLR4, nucleotide-binding oligomerization domain protein 1 (NOD1), or NOD2 (27), which are the receptors responsible for the recognition of bacterial LPS and other cell wall constituents. TLR4 has been shown to be expressed on resting NK cells, but at considerably lower levels than on monocytes (35), and IL-12 has been shown to be involved in the regulation of TLR4- and NOD1-dependent IFN-γ production (2). Therefore, it is possible that the expression of TLR4 and/or NOD1 is increased after IL-12-induced NK cell activation and consequently that NK cells respond more vigorously to LPS-induced activation. This will be investigated in future studies.
Based on the results presented here, we propose that NK cells may play an active and important role in the immune response to H. pylori infection. We showed that NK cells can be activated by H. pylori components across an epithelial layer. In the stomach mucosa of an infected individual, most likely H. pylori antigens are transported across the epithelium and released on the basolateral side or diffuse through the tight junctions that become loosened by the local inflammation. This is confirmed by previous studies in which H. pylori antigens have been visualized in the lamina propria by immunohistochemical staining of H. pylori-infected stomach mucosa (42, 49). Furthermore, upon encountering H. pylori, monocytes and dendritic cells in the stomach mucosa will release IL-12, which can activate other cells in the vicinity. This is supported by a study of cultured human gastric biopsy specimens that demonstrated a spontaneous release of IL-12 from biopsy specimens from H. pylori-infected subjects (3). The synergistic activation of NK cells by H. pylori components and physiological concentrations of IL-12 ensure an efficient activation and production of IFN-γ by the NK cells, which can potentiate antigen presentation to T cells and increase the activation of resident and recruited phagocytes. The importance of IL-12 and IFN-γ production in the stomach was recently shown in a mouse model of H. pylori infection in which mice that were either IL-12 or IFN-γ deficient failed to clear the infection (1).
In conclusion, we have shown that NK cells become activated by a combination of H. pylori lysate and IL-12 in vitro, and we believe that local activation of NK cells in the H. pylori-infected mucosa may have the ability to decrease the bacterial load and that it is therefore an important component of the defense against H. pylori infection.
Acknowledgments
Financial support for the present study was obtained from the Swedish Cancer Society and the Swedish Research Council. C.H.Y. was supported by the IVI fellowship program.
We thank the Blood Bank, Sahlgrenska University, Göteborg, Sweden, for providing buffy coats.
Editor: A. D. O'Brien
REFERENCES
- 1.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 2.Aseffa, A., A. Gumy, P. Launois, H. R. MacDonald, J. A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 169:3232-3241. [DOI] [PubMed] [Google Scholar]
- 3.Bauditz, J., M. Ortner, M. Bierbaum, G. Niedobitek, H. Lochs, and S. Schreiber. 1999. Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clin. Exp. Immunol. 117:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, S., V. Groh, J. Wu, A. Steinle, J. H. Phillips, L. L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727-729. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 1999. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J. Infect. Dis. 179:1523-1530. [DOI] [PubMed] [Google Scholar]
- 6.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 7.Bream, J. H., R. E. Curiel, C. R. Yu, C. E. Egwuagu, M. J. Grusby, T. M. Aune, and H. A. Young. 2003. IL-4 synergistically enhances both IL-2- and IL-12-induced IFN-gamma expression in murine NK cells. Blood 102:207-214. [DOI] [PubMed] [Google Scholar]
- 8.Carson, W. E., T. A. Fehniger, and M. A. Caligiuri. 1997. CD56bright natural killer cell subsets: characterization of distinct functional responses to interleukin-2 and the c-kit ligand. Eur. J. Immunol. 27:354-360. [DOI] [PubMed] [Google Scholar]
- 9.Cerwenka, A., and L. L. Lanier. 2001. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 1:41-49. [DOI] [PubMed] [Google Scholar]
- 10.Chakir, H., A. A. Camilucci, L. G. Filion, and J. R. Webb. 2000. Differentiation of murine NK cells into distinct subsets based on variable expression of the IL-12R beta 2 subunit. J. Immunol. 165:4985-4993. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, M. A., T. A. Fehniger, and M. A. Caligiuri. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633-640. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, M. A., T. A. Fehniger, S. C. Turner, K. S. Chen, B. A. Ghaheri, T. Ghayur, W. E. Carson, and M. A. Caligiuri. 2001. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97:3146-3151. [DOI] [PubMed] [Google Scholar]
- 13.DeBlaker-Hohe, D. F., A. Yamauchi, C. R. Yu, J. A. Horvath-Arcidiacono, and E. T. Bloom. 1995. IL-12 synergizes with IL-2 to induce lymphokine-activated cytotoxicity and perforin and granzyme gene expression in fresh human NK cells. Cell Immunol. 165:33-43. [DOI] [PubMed] [Google Scholar]
- 14.Eckenberg, R., J. L. Moreau, O. Melnyk, and J. Theze. 2000. IL-2R beta agonist P1-30 acts in synergy with IL-2, IL-4, IL-9, and IL-15: biological and molecular effects. J. Immunol. 165:4312-4318. [DOI] [PubMed] [Google Scholar]
- 15.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 16.Favre-Felix, N., M. Martin, E. Maraskovsky, A. Fromentin, M. Moutet, E. Solary, F. Martin, and B. Bonnotte. 2000. Flt3 ligand lessens the growth of tumors obtained after colon cancer cell injection in rats but does not restore tumor-suppressed dendritic cell function. Int. J. Cancer 86:827-834. [DOI] [PubMed] [Google Scholar]
- 17.Fukao, T., S. Matsuda, and S. Koyasu. 2000. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J. Immunol. 164:64-71. [DOI] [PubMed] [Google Scholar]
- 18.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2003. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J. Gen. Virol. 84:1961-1972. [DOI] [PubMed] [Google Scholar]
- 19.Gilfillan, S., E. L. Ho, M. Cella, W. M. Yokoyama, and M. Colonna. 2002. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat. Immunol. 3:1150-1155. [DOI] [PubMed] [Google Scholar]
- 20.Gobert, A. P., J. C. Bambou, C. Werts, V. Balloy, M. Chignard, A. P. Moran, and R. L. Ferrero. 2004. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J. Biol. Chem. 279:245-250. [DOI] [PubMed] [Google Scholar]
- 21.Hafsi, N., P. Voland, S. Schwendy, R. Rad, W. Reindl, M. Gerhard, and C. Prinz. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 173:1249-1257. [DOI] [PubMed] [Google Scholar]
- 22.Hamlet, A. K., K. I. Erlandsson, L. Olbe, A. M. Svennerholm, V. E. Backman, and A. B. Pettersson. 1995. A simple, rapid, and highly reliable capsule-based 14C urea breath test for diagnosis of Helicobacter pylori infection. Scand. J. Gastroenterol. 30:1058-1063. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa, Y., J. M. Kelly, J. A. Westwood, P. K. Darcy, A. Diefenbach, D. Raulet, and M. J. Smyth. 2002. Cutting edge: tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J. Immunol. 169:5377-5381. [DOI] [PubMed] [Google Scholar]
- 24.Herberman, R. B. 2002. Cancer immunotherapy with natural killer cells. Semin. Oncol. 29:27-30. [DOI] [PubMed] [Google Scholar]
- 25.Hida, N., T. Shimoyama, Jr., P. Neville, M. F. Dixon, A. T. Axon, T. Shimoyama, Sr., and J. E. Crabtree. 1999. Increased expression of IL-10 and IL-12 (p40) mRNA in Helicobacter pylori infected gastric mucosa: relation to bacterial cag status and peptic ulceration. J. Clin. Pathol. 52:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 27.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs, R., G. Hintzen, A. Kemper, K. Beul, S. Kempf, G. Behrens, K. W. Sykora, and R. E. Schmidt. 2001. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 31:3121-3127. [DOI] [PubMed] [Google Scholar]
- 29.Kelly, J. M., P. K. Darcy, J. L. Markby, D. I. Godfrey, K. Takeda, H. Yagita, and M. J. Smyth. 2002. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat. Immunol. 3:83-90. [DOI] [PubMed] [Google Scholar]
- 30.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 169:4450-4459. [DOI] [PubMed] [Google Scholar]
- 31.Lundin, B. S., C. Johansson, and A. M. Svennerholm. 2002. Oral immunization with a Salmonella enterica serovar Typhi vaccine induces specific circulating mucosa-homing CD4+ and CD8+ T cells in humans. Infect. Immun. 70:5622-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelboim, O., P. Malik, D. M. Davis, C. H. Jo, J. E. Boyson, and J. L. Strominger. 1999. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:5640-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQueen, K. L., and P. Parham. 2002. Variable receptors controlling activation and inhibition of NK cells. Curr. Opin. Immunol. 14:615-621. [DOI] [PubMed] [Google Scholar]
- 34.Moser, J. M., J. Gibbs, P. E. Jensen, and A. E. Lukacher. 2002. CD94-NKG2A receptors regulate antiviral CD8+ T cell responses. Nat. Immunol. 3:189-195. [DOI] [PubMed] [Google Scholar]
- 35.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 36.Obonyo, M., D. G. Guiney, J. Harwood, J. Fierer, and S. P. Cole. 2002. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect. Immun. 70:3295-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 192:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto, M., S. Kato, K. Oizumi, M. Kinoshita, Y. Inoue, K. Hoshino, S. Akira, A. N. McKenzie, H. A. Young, and T. Hoshino. 2002. Interleukin 18 (IL-18) in synergy with IL-2 induces lethal lung injury in mice: a potential role for cytokines, chemokines, and natural killer cells in the pathogenesis of interstitial pneumonia. Blood 99:1289-1298. [DOI] [PubMed] [Google Scholar]
- 39.Pierson, B. A., and J. S. Miller. 1996. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood 88:2279-2287. [PubMed] [Google Scholar]
- 40.Raghavan, S., M. Hjulstrom, J. Holmgren, and A. M. Svennerholm. 2002. Protection against experimental Helicobacter pylori infection after immunization with inactivated H. pylori whole-cell vaccines. Infect. Immun. 70:6383-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito, S., K. Nishikawa, T. Morii, M. Enomoto, N. Narita, K. Motoyoshi, and M. Ichijo. 1993. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int. Immunol. 5:559-563. [DOI] [PubMed] [Google Scholar]
- 42.Semino-Mora, C., S. Q. Doi, A. Marty, V. Simko, I. Carlstedt, and A. Dubois. 2003. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J. Infect. Dis. 187:1165-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 44.Smyth, M. J., D. I. Godfrey, and J. A. Trapani. 2001. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2:293-299. [DOI] [PubMed] [Google Scholar]
- 45.Soloski, M. J. 2001. Recognition of tumor cells by the innate immune system. Curr. Opin. Immunol. 13:154-162. [DOI] [PubMed] [Google Scholar]
- 46.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stromberg, E., A. Lundgren, A. Edebo, S. Lundin, A. M. Svennerholm, and C. Lindholm. 2003. Increased frequency of activated T-cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol. Med. Microbiol. 36:159-168. [DOI] [PubMed] [Google Scholar]
- 48.Su, R. C., S. K. Kung, J. Gariepy, B. H. Barber, and R. G. Miller. 1998. NK cells can recognize different forms of class I MHC. J. Immunol. 161:755-766. [PubMed] [Google Scholar]
- 49.Suzuki, T., M. Ito, N. Hayasaki, A. Ishihara, T. Ando, K. Ina, and K. Kusugami. 2003. Cytotoxic molecules expressed by intraepithelial lymphocytes may be involved in the pathogenesis of acute gastric mucosal lesions. J. Gastroenterol. 38:216-221. [DOI] [PubMed] [Google Scholar]
- 50.Tarkkanen, J., T. U. Kosunen, and E. Saksela. 1993. Contact of lymphocytes with Helicobacter pylori augments natural killer cell activity and induces production of gamma interferon. Infect. Immun. 61:3012-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Tol, E. A., H. W. Verspaget, A. S. Pena, C. V. Kraemer, and C. B. Lamers. 1992. The CD56 adhesion molecule is the major determinant for detecting non-major histocompatibility complex-restricted cytotoxic mononuclear cells from the intestinal lamina propria. Eur. J. Immunol. 22:23-29. [DOI] [PubMed] [Google Scholar]
- 52.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide: extraction with phenol-water and further applications of the procedure, p. 83-92. In R. Whitler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, N.Y.
- 53.Wu, C., J. Ferrante, M. K. Gately, and J. Magram. 1997. Characterization of IL-12 receptor β1 chain (IL-12Rβ1)-deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J. Immunol. 159:1658-1665. [PubMed] [Google Scholar]
- 54.Wu, C., X. Wang, M. Gadina, J. J. O'Shea, D. H. Presky, and J. Magram. 2000. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 165:6221-6228. [DOI] [PubMed] [Google Scholar]
- 55.Xing, Z., A. Zganiacz, J. Wang, and S. K. Sharma. 2001. Enhanced protection against fatal mycobacterial infection in SCID beige mice by reshaping innate immunity with IFN-gamma transgene. J. Immunol. 167:375-383. [DOI] [PubMed] [Google Scholar]